Abstract
Rift Valley fever (RVF) is an important viral zoonotic disease in Africa with periodic outbreaks associated with severe disease, death, and economic hardship. During the 2006–2007 outbreaks in Eastern Africa, postmortem and necropsy tissue samples from 14 animals and 20 humans clinically suspected of RVF were studied with histopathologic evaluation and immunohistochemical (IHC) assays. Six animal and 11 human samples had IHC evidence of Rift Valley fever virus (RVFV) antigens. We found that extensive hepatocellular necrosis without prominent inflammatory cell infiltrates is the most distinctive histopathologic change in liver tissues infected with RVFV. Pathologic studies on postmortem tissue samples can help establish the diagnosis of RVF, differentiating from endemic diseases with clinical manifestations similar to RVF, such as malaria, leptospirosis, or yellow fever.
Introduction
Rift Valley fever (RVF) is a zoonotic, arthropod-borne viral disease that affects domestic animals and humans. The disease is caused by the RVF virus (RVFV), a member of the genus Phlebovirus in the family Bunyaviridae. Rift Valley fever is reported mainly in regions of eastern and southern Africa.1–3 Outbreaks of RVF have also occurred in Egypt,4 Madagascar,5 and in the Arabian peninsula.6 From December 2006 to January 2007, an outbreak of RVF was identified in Kenya, Tanzania, and Somalia with several hundreds of animal and human deaths.7,8 Pathologic studies can help confirm the clinical diagnosis and further the understanding of the disease pathogenesis and are very useful in outbreak investigations. Previous pathologic studies of RVF infection on fatal human and animal cases are few, and have contained limited microscopic descriptions. Postmortem tissue samples were obtained from animal carcasses in the field and humans who died with suspected RVF during this outbreak. This report describes the histopathologic and immunohistochemical (IHC) findings of studies performed on human and animal postmortem tissue samples.
Materials and Methods
Various postmortem tissue samples from 14 animals (Table 1) and 20 humans (Table 2) clinically suspected of having RVF infection were fixed in formalin and shipped to the Infectious Diseases Pathology Branch, Centers for Disease Control and Prevention (CDC) for pathologic studies. The samples were embedded in paraffin and tissue sections were stained with hematoxylin and eosin for routine histopathologic evaluation. Immunohistochemical assays for RVFV were performed by using a colorimetric immunoalkaline phosphatase method as previously described.9 Briefly, IHC was performed on 3-μm sections that were deparaffinized, rehydrated, and placed in a Dako Autostainer (Dako, Carpinteria, CA). Sections were incubated for 1 hour with a polyclonal rabbit anti-RVFV antibody and a monoclonal mouse anti-RVFV antibody. Optimal dilutions of the antibodies had been determined by previous experiments at CDC on positive control tissue samples. After incubation with the primary antibody, slides were washed, and the LSAB2 universal alkaline phosphatase system (Dako) was used for colorimetric detection. Sections were then counterstained with Mayer's hematoxylin (Fisher Scientific International Inc., Hampton, NH). Appropriate positive and negative controls were run in parallel. Other IHC tests for Plasmodium falciparum,10 human herpes simplex viruses, and Leptospira spp.,11 were performed when indicated by compatible histopathologic findings.
Table 1.
Histopathologic findings and immunohistochemical (IHC) results of animal tissue samples
| No. | Animal | Tissue | Histopathologic findings | IHC result for RVF virus |
|---|---|---|---|---|
| 1 | Sheep | Liver | Necrosis with focal inflammation | Positive |
| 2 | Sheep | Spleen | Autolysis | Positive |
| 3 | Cow | Kidney, liver, spleen | Focal inflammation in kidney; autolysis in liver; no significant change in spleen | Negative |
| 4 | Cow | Liver | Autolysis | Positive |
| 5 | Cow | Liver | Necrosis | Positive |
| 6 | Cow | Liver, kidney, spleen, muscle, lung | Necrosis in liver; no significant change in other organs | Positive in liver |
| 7 | Cow | Kidney, liver | Focal inflammation in kidney; autolysis in liver | Negative |
| 8 | Cow | Liver | Autolysis | Positive |
| 9 | Cow | Liver, muscle | Focal inflammation in liver | Negative |
| 10 | Goat | Liver | Autolysis | Negative |
| 11 | Goat | Liver | Autolysis | Negative |
| 12 | Cow | Liver | No significant change | Negative |
| 13 | Goat | Liver | No significant change | Negative |
| 14 | Goat | Kidney | Focal inflammation | Negative |
Table 2.
Histopathologic findings and IHC results of human tissue samples*
| No. | Tissue | Histopathologic findings | IHC results |
|---|---|---|---|
| 1 | Liver | Extensive necrosis | Positive for RVF |
| 2 | Liver | Extensive necrosis | Positive for RVF |
| 3 | Liver | Extensive necrosis | Positive for RVF |
| 4 | Liver, lung, spleen | Fatty changes in liver; intra-alveolar edema in lung; no significant change in spleen | Negative for RVF |
| 5 | Liver, kidney | Autolysis | Negative for RVF |
| 6 | Liver | Extensive necrosis | Positive for RVF |
| 7 | Liver | Extensive necrosis | Positive for RVF |
| 8 | Lung, heart, liver, pancreas, kidney, skin | Extensive necrosis in liver; focal interstitial inflammation, acute tubular necrosis and fibrinous casts in kidney; diffuse alveolar damage in lung; autolysis in pancreas; no significant change in heart and skin | Positive for RVF in liver and kidney |
| 9 | Liver | No significant change | Negative for RVF and Plasmodium falciparum |
| 10 | Liver | No significant change | Negative for RVF and P. falciparum |
| 11 | Liver | Fatty metamorphosis | Negative for RVF, P. falciparum, Leptospira, and herpes simplex virus |
| 12 | Liver | No significant change | Negative for RVF and P. falciparum |
| 13 | Liver | Extensive necrosis | Positive for RVF |
| 14 | Liver | Extensive necrosis | Negative for RVF; positive for herpes simplex virus |
| 15 | Liver | Extensive necrosis | Positive for RVF |
| 16 | Liver | Extensive necrosis | Positive for RVF |
| 17 | Liver | Extensive necrosis | Positive for RVF |
| 18 | Liver | No significant change | Negative for RVF |
| 19 | Liver | Extensive necrosis | Positive for RVF |
| 20 | Muscle | Scattered intra-erythrocytic organisms; no other significant change | Negative for RVF; positive for P. falciparum |
IHC = immunohistochemical; RVF = Rift Valley fever.
Results
Liver was the most frequently available tissue from animals and humans; 12 of the 14 animal samples and 19 of the 20 human samples contained liver tissue (Tables 1 and 2). Only muscle tissue was available from one human specimen. The most significant histopathologic finding was extensive hepatocellular necrosis with scattered acidophilic bodies in three (25%) of the animal specimens (Figure 1A) and 12 (60%) of the human specimens (Figure 1C). There were no prominent inflammatory cell infiltrates seen in liver tissue in association with the necrosis. Extensive autolysis, present in samples from seven animals and one human, precluded an accurate histopathologic assessment. One human sample showed hepatocellular necrosis with abundant Cowdry type A intranuclear inclusions and multinucleated cells (Figure 1E) highly suspicious for a herpetic infection. Only two human kidney samples were available for examination of which one sample had undergone autolysis and was difficult to evaluate, whereas the other sample showed focal interstitial inflammation, acute tubular necrosis, and fibrinous casts in the tubular lumen (Figure 2A). Immunohistochemical evidence of RVFV was present in six animal samples with three showing extensive hepatocellular necrosis (Figure 1B), two showing hepatic autolysis, and one demonstrating splenic autolysis. For the human samples, IHC evidence of RVFV was found in 11 of the 12 (92%) human liver specimens that had extensive hepatocellular necrosis (Figure 1D). Immunostaining of viral antigens was observed mainly within necrotic and adjacent hepatocytes, and in scattered Kupffer cells in all 11 human samples with IHC evidence of RVFV. Immunostaining of RVFV antigens was also present in the focal renal tubular epithelial cells of the only human kidney sample without autolysis (Figure 2B). Human herpes simplex virus was identified by the IHC method in one RVFV-negative sample that also had hepatocellular necrosis (Figure 1F). Plasmodium falciparum was detected in the red blood cells of the case that only had skeletal muscle available for testing (Figure 2C and D).
Figure 1.
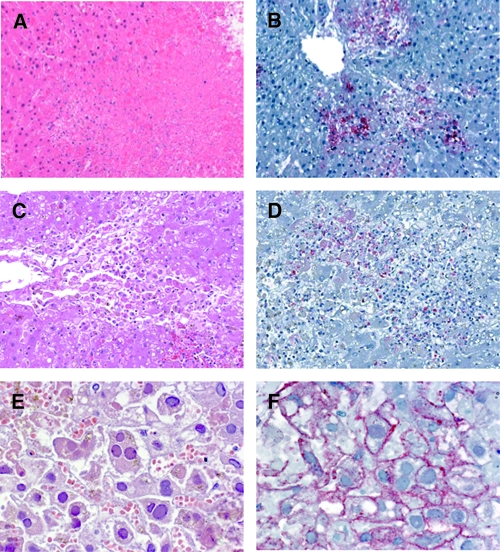
(A) Extensive hepatocellular necrosis with acidophilic bodies in a bovine liver. Hematoxylin-eosin staining. Original magnification, ×100. (B) Positive staining of Rift Valley fever (RVF) viral antigens in necrotic hepatocytes of the bovine liver. Immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain. Original magnification, ×100. (C) Extensive hepatocellular necrosis with acidophilic bodies in a human liver. Hematoxylin-eosin staining. Original magnification, ×200. (D) Positive staining of RVF viral antigens in necrotic hepatocytes of the human liver. Immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain. Original magnification, ×200. (E) Extensive hepatocellular necrosis with Cowdry type A intranuclear inclusions and multinucleated cells in a human liver. Hematoxylin-eosin staining. Original magnification, ×400. (F) Positive staining of human herpes simplex viral antigens in hepatocytes of the human liver. Immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain. Original magnification, ×400.
Figure 2.
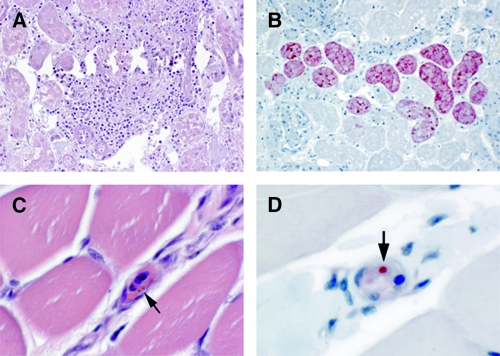
(A) Focal interstitial inflammation, acute tubular necrosis, and fibrinous casts in a human kidney. Hematoxylin-eosin staining. Original magnification, ×100. (B) Positive staining of Rift Vallye fever (RVF) viral antigens in renal tubules of a human kidney. Immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain. Original magnification, ×100. (C) Intra-erythrocytic parasites (arrow) in the blood vessel of a human muscle. Hematoxylin-eosin staining. Original magnification, ×630. (D) Positive staining of Plasmodium falciparum antigen in red blood cell (arrow) of a human muscle. Immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain. Original magnification, ×630.
Discussion
Limited pathologic studies of RVF infection, based on a few human cases, field animal samples, and experimental animal specimens, have been reported previously. Abdel-Wahab and others12 observed severe liver necrosis, interstitial pneumonia, and myocardial degeneration in postmortem tissues of two human cases. The RVFV was isolated from post-mortem samples of liver, cerebro-spinal fluid, pericardial and pleural fluid, and from a throat swab of these two cases. Arborio and Hall13 showed RVF viral antigen in fixed liver tissue of a human case by the immunoperoxidase method. The RVF viral antigen was present mainly in the cytoplasm of hepatocytes adjacent to areas of hepatocellular necrosis. They did not observe immunostaining in other cell types. Coetze reported massive diffuse necrosis of hepatocytes as the most characteristic histopathologic finding in new-born lambs infected with RVFV.14,15 Other less common findings included bile thrombi and intranuclear inclusions in hepatocytes. Lymphoid depletion in lymph nodes and spleen has been seen in many animal samples examined, and similar histopathologic findings have also been observed in cattle, calves, and aborted fetuses with RVFV infection.16 Autopsy samples from rhesus monkeys experimentally infected with RVFV displayed severe liver necrosis as the major microscopic finding.17,18 Van der Lugt and others19 studied the distribution of RVF viral antigens by immunoperoxidase in liver, spleen, lymph node, lung, and kidney of eight experimentally infected new-born lambs and four new-born lambs that died of RVF. Viral antigens were most prominent in the liver and were detected in the cytoplasm of hepatocytes at early stage of infection. Viral antigens were consistently present in the cytoplasm of large numbers of degenerated or necrotic hepatocytes and in acidophilic bodies at a later stage of infection. Immunostaining was rarely observed in the nucleus of hepatocytes and few cells stained positively in the spleen, lymph node, lung, and kidney. The authors concluded that hepatocytes constitute the primary site of RVFV replication in infected animals.
Our studies are consistent with other reports identifying extensive hepatocellular necrosis as the most prominent histopathologic change in animals and humans infected with RVFV and this histopathologic feature correlates well with the IHC test results. However, unlike some previous reports, we did not observe a particular zonal distribution or conspicuous inclusions. No prominent inflammatory reaction associated with necrosis was observed in the liver. Similar hepatic pathology can be seen in hemorrhagic fevers caused by other viruses, including yellow fever virus,20 Lassa fever virus,21 Crimean-Congo hemorrhagic fever virus,22 Marburg virus,23 and Ebola virus.24 As with the geographic ranges of more common febrile illnesses, many of these exotic infections occur sympatrically with RVFV in Africa; thus, specific diagnostic tests are critical to identify the causative agent. As we observed, the IHC assay can detect viral antigens in tissues, and is a specific diagnostic method for samples obtained from patients and animals dying from clinically suspected RVF. Abundant immunostaining was present within necrotic and neighboring hepatocytes, and in scattered Kupffer cells. In our studies, immunostaining for RVF viral antigens was seen in focal renal tubular epithelial cells in one single human kidney sample. This observation suggests possible excretion of RVF viral antigens in urine; however, its validity and clinical importance warrants further investigation. Madani and others6 reported the epidemiological, clinical, and laboratory characteristics of the RVF epidemic that occurred in Saudi Arabia, 2001. In their cohort study, renal impairment or failure was found in almost one-third of the patients with RVF. Whether this clinical observation is related to the renal damage by RVFV infection or a consequence of systemic shock is unknown. The RVFV antigen in animal kidney was described in a few reports,19,25 but has never been described in human sample. Our observation of RVFV antigen in the kidney is a novel finding, although it was seen in only one human sample in this report.
Localization of viral antigens can also elucidate the pathogenetic mechanisms involved in certain viral hemorrhagic fevers.22,26,27 Despite similar clinical manifestations among various viral hemorrhagic fevers, there are clear differences in their pathogenesis and clinical progression.28,29 The lytic virus-cell interaction suggests that the major pathogenesis of RVFV infection involves direct, virus-induced cellular necrosis, particularly in the liver. Unlike some other hemorrhagic fever viruses, such as Lassa fever virus, Crimean-Congo hemorrhagic fever virus, Marburg virus, and Ebola virus, no prominent immunostaining is seen in endothelial cells or Kupffer cells in liver infected with RVFV. Unfortunately, more thorough histopathologic and immunopathologic studies were hampered by autolysis of many of the animal samples, and the limited tissue types available for evaluation.
In sub-Saharan Africa, many other severe febrile illnesses can have similar presentations. Pathological studies of postmortem tissue samples are helpful in differentiating RVF from other endemic infections, especially when clinical manifestations of those infections are similar to RVF yet the infection is potentially treatable. The detection of human herpes simplex virus and P. falciparum, in our studies, illustrates the usefulness of pathologic examination in patients who die of febrile illness that resembles RVF, but in fact are caused by pathogens other than RVFV. Because of this, pathologic studies can contribute valuable information to outbreak detection and response.
Future opportunities for more complete examination of autopsy and necropsy tissue obtained from humans and animals during outbreaks of RVF will lead to improved understanding of this disease.
Footnotes
Authors' addresses: Wun-Ju Shieh, Chris D. Paddock, and Sherif R. Zaki, Infectious Disease Pathology Branch, Division of Viral and Rickettsial Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: wshieh@cdc.gov, cdp9@cdc.gov, and sxz1@cdc.gov. Edith Lederman, Division of Infectious Diseases, Naval Medical Center, San Diego, CA, E-mail: Edith.Lederman@med.navy.mil. Carol Y. Rao, Prevention and Response Branch, Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Atlanta, GA, E-mail: cnr3@cdc.gov. L. Hannah Gould, Enteric Diseases Epidemiology Branch, Division of Foodborne, Bacterial, and Mycotic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: dvj9@cdc.gov. Mohamed Mohamed and Janeth Mghamba, Epidemiology Section, Preventive Department, Tanzania Ministry of Health and Social Welfare, Dar es Salaam, Tanzania, E-mails: mahd67@yahoo.com and mashaka_2000@yahoo.com. Fausta Mosha, African Field Epidemiology Network, Tanzania Field Epidemiology and Laboratory Training Programme, Dar es Salaam, Tanzania, E-mail: fausta_mosha@yahoo.com. Peter Bloland, Office of the Director, National Center for Zoonotic, Vector-borne, and Enteric Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pbb1@cdc.gov. M. Kariuki Njenga and Robert F. Breiman, International Emerging Infections Program, Global Disease Detection Division, Centers for Disease Control and Prevention-Kenya, Nairobi, Kenya, E-mails: knjenga@ke.cdc.gov and rbeiman@ke.cdc.gov. David Mutonga and Amwayi A. Samuel, Ministry of Public Health and Sanitation, Department of Disease Control and Prevention, Field Epidemiology and Laboratory Training Programme (FELTP), Nairobi, Kenya, E-mails: doctordavidm2000@yahoo.com and amwayi2004@yahoo.com. Jeannette Guarner, Department of Pathology and Laboratory Medicine at Emory University, Emory University School of Medicine, Emory University Hospital, Atlanta, GA, E-mail: jguarne@emory.edu.
References
- 1.Scott GR, Heisch RB. Rift Valley fever and Rift Valley rodents. East Afr Med J. 1959;36:665–667. [PubMed] [Google Scholar]
- 2.Mundel B, Gear J. Rift valley fever. I. The occurrence of human cases in Johannesburg. S Afr Med J. 1951;25:797–800. [PubMed] [Google Scholar]
- 3.Swanepoel R. Studies on the epidemiology of Rift Valley fever. JS Afr Vet Assoc. 1976;47:93–94. [PubMed] [Google Scholar]
- 4.Imam IZ, Darwish MA. A preliminary report on an epidemic of Rift Valley fever (RVF) in Egypt. J Egypt Public Health Assoc. 1977;52:417–418. [PubMed] [Google Scholar]
- 5.Morvan J, Lesbordes JL, Rollin PE, Mouden JC, Roux J. First fatal human case of Rift Valley fever in Madagascar. Trans R Soc Trop Med Hyg. 1992;86:320. doi: 10.1016/0035-9203(92)90329-b. [DOI] [PubMed] [Google Scholar]
- 6.Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37:1084–1092. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Rift Valley fever outbreak–Kenya, November 2006–January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:73–76. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Outbreaks of Rift Valley fever in Kenya, Somalia and United Republic of Tanzania, December 2006–April 2007. Wkly Epidemiol Rec. 2007;82:169–178. [PubMed] [Google Scholar]
- 9.Wong KT, Shieh WJ, Kumar S, Norain K, Abdullah W, Guarner J, Goldsmith CS, Chua KB, Lam SK, Tan CT, Goh KJ, Chong HT, Jusoh R, Rollin PE, Ksiazek TG, Zaki SR. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. Group NVPW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genrich GL, Guarner J, Paddock CD, Shieh WJ, Greer PW, Barnwell JW, Zaki SR. Fatal malaria infection in travelers: novel immunohistochemical assays for the detection of Plasmodium falciparum in tissues and implications for pathogenesis. Am J Trop Med Hyg. 2007;76:251–259. [PubMed] [Google Scholar]
- 11.Zaki SR, Shieh WJ. Leptospirosis associated with outbreak of acute febrile illness and pulmonary haemorrhage, Nicaragua, 1995. The Epidemic Working Group at Ministry of Health in Nicaragua. Lancet. 1996;347:535–536. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Wahab KS, El Baz LM, El-Tayeb EM, Omar H, Ossman MA, Yasin W. Rift Valley Fever virus infections in Egypt: pathological and virological findings in man. Trans R Soc Trop Med Hyg. 1978;72:392–396. doi: 10.1016/0035-9203(78)90134-7. [DOI] [PubMed] [Google Scholar]
- 13.Arborio M, Hall WC. Diagnosis of a human case of Rift Valley fever by immunoperoxidase demonstration of antigen in fixed liver tissue. Res Virol. 1989;140:165–168. doi: 10.1016/s0923-2516(89)80094-9. [DOI] [PubMed] [Google Scholar]
- 14.Coetzer JA. The pathology of Rift Valley fever. I. Lesions occurring in natural cases in new-born lambs. Onderstepoort J Vet Res. 1977;44:205–211. [PubMed] [Google Scholar]
- 15.Coetzer JA, Ishak KG. Sequential development of the liver lesions in new-born lambs infected with Rift Valley fever virus. I. Macroscopic and microscopic pathology. Onderstepoort J Vet Res. 1982;49:103–108. [PubMed] [Google Scholar]
- 16.Coetzer JA. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J Vet Res. 1982;49:11–17. [PubMed] [Google Scholar]
- 17.Cosgriff TM, Morrill JC, Jennings GB, Hodgson LA, Slayter MV, Gibbs PH, Peters CJ. Hemostatic derangement produced by Rift Valley fever virus in rhesus monkeys. Rev Infect Dis. 1989;11((Suppl 4)):S807–S814. doi: 10.1093/clinids/11.supplement_4.s807. [DOI] [PubMed] [Google Scholar]
- 18.Peters CJ, Jones D, Trotter R, Donaldson J, White J, Stephen E, Slone TW., Jr Experimental Rift Valley fever in rhesus macaques. Arch Virol. 1988;99:31–44. doi: 10.1007/BF01311021. [DOI] [PubMed] [Google Scholar]
- 19.Van der Lugt JJ, Coetzer JA, Smit MM. Distribution of viral antigen in tissues of new-born lambs infected with Rift Valley fever virus. Onderstepoort J Vet Res. 1996;63:341–347. [PubMed] [Google Scholar]
- 20.Burke-Gaffney HJ. II. Yellow fever. Trop Dis Bull. 1965;62:73–75. [PubMed] [Google Scholar]
- 21.Walker DH, McCormick JB, Johnson KM, Webb PA, Komba-Kono G, Elliott LH, Gardner JJ. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982;107:349–356. [PMC free article] [PubMed] [Google Scholar]
- 22.Burt FJ, Swanepoel R, Shieh WJ, Smith JF, Leman PA, Greer PW, Coffield LM, Rollin PE, Ksiazek TG, Peters CJ, Zaki SR. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 23.Rippey JJ, Schepers NJ, Gear JH. The pathology of Marburg virus disease. S Afr Med J. 1984;66:50–54. [PubMed] [Google Scholar]
- 24.Zaki SR, Shieh WJ, Greer PW, Goldsmith CS, Ferebee T, Katshitshi J, Tshioko FK, Bwaka MA, Swanepoel R, Calain P, Khan AS, Lloyd E, Rollin PE, Ksiazek TG, Peters CJ. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179((Suppl 1)):S36–S47. doi: 10.1086/514319. [DOI] [PubMed] [Google Scholar]
- 25.Kamal SA. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol J. 2009;6:94. doi: 10.1186/1743-422X-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, Khabbaz RF, Ksiazek TG, Peters CJ. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 27.Geisbert TW, Jaax NK. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 28.Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17:399–403. doi: 10.1016/j.coi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Peters CJ, Liu CT, Anderson GW, Jr, Morrill JC, Jahrling PB. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev Infect Dis. 1989;11((Suppl 4)):S743–S749. doi: 10.1093/clinids/11.supplement_4.s743. [DOI] [PubMed] [Google Scholar]


