Abstract
Purpose
The tetraspan protein epithelial membrane protein-2 (EMP2) has been shown to regulate the surface display and signaling from select integrin pairs, and it was recently identified as a prognostic biomarker in human endometrial cancer. In this study, we assessed the role of EMP2 in human ovarian cancer.
Experimental Design
We examined the expression of EMP2 within a population of women with ovarian cancer using tissue microarray assay technology. We evaluated the efficacy of EMP2-directed antibody therapy using a fully human recombinant bivalent antibody fragment (diabody) in vitro and ovarian cancer xenograft models in vivo.
Results
EMP2 was found to be highly expressed in over 70% of serous and endometrioid ovarian tumors compared to non-malignant ovarian epithelium using a human ovarian cancer tissue microarray. Using anti-EMP2 diabody, we evaluated the in vitro response of 9 human ovarian cancer cell lines with detectable EMP2 expression. Treatment of human ovarian cancer cell lines with anti-EMP2 diabodies induced cell death and retarded cell growth, and these response rates correlated with cellular EMP2 expression. We next assessed the effects of anti-EMP2 diabodies in mice bearing xenografts from the ovarian endometrioid carcinoma cell line OVCAR5. Anti-EMP2 diabodies significantly suppressed tumor growth and induced cell death in OVCAR5 xenografts.
Conclusions
These findings indicate that EMP2 is expressed in the majority of ovarian tumors and it may be a feasible target in vivo.
Keywords: Epithelial membrane protein-2, ovarian cancer, antibody therapy, diabody, xenograft
INTRODUCTION
Ovarian cancer is the foremost cause of death from gynecological malignancy in the United States, with an estimated 21,550 new cases and 14,660 deaths in 2009 (1). Ovarian cancer is classified based on the histology of the tumor, clinical behavior, and epidemiology. Epithelial ovarian cancer is the most common type in origin, and it includes both serous and endometrioid tumors. Incidence of ovarian cancer generally increases with age, with the majority of cases occurring in postmenopausal women (2).
Screening for ovarian cancer has met with limited success (3–6). This is largely due to a lack of early detection markers, and efficient screening tools for surveillance typically lack sensitivity and specificity. In fact, almost 70% of patients with ovarian cancer are not diagnosed until they have reached the high stage of disease (7). Primary treatment of ovarian cancer is surgical resection and adjuvant chemotherapy, but recurrence is common (8, 9). Therefore, there is need for the development and validation of molecular markers sensitive to disease onset and progression in order to improve patient management as well as to point to new targets for drug design.
An emerging molecule in female reproductive cancers is the tetraspan protein epithelial membrane protein-2 (EMP2). In normal tissue, EMP2 has a discrete tissue distribution, with high expression in secretory endometrium (10, 11), lung alveolar epithelium (12), and in retinal pigmented epithelium within the eye (13). Dysregulation of EMP2 has been implicated in endometrial cancer, where EMP2 expression correlates with poor prognosis and survival (14), and EMP2 is the only known early diagnostic marker to predict endometrial hyperplasia progression to endometrial cancer (15). As recombinant antibodies have been successful in treating a variety of carcinomas and lymphomas (16–19), we recently developed an engineered anti-EMP2 diabody that binds to its second extracellular domain with high specificity and avidity (15). Treatment of endometrial adenocarcinoma cells with anti-EMP2 diabody resulted in a significant increase in apoptotic cell death in vitro and a reduction in tumor volume in vivo (15).
The basic biology of EMP2 provides insights into its potential role in reproductive epithelial carcinogenesis. EMP2 is a member of the growth arrest-specific gene 3/peripheral myelin protein 22 four-transmembrane protein family (20–22). It associates with integrin αvβ3 and focal adhesion kinase (FAK), and it can regulate αvβ3 integrin expression and localization, functions critical for its physiologic role in blastocyst implantation (10, 23, 24).
Assessment of public databases indicated that EMP2 mRNA may be up-expressed in serous and endometrioid ovarian tumors, including poorly differentiated and high grade cancers (25), and it may be selectively upregulated in carboplatin-resistant ovarian tumors (26). Thus, in the present study, we test the association of EMP2 expression using a human ovarian cancer tissue microarray (TMA). Next, we evaluated the ability of anti-EMP2 diabodies to alter cell growth and induce cytotoxicity in human ovarian cancer cell lines in vitro and in vivo. Our findings indicate that EMP2 protein is expressed in the majority of human ovarian carcinomas, and that anti-EMP2 diabody is effective in targeting human ovarian cancer cell lines and xenograft tumors. These results suggest that EMP2 has merit for further assessment as a potential target for imaging and therapy in ovarian cancer.
MATERIALS AND METHODS
Cell lines and cell culture
OVCA432 and OVCA433 (27) were generously provided by Dr. Robert Bast (M.D. Anderson Cancer Center, TX). A1847 (28) was a gift from Dr. Stuart Aaronson (Mount Sinai Medical Center, NY), and CAOV-3, ES-2, OV90, PA-1, OVCAR-5, IGROV-1, and SKOV-3 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultivated in appropriate medium supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 U/ml streptomycin (all from Invitrogen Life Technologies, Carlsbad, CA), incubated at 37°C in a humidified 5% CO2 and passaged every 7 days.
Anti-EMP2 reagents
Rabbit polyclonal anti-human EMP2 antibody has been described previously (13, 29). Human bivalent anti-EMP2 antibody fragments (diabodies) KS49 and KS83 were generated with specificity and avidity to both human and mouse EMP2 peptides and native cell-surface EMP2 protein. A10, a human diabody without known specificity, was used as a negative control. These diabodies have been described previously (15).
Tissue microarray analysis
Ovarian cancer patients who were surgically treated at Memorial Sloan-Kettering Cancer Center (from 1980 to 2004) had their tumor specimens banked according to Institutional Review Board (IRB) guidelines after they provided informed consent. A TMA was constructed as described previously (14). Initially, samples from 161 patients were present on the TMA (34 Borderline tumor patients; 53 low stage carcinoma patients [FIGO stages I and II]; 74 high stage carcinoma patients [FIGO stages III and IV]). Patients were excluded from our analyses if no clinical information could be obtained or if the targeted histology contained no relevant cells (i.e., benign nor malignant cells). Ultimately, data from 129 patients were utilized in our analyses: 21 borderline tumor patients; 34 low stage carcinoma patients; 74 high stage carcinoma patients. Tumor stage was classified according to International Federation of Gynecologists and Obstetricians (FIGO) classification. Table 1 summarizes clinical variables and patient groups.
Table 1. Clinical Variables and Patient Groups.
A TMA was constructed from 129 patients with archived paraffin tissue at the Memorial Sloan-Kettering Cancer Center.
| Clinical Characteristics |
All Patients |
Borderline tumor |
Low Stage CA1 |
High Stage CA |
|---|---|---|---|---|
| N | 129 | 21 (16%) | 34 (26%) | 74 (57%) |
| Vital Status | ||||
| Alive | 71 (55%) | 19 (27%) | 25 (35%) | 27 (38%) |
| Dead | 55 (43%) | 0 (0%) | 8 (15%) | 47 (85%) |
| Missing | 3 (2%) | 2 (67%) | 1 (33%) | 0 (0%) |
| Age | ||||
| Mean (Median) | 60.4 (61) | - | - | 60.4 (61) |
| Range | 36 ~ 79 | - | - | 36 ~ 79 |
| Histology | ||||
| Borderline tumor* | 21 (16%) | 21 (100%) | 0 (0%) | 0 (0%) |
| Clear Cell CA | 10 (8%) | 0 (0%) | 10 (100%) | 0 (0%) |
| Endometrioid CA | 14 (11%) | 0 (0%) | 12 (86%) | 2 (14%) |
| Mixed CA** | 9 (7%) | 0 (0%) | 3 (33%) | 6 (67%) |
| Serous CA | 75 (58%) | 0 (0%) | 9 (12%) | 66 (88%) |
| Grade | ||||
| 1 | 18 (14%) | 13 (72%) | 5 (28%) | 0 (0%) |
| 2 | 33 (26%) | 7 (21%) | 18 (55%) | 8 (24%) |
| 3 | 76 (59%) | 0 (0%) | 11 (14%) | 65 (86%) |
| Missing | 2 (2%) | 1 (67%) | 1 (33%) | |
CA=carcinoma
Borderline tumors consisted of a serous, intestinal mucinous and endometrioid types
Mixed epithelial carcinomas consisted of mixed epithelial tumors with a serous component
An independent validation sample, included 10 normal ovaries with no significant pathological changes and 10 ovary tumor samples, obtained from the UCLA Translational Pathology Core Laboratory with IRB approval. Samples were analyzed for the presence or absence of EMP2 expression by immunohistochemistry or western blot analysis as described below.
Immunohistochemistry
The immunohistochemical staining for EMP2 has been described previously (14). Briefly, formalin-fixed, paraffin-embedded tissue sections were heated at 95°C for 20 min in 0.1 M citrate buffer (pH 6.0) for antigen retrieval. The sections were incubated with rabbit anti-human EMP2 polyclonal antibody (1:400) in a humidified chamber overnight at 4°C. The corresponding preimmune serum was used as a negative control and was processed at the same condition. A biotinylated anti-rabbit secondary antibody was used from the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) following the manufacturer’s protocol. Antibody signal was detected using the Vector Laboratories DAB substrate kit (Vector Laboratories) according to the manufacturer’s instructions.
Immunohistochemical Scoring
We conducted a semi-quantitative analysis of the EMP2-stained ovarian cancer TMA, ovarian tumors, and normal ovary samples by two independent pathologists (RAS and MA). These pathologists were both blind to clinical information. We quantified EMP2 staining per TMA spot or whole tissue by considering the staining intensity (0 = below the level of detection, 1, weak; 2, moderate; and 3, strong) and the percentage of cells staining at each intensity level (0–100%). For each spot, we then calculated an integrated value of intensity combined with frequency was derived using the formula: [(3x) + (2y) + (1z)] / 100 where x, y, and z are % staining at intensity 3, 2, and 1, respectively. These values were used for comparing spot-level expression of EMP2 across different histopathologies.
Western blot analysis
Frozen normal and ovarian cancer tissue were obtained from the Tissue Procurement Laboratory Core at UCLA. 5 normal ovaries and 5 ovarian tumor samples were homogenized and lysed in ice-cold RIPA buffer containing a cocktail of EDTA-free protease inhibitors (Roche, Mannheim, Germany). After centrifugation at 14,000 × g for 10 min at 4°C, supernatants were collected and protein concentrations were measured using BCA protein assay kit (Pierce, Rockford, IL).
For experiments involving ovarian cancer cell lines, cells were washed in PBS, counted, and then lysed in Laemmli buffer. Samples were treated with peptide N-glycosidase F (PNGase; New England Biolabs, Beverly, MA) to deglycosylate the proteins as previously described (21). Equivalent cell lysates were separated on 18% SDS-PAGE gel, and proteins were transferred to nitrocellulose membrane.
EMP2 was detected using primary anti-human EMP2 (1:2000) antisera, and secondary horseradish-peroxidase-conjugated goat anti-rabbit IgG (Transduction Laboratories, Lexington, KY). Actin was detected as a loading control using primary monoclonal anti-human β actin (Sigma) and secondary horseradish-peroxidase-conjugated sheep anti-mouse IgG (Amersham, Piscataway, NJ). The secondary antibodies were detected using ECL detection reagents (Amersham). Specific bands were quantified by scanning and densitometric analyses using Scion Image software (Scion Corp., Frederick, MD).
Cell growth and cell death analysis
5×104 cells were placed in duplicate in a 12-well plate (Becton Dickinson, Franklin Lakes, NJ) and incubated with 20 µg/ml diabody A10 (control), KS49, or KS83 for 48hrs. After incubation, cells at 60% to 70% confluence were washed in PBS and trypsinized. Cell viability was determined by trypan blue exclusion. Cell growth was determined by comparing the ratio of final viable cells /initial cells using a hemocytometer.
In order to quantitate diabody-induced apoptosis, cells were harvested and stained using an annexin V-FITC apoptosis detection kit I according to the instructions of the manufacturer (BD Biosciences, Bedford, MA). Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility.
Tumor xenografts and treatment
Four- to six-week-old female BALB/c nude mice were purchased from Charles River Laboratories (Charles River, MA) for xenograft research. Following the National Academy of Science Guide for the Care and Use of Laboratory Animals, the mice were fed with a controlled light schedule (14L:10D) and controlled temperature range at the vivarium of University of California at Los Angeles. To obtain the solid tumors, 2 × 106 ovarian cancer cells suspended with 5% matrigel (BD Biosciences) were injected subcutaneously into the right or left shoulder flank of each mouse. On day 8, with the tumor diameter ~1 mm, therapy was started by injection twice a week with 1 mg/kg anti-EMP2 diabody KS83, KS49, control diabody A10, or a vehicle control (sterile saline). Four mice were utilized per group. Tumors were measured with Vernier calipers and tumor volumes were calculated by the formula (π/6 × larger diameter × smaller diameter). Mice were maintained until the tumor diameter reached 1.5cm or mice were moribund. Following treatment, all tumors were excised, formalin-fixed, and paraffin-embedded. Tumor sections were stained with hemotoxylin and eosin, EMP2, or 397p-FAK expression as described above.
Statistical analysis
Significant differences in EMP2 expression levels among various subgroups in the TMA were determined by the Mann-Whitney or Kruskal-Wallis rank sum test. For outcome analyses, spot expression levels were pooled using criteria described in a number of our publications (30–36). The Cox proportional hazards model (univariate and multivariate) was used to determine the significance of various factors related to recurrence or survival. The proportional hazards assumption was verified using Schoenfeld, martingale, and dfbeta residuals. LogRank and Fisher exact P-values were two-sided. A P < 0.05 was considered significant.
In addition to exploring the population as a continuous variable, we also dichotomized the population into relatively high versus relatively low EMP2 expressing patients. To find significant dichotomizing cut points, we used typically use either recursive partitioning, regression trees (available in the rpart software package) and/or by plotting log-rank p-values versus hazard ratios (31, 37). Survival curves were calculated using the Kaplan-Meier method and comparisons were done using the log-rank test. The Cox proportional hazards model (univariate and multivariate) was used to determine the significance of various factors related to survival. The proportional hazards assumption was verified using Schoenfeld, martingale, and dfbeta residuals. LogRank and Fisher exact P-values were two-sided and a P < 0.05 was considered significant.
Differences in the in vitro cell growth and cell death with diabodies and various in vivo experimental groups were evaluated using one-tailed Student’s unpaired t test at a 95% confidence level (GraphPad Prism version 3.0; GraphPad Software, La Jolla, CA).
RESULTS
EMP2 expression is associated with serous and endometrioid ovarian tumors
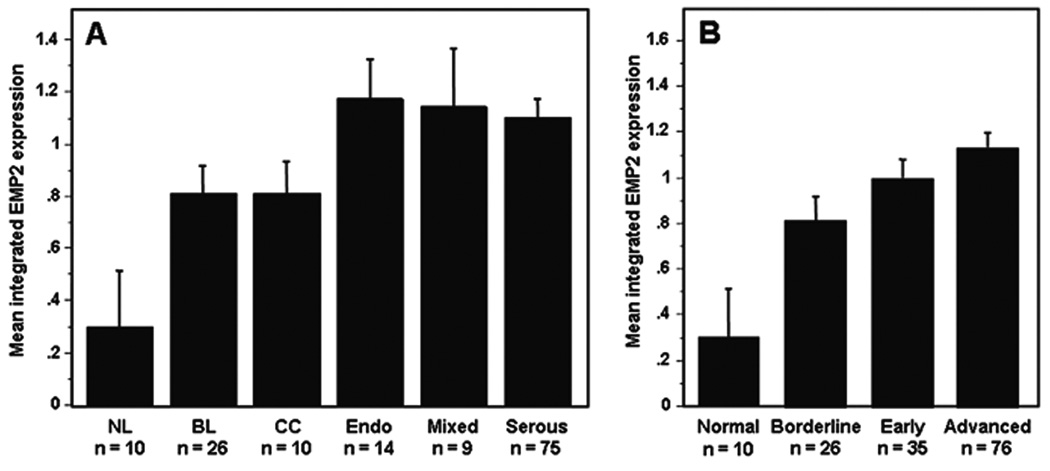
The expression of EMP2 in ovarian cancer was initially evaluated in a tissue microarray (TMA) containing samples from 129 ovarian cancer patients (Table 1). When we considered EMP2 expression level as a function of histology, in general, non-neoplastic ovarian epithelium expressed significantly lower levels of EMP2 than all malignant variants (Figure 1A). EMP2 was somewhat elevated in early and advanced stage cancer compared to borderline tumors (Figure 1B; P=0.210 and P=0.021, respectively) with trend towards slightly elevated levels in advanced compared to early stage ovarian malignancies (Figure 1B).
Figure 1. EMP2 expression stratified by histologic type and stage.
The mean integrated intensity of EMP2 protein expression for each category is shown using bar plots. The error bars represent the standard error of the mean; n is number of sample. (A) EMP2 expression was significantly increased in borderline (BL, P = 0.0088), clear cell (CC, P = 0.0233), endometriod (Endo, P = 0.0025), mixed (P = 0121), and serous (P = 0.0003) compared to non-malignant normal ovarian epithelium. (B) EMP2 expression was significantly higher in borderline (P = 0.0088), early stage (P = 0.0021) and advance stage tumors (P = 0.0003) compared to non-malignant normal ovarian epithelium. There was a trend towards higher EMP2 expression from borderline to early to advance stage tumors, however, these differences were not statistically significant.
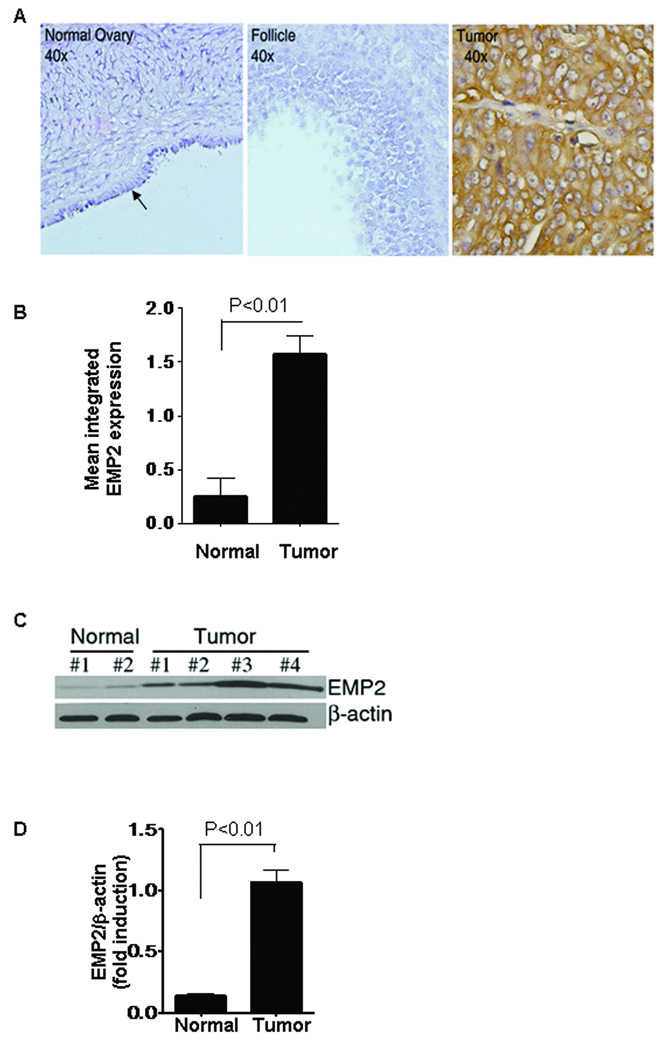
In order to validate the expression of EMP2 in ovarian cancer, we analyzed the expression of EMP2 in independent samples from the UCLA tissue procurement core facility within the Department of Pathology and Laboratory Medicine by both immunohistochemistry and western blot analysis. By immunohistochemistry, strong EMP2 expression was observed in 10 ovarian tumors (1 borderline tumor, 1 clear cell carcinoma, 5 endometrioid carcinoma, 1 mixed carcinoma and 3 serous carcinoma), while 10 normal ovaries showed a low to negligible staining pattern in both epithelial cells and follicle somatic cells (Figure 2A). The EMP2 staining pattern of the patients showed the same trends as TMA data (Figure 2B). To further verify these results, a western blot analysis was performed on an additional 5 normal ovary or 5 ovarian cancer specimens. EMP2 expression was significantly higher in the tumor specimens compared to the normal ovaries (Figure 2C, D).
Figure 2. EMP2 expression in human normal ovaries and ovarian tumors.
In an independent population of samples which included 10 normal ovaries and 10 ovary tumors obtained at UCLA, EMP2 expression was assessed by immunohistochemistry (A, B) and western blotting (C, D). (A) Negligible EMP2 staining was detected in the normal epithelial cells (arrow) of the ovary and ovarian follicle. By contrast, EMP2 was strongly expressed in an ovarian tumor (400× magnification). (B) Quantitation of EMP2 expression levels in samples stained by immunohistochemistry. Samples were scored from 0–3; results are the composite of all samples. EMP2 is detectably upregulated in ovarian tumors compared to benign ovaries. (C) 5 frozen normal ovaries and 5 ovarian tumors were homogenized and lysates prepared. Samples were separated by SDS-PAGE and probed for EMP2 expression by western blot analysis. A representative panel of 6 patients is shown. (D) Quantitation of composite EMP2 expression from samples measured by Western blot analysis. β-actin was used as an internal standard and the fold induction was measured using Scion Image software (Scion Corp., Frederick, MD). Analysis of ovarian cancer samples show that EMP2 expression is significantly increased ovarian cancer (P<0.01).
We next considered whether EMP2 expression levels were predictive of patient survival. However, whether as a continuous or dichotomized variable, EMP2 levels had no significant predictive value (P=0.5507; data not shown).
EMP2 is expressed in most ovarian cancer cell lines
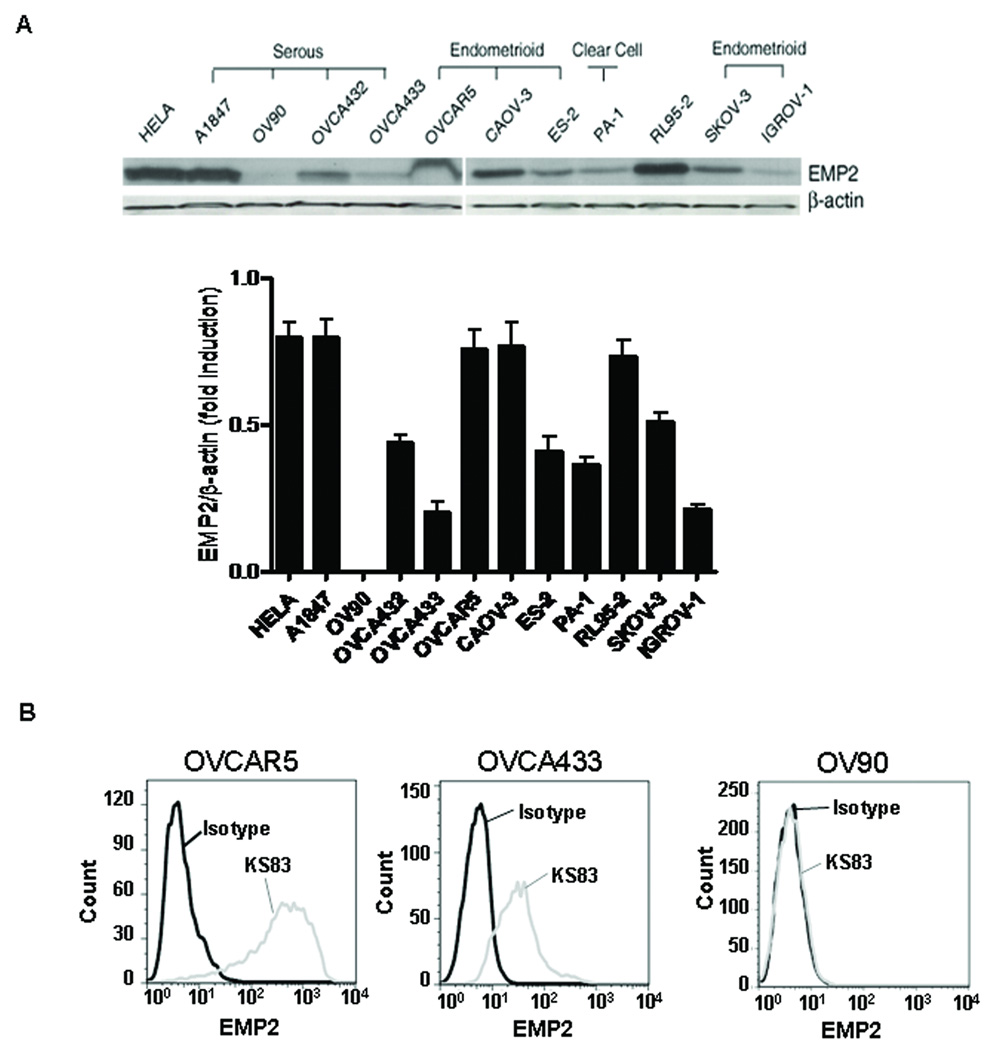
Similarly, in ovarian cancer cell lines, EMP2 is expressed in a number of serous derived primary ovarian tumors (OVCA432, OVCA433, A1847) as well as in ovarian endometrioid carcinoma cell lines (OVCAR-5, CAOV-3, ES-2, SKOV-3, IGROV-1). Positive control cell lines include the endometrial carcinoma cell line RL95-2 (10) and cervical cancer HELA cells. Overall, EMP2 expression was detected in all cells except the serous carcinoma cell line, OV90. Curiously, the one clear cell tumor (PA-1) was also EMP2 positive (Figure 3A). These findings indicate that, as in native ovarian tumors, the majority of ovarian cancer cell lines are EMP2 positive.
Figure 3. EMP2 expression in human ovarian cell lines.
(A) Equivalent amounts of cell lysates from all 12 cell lines, as indicated, were immunoblotted with EMP2 antisera. The same blot was re-probed using a monoclonal anti-β-actin antibody. The ratio of EMP2/β-actin was calculated by scanning quantities of EMP2 and β-actin. As a positive control, EMP2 expression was assessed in the cervical cancer cell line (HeLa) and the endometrial carcinoma cell line RL95-2. (B) To determine the surface expression of EMP2, ovarian cancer cells were stained and analyzed by flow cytometry. High EMP2 expression is detectable on the plasma membrane of OVCAR5 cells using an EMP2 diabody KS83. Lower levels of EMP2 were detected on the membrane of OVCA433 cells. As a negative control, no EMP2 was found on OV90 cell surface. An isotype control (diabody A10) was used to show specificity of binding.
In a number of cell types, EMP2 has been shown to reside within cytoplasmic compartments or on the plasma membrane. In order to assess the targetability of EMP2, we analyzed the surface expression of EMP2 in ovarian cancer cells using flow cytometry. In a number of cell lines including OVCAR5, CaOV3, and SKOV3, high levels of EMP2 were observed on the plasma membrane using anti-EMP2 antibody fragments. A representative cell line (OVCAR5) is depicted (Figure 3B). In addition, some cell lines expressed moderate/low levels of EMP2 were detected on the surface. These include OVCA433 (Figure 3B) and A1847 cells (data not shown). Finally, no surface expression of EMP2 was observed on. OV90 cells (Figure 3B). These results show that EMP2 may serve as an accessible therapeutic target for ovarian cancer.
Diabodies to EMP2 inhibit cell growth and promote cell death
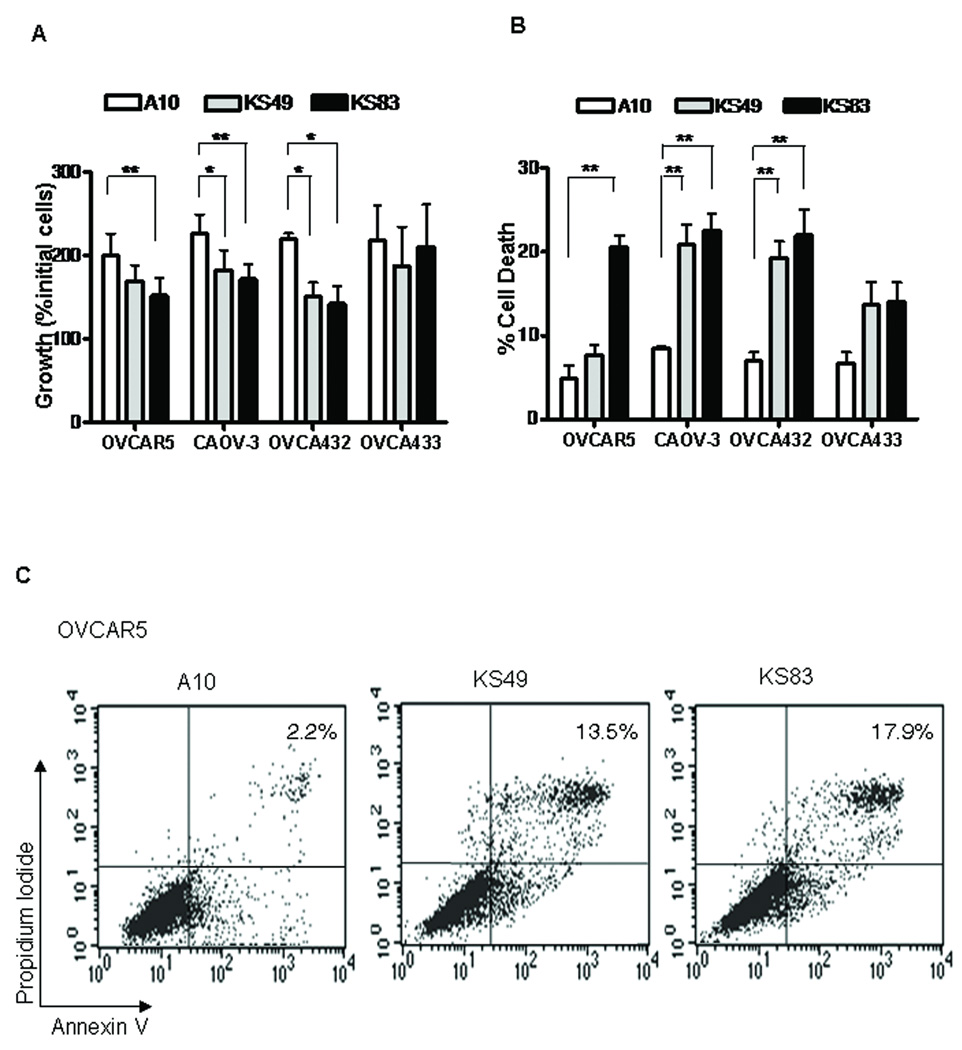
We have previously shown that our anti-EMP2 recombinant antibody fragments (diabodies) selectively bind EMP2 and induce apoptosis in a number of endometrial cancer cells (15). To determine if selective targeting of EMP2 may be an effective therapy in ovarian cancer, cell lines with high EMP2 expression (OVCAR5, CAOV-3, OVCA432) and an EMP2 low cell line (OVCA433) were utilized. Cells were treated with 20 µg/ml KS83, KS49 or control diabody A10 for 48 hrs. Significant cytostasis was observed in all three EMP2-high bearing ovarian cancer cell lines treated with anti-EMP2 diabodies KS83 or KS49 but not with control diabody A10 (Figure 4A). In contrast, negligible changes in cytostasis were observed in OVCA433 cells.
Figure 4. Ovarian cancer cells are responsive to EMP2 diabodies in vitro.
20µg/ml of diabody KS83 or KS49 were added to 4 ovarian cancer cell lines in duplicate for 48hrs. (A) Growth inhibition was calculated as the ratio of final viable cells /initial cells using hemocytometer. (B) Cell death was calculated as the ratio of blue (dead cells) to the total cells with trypan blue. (C) The rate of cell apoptosis was determined by flow cytometry. Comparison by Student’s t test, * P<0.05. ** P<0.01.
In order to correlate the decrease in cell number with an increase in cell death, dead cells were counted by trypan blue under the same experimental conditions. Consistent with the cell growth inhibition data above, diabody KS83 induced significant cell death in 3 of the 4 cell lines tested: OVCAR5, CAOV-3 and OVCA432 cells. KS49 induced a similar response in OVCAR5 and OVCA432 cells although the response was not as robust in CAOV-3 cells (Figure 4B). Finally, OVCA433 cells with low EMP2 expression on the plasma membrane did not significantly respond to anti-EMP2 therapy, suggesting that there is a threshold EMP2 level necessary for eliciting an efficient therapeutic response (Figure 4B).
To confirm that anti-EMP2 diabodies induced cell death via an apoptotic pathway, OVCAR5 cells were stained with propidium iodide and annexin V. 14% and 18% of cells were annexin and/or propidium iodide positive when treated with diabodies KS49 and KS83, respectively for only 24 hours. In contrast, less than 3% of cells were annexin and/or propidium iodide positive when treated with the control diabody A10 (Figure 4C).
In vivo tumor targeting
In order to test the efficacy of EMP2 immunotherapy in vivo, a mouse xenograft model was created using the ovarian cancer cell line OVCAR5. Female BALB/c nude mice were injected subcutaneously with OVCAR5 cells. On day 8, when tumors were approximately 1 mm in diameter, anti-EMP2 diabody KS83, KS49 and control A10 were injected intratumor twice a week, and progression of tumor size was measured using calipers. 0.9% saline was used as an additional negative control. By day 29, KS83 and KS49 significantly retarded OVCAR5 tumor growth (Figure 5A).
Figure 5. Anti-EMP2 diabodies retarded tumor formation in vivo.
(A) OVCAR5 cells were inoculated subcutaneously in nude mice, and tumor formation was monitored. At day 8 (arrow), mice were injected twice a week with 1 mg/kg of anti-EMP2 diabody KS83, KS49 and control diabody A10 or a vehicle control. Comparison by Student’s t test, * p<0.05. (B) At day 29, xenograft tumors were excised. Tumor histology was assessed by hemotoxylin and eosin staining (400× magnification). Insets represented the size of excised tumors (scale bar, mm). EMP2 expression was detected by (C) western blotting or (D) immunohistochemistry (200× magnification). Inset: negative staining with isotype control rabbit IgG.
All tumors were excised on day 29. Significantly, KS83 and KS49 both exhibited greater than a 2-fold difference in tumor size compared with A10 or sterile saline treatment (Figure 5B insets). Moreover, large areas of necrosis were observed in tumors treated with KS83 or KS49 but not with A10 or saline control (Figure 5B). These results suggest that anti-EMP2 immunotherapy reduces ovarian tumor load.
We next analyzed all treated tumors for EMP2 expression on day 30. As shown in Figure 5C, all tumors retained similar expression of EMP2, regardless of treatment. In order to confirm that EMP2 specific diabody treatment did not alter the distribution of EMP2, immunohistochemistry was performed on excised tumors. Detailed immunohistochemical analysis revealed that following diabody KS83 and KS49 treatments, the surface expression of EMP2 within the tumor was largely unaffected as compared to tumors treated with controls diabody A10 or sterile saline (Figure 5D). This suggests that increasing the dosage or length of treatment time with anti-EMP2 diabodies may be more effective at reducing the residual tumor.
DISCUSSION
In this study, we described the utilization of EMP2 as a therapeutic target in ovarian cancer. Previous studies have demonstrated the importance of EMP2 in human endometrial cancer (14, 15). However, to date, limited data exists on the role of EMP2 in ovarian cancer. In this study, we assessed the expression of EMP2 in an ovarian cancer TMA, and determined its suitability as a therapeutic target using anti-EMP2 recombinant antibody fragments.
Analysis of 129 ovarian carcinoma patients revealed that EMP2 expression was prevalent among serous and endometrioid tumors, although its expression was not predictive of overall survival probability. As these subtypes represent the majority (90–95%) of ovarian cancers diagnosed in North America, we further evaluated the potential of EMP2 to serve as a therapeutic target. Preliminary experiments in a panel of ovarian cancer cell lines demonstrated that EMP2 is highly expressed in the majority of cell lines. Moreover, incubation with recombinant EMP2 diabodies significantly inhibited cell growth and induced cell death both in vitro and in vivo.
EMP2 is a member of the tetraspan superfamily of proteins. The tetraspan family have been implicated in a multitude of processes including malignancy, regulation of the immune system, fertilization, and infectious disease processes (15, 38–40). Moreover, targeting of specific tetraspan proteins has been shown to induce the subsequent activation of an intracellular signal transduction cascade resulting in cell death, cell growth inhibition, antibody-dependent cellular cytotoxicity (ADCC), complement mediated cytotoxicity or activation of anti-tumor immune response (38, 41). Similarly, EMP2 in several cell types plays a role in growth control, invasion, metastasis and protein trafficking (20–22, 29). Biochemically, EMP2 can directly associate with integrin αvβ3 and focal adhesion kinase (FAK), and promote integrin-mediated FAK-Src activation (23, 24). Although the exact mechanism of EMP2 diabodies on ovarian cancer has yet to be elucidated, we predict that the diabodies dysregulate the integrin-FAK nexus, leading to apoptosis. Accordingly, it is possible that EMP2 contributes to malignant progression in part by augmenting integrin-mediated functions essential to tumor cell biology.
Human ovarian cancer cell xenografts in immune-deficient mice are useful research models for analyzing cell tumorigenicity and evaluation of therapeutics in ovarian cancer (42, 43). In the present study, treatment of OVCAR5 human ovarian cancer xenografts with anti-EMP2 diabody blocked tumor growth and induced tumor necrosis. These findings are similar to the effect of anti-EMP2 diabody on EMP2-positive human endometrial cancer xenografts (15). Anti-EMP2 diabodies lack detectable toxicity to normal tissues, including the lung, which physiologically express high levels of EMP2 (15). These findings suggest that in contrast to tumor cells, physiological expression of EMP2 is either inaccessible to anti-EMP2 antibody (perhaps due to tight junction sequestration (44), or its ligation does not interfere with critical functions required by these normal cell types. Additional studies will be required to further delineate the in vivo biodistribution and safety of anti-EMP2 therapy, and the efficacy of anti-EMP2 diabody (or other native or antibody fragments) for in vivo cytotoxicity of ovarian cancer cell lines.
In conclusion, EMP2 expression is a common feature of major subtypes of human ovarian carcinoma, and treatment of human ovarian cancer cell lines with human bivalent anti-EMP2 diabodies directly induced cell death and retarded cell growth both in vitro, and in tumor xenografts. These results suggest that EMP2 may be a potential target for ovarian cancer antibody therapy. Finally, reengineering of anti-EMP2 diabody fragments into a native antibody format may offer improved therapeutic benefits relative to pharmacokinetics, biodistribution, and effector functions (16, 45)
STATEMENT OF TRANSLATIONAL RELEVANCE
Ovarian cancer is the fifth leading cause of death from cancer in women and the leading cause of death from a gynecological cancer. Few modalities exist for its treatment, and like most cancers new treatments are needed. Epithelial membrane protein-2 (EMP2) is a tetraspan protein whose expression was previously shown to be an independent, prognostic indicator for endometrial cancer. In this study, we analyze the expression of EMP2 in ovarian cancer and determine its utility as a therapeutic target for disease. Using recombinant bivalent antibody fragments (diabody) to EMP2, we test their cytotoxic efficacy on a panel of human ovarian cancer cell lines in vitro and in xenografts in vivo. This study provides a preclinical assessment of antibody-targeting of EMP2 for treatment of ovarian cancer and further justifies its development as a treatment strategy for other EMP2-expressing cancers.
ACKNOWLEDGEMENTS
We are grateful to Dr. Robert C. Bast (The University of Texas M. D. Anderson Cancer Center, TX) for cell lines OVCA432 and OVCA433; and to Dr. Stuart Aaronson (Mount Sinai Medical Center, NY) for A1847.
Financial Support: NIH grants HD48540 (J. Braun), R21 CA131756 (M. Wadehra), CA016042 (University of California at Los Angeles Jonsson Comprehensive Cancer Center flow cytometry core); Iris Cantor Seed Grant and U54 CA119367 (M. Wadehra); The Early Detection Research Network NCI CA-86366 (LG); and Oppenheimer Family Foundation Grant Center for the Prevention of Eye Disease (L.K. Gordon)
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih Ie M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mironov S, Akin O, Pandit-Taskar N, Hann LE. Ovarian cancer. Radiol Clin North Am. 2007;45:149–166. doi: 10.1016/j.rcl.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Nick AM, Sood AK. The ROC 'n' role of the multiplex assay for early detection of ovarian cancer. Nat Clin Pract Oncol. 2008;5:568–569. doi: 10.1038/ncponc1214. [DOI] [PubMed] [Google Scholar]
- 6.Visintin I, Feng Z, Longton G, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 9.Vasey PA. Ovarian cancer: front-line standard treatment in 2008. Ann Oncol. 2008;19 Suppl 7:vii61–vii66. doi: 10.1093/annonc/mdn479. [DOI] [PubMed] [Google Scholar]
- 10.Wadehra M, Dayal M, Mainigi M, et al. Knockdown of the tetraspan protein epithelial membrane protein-2 inhibits implantation in the mouse. Dev Biol. 2006;292:430–441. doi: 10.1016/j.ydbio.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Wadehra M, Mainigi M, Morales SA, et al. Steroid hormone regulation of EMP2 expression and localization in the endometrium. Reprod Biol Endocrinol. 2008;6:15. doi: 10.1186/1477-7827-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlin K, Mager EM, Allen L, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 13.Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003;74:106–112. doi: 10.1016/s0014-4800(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 14.Wadehra M, Natarajan S, Seligson DB, et al. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer. 2006;107:90–98. doi: 10.1002/cncr.21957. [DOI] [PubMed] [Google Scholar]
- 15.Shimazaki K, Lepin EJ, Wei B, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367–7377. doi: 10.1158/1078-0432.CCR-08-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi H, Asano R, Tsumoto K, et al. A highly effective and stable bispecific diabody for cancer immunotherapy: cure of xenografted tumors by bispecific diabody and T-LAK cells. Cancer Immunol Immunother. 2004;53:497–509. doi: 10.1007/s00262-003-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura N, Kawai S, Kinoshita Y, et al. 2D7 diabody bound to the alpha2 domain of HLA class I efficiently induces caspase-independent cell death against malignant and activated lymphoid cells. Biochem Biophys Res Commun. 2004;325:1201–1209. doi: 10.1016/j.bbrc.2004.10.163. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Stuckert P, Bosch I, Marks JD, Marasco WA. Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther. 2001;8:555–565. doi: 10.1038/sj.cgt.7700337. [DOI] [PubMed] [Google Scholar]
- 19.Orita T, Tsunoda H, Yabuta N, et al. A novel therapeutic approach for thrombocytopenia by minibody agonist of the thrombopoietin receptor. Blood. 2005;105:562–566. doi: 10.1182/blood-2004-04-1482. [DOI] [PubMed] [Google Scholar]
- 20.Wadehra M, Goodglick L, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004;15:2073–2083. doi: 10.1091/mbc.E03-07-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–41100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- 22.Wadehra M, Su H, Gordon LK, Goodglick L, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003;107:129–136. doi: 10.1016/s1521-6616(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 23.Morales SA, Mareninov S, Wadehra M, et al. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009;50:462–469. doi: 10.1167/iovs.07-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadehra M, Forbes A, Pushkarna N, et al. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005;287:336–345. doi: 10.1016/j.ydbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters D, Freund J, Ochs RL. Genome-wide transcriptional analysis of carboplatin response in chemosensitive and chemoresistant ovarian cancer cells. Mol Cancer Ther. 2005;4:1605–1616. doi: 10.1158/1535-7163.MCT-04-0311. [DOI] [PubMed] [Google Scholar]
- 27.Bast RC, Jr., Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eva A, Robbins KC, Andersen PR, et al. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982;295:116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- 29.Wang CX, Wadehra M, Fisk BC, Goodglick L, Braun J. Epithelial membrane protein 2, a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001;97:3890–3895. doi: 10.1182/blood.v97.12.3890. [DOI] [PubMed] [Google Scholar]
- 30.Krysan K, Merchant FH, Zhu L, et al. COX-2-dependent stabilization of survivin in non-small cell lung cancer. Faseb J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- 31.Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seligson D, Horvath S, Huerta-Yepez S, et al. Expression of transcription factor Yin Yang 1 in prostate cancer. Int J Oncol. 2005;27:131–141. [PubMed] [Google Scholar]
- 33.Seligson DB, Hongo F, Huerta-Yepez S, et al. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res. 2007;13:6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 34.Seligson DB, Horvath S, McBrian MA, et al. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174:1619–1628. doi: 10.2353/ajpath.2009.080874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen D, Chang HR, Chen Z, et al. Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun. 2005;326:218–227. doi: 10.1016/j.bbrc.2004.10.214. [DOI] [PubMed] [Google Scholar]
- 36.Shen D, Nooraie F, Elshimali Y, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Minin V, Huang Y, Seligson DB, Horvath S. Statistical methods for analyzing tissue microarray data. J Biopharm Stat. 2004;14:671–685. doi: 10.1081/BIP-200025657. [DOI] [PubMed] [Google Scholar]
- 38.Caplan MJ, Kamsteeg EJ, Duffield A. Tetraspan proteins: regulators of renal structure and function. Curr Opin Nephrol Hypertens. 2007;16:353–358. doi: 10.1097/MNH.0b013e328177b1fa. [DOI] [PubMed] [Google Scholar]
- 39.Hemler ME. Targeting of tetraspanin proteins--potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimazaki K, Chan AM, Moniz RJ, et al. Blockade of epithelial membrane protein 2 (EMP2) abrogates infection of Chlamydia muridarum murine genital infection model. FEMS Immunol Med Microbiol. 2009 doi: 10.1111/j.1574-695X.2008.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271–278. doi: 10.1007/s12026-007-0073-4. [DOI] [PubMed] [Google Scholar]
- 42.Garson K, Shaw TJ, Clark KV, Yao DS, Vanderhyden BC. Models of ovarian cancer--are we there yet? Mol Cell Endocrinol. 2005;239:15–26. doi: 10.1016/j.mce.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10:1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Latorre IJ, Frese KK, Javier RT. Tight Junction Proteins and Cancer. In: Gonzalez-Mariscal L, editor. Tight Junctions. New York: Springer US; 2006. pp. 116–134. [Google Scholar]
- 45.Olafsen T, Tan GJ, Cheung CW, et al. Characterization of engineered anti-p185HER-2 (scFv-CH3)2 antibody fragments (minibodies) for tumor targeting. Protein Eng Des Sel. 2004;17:315–323. doi: 10.1093/protein/gzh040. [DOI] [PubMed] [Google Scholar]