Abstract
We analyzed the extent of livestock involvement in the latest Rift Valley fever (RVF) outbreak in Kenya that started in December 2006 and continued until June 2007. When compared with previous RVF outbreaks in the country, the 2006–07 outbreak was the most extensive in cattle, sheep, goats, and camels affecting thousands of animals in 29 of 69 administrative districts across six of the eight provinces. This contrasted with the distribution of approximately 700 human RVF cases in the country, where over 85% of these cases were located in four districts; Garissa and Ijara districts in Northeastern Province, Baringo district in Rift Valley Province, and Kilifi district in Coast Province. Analysis of livestock and human data suggests that livestock infections occur before virus detection in humans, as supported by clustering of human RVF cases around livestock cases in Baringo district. The highest livestock morbidity and mortality rates were recorded in Garissa and Baringo districts, the same districts that recorded a high number of human cases. The districts that reported RVF in livestock for the first time in 2006/07 included Kitui, Tharaka, Meru South, Meru central, Mwingi, Embu, and Mbeere in Eastern Province, Malindi and Taita taveta in Coast Province, Kirinyaga and Murang'a in Central Province, and Baringo and Samburu in Rift Valley Province, indicating that the disease was occurring in new regions in the country.
Introduction
Although Rift Valley Fever (RVF)-like disease was first documented in Kenya in 1912, the virus was not isolated and recognized as the etiological agent of the disease until 1931.1 The RVF disease in livestock is characterized by abortions and perinatal mortality, and in humans primarily as an influenza-like illness with occasional cases (< 8%) of encephalitis, retinitis, and generalized hemorrhagic syndrome.2,3 Periodic severe RVF outbreaks involving livestock and humans have occurred in Africa following heavy rainfall and flooding. Large, severe RVF outbreaks include the 1977–1979 outbreak in Egypt that affected over 200,000 people and resulted in over 600 deaths, and the 1997–1998 outbreak in East Africa (Kenya, Somalia, and Tanzania) that affected over 100,000 people with over 450 deaths in Kenya alone.4,5 Outbreaks have also occurred in Mauritania, Senegal, Sudan, Madagascar, South Africa, and in the Middle Eastern countries of Saudi Arabia and Yemen.6–8 Risk factor studies have suggested that most severe human infections are the result of direct or indirect contact with blood, secretions, or tissue of infected animals during slaughter, food preparation, assisting with animal births, or conducting veterinary procedures.9,10 However, it is important to emphasize that mosquitoes play an important role in RVF transmission to both humans and livestock.3
In livestock, sheep are more susceptible than cattle and goats, whereas indigenous African zebu cattle appear to be more resistant than exotic cattle breeds.11,12 Infected camels have low (< 2%) mortality and occasional abortions.13 In certain regions of East Africa, RVF is endemic with recurrent low level virus activity during non-epidemic periods infecting 1–3% of livestock herds annually.14 In addition, RVF virus neutralizing antibodies have been demonstrated among wildlife, including African buffalo, black rhino, lesser kudu, impala, African elephant, Kongoni, and waterbuck.15
In October 2006, the computer model that analyzes data from satellite images, jointly managed by United States National Aeronautics and Space Administration, United States Department of Agriculture, and the Global Emerging Infection Surveillance program of the United States Department of Defense, predicted a RVF outbreak in parts of sub-Saharan Africa. The prediction was based on expected elevated temperatures in the Pacific and Indian oceans that indicated heavy rains, elevated humidity, and cloud cover favoring increased population of mosquitoes that support and spread RVF virus.16 In December 2006, cases of RVF first appeared in Northeastern and Coast provinces of Kenya after unusually heavy rains that caused floods and a subsequent increase in mosquito populations.9 During the following 3 months, RVF outbreaks were reported in 29 of the 69 districts in Kenya, with the largest number of human cases coming from Garissa and Ijara districts in Northeastern province, Kilifi district in Coast province, and Baringo district in Rift Valley province. This work describes the extent of the 2006–2007 RVF outbreaks in livestock in Kenya. Because the RVF outbreak had never been reported in Baringo district, the survey for human and livestock cases there was used to evaluate the clustering of human and livestock cases in a region where RVF was not endemic.
Material and Methods
Case definitions and sampling.
Data regarding RVF disease in livestock were collected throughout Kenya in two ways—through targeted (risk-based) surveys in regions identified as vulnerable to RVF outbreaks based on susceptibility mapping using RVF historical data and through disease investigations by the veterinary field officers in response to reports of suspect cases. A suspected RVF-infected herd was defined as any herd in which there were reports of abortions and/or still births, deaths among young ruminants, or hemorrhagic syndrome occurring among individual animals.
Targeted surveys.
Targeted (risk-based) surveys were carried out in areas identified as high risk for RVFV activity. Criteria used to identify high-risk areas included historical occurrence of RVF, ecological receptiveness for the vector, proximity to known infected areas, and areas experiencing increased rainfall and flooding between October and December 2006. In these areas, a sampling criterion was developed to identify suspected RVF-infected herds and specimens taken. The sampling criteria included presence of abortions, mortalities in the young lambs, hemorrhagic syndrome, and herds in homesteads where human illness was reported. Using participatory disease surveillance through semi-structured interviews, survey teams identified high-risk geographic locations in districts. In the identified areas, suspected herds were identified through additional interviews with the community and key informants, such as local veterinary staff and provincial administrators. For herds meeting the sampling criteria, outbreak investigation questionnaires were administered to the herd owners and specimens taken for laboratory testing. In areas visited where no herds met the sampling criteria, zero report forms were filled but specimens were not collected.
A total of 20 serum specimens were collected per herd. In areas where 20 or more animals had aborted or had other clinical signs compatible with RVF, all 20 specimens were collected from sick or recovered animals. In areas where less than 20 animals had developed symptoms suggestive of RVF, specimens were collected from all sick and recovered animals, and additional specimens randomly taken from apparently healthy animals within the herd. Specimens obtained included EDTA blood from animals with an accompanying fever, sera, and tissues (liver, spleen, lymph nodes, and brain) in glycerol buffered saline where carcasses were encountered. In a mixed herd, specimens were collected from all representative species. Investigation personnel were required to wear protective clothing during herd examination and sampling, and to adhere to routine biosafety precautions.
Additionally, reports by farmers and livestock traders of disease in livestock meeting the suspect herd criteria were investigated by the field staff of the Department of Veterinary Services and specimens were taken for laboratory testing.
Intensive surveys were conducted in Garissa District of Northeastern Province and Baringo District in Rift Valley province starting from mid-January 2007. The Garissa survey was designed to capture the epidemiology of the RVF in a region that was the first focal center of the 1997–1998 and the 2006–2007 outbreaks. The survey in Baringo was designed to determine the pattern of transmission in human and livestock in a region that had never before reported RVF cases. In both districts survey questionnaires were administered to owners of herds meeting suspect herd criteria and data entered into an Access database for analysis using SAS (version 9.2, Cary, NC). Geographic coordinates for the areas visited in the country were captured using the global positioning system (GPS) reader. Waypoints were downloaded from the GPS using Ozi-explorer software and mapped with the Arcview GIS 3.2 program. All animal specimens were preserved on ice and submitted to the Central Veterinary Laboratories, Kabete, for RVF testing. In Baringo, human cases identified through active surveillance were linked to livestock cases in a map displaying soil types using geographic coordinates taken during the survey for livestock cases and centroid points from the sub-locations where human cases resided.22,23 Human cases were defined as described elsewhere.22
Detection of virus antigen, IgM, and IgG detection in sera.
Animal sera were tested for the presence of RVFV antigen or immunoglobulin M (IgM) or IgG antibodies reactive with the RVF virus. The RVFV antigen-capture assays were performed in an enzyme-linked immunosorbent assay (ELISA) format as described.10,17 The RVFV assay used polyclonal hyperimmune ascitic fluid raised against RVFV strain Zigzag 501 as the capture antibody and rabbit hyperimmune serum against RVF virus 501 as the detector antibody. The IgM antibody titers were determined by IgM antibody-capture ELISA, with RVFV-infected cell slurry prepared as described.8,21 The IgG antibody titers were determined by using RVFV-infected cell antigens in an ELISA format similar to that described previously.21
Real-time reverse transcription -polymerase chain reaction (RT-PCR).
The two-step real-time RT-PCR was conducted as described previously.18,19 Briefly, viral RNA was extracted directly from animal serum and PCR reaction set up using a single primer and probe set [RVFL-2912fwdGG (5′-TGAAAATTCCTGAGACACATGG-3′), RVFL-2981revAC (5′-ACTTCCTTGCATCATCTGATG-3′), and RVFL-probe-2950 (5′-CAATGTAAGGGGCCTGTGTGGACTTGTG-3′) labeled at the 5′ end with the reporter dye FAM and at the 3′ end with the quencher BHQ1] annealing in a highly conserved domain located on the virus L segment.
Results
Laboratory findings and extent of the outbreak.
Animal specimens were received from 54 of 69 districts in the country and RVF confirmed in 29 districts. Of the 4,553 specimens (from cattle, sheep, goats, and camels) received at the central veterinary laboratory, 4,162 (91.4%) were tested for RVF by at least one of the following methodologies; IgG antibody test, IgM antibody test, viral antigen detection detected by ELISA, or viral RNA detection using real-time RT-PCR (qRT-PCR). Any herd that reported abortions or death of animals and had ≥ 2 cases positive for viral RNA, antigen, or antibodies (IgM or IgG) was considered positive for RVF disease. This confirmed herd definition may represent overestimation because it is possible that some animals that were positive for RVF virus IgG antibodies were infected before the 2006–2007 epizootic. Over 97% (4,041 of 4,162) of the specimens were tested by IgG antibody, IgM antibody, or antigen detection tests, whereas only 14.4% (601 of 4,162) were tested by qRT-PCR. A total of 29 districts across six of the eight provinces in Kenya reported RVF positive herds (Figure 1, Table 1). Of these, cases positive for RVF virus antigen, RNA or IgM, indicating presence of acute RVF disease were identified in 20 districts. Previous studies have shown that RVF antigens and RNA are effective for diagnosis during the first week of infection, whereas IgM antibodies last for approximately 6 weeks20; only Western and Nyanza provinces were negative for RVF disease. A total of 260 livestock specimens tested from four districts in Western province were negative for RVF virus. Nyanza province, which had never previously reported RVF, had one out of 150 specimens from two districts positive by IgM. Because the positive herd definition required at least two positive animals, Nyanza was classified free of the disease (Table 1).
Figure 1.
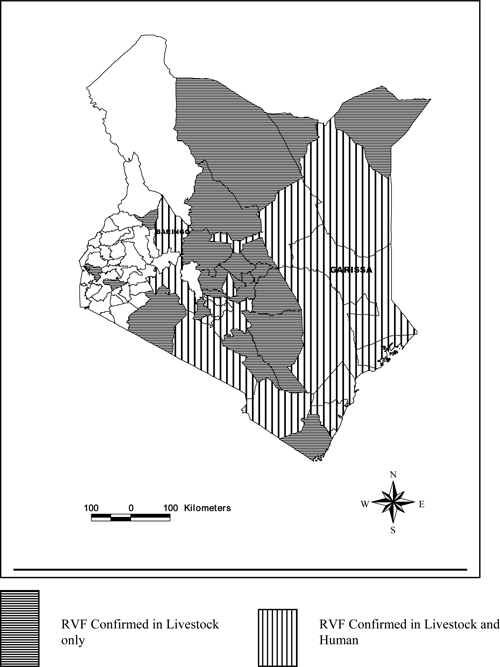
A map of Kenya showing districts that confirmed outbreak of Rift Valley fever in humans and animals in 2006/07.
Table 1.
Test results of livestock (cattle, sheep, and goats) survey in 27 districts where Rift Valley fever (RVF) cases were confirmed by enzyme-linked immunosorbent assay (ELISA) during the RVF outbreak in Kenya, 2006–2007†
| Province | District | Number IgM positive [Total] | Percent IgM | Number IgG positive [Total] | Percent IgG | Both IgM and IgG positive | Percent IgM and IgG* |
|---|---|---|---|---|---|---|---|
| Rift Valley | Baringo | 32 [171] | 18.7 | 26 [135] | 19.2 | 21 | 56.8 |
| Kajiado | 6 [125] | 4.8 | 11 [91] | 12.1 | 5 | 41.7 | |
| Laikipia | 0 [95] | 0.0 | 4 [95] | 4.2 | 0 | 0.0 | |
| Nakuru | 0 [176] | 0.0 | 7 [176] | 3.9 | 0 | 0.0 | |
| Samburu | 4 [97] | 4.1 | 2 [97] | 2.1 | 2 | 50.0 | |
| Eastern | Embu | 4 [95] | 4.2 | 5 [95] | 5.6 | 2 | 28.6 |
| Isiolo | 0 [42] | 0.0 | 4 [42] | 9.5 | 0 | 0.0 | |
| Kitui | 16 [107] | 14.9 | 6 [37] | 16.2 | 2 | 10.0 | |
| Machakos | 3 [125] | 2.4 | 13 [125] | 10.4 | 1 | 6.7 | |
| Mbeere | 3 [113] | 2.6 | 6 [113] | 5.3 | 2 | 28.6 | |
| Meru Central | 5 [42] | 11.9 | 1 [42] | 2.3 | 1 | 20.0 | |
| Mwingi | 4 [29] | 13.7 | 4 [29] | 13.7 | 4 | 100 | |
| Tharaka | 1 [81] | 1.2 | 5 [80] | 6.2 | 0 | 0.0 | |
| Northeastern | Garissa | 4 [117] | 3.4 | 22 [105] | 20.9 | 1 | 4.0 |
| Mandera | 0 [68] | 0.0 | 5 [68] | 7.4 | 0 | 0.0 | |
| Wajir | 1 [194] | 0.5 | 15 [194] | 7.7 | 1 | 6.7 | |
| Central | Kiambu | 0 [61] | 0.0 | 2 [61] | 3.3 | 0 | 0.0 |
| Kirinyaga | 3 [93] | 3.2 | 3 [93] | 3.2 | 0 | 0.0 | |
| Maragua | 8 [95] | 8.4 | 5 [95] | 5.2 | 1 | 8.3 | |
| Muranga | 5 [67] | 7.4 | 12 [67] | 17.9 | 2 | 13.3 | |
| Thika | 7 [52] | 13.4 | 11 [52] | 21.1 | 7 | 63.6 | |
| Coast | Kilifi | 2 [72] | 2.7 | 0 [0] | 0.0 | 0 | 0.0 |
| Kwale | 1 [51] | 1.9 | 4 [51] | 7.8 | 0 | 0.0 | |
| Malindi | 1 [30] | 3.3 | 6 [30] | 20.0 | 1 | 16.7 | |
| Mombasa | 5 [111] | 4.5 | 7 [111] | 6.3 | 2 | 20.0 | |
| Taita Taveta | 4 [360] | 1.1 | 26 [360] | 7.2 | 0 | 0.0 | |
| Nairobi | Nairobi | 2 [26] | 7.6 | 0 [26] | 0.0 | 0 | 0.0 |
| TOTAL | 126 [2996] | 4.2 | 223 [2849] | 7.8 | 56 | 19.1 |
This represents specimens that tested positive for both IgM and IgG antibodies.
Additional two districts were considered positive as samples tested positive by polymerase chain reaction (PCR) (Nyeri 2/8; Meru south 7/12).
Districts with ≤ 2 serological positive specimens (Bondo, Rachuonyo, Koibatek, and Marsabit) were considered negative. All specimens from 21 other districts were negative for RVF.
Districts reporting outbreaks for the first time were Kitui, Tharaka, Meru South, Meru central, Mwingi, Embu, and Mbeere in Eastern province, Malindi and Taita taveta in Coast province, Kirinyaga and Murang'a in Central province and Baringo and Samburu in Rift valley province. The 2006–2007 outbreak appears to have been more widely distributed in the Eastern province being reported for the first time in 7 of the 13 districts in the province and confirmed in 9 (69%) of the districts.
For the purpose of analysis of data collected through the targeted surveys, specimens collected from one administrative sub-location were considered as a single herd even if collected from animals owned by different farmers. The mean number of herds sampled per district was 5.8 (SE ± 0.5, range = 1–14), whereas the mean number of confirmed positive herds found per district was 2.8 (SE ± 0.31, range 1–9). In the 29 districts where positive herds were detected, Taita Taveta reported the highest number (N = 9) followed by Baringo (N = 6), Maragua (N = 5), Muranga (N = 5), Nairobi (N = 5), and Nakuru (N = 5). The RVF infection among camels was confirmed in six herds located in the four districts of Mandera, Garissa, Isiolo, and Wajir in Northeastern province.
Focused survey results.
Intensive survey was conducted in Garissa district to assess the virus transmission pattern in a region that was the first major focal center of the last two major outbreaks; and in Baringo district to determine whether clustering of human and livestock occurred in this region that had never reported RVF cases before. In Garissa district, 79 herds of livestock drawn from 15 administrative sub-locations in the district were assessed, including 34 herds of cattle, 50 herds of sheep, 55 herds of goats, and 19 herds of camels. Thirteen of the 15 (86.7%) sub-locations reported herds with clinical signs consistent with RVF. Abortions were reported in cattle, camels, sheep, and goats (Table 2). The survey suggested that livestock abortions started in mid-October 2006, over 4 weeks before the first human case in the district.21 The last suspect RVF herd in the district was reported around January 21, 2007, approximately the same time the last human case in the district was reported.
Table 2.
Survey results of clinical signs associated with Rift Valley fever (RVF) infection in livestock from Garissa District, Northeastern Province
| Clinical signs | Number (%) from a total of 79 herds |
|---|---|
| Abortion | 30 (38%) |
| Hemorrhage | 7 (9%) |
| Mortality | 11(14%) |
| None | 49 (62%) |
In Baringo district, 30 herds drawn from 14 sub-locations were assessed, most consisting of cattle, sheep, and goats. Positive herds were identified in six sub-locations, all located in the lowland semiarid regions and confined to Marigat and Mukutani divisions (Figures 2 and 3B). The seroprevalence of RVF virus IgM among livestock was 25.2% (36 of 143), whereas IgG was 27.8% (22 of 79), as shown in Table 3. It is important to note that of the 22 specimens that tested positive for IgG antibodies, 21 (95.4%) were also positive for IgM antibodies, indicating that this was a naive ecology that had no prior virus activity. Sintaan sub-location had the highest proportion of the positive specimens (18 of 36, 50%) followed by Longewan (10 of 36, 27.8%), whereas Kapkuikui had no positive specimens (0 of 19). By species, the seroprevalence of RVF virus IgM was highest in sheep sampled (38.8%) followed by goats (14%) and cattle (10.5%) (Table 4).
Figure 2.

Distribution of the livestock cases for acute Rift Valley fever (RVF) virus infection by antiviral IgM antibodies in seven administrative sublocations affected by the outbreak. The highest proportion of acute livestock cases was in Sintaan and Longewan sub-locations.
Figure 3.

(A) Spatial distribution (dot density) of Rift Valley fever (RVF) confirmed cases in humans and livestock herds, overlain on the various soil types of Baringo district.26 The red circles (o) represent the GPS coordinates of livestock confirmed herd cases distributed randomly in the sub-location (1 dot = 1%). The black cross sign (+) represents the Global Positioning System (GPS) coordinates of confirmed human cases. The gold color represents regions carrying the Solonchak soil type where most of the RVF cases were located. (B). Map of Baringo district showing the 13 administrative divisions. Most of the RVF cases were concentrated in Marigat and Mukutani divisions.
Table 3.
Test results of specimens from livestock collected from the 8 affected sub-locations of Baringo District, Rift Valley Province*
| Division | Sub-location | No. of specimens | RVF IgM ELISA | RFV IgG ELISA | Both IgM and IgG positive (%) | RVF RT-PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Positive (%) | Negative | Total | Positive (%) | Negative | Total | Positive (%) | Negative | ||||
| Makutani | Keserian | 27 | 23 | 4 (17.3) | 19 | 10 | 1 (10) | 9 | 0 | 20 | 0 | 20 |
| Marigat | Maji Ndege | 24 | 24 | 1 (4.2) | 23 | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Marigat | Sintaan | 25 | 24 | 18 (75) | 6 | 24 | 11 (45.8) | 13 | 11 (45.8) | 15 | 1 (6.7) | 14 |
| Marigat | Longewan | 26 | 22 | 10 (45.4) | 12 | 22 | 9 (40.9) | 13 | 9 (50) | 0 | 0 | 0 |
| Marigat | Kipkuikui | 20 | 19 | 0 | 19 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
| Marigat | Meisori | 20 | 19 | 1 (5.2) | 18 | 20 | 1 (5) | 19 | 1 (5) | 0 | 0 | 0 |
| Marigat | Sandai | 14 | 12 | 2 | 10 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
| Makutani | Logumgum | 24 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Total | 180 | 143 | 36 (25.2) | 107 | 79 | 22 (27.8) | 57 | 21 (26.6) | 47 | 1 (2.1) | 46 | |
ELISA = enzyme-linked immunosorbent assay; RT-PCR = reverse transcription-polymerase chain reaction; ND = indicated no laboratory data was obtained.
Table 4.
Number of confirmed Rift Vallely fever (RVF) cases in humans, sheep, goats, and cattle from various sub-locations in Baringo District, Rift Valley Province*
| Sub-location | Suspected human and livestock cases | Confirmed cases and proportion (%) positive | ||||||
|---|---|---|---|---|---|---|---|---|
| Human | Sheep | Goats | Cattle | Human | Sheep | Goats | Cattle | |
| Sintaan | 27 | 21 | 3 | 0† | 20 (23) | 16 (61.5) | 2 (25) | 0 |
| Logumgum | 26 | ND | ND | ND | 17 (19) | – | – | – |
| Longewan | 13 | 17 | 3 | 2 | 11 (13) | 6 (23.1) | 3 (37.5) | 1 (50) |
| Keserian | 12 | 6 | 16 | 1 | 11 (13) | 3 (11.5) | 1 (12.5) | 0 |
| Kipkuikui | 0 | 6 | 13 | 0 | 0 | 0 | 0 | 0 |
| Meisori | 0 | 9 | 10 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Sandai | 0 | 0 | 2 | 10 | 0 | 0 | 1 (12.5) | 1 (50) |
| Maji Ndege | 0 | 8 | 10 | 6 | 0 | 1 (3.8) | 0 | 0 |
| Eldume | 15 | 0 | 0 | 0 | 5 (6) | 0 | 0 | 0 |
| Kailer | 11 | 0 | 0 | 0 | 9 (10) | 0 | 0 | 0 |
| Lorok | 3 | 0 | 0 | 0 | 2 (2) | 0 | 0 | 0 |
| Perkerra | 2 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| Rabai | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salabani | 2 | 0 | 0 | 0 | 2 (2) | 0 | 0 | 0 |
| Sibilo | 4 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| Others | 43 | 0 | 0 | 0 | 9 (10) | 0 | 0 | 0 |
| Total | 169 | 67 | 57 | 16 | 88 (52.1) | 26 (38.8) | 8 (14) | 2 (12.5) |
ND = indicated no laboratory data was obtained.
A zero reading indicates that no cases were found in herds sampled before February 12 when the investigation was conducted in the region. However, it is possible that RVF cases emerged later during the outbreak, which lasted until March 31, 2007.
Clustering of human and livestock cases in Baringo district.
There were a total of 169 suspected human cases from over 11 sub-locations,22 with 52.1% confirmed by laboratory testing. When mapped together, the human and livestock cases in Baringo district showed clustering by sub-location (Figure 3A and Table 4). Sintaan, Longewan, and Keserian sub-locations together had 47% of the confirmed human cases and > 85% of the livestock cases (Table 4). Whereas all five major soil types, i.e., solonetz, planesol, chemozems, and solonchaks found elsewhere in Kenya were also present in Baringo district, almost all human and livestock cases were found within or in close proximity to solonchak soils, in agreement with the findings of a countrywide study analyzing the role of soil types with occurrence of RVF cases (Reference 21; Figure 3A).
Clinical signs.
The livestock survey questionnaires were completed and returned from 12 districts. In sheep, goats, and cattle, the main clinical signs reported were mortality, hemorrhagic syndrome (including bloody discharge from nostrils or mouth, and hematuria), and abortion. In 30% of the suspected infected herds, abortions and mortality were observed in all species. Other clinical signs in cattle, sheep, and goats were dyspnoea, coughing, bloody discharge, anorexia, weakness, and generalized nasal and oral discharge. In camels, the clinical signs included fever, swollen lymph nodes, abortion in approximately 10% of the pregnant animals, and lacrimation. Over 62% of livestock herds in regions with severe RVF outbreaks reported no clinical signs suggestive of RVF (Table 2). The crude morbidity and mortality rates were highest among goats at 4.6% and 1.9%, followed by sheep at 1.5% and 0.3%, respectively. The highest crude morbidity and mortality rates were reported in Baringo at 26.8% and 19.8% in goats and 18.1% and 7.1% in sheep, respectively. This probably reflected the fact that this was the first RVF outbreak in the district.
Discussion
The 2006–2007 livestock RVF outbreak in Kenya resulted in widespread infection of livestock across six of the eight provinces, and in 29 of the 69 administrative districts in the country. In contrast, over 85% of the detected RVF human cases occurred in three districts; Garissa in Northeastern Province, Baringo in Rift Valley Province, and Kilifi in Coast Province.21,22 Although the livestock data does not suggest more extensive livestock disease in the districts reporting a high number of severe human cases, it does suggest that requisite levels of livestock infection may be necessary before virus causes severe disease in a detectable number of humans. A limitation of this interpretation is the possibility that the passive surveillance used across the country may have missed many mild RVF cases in regions where the link between human and livestock diseases may have been more difficult to make. However, the link between livestock and human RVF cases is supported by the clustering of human RVF cases around livestock cases in Baringo district within just six sub-locations. Livestock disease in the district (Baringo) started in late December 2006, although the first human case in the district was reported on January 25, 2007. Unfortunately, livestock cases were detected after human cases in most regions of the country, in part, because of inadequate surveillance in livestock and making the exact interval between onset of livestock disease and human cases hard to determine. Limited resources severely hampered the potential for much earlier detection of the epizootic, which would have provided public health officials more time to implement effective public health control measures.
The sequential pattern of occurrence of the RVF outbreaks in the country in 2006–2007 was similar to that observed in the 1997–1998; with the index foci located at flood-prone Northeastern province before spreading to the Coast and Rift Valley provinces. In fact, vulnerability mapping of the country using historical RVF data collected since 1912 showed recurrence of disease in the same locations, indicating that targeted control measures such as livestock vaccination in high-risk areas before outbreak onset can minimize the impact of RVF.23 Unfortunately, Kenya and the international community did not have contingency plans for response to RVF in Kenya when the alert on possible RVF outbreak in the region was issued, which delayed the implementation of response activities. The 2006–2007 epizootic was the most extensive in Kenya, occurring in 12 new districts that previously had not reported RVF epizootics. An example of this was Baringo district, which reported the highest proportion of recent livestock cases of RVF. In addition, a sero-survey conducted shortly after the outbreak reported 20% of human population in Baringo positive for IgM antibodies indicating recent infections, the highest of any district surveyed.21 It is possible that this outbreak occurred de novo in Baringo because there was no possibility of routing of animals from Northeastern and Coast provinces infected with RVF earlier through the district. However, it is impossible to rule out the possibility that the disease was transmitted to Baringo through movement of infected mosquitoes.
Determining the extent of livestock involvement of RVF outbreak in each district of the country as attempted in this study enables mapping of vulnerable regions that can be targeted for aggressive prevention and control measures when outbreaks are predicted in the future. The finding that livestock cases preceded human cases in the same locations further emphasizes the importance of controlling the disease in animals to prevent human infections. Perhaps the most effective control RVF measures will always be public education, closure of livestock markets, ban on livestock slaughtering, and ban on movement of livestock from affected districts. In Kenya, public education messages should be developed in different languages because of heterogeneity of communities living in RVF prone regions and the messages should be disseminated by radio, a widely used medium for most parts of the country. In addition, village elders, chiefs, and religious leaders should be educated through public meetings.24,25 This approach was a critical intervention in the 2006–2007 outbreak because the outbreak occurred a week before the Muslim Eid al-Adha holiday, during which an estimated 20,000 animals or more would have been slaughtered in Northeastern Province. There was widespread compliance with a slaughter ban because of the support by religious leaders, which may have significantly reduced the human cases. An integrated vector-control strategy can be attempted but it is likely to be unsuccessful because of cost, difficulties in accessing the flooded areas, and the sheer expansiveness of affected areas. Most experts agree that livestock vaccination should not be attempted once the outbreak starts because of the associated risk of mechanical spread of the virus and possibility of virus transmission by mosquitoes when the live attenuated vaccines are used.27
The nomadic lifestyle and traditional belief of the communities living in arid and semi-arid lands where most outbreaks occur may play a major role in the spread of RVF epizootics. With the onset of rains, many pastoralists move their herds to areas with new grass growth and water-filled dambos (temporary water bodies). However, as the rains continue, counter movement of the herds away from the increasing number of mosquitoes around the flooded areas occurs. These movements have the potential of introducing infected animals into uninfected areas. At the peak of the floods, movement of the herds is hindered, as is the ability to identify and transfer ill people to hospitals for treatment. The common practice among most pastoralist communities in Kenya is to slaughter and consume the meat of an ill or recently dead animal, to salvage the value of the protein of that animal. With RVF, contact with animal products of a sick animal, or consumption of improperly prepared meat from sick animals is the most significant risk factor for human infection.10,27 Even after extensive public education throughout the country in the 2006 epizootic, spread of the disease in Baringo district epizootic that occurred over 6 weeks after the Garissa epizootic was still attributed, in part, to eating sick or dead animals. There is also evidence suggesting that consumption of fresh milk from infected animals may also be a risk factor for disease transmission.10
Acknowledgments
We thank the entire RVF response team that included staff from Kenya Ministry of Livestock and Fisheries Development, Kenya Ministry of Health, Kenya Medical Research Institute, and Food and Agriculture organization. The Special Pathogens Branch in the Division of Viral and Richettsial Diseases at the Centers for Disease Control provided equipment and reagents for PCR and immunodiagnosis.
Disclaimer: The findings and conclusions in this paper are by the authors and should not be construed to represent the Centers for Disease Control and Prevention determination or policy.
Footnotes
Authors' addresses: Peninah Munyua, Rees M. Murithi, Peter M. Ithondeka, Joseph Macharia, Joseph Musaa, and Jane Githinji, Kenya Ministry of Livestock Development, Nairobi, Kenya, E-mails: munyuap@gmail.com, murithimbabu@yahoo.com, peterithondeka@yahoo.com, jmmacharia@excite.com, jmusaa@yahoo.com, and janejackim@yahoo.com. Sherrilyn Wainwright, United States Department of Agriculture, Fort Collins, CO, E-mail: Sherrilyn.H.Wainwright@aphis.usda.gov. Peter Bloland, National Center for Zoonosis, Vector-borne, and Enteric Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pbb1@cdc.gov. David Mutonga, Ministry of Public Health and Sanitation, Nairobi, Kenya, E-mail: davidmutonga@yahoo.com. Allen Hightower, Robert Breiman, and M. Kariuki Njenga, Global Disease Detection Program, Centers for Disease Control and Prevention–Kenya, Nairobi, Kenya, E-mails: awh1@cdc.gov, rbreiman@ke.cdc.gov, and knjenga@ke.cdc.gov.
References
- 1.Bishop DH, Calisher CH, Casals J, Chumakov MP, Gaidamovich SY, Hannoun C, Lvov DK, Marshall LD, Oker-Blom N, Pettersson RF, Porterfield JS, Russell PK, Shope RE, Westaway EG. Bunyaviridae. Intervirology. 1980;14:125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- 2.Daubney R, Hudson JR, Granham PC. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- 3.Meegan JM, Bailey CH. In: The Arboviruses: Epidemiology and Ecology. Monath TP, editor. Volume 4. Boca Raton, FL: CRC Press; 1988. pp. 51–76. (Rift Valley fever). [Google Scholar]
- 4.Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster L, Henderson A, Khan AS, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak BL, Ryan M, Ksiazek TG, Arthur RR, Ndikuyeze A, Agata NN, Peters CJ. An outbreak of Rift Valley fever in northeastern Kenya, 1997–1998. Emerg Infect Dis. 2002;8:138–144. doi: 10.3201/eid0802.010023. World Health Organization Hemorrhagic Fever Task Force. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Akkad AM. Rift Valley fever in Egypt, October–December 1977. J Egypt Public Health Assoc. 1978;53:137–146. [PubMed] [Google Scholar]
- 6.Zeller HG, Fontenille D, Traore-Lamizana M, Thiongane Y, Digoutte JP. Enzootic activity of Rift Valley fever virus in Senegal. Am J Trop Med Hyg. 1997;56:265–272. doi: 10.4269/ajtmh.1997.56.265. [DOI] [PubMed] [Google Scholar]
- 7.Al-Afaleq AI, Abu Elzein EM, Mousa SM, Abbas AM. A retrospective study of Rift Valley fever in Saudi Arabia. Rev Sci Tech. 2003;22:867–871. doi: 10.20506/rst.22.3.1436. [DOI] [PubMed] [Google Scholar]
- 8.Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, Khan AS, Rollin PE, Swanepoel R, Ksiazek TC, Nichol ST. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–2001. Emerg Infect Dis. 2002;8:1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amwayi SA, Gould LH, Sharif SK, Nguku PM, Omolo J, Mutonga D, Rao C, Lederman E, Sshnable D, Sang R, Paweska JT, Katz M, Hightower A, Kariuki Njenga M, Feikin DR, Breiman RF. Risk factors for severe Rift Valley fever infection in Kenya. Am J Trop Med Hyg. 2010;83((Suppl 2)):14–21. doi: 10.4269/ajtmh.2010.09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) RVF fact sheet. 2007. http://www.who.int/mediacentre/factsheet/fs207/en/index.html Available at. Accessed August 21, 2008.
- 11.Swanepoel R, Coetzer JAW. In: Infectious Diseases of Livestock. Second edition. Coetzer JA, Tustin RC, editors. Volume 2. Oxford, UK: Oxford University Press; 2004. pp. 1037–1070. (Rift Valley fever). [Google Scholar]
- 12.Davies FG. Observations on the epidemiology of Rift Valley fever in Kenya. J Hyg Camb. 1975;75:219–229. doi: 10.1017/s0022172400047252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenya Ministry of Livestock Development . Annual Report on Livestock Health. Nairobi; Kenya: 1998. [Google Scholar]
- 14.Davies FG, Kilelu D, Linthicum KJ, Pegram RG. Patterns of Rift Valley fever activity in Zambia. Epidemiol Infect. 1992;108:185–191. doi: 10.1017/s0950268800049633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, Van Vuren PJ, Manyibe T, Macharia JM, Ksiazek TG, Feikin DR, Breiman RF, Kariuki Njenga M. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–1269. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Agriculture Organization (FAO) Possible RVF activity in the Horn of Africa. EMPRES Watch. 2006 http://www.fao.org/docs/eims/upload/217874/EW_hornafrica_nov06_rvf.pdf Nov 2006. Available at. Accessed June 20, 2008. [Google Scholar]
- 17.Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, Burt FJ, Leman PA, Khan AS, Rowe AK, Mukun R, Sanchez A, Peters CJ. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179:S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 18.Bird BH, Bawiec DA, Ksiazek TG, Shoemaker TR, Nichol ST. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J Clin Microbiol. 2007;45:3506–3513. doi: 10.1128/JCM.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird BH, Githinji JW, Macharia JM, Kasiiti JL, Murithi RM, Gacheru SG, Musaa JO, Towner JS, Reeder SA, Oliver JB, Erickson BR, Morgan LT, Khristova ML, Hartman AL, Comer JA, Rollin PE, Ksiazek TG, Nichol ST. Multiple virus lineages sharing recent common ancestry were associated with a large Rift Valley fever outbreak among livestock in Kenya during 2006–2007. J Virol. 2008;82:11152–11166. doi: 10.1128/JVI.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paweska JT, Burt FJ, Anthony F, Smith SJ, Grobbelaar AA, Croft JE, Ksiazek TG, Swanepoel R. IgG-sandwich and IgM-capture enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in domestic ruminants. J Virol Methods. 2003;113:103–112. doi: 10.1016/s0166-0934(03)00228-3. [DOI] [PubMed] [Google Scholar]
- 21.Nguku P, Sharif SK, Mutonga D, Amwayi S, Omollo J, Mohammed O, Farnon EC, Gould LH, Lederman E, Rao C, Sang R, Schnabel D, Feikin DR, Hightower A, Njenga MK, Breiman RF. Investigation of a major outbreak of Rift Valley fever in Kenya, 2006–2007: clues and enigmas concerning Rift Valley fever outbreaks and their prevention. Am J Trop Med Hyg. 2010;83((Suppl 2)):5–13. doi: 10.4269/ajtmh.2010.09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Rift Valley fever outbreak - Kenya, November 2006–January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:73–76. [PubMed] [Google Scholar]
- 23.Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect May. 2010;18:1–9. doi: 10.1017/S0950268810001020. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Sang R, Kioko E, Lutomiah J, Warigia M, Ochieng C, O'Guinn M, Lee JS, Cheruiyot P, Koka H, Godsey M, Hoel D, Hanafi H, Miller B, Schnabel D, Breiman RF, Richardson J. Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am J Trop Med Hyg. 2010;83((Suppl 2)):28–37. doi: 10.4269/ajtmh.2010.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nzietchueng S, Bett B, Njogu G, Jost C, Mariner J. Participatory assessment of Rift Valley surveillance and response activities. Learning the Lessons of Rift Valley Fever: Improved Detection and Mitigation of Outbreaks. Nairobi, Kenya; ILRI: 2007. pp. 21–57. [Google Scholar]
- 26.Government of Kenya . Exploratory Soil Survey Report. Number E1. Kenya Soil survey; Nairobi: 1982. [Google Scholar]
- 27.Turell MJ, Rossi CA. Potential for mosquito transmission of attenuated strains of Rift Valley fever virus. Am J Trop Med Hyg. 1991;44:278–282. doi: 10.4269/ajtmh.1991.44.278. [DOI] [PubMed] [Google Scholar]
- 28.Olaleye BO. Rift Valley fever in Nigeria: infections in humans. Rev Sci Tech Off Into Epic. 1996;15:923–935. doi: 10.20506/rst.15.3.967. [DOI] [PubMed] [Google Scholar]


