Abstract
Alkaline phosphatase and acid phosphatase are two major enzymatic measures of osteoblastic and osteoclastic activity, respectively. As a result, the preservation of the enzymes in bone specimens to near in vivo accuracy is essential. Despite standardization of the staining process, several factors related to the storage of blocks and slides before sectioning and staining impact the level of enzymes detected in the tissue. Block condition (intact, faced, or unstained) as well as environment (temperature and length of time in storage) affect alkaline phosphatase preservation while the acid phosphatase enzyme remains unaffected. We conclude that to optimally preserve alkaline phosphatase enzyme, methacrylate-embedded undecalcified murine bones should be stored as intact blocks. After sectioning, the faced blocks should be stored at 4°C for optimal enzyme staining of future sections. Furthermore, it is best to stain sections immediately after sectioning.
Keywords: alkaline phosphatase, bone, enzymatic preservation, ethanol, histology
Introduction
Since the 1930s when Robison first hypothesized the role of alkaline phosphatase in bone formation (1), there has been a continued struggle to accurately stain for the enzyme. In 1939, George Gomori (2) demonstrated that paraffin embedding did not destroy alkaline phosphatase activity in soft tissue, but 10 years later his technique still was being questioned (3). The Menten modification for alkaline phosphatase staining, which became accepted by the late 1960s (4,5), is based on hydrolysis of naphthyl phosphate resulting in the naphthol coupling to an azo dye and depositing an insoluble pigment at the site of the enzyme.
Like alkaline phosphatase, acid phosphatase is an enzyme that has been studied extensively, and articles have been published on the topic since the 1950s. In 1964, Farnes and Barker (6) applied the enzyme stain to the analysis of the composition of different cell types in bone marrow. Although the preservation of acid phosphatase staining has been studied in regard to fixation and processing (7–12), embedding material (13–19), and substrates used (20–26), there has not been a study to determine the storage conditions of blocks and unstained slides for optimal preservation of the enzyme. Accurate representation of alkaline and acid phosphatase enzymes in bone requires attention to more than the fixation, processing, and staining protocols. As demonstrated here, the storage conditions of the blocks and slides play a crucial role in whether alkaline phosphatase activity will be demonstrated.
Materials and Methods
Specimens
Intact tibiae and femurs from 12- to 16-week-old GATA-1-deficient mice (kindly donated by Dr. Stuart Orkin) that are maintained on the C57BL/6 background were used for this study because they exhibit increased indices for bone formation and resorption (27). The bones were stripped of soft tissue, and placed in ~20 mL of 70% ethyl alcohol (EtOH) for 60 h at 4°C (approximately a 1:20 specimen:fixative ratio). Table 1 summarizes the processing schedule.
Table 1.
MMA processing schedule
| Solution | Time in solution | Temp |
|---|---|---|
| Fixative (70% EtOH) | 60 h | 4°C |
| 70% acetone | 24 h | 4°C |
| 90% acetone | 1 h | 4°C |
| 100% acetone | 1 h | 4°C |
| 100% acetone | 1 h | 4°C |
| 85% MMA infiltration solution (vacuum desiccator) | 48 h | 4°C |
| MMA embedding solution (waterbath in radiant oven) | 48 h | 37°C |
Tissue Processing/Handling
After fixation, tibiae and femurs were transferred to 70% acetone for 24 h. After 24 h, the tibiae and femurs were dehydrated in graded acetones (90% 1X, 100% 2X for 1 h each). All of the aforementioned steps were performed at 4°C.
As has been detailed elsewhere (28–30), after dehydration, tibiae and femurs were placed in a 20-mL glass vial containing 15 mL of infiltration medium containing 85% destabilized methylmethacrylate (MMA; Sigma, St. Louis, MO), 15% dibutyl phthalate (Sigma), and 0.15% benzoyl peroxide (Polysciences, Inc., Warrington, PA) and placed in a vacuum desiccator.
After 48 h of infiltration in a vacuum desiccator at 4°C, tibiae and femurs were removed from infiltration MMA and were placed on prepolymerized bases/layers, covered with 8 mL of fresh catalyzed MMA, and incubated for 2 d in a water bath, which was placed in a 37°C radiant heat oven (Labline, Melrose Park, IL). Glass vials were removed from the oven, cooled at −20°C for 1 h, and the specimen blocks were removed by breaking the glass vials.
Storage of Slides and Blocks
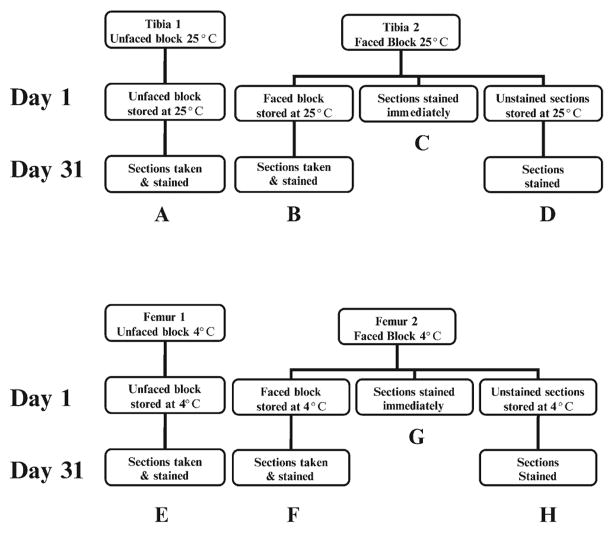
Figure 1 is a flow diagram that summarizes the groups examined for this study. From each animal, one femur was stored at 4°C in a faced block and the other femur was stored at 4°C in an unfaced block. Likewise, one tibia was stored at room temperature in a faced block and the other tibia was stored at room temperature in an unfaced block. From each faced block, some sections were mounted on slides and stored at room temperature or 4°C for 1 month whereas others were sectioned and stained immediately. For the purpose of this study, we considered room temperature to be 25°C. In addition, both the faced blocks used above and unfaced blocks were stored at 25°C or at 4°C for 1 month. At the end of 1 month, the unfaced blocks were sectioned, and faced blocks were further sectioned and immediately stained as described to follow.
Figure 1.
Flow diagram of storage conditions. Letters refer to the photographs of stained sections found in Figure 2.
Facing Blocks and Sectioning
To face blocks, the MMA blocks containing bone were trimmed and sanded to the center of the longitudinal bone on the Buehler Metaserv Grinder-Polisher (Buehler UK Ltd, Coventry, England). For these studies, 4-μm sections were obtained with the use of a Leica™ 2165 Microtome (Leica, Heidelberg, Germany), and a tungsten-carbide knife, D-profile (Dorn/Hart Microedge, Villa Park, IL). Sections were then placed on gelatin-chromium-alum coated slides and incubated overnight at 37°C to adhere sections to slides as previously described (28,31).
Alkaline Phosphatase Staining
For alkaline phosphatase staining, sections mounted on gelatin/chromium/alum–coated slides were deplastified in two changes of 100% acetone (5 min each) and then rehydrated in 70% EtOH, 40% EtOH, and distilled water (one change each). After rehydration, slides were incubated at room temperature for 60 min in Tris buffer, pH 9.4.
The staining solution was prepared as described by Baron et al. (31): 40 mg of naphthol ASBI phosphate (Sigma) was dissolved in 2 mL of dimethylformamide (Sigma). Separately, 40 mg of Fast Blue RR salt (Sigma) was dissolved in 2 mL of dimethylformamide. To prepare the final staining solution, 2 mL of naphthol ASBI solution was combined with 2 mL of Fast Blue RR salt solution and 0.195 g of MgCl2 catalyst. This was then added to 35 mL of Tris buffer (12.1 g Trisma Base [Sigma] dissolved in 500 mL dH2O, pH 9.4). The solution was filtered before use and was prepared fresh as the substrate deteriorates over time. After incubation in Tris buffer, slides were then incubated for 60 min in the staining solution in a 37°C oven. After incubation in stain, slides were rinsed in distilled water and mounted with aqueous Crystal Mount™ (Biomeda Corp., Foster City, CA). Once dried, the slides were coverslipped with Mounting Medium™ (Richard-Allan Scientific, Kalamazoo, MI). Alkaline phosphatase–rich structures are stained a dark purple color.
Acid Phosphatase Staining
Acid phosphatase staining was performed on sections attached to gelatin/chromium/alum-coated slides that were deplastified as described for alkaline phosphatase staining. Stock solutions of pararosaniline, sodium nitrite, naphthol ASTR phosphate, acetate buffer, and manganese sulfate were prepared and combined to make the acid phosphatase stain as described by Baron et al (31). After rehydration, slides were blotted dry around sections and several drops of stain were placed on each section to cover it entirely. Slides were incubated in a moisture chamber in a 37°C radiant heat oven for 1 h. After incubation, slides were rinsed for 2 min in running distilled water, and then placed in 70% EtOH for 30 min at room temperature. Slides were then washed 2X in distilled water for 2 min each and counterstained with toluidine blue (Fisher Scientific, Pittsburgh, PA), pH 3.7, for 2 min. Counterstaining was followed by a quick dehydration. Slides were dipped in tertiary butyl alcohol (butanol) 2X, butanol/toluene (50:50) 1X, and toluene 2X, before being mounting with Mounting Medium™. Osteoclasts that are rich in acid phosphatase will appear red.
Results
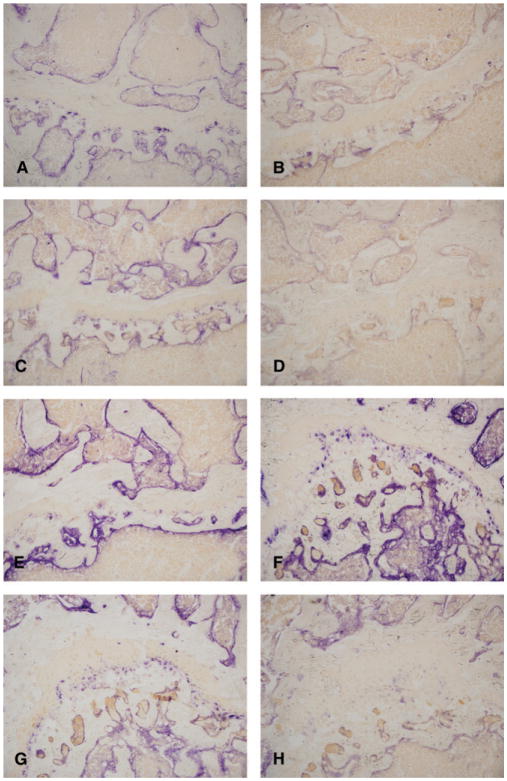
For this study, we believed it would be best to compare the effects of storage conditions on enzymatic preservation in bones taken from the same animals to eliminate interanimal variation. Because we needed to have four bones stored from each mouse (see Figure 1), we first confirmed that staining results were comparable in tibiae and femurs. The similarity in staining quality between femurs and tibiae can be detected by examining the initial section taken from the femur (Figure 2G) and tibia (Figure 2C), which appear virtually identical in terms of alkaline phosphatase staining.
Figure 2.
Alkaline phosphatase stain. (A) Section taken from an unfaced block after 1 month storage at 25°C. (B) Section taken from a faced block at the end of 1 month storage at 25°C. (C) Section taken from a faced block at the beginning of 1 month, before storage at 25°C. (D) Section stored on slide, unstained at 25°C for 1 month. (E) Section from unfaced block after 1 month storage at 4°C. (F) Section from a faced block after 1 month of storage at 4°C. (G) Section from a faced block before storage at 4°C. (H) Section stored on slide, unstained at 4°C for 1 month. Original magnification for all micrographs, ×200.
For the remainder of this study, our results are focused on comparing several storage conditions. These storage conditions are diagrammed in Figure 1, and representative micrographs from the same animal are shown in Figure 2. Panels A–H on Figure 1 correspond with the identical letters in Figure 2 and Table 2. The results of this study focus on five main comparisons of enzyme activity. First, the change in activity from the first section taken (C and G) to the section cut 1 month later (from the same block, B and F) was compared to determine whether enzymatic preservation was altered when blocks were faced and stored (C vs. B at 25°C and G vs. F at 4°C). Second, the difference in enzyme staining that occurred between the first section taken (C and G) and the section that was stored on the slide and stained 1 month later (D and H) was examined to determine whether enzymatic levels were altered in unstained slides that were stored as opposed to being stained immediately (C vs. D at 25°C and G vs. H at 4°C). Third, we compared the first and second storage conditions, which examined the difference between the section that had been stored on a slide for a month before staining (D and H) and the section taken from the same block 1 month after it had been faced (B and F) to determine whether the enzyme was better preserved in a faced block or in a stored slide (B vs. D at 25°C and F vs. H at 4°C). Fourth, the sections taken from an unfaced block (A and E) were compared with the first sections (C and G) taken that were stained immediately (A vs. C at 25°C and E vs. G at 4°C). Fifth, we compared the sections that were taken from a block 1 month after being faced (B and F) to the sections taken from an unfaced block (A and E) containing a bone taken from the same animal to determine whether enzyme was better preserved in faced or unfaced blocks (B vs. A at 25°C and F vs. E at 4°C). Furthermore, as indicated previously, we compared the effect of temperature (4°C vs. 25°C) on each storage condition. All comparisons were qualitative, including the presence or absence of stain and the intensity of staining. The results were comparable between each mouse in the study; representative slides taken from a single mouse can be found in Figure 2 (all pictures were taken at 200× magnification).
Table 2.
Summary of alkaline phosphatase staining results after storage
| Grading |
|||
|---|---|---|---|
| 0 = same as control |
|||
| Figure panel | Storage condition | Temp (°C) | >0 = better than control |
| <0 = worse than control | |||
| A | Unfaced block | 25 | 0 |
| B | Faced block | 25 | −1 |
| C (control 25°C) | None (control) | 25 | 0 |
| D | Unstained slides | 25 | −2 |
| E | Unfaced block | 4 | 1 |
| F | Faced block | 4 | 1+ |
| G (control 4°C) | None (control) | 4 | 0 |
| H | Unstained slides | 4 | −1 |
The first comparison of the initial (C and G) and subsequent sections of a faced block (B and F) showed that, at 4°C, the alkaline phosphatase preservation in the faced block was as good or better than that seen in sections stained immediately from the initial cut (Figure 2G vs. 2F). However, the faced block stored at 25°C had obviously decreased alkaline phosphatase activity one month later (Figure 2C vs. 2B).
The second comparison between the slides stained immediately (C and G) and those stained after storage for 1 month (D and H) demonstrated that storage of slides at either temperature had less alkaline phosphatase activity than the slides stained immediately (Figure 2C vs. 2D and Figure 2G vs. 2H). Importantly, storage of the slides at 4°C (Figure 2H) better preserved the alkaline phosphatase activity than did storage at 25°C (Figure 2D).
The third comparison between the enzyme preservation after a month in the faced block (B and F) and the preservation in the stored slides (D and H) showed that the preservation in the faced block and stored slides was greater in those stored at 4°C (Figure 2F and 2H). The slides stored for 1 month at 4°C before staining showed decreased preservation as compared with the sections taken from the faced block after 1 month of storage (Figure 2F vs. 2H). Importantly, the faced block stored at 25°C had significantly less alkaline phosphatase preservation than the faced block stored at 4°C (Figure 2B vs. 2F and Figure 2D vs. 2H).
In the fourth comparison, the sections taken from the unfaced block (A and E) were compared with the sections stained immediately after removal from the faced block (C and G). At 25°C, the staining intensity of sections stained immediately (Figure 2C) and those taken from the unfaced block (Figure 2A) appeared identical. At 4°C the section taken from the unfaced block stored for 1 month (Figure 2E) appeared to have a darker alkaline phosphatase stain than the sections stained immediately (Figure 2G).
The last comparison between the preservation in faced blocks (B and F) compared with unfaced blocks (A and E) demonstrated that the faced (Figure 2E) and unfaced blocks (Figure 2F) stored at 4°C were either similar with regard to alkaline phosphatase staining or the staining in the faced block might be slightly better (Figure 2E vs. 2F). Among those stored at 25°C, the unfaced block (Figure 2A) resulted in much better preservation than did the faced block (Figure 2B); however, the unfaced block stored at 25°C still had less alkaline phosphatase activity than the unfaced block stored at 4°C (Figure 2A vs. 2E).
A summary of these alkaline phosphatase results can be found in Table 2. Unlike alkaline phosphatase, the acid phosphatase enzyme was equally well preserved during the month in each storage condition (results not shown).
Discussion
To ensure the quality of the comparison between tibiae and femur results, a pilot study was performed (results not shown) in which both tibiae and femur were stored at 1, 3, and 6 months in both faced and unfaced blocks at 4°C and at 25°C. The study, including approximately 60 murine bones, found that the rate of enzyme decomposition was identical in both tibiae and femurs when exposed to the same conditions. The decrease in alkaline phosphatase enzyme activity was visually apparent after 1 month and continued to decrease after 3 and 6 months, whereas the acid phosphatase activity remained unchanged. In the interest of simplifying the results, we presented this study with a smaller sample size and only compared the results at the 1 month point. In addition, each storage condition was represented within the same animal to reduce the likelihood of biased results due to inter-individual variation.
There are two intriguing findings upon which we will speculate here. The first is the observation that storage of faced and unfaced blocks at 4°C for 1 month before sectioning resulted in better alkaline phosphatase staining than in sections cut and stained immediately. Several theories were considered, but none substantiated, to explain the results that were found. Although temperature has not formerly been proven to have a conclusive effect on alkaline phosphatase preservation in tissues (32), our results suggest that alkaline phosphatase is better preserved at 4°C as compared with 25°C. The polymerization of methacrylate is an exothermic reaction (33) that may continue beyond the time at which the block is typically cut. Thus, although not conclusive, it is possible that storage at 4°C reduces the negative effect of heat (resulting from the continued exothermic polymerization during storage) and therefore allows for better preservation of alkaline phosphatase. This theory would explain why staining in conditions E, F, and H were better than A, B, and D, respectively and may also explain why staining in E and F was better than in the control sections (G). The second unanticipated finding is that at 4°C it appears that storage of faced blocks (F) as opposed to unfaced blocks (E) results in slightly better alkaline phosphatase staining. In critically analyzing the difference in storage conditions, we can identify at least two possible factors: exposure of the tissue to air (faced block) and exposure of the tissue to humidity (faced block). However, the mechanisms whereby alkaline phosphatase staining is enhanced in the 4°C faced blocks remain unclear.
Conclusions
In summary, storage conditions can impact the detection of some enzymes. Here, we found that acid phosphatase activity was unaltered by the storage conditions examined, whereas alkaline phosphatase activity was significantly impacted in the same specimens. Thus, for the best alkaline phosphatase preservation, MMA-embedded murine bones should be stored in unfaced or faced blocks at 4°C. Although alkaline phosphatase staining was best in unfaced and faced blocks stored at 4°C and sectioned 1 month later, unfaced blocks stored at 25°C and sectioned 1 month later resulted in similar staining to sections stained immediately after sectioning. The pilot study whose data are not shown found that unfaced blocks stored at 25°C did not lose enzyme activity after six months, resulting in the conclusion that if blocks must be stored at 25°C, it would be best to store them unfaced. However, if the block has been faced and it is necessary to return for more sections at a later date, it would be best to store the block at 4°C. Furthermore, for optimal alkaline phosphatase enzyme staining, slides should be stained immediately, because even 4°C storage temperature does not adequately protect the alkaline phosphatase enzyme on an unstained stored slide.
Acknowledgments
The authors thank Mrs. Christiane E. Coady, H.T. for her technical assistance and Mrs. Tracy Nelson for maintenance of the mouse colony. The authors also thank Dr. Stuart Orkin for the donation of GATA-1 deficient mice.
This work was supported by the Department of Orthopaedics and Rehabilitation at Yale University School of Medicine and by NIH AR46032, the Physiology Core of the Yale Core Center for Musculoskeletal Disorders.
References
- 1.Robison R, Soames KM. Calcification in vitro. Lister Institute; London: 1930. CCXVI: The possible significance of hexosephosphoric esters in ossification. VIII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomori G. Microtechnical determination of phosphatase in tissue sections. Proc Soc Exp Biol Med. 1939;42:23–29. [Google Scholar]
- 3.Jacoby F, Martin BF. The histochemical test for alkaline phosphatase. Nature. 1949;163:875. doi: 10.1038/163875a0. [DOI] [PubMed] [Google Scholar]
- 4.Pearse AGE. Histochemistry, Theory and Applied. 3. Boston: Little Brown; 1968. [Google Scholar]
- 5.Morrison GR, Karl IE, Schwartz R, Shank RE. The quantitative histochemistry of the normal human liver lobule. J Clin Lab Med. 1965;65:248–256. [PubMed] [Google Scholar]
- 6.Farnes P, Barker BA. Cytochemical studies of human bone marrow fibroblast-like cells. Am J Pathol. 1964;44:481–489. [PMC free article] [PubMed] [Google Scholar]
- 7.Manning JP, Butler MC. Observations on the preservation of acid phosphatase in hard tissues with simultaneous fixation and decalcification and carbowax embedding. Acta Histochem. 1965;22:383–384. [PubMed] [Google Scholar]
- 8.Doty SB, Schofield BH. Enzyme histochemistry of bone and cartilage cells. Prog Histochem Cytochem. 1976;8:1–38. doi: 10.1016/s0079-6336(76)80010-1. [DOI] [PubMed] [Google Scholar]
- 9.Beckstead JH, Halverson PS, Ries CA, Bainton DF. Enzyme histochemistry and immunohistochemistry on biopsy specimens of pathologic human bone marrow. Blood. 1981;57:1088–1098. [PubMed] [Google Scholar]
- 10.Gruber HE, Marshall GJ, Nolasco LM, Kirchen ME, Rimoin DL. Alkaline and acid phosphatase demonstration in human bone and cartilage: Effects of fixation interval and methacrylate embedments. Stain Technol. 1988;63:299–306. doi: 10.3109/10520298809107604. [DOI] [PubMed] [Google Scholar]
- 11.Kacena MA, Troiano NW, Coady CE, Horowitz MC. HistoChoice as an alternative to formalin fixation of undecalcified bone specimens. Biotech Histochem. 2004;79:185–190. doi: 10.1080/10520290400015506. [DOI] [PubMed] [Google Scholar]
- 12.Laboux O, Dion N, Arana-Chavez V, Ste-Marie LG, Nanci A. Microwave irradiation of ethanol-fixed bone improves preservation, reduces processing time, and allows both light and electron microscopy on the same sample. J Histochem Cytochem. 2004;52:1267–1275. doi: 10.1177/002215540405201003. [DOI] [PubMed] [Google Scholar]
- 13.Troyer H, Nusbickel FR. Enzyme histochemistry of undecalcified bone and cartilage embedded in glycol methacrylate. Acta Histochem. 1975;53:198–202. [PubMed] [Google Scholar]
- 14.Vykoupil KF, Thiele J, Georgii A. Histochemical and immunohistochemical techniques on acrylate embedded bone biopsies. Blut. 1976;32:215–218. doi: 10.1007/BF00995915. [DOI] [PubMed] [Google Scholar]
- 15.Horton WA, Dockery N, Sillence D, Rimoin DL. An embedding method for histochemical studies of undecalcified skeletal growth plate. Stain Technol. 1980;55:19–29. doi: 10.3109/10520298009067891. [DOI] [PubMed] [Google Scholar]
- 16.Van Noorden CJ, Vogels IM. Enzyme histochemical reactions in unfixed and undecalcified cryostat sections of mouse knee joints with special reference to arthritic lesions. Histochemistry. 1986;86:127–133. doi: 10.1007/BF00493377. [DOI] [PubMed] [Google Scholar]
- 17.Cole AA, Walters LM. Tartrate-resistant acid phosphatase in bone and cartilage following decalcification and cold-embedding in plastic. J Histochem Cytochem. 1987;35:203–206. doi: 10.1177/35.2.3540104. [DOI] [PubMed] [Google Scholar]
- 18.Aaron JE, Carter DH. Rapid preparation of fresh-frozen undecalcified bone for histological and histochemical analysis. J Histochem Cytochem. 1987;35:361–369. doi: 10.1177/35.3.2434557. [DOI] [PubMed] [Google Scholar]
- 19.Onetti Muda A, Riminucci M, Bianco P. Freeze-drying of bone tissue: Immunocytochemistry and enzyme histochemistry on paraffin embedded and low-temperature resin embedded specimens. Histochemistry. 1992;98:283–288. doi: 10.1007/BF00270011. [DOI] [PubMed] [Google Scholar]
- 20.Kaplow LS, Burstone MS. Cytochemical demonstration of acid phosphatase in hematopoietic cells in health and in various hematological disorders using azo dye techniques. J Histochem Cytochem. 1964;12:805–811. doi: 10.1177/12.11.805. [DOI] [PubMed] [Google Scholar]
- 21.Jeffree GM. The histochemical differentiation of various phosphatases in a population of osteoclasts by a simultaneous coupling method using different diazonium salts, with observations on the presence of inhibitors in stable diazonium salts. Histochem J. 1970;2:231–242. doi: 10.1007/BF01003472. [DOI] [PubMed] [Google Scholar]
- 22.McDonald DF, Schofield BH, Geffert MA, Coleman RA. A comparative study of new substrates for the histochemical demonstration of acid phosphomonoesterase activity in tissues which secrete acid phosphatase. J Histochem Cytochem. 1980;28:316–322. doi: 10.1177/28.4.6246163. [DOI] [PubMed] [Google Scholar]
- 23.Chappard D, Alexandre C, Riffat G. Histochemical identification of osteoclasts. Review of current methods and reappraisal of a simple procedure for routine diagnosis on undecalcified human iliac bone biopsies. Basic Appl Histochem. 1983;27:75–85. [PubMed] [Google Scholar]
- 24.Mostafa YA, Meyer RA, Jr, Latorraca R. A simple and rapid method for osteoclast identification using a histochemical method for acid phosphatase. Histochem J. 1982;14:409–413. doi: 10.1007/BF01011853. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Sanghvi R, Burnell JM, Howard GA. Simultaneous demonstration of bone alkaline and acid phosphatase activities in plastic-embedded sections and differential inhibition of the activities. Histochemistry. 1987;86:559–565. doi: 10.1007/BF00489547. [DOI] [PubMed] [Google Scholar]
- 26.Andersson GN, Marks SC., Jr Tartrate-resistant acid ATPase as a cytochemical marker for osteoclasts. J Histochem Cytochem. 1989;37:115–117. doi: 10.1177/37.1.2461980. [DOI] [PubMed] [Google Scholar]
- 27.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, et al. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- 28.Kacena MA, Troiano NW, Wilson KM, Coady CE, Horowitz MC. Evaluation of two different methylmethacrylate processing, infiltration, and embedding techniques on the histological, histochemical, and immunohistochemical analysis of murine bone specimens. J Histotechnol. 2004;27:119–130. [Google Scholar]
- 29.Kacena MA, Troiano NW, Coady CE, Horowitz MC. Decalcification of mounted bone sections enhances immunohistochemical staining. J Histotechnol. 2003;26:105–109. [Google Scholar]
- 30.Kacena MA, Troiano NW, Coady CE, Horowitz MC. Effects of ethanol post-fixation on the histological, histochemical, and immunohistochemical analysis of murine bone specimens. J Histotechnol. 2004;27:15–20. [Google Scholar]
- 31.Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker R, editor. Bone Histomorphometry: Techniques and Interpretation. CRC Press Inc; Boca Raton, FL: 1983. [Google Scholar]
- 32.McComb RB, Bowers GN, Jr, Posen S. Alkaline Phosphatase. Plenum Press; New York: 1979. [Google Scholar]
- 33.Erben RG. Embedding of bone samples in methylmethacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem. 1997;45:307–313. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]