Abstract
Aims
Repeated treatment with morphine increases antinociceptive effects of delta opioid agonists in rodents by a mechanism that may involve increased cell-surface expression of delta receptors. The present study evaluated effects of repeated morphine treatment on behavioral effects of the delta agonist SNC80 and the mu agonist fentanyl in rhesus monkeys. Main
Methods
In an assay of schedule-controlled responding, three monkeys responded for food reinforcement under a fixed-ratio 30 schedule. In an assay of thermal nociception, tail-withdrawal latencies were evaluated in three monkeys using thermal stimulus intensities of 48 and 54°C. In both assays, the effects of SNC80 (0.032-3.2 mg/kg) and fentanyl (0.001-0.056 mg/kg) were evaluated after repeated treatment with saline or a regimen of morphine doses modeled on the regimen that enhanced delta agonist antinociception and apparent delta receptor availability in previous rodent studies.
Key Findings
Both SNC80 and fentanyl dose-dependently decreased rates of schedule-controlled responding, and repeated morphine treatment did not significantly alter these effects. In the assay of thermal nociception, SNC80 had little effect on tail-withdrawal latencies from water heated to 48 or 54°C, whereas fentanyl increased tail withdrawal latencies at both temperatures. Repeated morphine tended to increase the antinociceptive effects of SNC80 and to decrease the antinociceptive effects of fentanyl, but these effects of repeated morphine were small and were significant only at the higher stimulus intensity (54°C).
Significance
These results provide limited support for the proposition that prior stimulation of mu receptors selectively increases the antinociceptive effects of delta agonists in rhesus monkeys.
Keywords: delta opioid agonist, delta opioid receptor, antinociception, SNC80, morphine, monkey
INTRODUCTION
Delta opioid receptors constitute one of the three main families of opioid receptors, and drugs that target delta receptors are being evaluated for a range of applications including the treatment of pain, depression and drug abuse (Jutkiewicz and Woods 2004; Negus 2004; Ossipov et al. 2004). Under basal, homeostatic conditions, delta agonists produce relatively weak effects in assays of acute nociception despite the existence of delta receptors in primary afferent neurons and in spinal and supraspinal neural circuits thought to be important in nociception (Mansour et al. 1988; Mennicken et al. 2003; Ossipov et al. 2004). However, the antinociceptive effectiveness of delta agonists can be increased by inflammation and associated states of allodynia/hyperalgesia (Stewart and Hammond 1994; Fraser et al. 2000; Gallantine and Meert 2005; Rowan et al. 2009). These findings have been interpreted to suggest that many delta receptors are functionally inactive under basal conditions and require activation by challenges such as inflammation. For example, inflammation-related increases in delta agonist-induced antinociception in rodents were correlated with increased trafficking of delta opioid receptor-like immunoreactivity (DOR-IR) from cytoplasmic compartments to the cell surface, suggesting that increased delta agonist effects resulted from increased cell-surface availability of delta receptors (Cahill et al. 2003; Gendron et al. 2006; Cahill et al. 2007).
The effects of inflammation on delta agonist-induced antinociception and DOR-IR trafficking in rodents are reduced or eliminated in mu opioid receptor knockout mice, suggesting that mu receptors are necessary and that activation of delta receptors may require prior stimulation of mu receptors by endogenous opioids (Morinville et al. 2003; Gendron et al. 2007b). In support of this possibility, repeated pretreatment of rodents with exogenous mu agonists such as morphine produced both an inflammation-like trafficking of spinal DOR-IR to the cell surface and an enhancement of antinociceptive effects produced by spinal administration of the peptidic agonist [D-Ala2]-deltorphin-II (deltorphin II) (Cahill et al. 2001; Morinville et al. 2003; Gendron et al. 2007a). Moreover, these findings have stimulated other studies to examine the generality of mu agonist-induced modulation of delta receptor function (Hack et al. 2005; Morgan et al. 2009).
The purpose of the present study was to evaluate mu agonist-induced modulation of delta agonist-induced antinociception in nonhuman primates. As in rodents, the antinociceptive effects of delta agonists in rhesus monkeys are relatively weak under basal conditions, but are enhanced under conditions of inflammation-related thermal allodynia (Negus et al. 1994; Butelman et al. 1995; Negus et al. 1998; Brandt et al. 2001). However, the degree to which this effect might require mu opioid receptors has not been extensively examined. Notably, the antiallodynic effects of the nonpeptidic delta agonist SNC80 [((+)-4-[(aR)-a-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] in rhesus monkeys were blocked by the delta-selective antagonist naltrindole, but not by a mu-selective dose of the opioid antagonist quadazocine, suggesting that mu receptors were not necessary for delta agonist-induced antiallodynia in monkeys (Brandt et al. 2001). The present study was designed to test the hypothesis that prior stimulation of mu receptors would be sufficient to increase delta agonist-induced antinociception in non-human primates. Accordingly, the antinociceptive effects of SNC80 in rhesus monkeys were evaluated after repeated treatment with saline or a regimen of morphine modeled on that which increased delta agonist-induced antinociception in rodents (Cahill et al. 2001; Morinville et al. 2003). To assess pharmacologic and behavioral selectivity, the effects of repeated saline and morphine were also evaluated on antinociception produced by the mu agonist fentanyl, and on rate-decreasing effects of SNC80 and fentanyl in an assay of schedule-controlled responding maintained by food reinforcement. Our hypothesis predicted that repeated morphine would selectively enhance SNC80-induced antinociception without altering fentanyl-induced antinociception or rate-decreasing effects of either SNC80 or fentanyl.
MATERIALS AND METHODS
Subjects
Three adult male rhesus monkeys (Macaca mulatta) were used in studies of schedule-controlled responding, and one adult male and two adult female monkeys were used in studies of thermal nociception. Previous studies have found that groups of 3 subjects were sufficient to show enhancement of delta agonist-induced antinociception by inflammation (Brandt et al. 2001) or enhancement of mu agonist-induced antinociception by delta agonists (Negus et al. 2009). Subjects weighed 4.6-12.4 kg during the course of these studies. All monkeys had prior exposure to drugs (primarily dopaminergic and opioid compounds) and to the behavioral procedures in which they were tested. The subjects were individually housed, and water was freely available. Their diet consisted of Purina Lab Diet Fiber-Plus Monkey Biscuits #5049 (PMI Feeds, Inc., St. Louis, MO) supplemented with fresh fruit twice weekly. In addition, monkeys in the assay of schedule-controlled behavior could earn additional food pellets during experimental sessions. A 12 h light/12 h dark cycle was in effect (lights on from 7AM-7PM).
Animal maintenance and research were conducted in accordance with the guidelines provided by the NIH Committee on Laboratory Animal Resources. The facility was licensed by the United States Department of Agriculture and accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was monitored daily by technical and veterinary staff. Monkeys had visual, auditory and olfactory contact with other monkeys throughout the study. Monkeys also had access to puzzle feeders, mirrors and chew toys to provide environmental enrichment. Nature videotapes or music were played daily in all housing rooms.
Behavioral and Pharmacological Procedures
Behavioral studies were conducted using two procedures: (1) an assay of schedule-controlled responding for food presentation, and (2) an assay of thermal nociception using warm water as the noxious stimulus. These procedures have been used extensively by our laboratory to examine the effects of other opioids and of delta, mu and kappa opioid agonist interactions in rhesus monkeys (Stevenson et al. 2003; Stevenson et al. 2005; Negus et al. 2008; Pereira Do Carmo et al. 2008). In each assay, the potency and time course of morphine alone (0.32-5.6 mg/kg) was determined initially to provide an empirical basis for selection of doses used as repeated treatments. Subsequently, the effects of the selective delta opioid agonist SNC80 (0.032-3.2 mg/kg) were studied after a regimen of repeated treatment with either morphine or saline. For the repeated morphine treatment, morphine doses of 1.0, 1.6, 2.0 and 3.0 mg/kg were administered 48hr, 36hr, 24hr and 12hr, respectively, before determination of cumulative SNC80 dose-effect curves. This regimen of repeated treatments was modeled on previous studies conducted in rats and mice (Cahill et al. 2001; Morinville et al. 2003). Specifically, these studies reported increased expression of DOR-IR on neuronal membranes and enhanced delta agonist-mediated antinociception after repeated treatment at 12 hr intervals with four morphine doses equivalent to 1, 1.6, 2.0 and 3.0 times the morphine antinociceptive ED50 value. For repeated saline treatment, saline injections were administered at the same 12 hr intervals before testing with SNC80. The order of saline and morphine treatments was counterbalanced across monkeys, and tests were conducted at least two weeks apart. After testing with SNC80 was completed, the effects of the selective mu opioid receptor agonist fentanyl (0.001-0.056 mg/kg) were also evaluated after repeated saline and repeated morphine. Previous studies have shown that the effects of SNC80 and fentanyl are mediated by delta and mu opioid receptors, respectively, in these procedures in rhesus monkeys (Negus et al. 1993a; Negus et al. 1998; Brandt et al. 2001; Negus et al. 2002).
Assay of Schedule-Controlled Responding
Experiments were conducted in each monkey’s home cage (dimensions: 61 × 66 × 86 cm or 76 × 76 × 86 cm). The home cages of all monkeys were modified to include an operant response panel (28 × 28 cm) mounted on the front wall. Three square translucent response keys (6.4 × 6.4 cm) were arranged 2.54 cm apart in a horizontal row 3.2 cm from the top of the operant panel. Each key could be transilluminated by red, green or yellow stimulus lights. The operant panel also supported an externally-mounted pellet dispenser (Gerbrands, Model G5210; Arlington, MA) that delivered 1 gm banana-flavored food pellets (PMI Feeds, Inc., St. Louis, MO) to a food receptacle mounted on the cage beneath the operant response panel. The panel was controlled by a MED-PC interface and an IBM compatible computer programmed in MEDSTATE Notation (MED Associates, Inc., East Fairfield, VT).
Training sessions consisted of five consecutive, 15-min cycles, each of which consisted of two components: a 10-min timeout period followed by a 5-min response period. During the timeout period, no stimulus lights were illuminated and responding had no scheduled consequences. During the response period, the center key was transilluminated yellow, and the subjects could respond for up to 10 food pellets under a fixed-ratio 30 (FR30) schedule of reinforcement. If all 10 food pellets were delivered before 5 min elapsed, the lights were turned off, and responding had no scheduled consequences for the remainder of that response period. All monkeys were trained until they responded at rates greater than 1.0 responses per sec during all five cycles for 10 consecutive days, and we have shown previously that monkeys respond at relatively stable rates across successive response periods in this procedure (e.g. (Pereira Do Carmo et al. 2008). Training sessions were also conducted between test sessions, and a test session was conducted in a monkey only after a training session in which that monkey responded at rates greater than 1.0 responses per sec for all five cycles.
Test sessions were conducted using either a time course procedure or a cumulative dosing procedure. Each session began with a 15 min cycle during which no drugs were administered and baseline response rates were determined. Subsequently, in the time course procedure, a single dose of morphine (0.32-5.6 mg/kg) was administered at the start of the experiment, and 5-min response periods identical to those described above were initiated 10, 30, 100 and 300 min after the injection. After treatment with the highest morphine dose (5.6 mg/kg), an additional 5-min response period was initiated 24 hr after morphine. Morphine doses were tested in an irregular order that varied across monkeys, and tests were separated by at least one week. In the cumulative dosing procedure, the initial baseline cycle was followed by up to five additional 15 min cycles. Increasing doses of SNC80 (0.032-3.2 mg/kg) or fentanyl (0.001-0.032 mg/kg) were administered at the start of the time out period for each cycle. Each dose increased the total cumulative dose in increments of 0.25 or 0.5 log units. Cumulative dosing test sessions with SNC80 and fentanyl were preceded by a regimen of repeated saline or morphine treatment as described above. Each test of repeated saline or morphine followed by cumulative SNC80 or fentanyl was separated by at least two weeks.
Assay of Thermal Nociception
Monkeys were seated in acrylic restraint chairs so that their tails hung down freely. The bottom 10 cm of each monkey’s shaved tail was immersed in a thermal container of warm water. If the subject did not withdraw its tail within 20 s, the tail was removed from the water by the experimenter, and a latency of 20 s was assigned to that measurement. During each cycle of measurements, tail-withdrawal latencies were measured from water heated to 38, 48 and 54°C. The order in which the temperatures were presented varied from one set of measurements to the next. Experiments were conducted no more than twice a week. A stopwatch was used to measure and record time intervals.
Each test session consisted of multiple cycles. Before the first cycle, baseline latencies to tail-withdrawal from 38, 48 and 54°C water were determined. Testing continued only if tail withdrawal from 38°C water did not occur before the 20 s cutoff, and if tail withdrawal occurred in ≤3 s from 54°C water. This criterion was met during every session with every monkey in this study. As in the assay of schedule-controlled responding, test sessions were conducted using either a time course procedure or a cumulative dosing procedure. In the time course procedure, a single dose of morphine (0.32-5.6 mg/kg) was administered at the start of the session, and tail withdrawal latencies from 38, 48 and 54°C water were redetermined 10, 30 and 100 min after the injection. Later time points were not evaluated in this assay to limit the duration of restraint in the primate chair. Morphine doses were tested in an irregular order that varied across monkeys, and tests were separated by at least one week. In the cumulative dosing procedure, test sessions consisted of consecutive 15-min cycles. Increasing doses of SNC80 (0.032-3.2 mg/kg) or fentanyl (0.001-0.056 mg/kg) were administered at the start of each cycle, and tail-withdrawal latencies were redetermined during the last five minutes of each cycle. Each dose increased the total cumulative dose in increments of 0.25 or 0.5 log units. Cumulative dosing tests with SNC80 and fentanyl were preceded by a regimen of repeated saline or morphine treatment as described above. Each test of repeated saline or morphine followed by cumulative SNC80 or fentanyl was separated by at least two weeks.
Data Analysis
For the assay of schedule-controlled responding, response rates from each cycle were expressed as Percent Control Rate using the equation “% Control Rate = (Test Rate/Control Rate) * 100”, where the Test Rate was the absolute response rate in responses/sec during each cycle of a test session, and Control Rate was the response rate during the baseline cycle at the beginning of the test session. For the assay of thermal nociception, drug effects were expressed as Percent Maximum Possible Effect (%MPE) using the following equation “%MPE = (Test Latency-Control Latency)/(20-Control Latency)*100”, where Test Latency was the tail-withdrawal latency from 48 or 54°C water obtained during each cycle after drug administration, and Control Latency was the latency obtained from water heated to that temperature during the baseline determinations at the beginning of the test session.
For morphine, the ED50 was defined as the log dose that reduced response rates to 50% Control Rate or increased tail withdrawal latencies to 50% MPE at the time of approximate peak effect (100 min). Individual ED50s were calculated by interpolation when only two data points were available (one below and one above 50% control response rate) or by linear regression when at least three data points were available from the linear portion of the log dose-effect curve. Individual data were averaged to yield mean ED50 values and 95% confidence limits, and means and confidence limits were converted from log to linear values for presentation.
Baseline response rates and tail withdrawal latencies after repeated treatment with saline or morphine were compared by T-test. Data for SNC80 and fentanyl cumulative dose-effect curves were analyzed by two-way analysis of variance, with dose of test drug (SNC80 or fentanyl) as one factor and repeated treatment condition (saline or morphine) as the other factor. A significant main effect was followed by the Holm-Sidak post hoc test. For all statistical tests, the criterion for significance was set at p<0.05.
Drugs
SNC80 was synthesized by Drs. Kenner C. Rice and John E. Folk (National Institutes of Health, Bethesda, MD). Fentanyl HCl and morphine sulfate were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). SNC80 was dissolved in 2-3% lactic acid to a final concentration of 50 mg/ml, and dilutions were made with sterile water. Fentanyl and morphine were dissolved in sterile water. All drugs were administered i.m. in the thigh, and doses were determined based on the free base or salt forms listed above.
RESULTS
Effects of Morphine Alone
In the assay of schedule-controlled responding, mean control response rates ± SEM during determination of effects produced by morphine alone were 2.80 ± 0.07 responses/sec. Figure 1 shows that morphine produced a dose- and time-dependent decrease in rates of schedule-controlled responding. Peak effects of the highest doses of 3.2 and 5.6 mg/kg morphine were obtained after 100 min, and these effects were sustained for at least 300 min. The effects of 5.6 mg/kg morphine were no longer apparent after 24 hr. The morphine ED50 value (95% CL) determined at the time of peak effect (100 min) was 1.29 (0.89-1.80) mg/kg.
Figure 1. Effects of morphine alone in the assay of schedule-controlled responding.
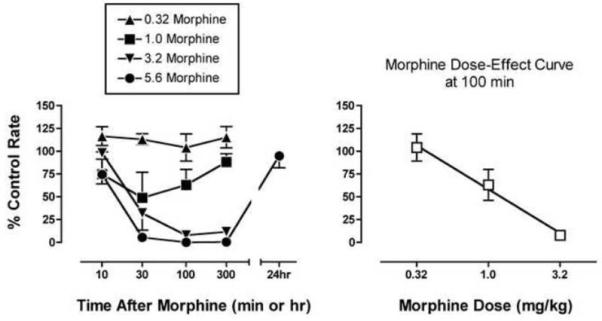
The left panel shows the time course of effects produced by 0.32-5.6 mg/kg morphine. Abscissa: Time after morphine administration in min or hr (log scale). Ordinate: Percent control response rate. The right panel shows the morphine dose-effect curve as the time of peak effect (100 min). Abscissa: Dose morphine in mg/kg (log scale). Ordinate: Percent control response rate in responses per second. All points show mean data ± SEM from three monkeys.
In the assay of thermal nociception, mean tail withdrawal latencies ± SEM from 48 and 54°C water during determination of effects produced by morphine alone were 0.97 ± 0.31 sec and 0.76 ± 0.18 sec, respectively. Figure 2 shows that morphine produced a dose- and time-dependent increase in %MPE at both temperatures. As in the assay of schedule-controlled responding, peak effects of the highest doses of 3.2 and 5.6 mg/kg morphine were obtained after 30 to 100 min. The morphine ED values (95% CL) determined at the time of peak effect (100 min) were 0.82 (0.62-1.05) and 2.36 (2.09-2.62) mg/kg at temperatures of 48 and 54°C, respectively.
Figure 2. Effects of morphine alone in the assay of thermal nociception.
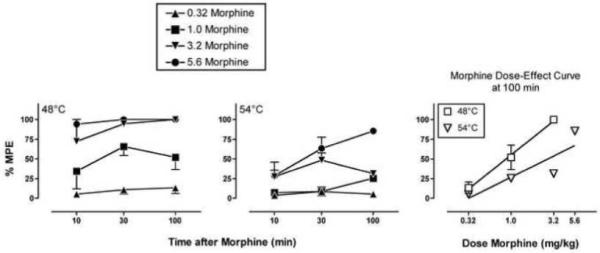
The left and center panels show the time course of effects produced by 0.32-5.6 mg/kg morphine at thermal stimulus intensities of 48 and 54°C, respectively. Abscissae: Time after morphine administration in minutes (log scale). Ordinates: Percent maximum possible effect (%MPE). The right panel shows the morphine dose-effect curve at each stimulus intensity at the time of peak effect (100 min). Abscissa: Dose morphine in mg/kg (log scale). Ordinate: %MPE. All points show mean data ± SEM from three monkeys.
Because the morphine ED50 values in the assays of schedule-controlled responding and 48°C thermal nociception were approximately 1.0 mg/kg, the dosing regimen selected for repeated morphine started at 1.0 mg/kg. Based on the dosing regimen described in previous rodent studies (Cahill et al. 2001; Morinville et al. 2003), the overall regimen of repeated morphine consisted of morphine doses of 1.0, 1.6, 2.0 and 3.0 mg/kg administered at 12hr intervals.
Effects of Repeated Morphine on Behavioral Effects of SNC80 and Fentanyl
In the assay of schedule controlled behavior, repeated morphine produced a significant decrease in baseline response rates from a mean ± SEM of 2.87 ± 0.04 responses/sec after repeated saline to 1.67 ± 0.29 responses/sec after repeated morphine (p=0.047). However, all monkeys responded at rates greater than 1 response per second and earned all available food pellets. Figure 3 shows that, when data were normalized to these different baseline response rates, repeated morphine did not alter dose-effect curves for either the delta agonist SNC80 or the mu agonist fentanyl. Both SNC80 and fentanyl produced dose-dependent decreases in response rates after repeated saline treatment, and these dose-effect curves were not significantly altered after repeated morphine treatment (see figure legend for details of statistical analysis).
Figure 3. Effects of repeated saline or repeated morphine on the rate-decreasing effects of SNC80 and fentanyl in the assay of schedule-controlled responding.
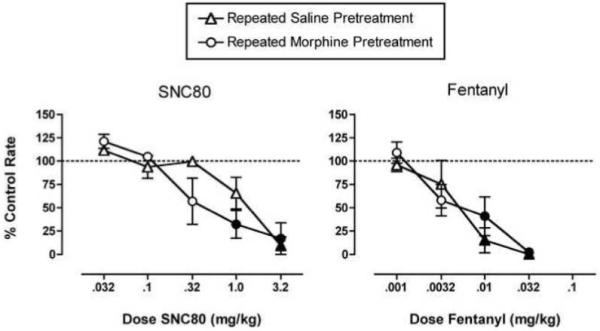
Abscissae: Dose SNC80 (left panel) or fentanyl (right panel) in mg/kg (log scale). Ordinates: Percent control response rate. All points show mean data ± SEM in three monkeys. In the left panel, two-way analysis of variance indicated that there was a significant main effect of SNC80 dose (F(5,24)=20.79, p<0.001) but not a significant main effect of morphine treatment (F(1,24)=1.34, p=0.26) or a significant interaction (F(5,24)=1.97, p=0.12). Similarly, in the right panel, there was a significant main effect of fentanyl dose (F(4,20)=22.88, p<0.001) but not a significant main effect of morphine treatment (F(1,20)=0.33, p=0.57) or a significant interaction (F(4,20)=0.74, p=0.58). Filled points show doses of SNC80 or fentanyl that produced effects significantly different from control as determined by the Holm-Sidak test (p<0.05).
In the assay of thermal nociception, there was a trend for repeated morphine to increase tail withdrawal latencies, and one monkey did not withdraw its tail from 48°C water when repeated morphine was administered prior to determination of the fentanyl dose-effect curve. However, the effect of repeated morphine on baseline tail withdrawal latencies did not achieve statistical significance. At 48°C, mean ± SEM baseline tail withdrawal latencies were 1.13±0.25 sec and 5.02 ±2.71 sec after repeated saline and morphine, respectively (p=0.32). At 54°C, mean ± SEM baseline tail withdrawal latencies were 0.95 ± 0.20 and 3.55 ± 1.25 sec after repeated saline and morphine, respectively (p=0.21).
Figure 4 shows effects of repeated saline or morphine on the antinociceptive effects of SNC80 and fentanyl. After repeated saline treatment, SNC80 had little or no effect on tail withdrawal latencies from water heated to either 48 or 54°C. Tail withdrawal latencies at both temperatures tended to be longer when SNC80 was administered after repeated morphine. However, the effect of repeated morphine was statistically significant only at 54°C, there was no interaction between morphine treatment and SNC80 dose, and even after repeated morphine, no dose of SNC80 produced a significant antinociceptive effect at either temperature (see figure legend for details of statistical analysis), Figure 5 shows that repeated morphine produced a robust enhancement in the antinociceptive effects of SNC80 at 54°C in only one of three monkeys tested. When fentanyl was administered after repeated saline treatment, it produced dose-dependent increases in tail-withdrawal latencies from water heated to both 48 and 54°C. Repeated morphine had no effect on the fentanyl dose-effect curve at 48°C, but at the higher temperature of 54°C, morphine treatment significantly reduced fentanyl antinociception. There was no interaction between morphine treatment and fentanyl dose (see figure legend for details of statistical analysis). In addition, after repeated morphine, fentanyl doses up to 0.056 mg/kg failed to produce a significant antinociceptive effect at 54°C. Overall, then, repeated morphine produced a slight enhancement of the antinociceptive effects of SNC80 and a slight decrease in the effects of fentanyl at a stimulus intensity of 54°C.
Figure 4. Effects of repeated saline or repeated morphine on the antinociceptive effects of SNC80 and fentanyl in the assay of thermal nociception.
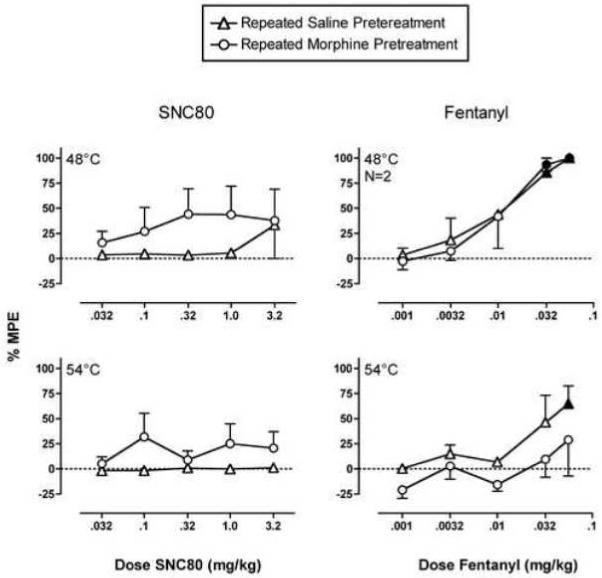
Abscissae: Dose SNC80 (left panels) or fentanyl (right panels) in mg/kg (log scale). Ordinates: Percent maximum possible effect at 48°C (top panels) or 54°C (bottom panels). All points show mean data ± SEM in three monkeys except the upper right panel (N=2; one monkey was not included in this comparison, because it did not withdraw its tail from 48°C water after repeated morphine treatment). In the upper left panel, two-way analysis of variance indicated that there was not a significant main effect of SNC80 dose (F(5,24)=0.86, p=0.52) or of morphine treatment (F(1,24)=3.22, p=0.08), nor was there a significant interaction (F(5,24)=0.41, p=0.84). In the lower left panel, there was not a significant main effect of SNC80 dose (F(5,24) = 0.66, p=0.66), but there was a significant main effect of morphine treatment (F(1,24)=6.40, p=0.018); the interaction was not significant (F(5,24)=0.70, p=0.63). In the upper right panel, there was a significant main effect of fentanyl dose (F(5,12)=24.50, p<0.001) but not a significant main effect of morphine treatment (F(1,12)=0.06, p=0.80) or a significant interaction (F(5,12)=0.13, p=0.98). In the lower right panel, there was a significant main effect of fentanyl dose (F(5,24)=3.76, p=0.012) and a significant main effect of morphine treatment (F(1,24) = 5.41, p=0.029); however, the interaction was not significant (F(5,24) = 0.39, p=0.85). Filled points show doses of SNC80 or fentanyl that produced effects significantly different from control as determined by the Holm-Sidak post hoc test (p<0.05). However, post hoc analysis indicated that effects of repeated morphine treatment were not significant at any dose of SNC80 or fentanyl at either stimulus intensity (p>0.05).
Figure 5. Effects of repeated saline or repeated morphine on the antinociceptive effects of SNC80 in individual monkeys tested at 54°C.
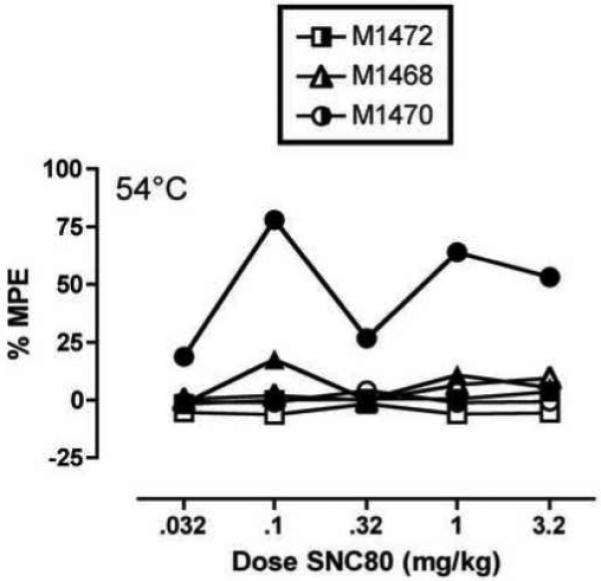
Abscissae: Dose SNC80 in mg/kg (log scale). Ordinates: Percent maximum possible effect at 54°C (bottom panels). Data from individual monkeys are indicated by different symbol shapes. Open symbols show data obtained after repeated saline, and closed symbols show data after repeated morphine.
DISCUSSION
The present results provide, at best, only weak support for the hypothesis that prior stimulation of mu opioid receptors is sufficient to promote delta receptor availability and enhance the antinociceptive effects of delta agonists in rhesus monkeys. Repeated morphine pretreatment produced a statistically significant and selective enhancement of the thermal antinociceptive effects of the delta agonist SNC80, but this effect was small, unrelated to SNC80 dose, and evident at only one of the two thermal stimulus intensities evaluated. Although previous studies have reliably found that repeated pretreatment with mu agonists can enhance the antinociceptive effects of intrathecally administered delta agonists in rodents (Cahill et al. 2001; Morinville et al. 2003), the present results suggest that this phenomenon may not extend to a robust enhancement of antinociception produced by systemically administered delta agonists in rhesus monkeys.
Effects of SNC80 and fentanyl after repeated saline treatment
Effects of the selective delta agonist SNC80 and the selective mu agonist fentanyl administered after repeated saline pretreatment are consistent with previous studies with these compounds in rhesus monkeys (Negus et al. 1993a; Negus et al. 1998; Negus et al. 2002; Stevenson et al. 2003). Thus, in agreement with previous studies, SNC80 dose-dependently decreased rates of schedule-controlled responding while having little effect on thermal nociception. To assess thermal nociception, the present study used two thermal stimulus intensities, including a relatively low intensity stimulus of 48°C. This is near the threshold for eliciting tail withdrawal by rhesus monkeys (Negus et al. 1993b; Walker et al. 1993), and this relatively low stimulus intensity was used in this study in an effort to maximize sensitivity to any antinociceptive effects of SNC80. However, even at this relatively low stimulus intensity, SNC80 at doses up to 3.2 mg/kg produced little or no antinociceptive effect. Higher doses were not administered to minimize risk of convulsant effects (Danielsson et al. 2006). In contrast to SNC80, fentanyl reduced rates of schedule-controlled responding and produced dose-dependent antinociception at stimulus intensities of both 48 and 54°C. This is consistent with the characterization of fentanyl as a high-efficacy mu opioid receptor agonist (Gatch et al. 1995; Emmerson et al. 1996; Selley et al. 1998; Negus and Mello 1999).
Effects of acute and repeated morphine on response rates and tail-withdrawal latencies
The potency and time course of morphine were assessed initially to determine morphine ED50 values in these assays and these monkeys prior to initiating repeated treatment. Morphine effects were similar to those described previously in rhesus monkeys (e.g. (Pereira Do Carmo et al. 2008). The morphine ED50 was approximately 1.0 mg/kg in the assays of schedule-controlled responding and 48°C tail withdrawal, and as a result, this dose was used as the starting point for repeated treatment modeled on the regimen used in previous studies in rodents (Cahill et al. 2001; Morinville et al. 2003). Agonist effects produced by repeated morphine (1.0, 1.6, 2.0 and 3.0 mg/kg at 12 hr intervals) had largely but not completely dissipated by 12 hr after the last dose. Thus, baseline response rates in the assay of schedule-controlled responding were significantly reduced after repeated morphine, but all monkeys responded at sufficiently high rates (> 1.0 response/second) to earn all available food pellets. Similarly, there was a trend for repeated morphine to slightly increase tail-withdrawal latencies, but this effect was not statistically significant, and with one exception (1 monkey tested at the 48°C stimulus intensity in the fentanyl experiment), all monkeys displayed baseline tail-withdrawal latencies below 5 sec from water heated to 48 or 54°C 12 hr after repeated morphine. These findings generally agree with previous rodent studies, wherein repeated morphine slightly decreased nociceptive scores to intraplantar formalin injections but did not significantly alter baseline tail-flick or hot-plate responses to 52°C thermal stimuli (Cahill et al. 2001; Morinville et al. 2003).
Effects of repeated morphine on rate-decreasing and antinociceptive effects of SNC80 and fentanyl
Repeated morphine slightly enhanced the antinociceptive effects of SNC80 and slightly attenuated the antinociceptive effects of fentanyl at 54°C. Moreover, repeated morphine did not significantly alter the rate-decreasing effects of either SNC80 or fentanyl in the assay of schedule-controlled responding. These results provide some support for the hypothesis that prior stimulation of mu receptors can produce a pharmacologically and behaviorally selective enhancement in delta agonist-induced antinociception in rhesus monkeys. Moreover, these results extend previous studies reporting that repeated treatment with morphine and other mu agonists enhanced the antinociceptive effects of the delta agonist deltorphin II administered intrathecally in mice and rats (Cahill et al. 2001; Morinville et al. 2003; Gendron et al. 2007a). However, these conclusions should be tempered by at least four caveats.
First, the effects of repeated morphine on SNC80-induced antinociception at 54°C were small. Although analysis of variance indicated a significant effect of repeated morphine treatment on SNC80-induced antinociception at 54°C, post hoc analysis failed to reveal a significant effect of morphine treatment at any dose of SNC80, and maximal antinociception failed to exceed a mean of 35%MPE at any dose of SNC80. Second, repeated morphine treatment did not significantly alter SNC80-induced antinociception at the lower stimulus intensity of 48°C, although there was a trend for enhancement at this stimulus intensity as well. Third, observation of individual data indicated that repeated morphine produced a robust enhancement in SNC80-induced antinociception in only one of three monkeys tested. Finally, we have reported previously that simultaneous administration of delta and mu agonists produces synergistic antinociceptive effects but only additive or sub-additive rate-decreasing effects in the assay of schedule-controlled responding (Stevenson et al. 2004; Stevenson et al. 2005; Negus et al. 2009). Thus, to the degree that repeated morphine produced residual mu agonist effects at the time of delta agonist testing, it is possible that any enhancement of delta agonist antinociception resulted from synergy produced by simultaneous stimulation of mu and delta receptors rather than from activation of delta receptors produced by prior stimulation of mu receptors. In view of these considerations, the present data provide at best only weak support for the hypothesis that prior stimulation of mu receptors is sufficient to increase antinociceptive effects of delta agonists in rhesus monkeys.
The relatively weak effect of repeated morphine on delta agonist-induced antinociception in the present study as compared to previous studies in rodents likely reflects parametric differences in experimental variables. Three of those will be briefly discussed here. First, it is possible that the regimen of repeated morphine treatment was not optimal to fully promote delta receptor availability and enhance delta agonist-induced antinociception in rhesus monkeys. Thus, enhancement of delta agonist-induced antinociception in rats was found to be extremely sensitive to such parameters as the number and magnitude of morphine doses and the interval between conclusion of morphine dosing and delta agonist testing (Morinville et al. 2003; Gendron et al. 2007a). The present study employed a morphine dosing regimen modeled after the most effective regimen used in rodents, but it is possible that a different regimen is required to produce maximal effects in rhesus monkeys.
Second, the present study used a different delta agonist (SNC80 vs. deltorphin II) and route of administration (systemic vs. intrathecal) than previous studies in rodents, and it is possible that effects of repeated morphine pretreatment do not extend to these conditions. In support of the proposition that route of administration is important, a recent study reported that repeated morphine treatment failed to enhance the antinociceptive effects of deltorphin II administered into the ventral periaqueductal gray in rats, although the locomotor depressant effects of deltorphin II administered into this region were enhanced (Morgan et al. 2009). The effect of repeated morphine on antinociception produced by SNC80 or other delta agonists in rodents has not been reported. However, the rationale for initially testing delta agonist antinociception after repeated morphine was that inflammation-induced enhancement of deltorphin II-induced antinociception was present in wild-type mice but not in mu receptor-knockout mice (thereby suggesting a role for mu receptor stimulation in enhancing delta agonist antinociception--see Introduction). Notably, inflammation-induced enhancement of antinociception produced by intrathecal SNC80 is also eliminated in mu receptor-knockout mice, suggesting that mu receptor stimulation may also contribute to enhanced antinociception of non-peptidic delta agonists like SNC80 in mice. Taken together, these findings suggest that effects of mu receptor stimulation may be especially important for modulating antinociceptive effects of intrathecally administered delta agonists.
A third potentially important difference between the present study and previous studies is the species of experimental subject. There are well established species differences in the density, distribution and function of delta, mu and kappa opioid receptors, and for delta receptors, these differences are especially prominent in spinal cord (Mansour et al. 1988; Mennicken et al. 2003). Thus, rodents display diffuse distribution of delta receptors throughout the dorsal and ventral horn, whereas non-human and human primates show a phylogenetically increasing concentration of delta receptors to the superficial laminae of the dorsal horn, a region know to be critical for processing nociceptive stimuli. These species differences in delta receptor distribution may contribute to species differences in delta receptor effects and modulation of those effects by prior treatment with mu agonists.
CONCLUSION
In summary, the present study found that repeated morphine pretreatment produced a weaker and less reliable enhancement of delta agonist-induced antinociception in monkeys than has been reported previously in rodents. These findings suggest that mu receptor-mediated promotion of delta receptor availability and subsequent enhancement of delta agonist-induced antinociception may occur under a relatively limited range of conditions.
ACKNOWLEDGEMENTS
This work was supported by Grant RO1-DA11460 from the National Institute on Drug Abuse, National Institutes of Health. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. These funding sources had no involvement in any aspect of the study. The authors would like to thank Katrina Schrode and Ember Morrissey for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. Journal of Pharmacology and Experimental Therapeutics. 2001;296(3):939–946. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Gatch MB, Chang KJ, Woods JH. BW373U86, a delta-opioid receptor agonist, reverses bradykinin-induced thermal allodynia in rhesus monkeys. European Journal of Pharmacology. 1995;277(2-3):285–287. doi: 10.1016/0014-2999(95)00134-7. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends in Pharmacological Sciences. 2007;28(1):23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101(1-2):199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. Journal of Neuroscience. 2001;21(19):7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson I, Gasior M, Stevenson GW, Folk JE, Rice KC, Negus SS. Electroencephalographic and convulsant effects of the delta opioid agonist SNC80 in rhesus monkeys. Pharmacology, Biochemistry and Behavior. 2006;85(2):428–434. doi: 10.1016/j.pbb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. Journal of Pharmacology and Experimental Therapeutics. 1996;278(3):1121–1127. [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. British Journal of Pharmacology. 2000;129(8):1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97(1):39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Negus SS, Butelman ER, Mello NK. Antinociceptive effects of cocaine/opioid combinations in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1995;275(3):1346–1354. [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144(1):263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. Journal of Neuroscience. 2006;26(3):953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150(4):807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. Journal of Neuroscience. 2005;25(12):3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Woods JH. Antidepressant-like effects of delta opioid receptor agonists. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. Marcel Dekker; New York: 2004. pp. 355–371. [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends in Neurosciences. 1988;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. Journal of Comparative Neurology. 2003;465(3):349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Ashley MD, Ingram SL, Christie MJ. Behavioral consequences of delta-opioid receptor activation in the periaqueductal gray of morphine tolerant rats. Neural Plast. 2009;2009:516328. doi: 10.1155/2009/516328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. Journal of Neuroscience. 2003;23(12):4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Delta Opioids and Substance Abuse. In: Chang K-J, Porreca F, Woods JH, editors. The Delta Receptor. Marcel Dekker, Inc.; New York: 2004. pp. 401–430. [Google Scholar]
- Negus SS, Bear AE, Folk JE, Rice KC. Role of delta opioid efficacy as a determinant of mu/delta opioid interactions in rhesus monkeys. Eur J Pharmacology. 2009;602(1):92–100. doi: 10.1016/j.ejphar.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. Journal of Pharmacology and Experimental Therapeutics. 1993a;267(2):896–903. [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Al Y, Woods JH. Prostaglandin E2-induced thermal hyperalgesia and its reversal by morphine in the warm-water tail-withdrawal procedure in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1993b;266(3):1355–1363. [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1994;270(3):1025–1034. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1998;286(1):362–375. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. Journal of Pharmacology and Experimental Therapeutics. 1999;290(3):1132–1140. [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW. Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol. 2008;16(5):386–399. doi: 10.1037/a0013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Zuzga DS, Mello NK. Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50,488 by quadazocine. J Pain. 2002;3:218–226. doi: 10.1054/jpai.2002.124734. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Vanderah T, Porecca F. The Delta Opioid Receptor Subtypes and Pain Modulation. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. Marcel Dekker; New York: 2004. pp. 297–329. [Google Scholar]
- Pereira Do Carmo GP, Polt R, Bilsky EJ, Rice KC, Negus SS. Behavioral pharmacology of the mu/delta opioid glycopeptide MMP2200 in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2008;326(3):939–948. doi: 10.1124/jpet.108.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. Peripheral delta opioid receptors require priming for functional competence in vivo. European Journal of Pharmacology. 2009;602(2-3):283–287. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q. Childers. Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GPTgammaS binding in mMOR-CHO cells and rat thalamus. Journal of Pharmacology and Experimental Therapeutics. 1998;285:496–505. [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 [(+)-4-[({alpha}R)-{alpha}-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] and heroin. Journal of Pharmacology and Experimental Therapeutics. 2005;314(1):221–231. doi: 10.1124/jpet.104.082685. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Linsenmayer DC, Folk JE, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: effects of delta + mu and delta + kappa agonists on schedule-controlled responding and thermal nociceptionn. Journal of Pharmacology and Experimental Therapeutics. 2003;307(3):1054–1064. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Wentland MP, Bidlack JM, Mello NK, Negus SS. Effects of the mixed-action kappa/mu opioid agonist 8-carboxamidocyclazocine on cocaine- and food-maintained responding in rhesus monkeys. European Journal of Pharmacology. 2004;506(2):133–141. doi: 10.1016/j.ejphar.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Stewart J, Hammond DL. Activation of spinal delta-1 or delta-2 opioid receptors reduces carrageenan-induced hyperalgesia in the rat. Journal of Pharmacology and Experimental Therapeutics. 1994;268:701–708. [PubMed] [Google Scholar]
- Walker EA, Butelman ER, Decosta BR, Woods JH. Opioid thermal antinociception in rhesus monkeys: Receptor mechanisms and temperature dependency. Journal of Pharmacology and Experimental Therapeutics. 1993;267:280–286. [PubMed] [Google Scholar]


