Abstract
In January 2007, an outbreak of Rift Valley fever (RVF) was detected among humans in northern Tanzania districts. By the end of the outbreak in June, 2007, 511 suspect RVF cases had been recorded from 10 of the 21 regions of Tanzania, with laboratory confirmation of 186 cases and another 123 probable cases. All confirmed RVF cases were located in the north-central and southern regions of the country, with an eventual fatality rate of 28.2% (N = 144). All suspected cases had fever; 89% had encephalopathy, 10% hemorrhage, and 3% retinopathy. A total of 169 (55%) of the 309 confirmed or probable cases were also positive for malaria as detected by peripheral blood smear. In a cohort of 20 RVF cases with known outcome that were also positive for human immunodeficiency virus, 15 (75%) died. Contact with sick animals and animal products, including blood, meat, and milk, were identified as major risk factors of acquiring RVF.
Introduction
Rift Valley fever (RVF) virus belongs to the Phlebovirus genus and Bunyaviridae family of viruses and causes a zoonotic disease that is characterized by periodic and severe outbreaks in humans and animals.1,2 Data suggest that in the interepidemic period, the virus is maintained within the eggs of Aedes mosquitoes embedded in soils where previous RVF outbreaks have occurred, whereas other findings suggest that the virus may also be maintained by cryptic cycling between domestic livestock or wild herbivores and mosquitoes.3 Heavy rainfall and flooding provide an environment for Aedes mosquitoes to rapidly multiply and become the predominant mosquito population, which results in extensive livestock transmission and amplification of the virus.4 After an initial burst of transmission, other mosquito species and biting insects can become infected and transmit the virus among animals and from animals to humans.4
Human RVF outbreaks are primarily characterized by mild, acute febrile illness with spontaneous recovery, although in a small number of cases (< 8%) the disease can be associated with severe jaundice, rhinitis, encephalitis, and hemorrhagic manifestations. Humans become infected through mosquito bites or through contact with products from infected animals.4 Outbreaks of RFV have been reported most frequently in East Africa, especially Kenya, Somalia, and Tanzania, with the last major outbreak in the region recorded in 1997–1998.5 However, outbreaks have also been reported in other African countries including Egypt, South Africa, Madagascar, and Senegal.6–10 In 2000, the first RVF outbreak outside of Africa was reported in Saudi Arabia and Yemen.11,12
In December 2006, a large number of cases of RVF among animals and humans were reported in Kenya, raising concerns that the disease would emerge in Tanzania.13,14 Of particular concern were the northern zone regions along the border with Kenya, which were experiencing heavy rainfall and flooding. An investigation team led by Tanzanian recent graduates and residents of the Kenya Field Epidemiology and Laboratory Training Program established a surveillance system for rapid detection of cases of RVF in northern Tanzania. Here, we report the extent and magnitude of the outbreak in Tanzania, and characterize factors relevant to public health interventions for controlling the spread of RVF.
Materials and Methods
Cases.
Cases suspected of RVF were initially reported through preexisting national surveillance systems based on the World Health Organization's (WHO) integrated disease surveillance and response strategy. A standardized case definition, distributed to all health facilities within the districts, was used to identify suspect cases. Information such as medical history and demographic data, risk factors for RVF virus infection, and clinical manifestations were obtained for each suspect case using a standardized semi-structured questionnaire. Laboratory specimens and results were also obtained.
A suspected cases was defined as a person of any age presenting with symptoms of fever (or axillary temperature > 37.5), headache, muscle pain or nausea, and/or direct contact with sick or dead animals or the animals products; or direct contact with body fluids of an infected person; or resident in or recent travel to an area where RVF activity in animals or humans was confirmed.
A probable RVF case was defined as a suspected case that presented with unexplained bleeding (bloody stool, vomiting blood, coughing blood, bleeding from gums, nose, vagina, skin, or eyes), deterioration of vision, or decreased consciousness.
A confirmed case was any suspected or probable case with laboratory confirmation of RVF by detection of viral immunoglobulin M (IgM) antibodies by enzyme-linked immunosorbent assay (ELISA), or detection of viral RNA by real-time reverse transcriptase polymerase chain reaction (RT-PCR); or detection of viral antigens in biopsy tissues by immunohistochemistry.
Laboratory testing.
Serum or plasma specimens were transported to the Kenya Medical Research Institute/Centers for Disease Control and Prevention (KEMRI/CDC) laboratory in Nairobi and tested for IgM and IgG antibodies by ELISA and for viral RNA by real-time RT-PCR as described previously.15–17 Liver tissues from biopsies or autopsy specimens were tested for viral RNA by real-time RT-PCR. Histopathologic evaluation and testing of liver biopsies for presence for RVF antigens by immunohistochemistry were performed at the Infectious Diseases Pathology Branch at the CDC in Atlanta, GA.
For IgM antibodies patient sera were added onto plates coated with goat antiserum against human μ-chain of IgM (ICN Pharmaceuticals, Costa Mesa, CA). After incubation and washing, RVF virus antigens were added, followed by mouse anti-RVF antibodies. Viral antigen binding was detected using horseradish peroxidase conjugated goat anti-mouse IgG (H + L chain; Zymed Laboratories, San Francisco, CA) followed by the addition of 2,2′-azino di-ethyl-benzothiazoline-sulfonic acid substrate (Kirkgaard and Perry Laboratories, Gaithersburg, MD). Optical density (OD) was determined at 405 nm, and the mean OD readings of triplicate dilutions was converted into a percentage of high-positive control serum (PP) value using the equation: (mean net OD of test sample/mean net OD of high-positive control) × 100.
The one-step real-time RT-PCR was performed using a method described previously.16,17 Primers and probes targeting the G2 gene of the virus were used.
Immunohistochemistry.
Immunohistochemical (IHC) tests using a multi-step indirect immunoalkaline phosphatase technique were performed.18,19 The primary antibodies used in these tests included a polyclonal rabbit anti-RVF virus antibody and a mouse anti-RVF virus antibody. Appropriate positive and negative controls were run in parallel with test samples.
Human immunodeficiency virus (HIV) Testing.
Before the diagnosis of RVF was established, patients that presented with central nervous system (CNS) symptoms were suspected of meningitis and tested for common etiologies. Cerebrospinal fluid was tested for bacterial meningitis (Streptococcus spp., ;Neisseria meningitides, and Haemophilus influenzae) and fungus (Cryptococcus spp.) using the microscopy and culture. Because many cases in the affected regions routinely tested negative for bacterial meningitis, the Tanzania Ministry of Health and Social Welfare (MOHSW), had adopted a policy of testing such cases for both Cryptococcus spp. and HIV. The standard Tanzania MOHSW HIV testing algorithm used Capillus HIV-1/HIV-2 (Cambridge Diagnostics Ireland Limited, Galway, Ireland) as the first test followed by the Determine HIV-1/2 (Abbott Diagnostic Division, Hoofdorp, Netherlands) test for confirmation of a reactive Capillus test. The Capillus HIV-1/HIV-2 test is a rapid particle agglutination test that detects both anti-HIV IgM and IgG antibodies, thus being capable of detecting seroconverters earlier.20 The Determine HIV-1/2 test is a rapid immunochromatographic test detect both antigens (P24 antigen) and antibodies to the HIV virus.21
Epidemiologic analysis.
Descriptive epidemiology was used to summarize the demographic data and behaviors representing potential risk factors among the cases and data analyzed using Epi Info Software version 3.3.2 (CDC, Atlanta, GA).
Results
Index case.
The index case was a 39 year-old male from the Maasai tribe living at Terati village, Simanjiro District in the Manyara Region, approximately 50 km south of the Kenyan border. He was a herdsman and small-scale farmer. He had butchered a goat, which had died of an illness approximately 3 days before the onset of his symptoms. There were previous reports of abortions in livestock and increased deaths among young animals in this area. On January 24, 2007, the patient developed a high fever, accompanied by headache, muscle pain, and nausea. Within several days, he developed abdominal swelling, vomiting, chest pain, and bleeding from the nose, ears, and the rectum. On January 31, 2007, he lost consciousness and was admitted to Mount Meru Hospital in the Arusha region. A blood smear taken for malaria was negative and the patient was treated with quinine, penicillin, chloramphenicol, and furosemide but died on the same day. A postmortem liver biopsy was sent to KEMRI/CDC in Nairobi, which tested positive for RVF RNA by real-time RT-PCR. His wife and elder sister who lived in the same compound began experiencing flu-like illnesses (fever, malaise, and headache) and tested positive for IgM antibodies against RVF. A brother who participated in the butchering of the goat and cared for the index case was reportedly sick with similar symptoms, but disappeared from the area before diagnostic specimens could be collected.
Extent of the outbreak.
Between February and June 2007, other regions of Tanzania including Manyara, Tanga, Dodoma, Morogoro, Dar es Salaam, Coast, Iringa, Mwanza, and Singida reported suspected cases of RVF (Figure 1). Cases were reported until mid-June 2007. All cases (N = 9) reported in the eastern coastal region of the country (Muheza, Rufiji, Kibaha, Bagamoyo, and Mkuranga districts) had a history of recent travel to affected regions (Figure 1). No cases were reported in the semi-temperate western highlands. By June 15, 2007, 511 suspected cases of RVF had been detected in Tanzania with 144 deaths reported, indicating a case fatality rate of 28.1%. Of 511 suspected RVF cases, 186 (36.4%) were laboratory confirmed and another 123 (24%) classified as probable. All the fatal cases met the confirmed or probable case definition, giving a case fatality rate of 47% among the confirmed and probable cases (N = 309). The largest number of suspected RVF cases (N = 186) were reported from the Dodoma region.
Figure 1.
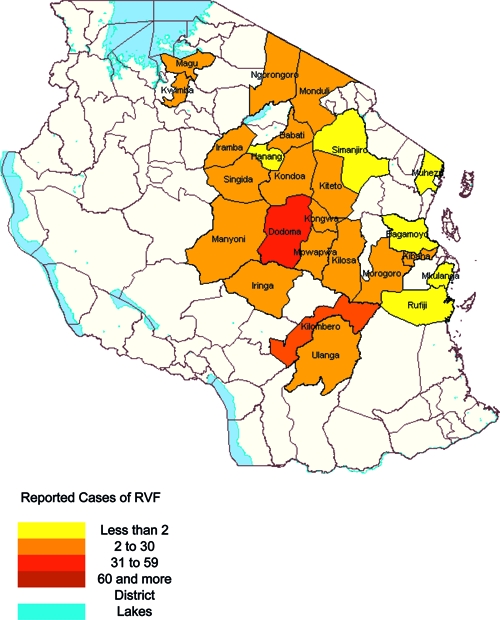
Map of Tanzania showing the districts that reported the Rift Valley fever (RVF) cases in the year 2007.
The peak of the outbreak occurred between late March and April (Figure 2). All suspected cases had fever; 89% had encephalopathy, 10% had hemorrhage, and 3% had retinopathy. A total of 169 (55%) of confirmed and probable cases had evidence of concurrent malaria based on peripheral blood smears. More males (191/309) than females were affected, with the highest proportions of cases (22.6%) observed in the 31–39 years of age group (Figure 3). Patients were hospitalized within an average of 5 days after onset of illness and they remained ill with RVF-associated symptoms for an average of 28 days (range of 2 to 120 days) before death or discharge.
Figure 2.
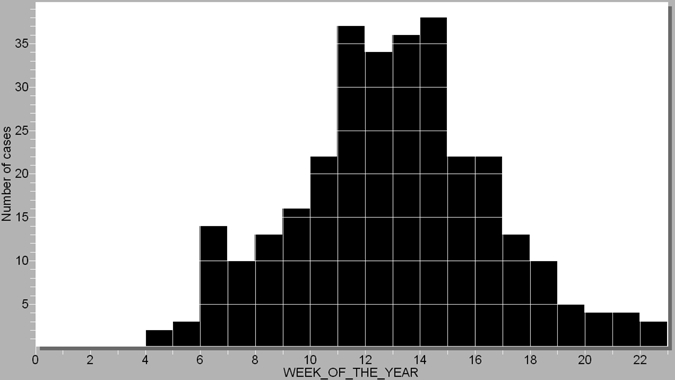
Epicurve of the Rift Valley fever (RVF) outbreak in Tanzania from January 24, 2007 to June 15, 2007. This is based on the confirmed and probable RVF cases.
Figure 3.

Age distribution of confirmed and probable Rift Valley fever (RVF) cases.
Out of 115 cases interviewed, 46 (40%) reported having contact with animal products including meat and milk from sick animals. These included 32 (28%) who had recently slaughtered a sick animal and 12 (10%) that contact with blood of a sick animal during food preparation.
Laboratory results.
The RVF viral RNA was detected in liver tissues by RT-PCR on February 2, 2007 from the index case. Of 511 suspected RVF cases, 186 (36.4%) were laboratory confirmed and another 123 (24%) classified as probable. The entire 186 laboratory confirmed cases were positive for RVF IgM antibodies by ELISA, whereas 8 cases (4.3%) were also positive for viral RNA by RT-PCR. The 123 were classified as probable because of presence of unexplained bleeding and ruling out other possible etiologies.
Histology and immunohistochemistry.
Liver tissues were obtained from 13 of 186 confirmed patients who died. Histological examination of the livers revealed extensive hepatocellular necrosis with acidophilic material in the cytoplasm (Figure 4A, arrows) in 7 patients. In addition, IHC staining for RVF viral antigens was demonstrated within hepatocytes and Kupffer cells for each of these 7 patients (Figure 4B, arrows). Serum was obtained from 4 of the 13 fatal cases, and all were negative for RVF IgM and IgG antibodies.
Figure 4.
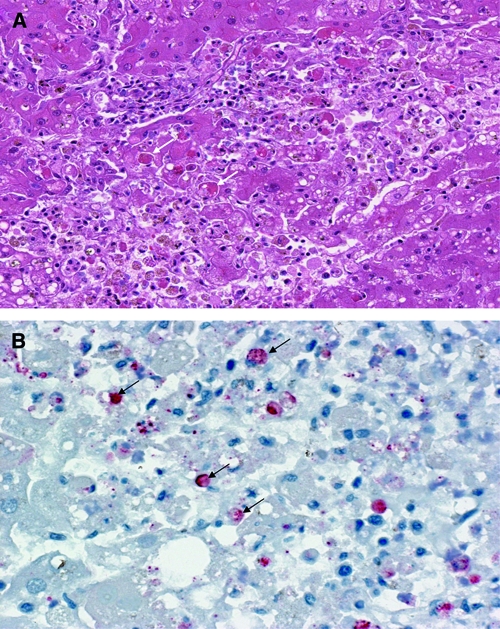
(A) Histopathological and (B) immunohistochemical analyses of the liver tissues from Rift Valley fever (RVF) cases during the outbreak in Tanzania, 2007. (A) Shows liver section with hematoxylin and eosin stain revealing extensive hepatocellular necrosis with acidophilic material in the cytoplasm (arrows). (B) Shows liver section with immunohistochemical staining for RVF viral antigens showing positive immunoreactivity in hepatocytes and Kupffer cells (arrows).
Effect of HIV status on RVF infection.
Out of the 309 confirmed or probable cases, the HIV status of 73 cases was known (21 cases HIV-positive and 52 cases HIV-negative). Of the 21 RVF confirmed HIV-positive cases, the outcome of one case could not be determined. Of the remaining 20 cases, 15 died (case fatality rate = 75%). Of the 52 RVF confirmed HIV-negative cases 7 died (case fatality rate = 13.4%). The case fatality rate for RVF plus HIV infection was significantly higher than the case fatality of the RVF-positive but HIV-negative cases (P < 0.001). All the HIV-positive cases were hospitalized within an average of 6 days after onset of illness and they remained ill with RVF-associated symptoms for an average of 33 days (range 4–120 days) before death or discharge.
Consistent with the high rate of encephalopathy (88.9%) reported for all RVF confirmed or probable cases in the country, all 73 patients whose HIV status was known presented with CNS symptoms, including severe headaches, convulsions, and coma.
Discussion
This is the largest RVF outbreak documented in Tanzania. It affected 10 of 21 regions of the country and 25 of 126 districts, resulting in over 300 suspected cases and a case fatality rate of 47%, which is higher than previously reported in the two recent outbreaks of 2000–2001 in Saudi Arabia (14%) and 2006–2007 in Kenya (26.4%).22 Because most (98–99%) RVF infections result in uncomplicated disease,23 the high case-fatality rate found among the confirmed and probable RVF cases during this investigation suggests that the investigating teams identified primarily severe cases. Because the survey could not detect a large number of non-severe or asymptomatic RVF infection that did not seek medical attention, we are not able to estimate the overall number of people infected during this outbreak. During this outbreak, young to middle-aged males accounted for a disproportionate number of infections, most likely caused by direct handling of animals and practices such as slaughtering that put males within these age ranges at higher risk of direct contact with bodily fluids from infected animals.
A unique finding during this outbreak was the high proportion of cases presenting with CNS symptoms, especially encephalopathy. This contrasts with the nearly simultaneous outbreak in neighboring Kenya, where the proportion of cases with encephalitis-like presentation was much lower (< 10%).24 It is possible that this represented passive surveillance bias, if a higher proportion of patients with central nervous system manifestations sought hospital care and/or if their illnesses were recognized by clinicians or surveillance officers as being unusual and worthy of reporting when compared with patients with RVF with less severe clinical manifestations. A high rate of encephalopathy was also noted during the RVF outbreak in Saudi Arabia where 50% of patients presented with CNS findings, compared with 28% of patients presenting with bleeding manifestations.25 It is possible that the high proportion of cases with CNS abnormalities in Tanzania was caused by co-factors such as concurrent malaria or HIV, and perhaps an interaction of immune-mediated and/or direct cytopathic effects of the virus. Unfortunately, we were not able to obtain brain tissue for histopathologic evaluation and IHC testing. Whereas only 3% of patients presented with retinopathy, less than the 10% reported in the Saudi Arabia outbreak, the low detection may have been because retinopathy is usually a late sequel of RVF infection and may have been missed in the Tanzania outbreak.25
Laboratory specimens were collected > 7 days following onset of symptoms for greater than half (54%) of the confirmed cases, resulting in a limited number of patients in which RVF RNA could be detected by RT-PCR. In contrast, real-time RT-PCR detected RVF RNA in 63.2% of Kenyan cases collecting between 3 and 7 days,17 showing that rapid collection of specimens in suspect cases improves sensitivity of this diagnostic method.
Previous studies have suggested a close association between climate variability and RVF outbreaks in East Africa.26 Because analysis of historical data indicated that most RVF outbreaks occurred during warm El Niño/Southern Oscillation (ENSO) event periods, the United States Department of Agriculture and United States Department of Defense issued an RVF alert for the region in mid-September 2006. The eastern coastal plain of mainland Tanzania has a tropical hot and humid climate with temperatures averaging 26.7°C and rainfall varying from 1,016 to 1,930 mm (40 to 76 in). The inland plateau that was heavily affected by the RVF outbreak is usually hot and dry, with rainfall averaging from 508 to 762 mm (20 to 30 in).27 During the period between January and June 2007, there was excessive rainfall of an average of 1,720 mm (2- to 3-fold higher than usual) that led to flooding in most parts of the plateau of Tanzania. This above-normal precipitation with subsequent changes in vegetation can be captured by mapping ecological conditions using satellite imagery and developing a normalized difference vegetation index (NDVI). The NDVI for February 2007 showed a significant increase in vegetative cover in the northern central region of Tanzania (the epicenter of the outbreak) indicative of increased rainfall when compared with maps of the same region from October 2006. This anomalous rainfall flooded mosquito-breeding habitats most likely leading to mass hatching of Aedes mosquitoes capable of transmitting RVF virus acquired from transovarial infection or by biting infected vertebrates and/or humans. Because the country's livestock population includes 17 million cattle, 11 million goats, and 3.6 million sheep, most of which are located in the north and central regions of the country, it is plausible that heavy rainfall, accompanied by flooding within an area of high density of livestock, created the permissive environment for an RVF outbreak.
Acknowledgments
We thank the Tanzania RVF investigation team, including staff from the Epidemiology and Disease Control Section within MOHSW, members of the Regional Health Management Team from Arusha, Manyara, Tanga, Dodoma, Dar es Salaam, Morogoro, Coast, Iringa, Mwanza, and Singida Regions. We also thank the WHO Country office, WHO AFRO, and WHO Headquarters for both technical and financial support during the outbreak. The CDC (Tanzania, Kenya, and Atlanta) also provided valuable technical and material support to facilitate transportation and testing of specimens. We also appreciate the assistance given from various organizations including AFENET, Red Cross Society-Tanzania, UNICEF, and other UN organizations throughout the country.
Footnotes
Authors' addresses: Mohamed Mohamed, Fausta Mosha, Janeth Mghamba, Peter Mmbuji, and Raphael Kalinga, Tanzania Ministry of Health and Social Work, Dar es Salaam, Tanzania, E-mails: mahd67@yahoo.com, Fausta_mosha@yahoo.com, mashaka_2000@yahoo.com, pmmbuji@yahoo.com, and rkalinga@yahoo.com. Sherif R. Zaki and Wun-Ju Shieh, Infectious Disease Pathology Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: sxl@cdc.gov and wbs9@cdc.gov. Sylvia Omulo, Solomon Gikundi, Robert F. Breiman, and M. Kariuki Njenga, Global Disease Detection Division, Centers for Disease Control and Prevention–Kenya, Nairobi, Kenya, E-mails: somulo@ke.cdc.gov, sgikundi@ke.cdc.gov, rbreiman@ke.cdc.gov, and knjenga@ke.cdc.gov. Peter Bloland, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pbb1@cdc.gov. Nordin Zeidner, National Center for Zoonotic, Vector-borne, and Enteric Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: naz2@cdc.gov.
References
- 1.Peters CJ, Linthicum KJ. In: CRC Handbook Series in Zoonoses. Section B: Viral Zoonoses. Second edition. Beran GW, editor. Boca Raton, FL: CRC Press Inc; 1994. pp. 125–138. (Rift Valley fever). [Google Scholar]
- 2.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley fever. Bull World Health Organ. 1985;63:941–943. [PMC free article] [PubMed] [Google Scholar]
- 3.Linthicum KJ, Davies FG, Kairo DA. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from diptera collected during inter-epizootic period in Kenya. J Hyg Camb. 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies FG, Highton RB. Possible vectors for Rift Valley fever in Kenya. Trans R Soc Trop Med Hyg. 1980;74:815–825. doi: 10.1016/0035-9203(80)90213-8. [DOI] [PubMed] [Google Scholar]
- 5.Woods C, Karpati A, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster E, Henderson A, Khan A, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak B, Ryan M, Ksiazek T, Ray R, Ndikuyeze A, Agata N, Peters C. An outbreak of Rift Valley fever in northeastern Kenya, 1997–1998. Emerg Infect Dis. 2002;8:138–144. doi: 10.3201/eid0802.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meegan JM. Rift Valley fever in Egypt: an overview of epizootics in 1977 and 1978. Contrib Epidemiol Biostat. 1981;3:100–113. [Google Scholar]
- 7.Gerdes GH. Rift Valley fever. Rev Sci Tech. 2004;23:613–623. doi: 10.20506/rst.23.2.1500. [DOI] [PubMed] [Google Scholar]
- 8.Zeller HG, Fontenille D, Traore-Lamizana M, Thiongane Y, Diguotte J-P. Enzootic activity of Rift Valley fever virus in Senegal. Am J Trop Med Hyg. 1997;56:265–272. doi: 10.4269/ajtmh.1997.56.265. [DOI] [PubMed] [Google Scholar]
- 9.Saluzzo JF, Diguotte JF, Chartier C, Martinez D, Bada R. Focus of Rift Valley fever transmission in southern Mauritania. Lancet. 1987;1:504. doi: 10.1016/s0140-6736(87)92110-6. [DOI] [PubMed] [Google Scholar]
- 10.El Akkad AM. Rift Valley fever outbreak in Egypt, October–December 1977. J Egypt Public Health Assoc. 1978;53:123–128. [PubMed] [Google Scholar]
- 11.Al-Afaleq AI, Abu Elzein EM, Mousa SM, Abbas AM. A retrospective study on Rift Valley fever in Saudi Arabia. Rev Sci Tech. 2003;22:867–871. doi: 10.20506/rst.22.3.1436. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, Khan AS, Rollin PE, Swanepoel R, Ksiazek TG, Nichol ST. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–2001. Emerg Infect Dis. 2002;12:1415. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Rift Valley fever outbreak - Kenya, November 2006–January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:73–76. [PubMed] [Google Scholar]
- 14.World Health Organization Outbreak of Rift Valley fever in Kenya, Tanzania, and Somalia, December 2006–April 2007. Wkly Epidemiol Rec. 2007;82:169–180. [PubMed] [Google Scholar]
- 15.Paweska JT, Burt FJ, Swanepoel R. Validation of IgG-sandwich and IgM-capture ELISA for the detection of antibody to Rift Valley fever virus in humans. J Virol Methods. 2005;124:173–181. doi: 10.1016/j.jviromet.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Drosten C, Gottig S, Schiling M, Jasper M, Panning H, Schmitz S, Gunther S. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus and yellow fever virus by real-time reverse transcriptase-PCR. J Clin Microbiol. 2002;40:2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Njenga MK, Paweska J, Wanjala R, Rao C, Weiner M, Omballa V, Luman E, Mutonga D, Sharif S, Panning M, Drosten C, Feikin D, Breiman RF. Using field quantitative real-time PCR test to rapidly identify highly viremic Rift Valley fever cases. J Clin Microbiol. 2009;47:1166–1171. doi: 10.1128/JCM.01905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong KT, Shieh WJ, Kumar S, Norain K, Abdullah W, Guarner J, Goldsmith CS, Chua KB, Lam SK, Tan CT, Goh KJ, Chong HT, Jusoh R, Rollin PE, Ksiazek TG, Zaki SR. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. Group NVPW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shieh W-J, Paddock C, Lederman E, Rao C, Gould H, Mohamed M, Mosha F, Mghamba J, Bloland P, Njenga MK, Mutonga D, Amwayi S, Guarner J, Breiman RF, Zaki S. Pathologic studies of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am J Trop Med Hyg. 2010;83((Suppl 2)):38–42. doi: 10.4269/ajtmh.2010.09-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramalingam S, Kanangai R, Raj AA, Jesudason MV, Sridharan G. Rapid particle agglutination test for human immunodeficiency virus: hospital-based evaluation. J Clin Microbiol. 2002;40:1553–1554. doi: 10.1128/JCM.40.4.1553-1554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Berk GE, Friesen PH, Regez RM, Rietra PJ. Evaluation of rapid immunoassay determine HIV1/2 for detection of human immunodeficiency virus type 1 and 2. J Clin Microbiol. 2003;41:3868–3869. doi: 10.1128/JCM.41.8.3868-3869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian Peninsula. Int J Antimicrob Agents. 2003;21:153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 23.Meegan JM, Watten RH, Laughlin LW. Clinical experience with Rift Valley fever in humans during the 1977 Egyptian epizootic. Contrib Epidemiol Biostat. 1981;3:114–123. [Google Scholar]
- 24.Nguku P, Sharif SK, Mutonga D, Amwayi S, Omolo J, Mohammed O, Farnon EC, Gould LH, Lederman E, Rao C, Sang R, Schnabel D, Feikin DR, Hightower A, Kariuki Njenga M, Breiman RF. Investigation of a major outbreak of Rift Valley fever in Kenya, 2006–2007: clues and enigmas concerning Rift Valley fever outbreaks and their prevention. Amer J Trop Med Hyg. 2010;83((Suppl 2)):5–13. doi: 10.4269/ajtmh.2010.09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Hazmi M, Ayoola E, Abdurahman M, Banzal S, Ashraf J, El-Bushra A, Hazmi A, Abdullah M, Abbo H, Elamin A, Al-Sambaing E, Gadour M, Menno C, Hama M, Rohm I, Hafez M, Jambavalikar M, Arishi H, Aqeel A. Epidemic Rift Valley fever in Saudi Arabia: a clinical study of severe illness in humans. Clin Infect Dis. 2003;36:245–252. doi: 10.1086/345671. [DOI] [PubMed] [Google Scholar]
- 26.Anyamba A, Linthicum KJ, Tucker CJ. Climate-disease connections: Rift Valley fever in Kenya. Cad Saude Publica. 2001;17((Suppl)):133–140. doi: 10.1590/s0102-311x2001000700022. [DOI] [PubMed] [Google Scholar]
- 27.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty P, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Linthicum KJ. Prediction of Rift Valley fever outbreak in the horn of Africa 2006–2007. Proc Natl Acad Sci USA. 2009;106:955–959. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]


