Introduction
Leukemia represents the most common pediatric malignancy, accounting for approximately 30% of all cancers in children less than 20 years of age. Acute lymphoblastic leukemia (ALL) is the most frequent, comprising approximately 23% of childhood cancers. Acute myeloid leukemia (AML) accounts for approximately 4% of pediatric cancer diagnoses and 20% of childhood leukemia. Chronic myelogenous leukemia (CML) is rare and accounts for approximately 1% of all pediatric cancer, although it comprises 10% of leukemia in older adolescents. Juvenile myelomonocytic leukemia (JMML) is infrequent making up about 2% of leukemia and 25% of myelodysplastic syndrome in childhood [1]. Most children diagnosed with leukemia are cured without hematopoietic stem cell transplantation (HSCT), but for some, high-risk subgroups, allogeneic HSCT plays an important role in their therapeutic approach.
Acute Lymphoblastic Leukemia (ALL)
Prognostic Variables and Risk Stratification at Diagnosis
Clinical and biologic features are used to subtype, risk-stratify and assign therapy at diagnosis. Initial risk group assignment is made based on age, peripheral white blood cell count (WBC), central nervous system (CNS) involvement, and phenotype [2]. Phenotypic classification is determined by flow cytometry of lineage-associated cell surface markers. The majority of ALLs are of precursor B-cell (pre-B) phenotype (CD10, CD19, HLA-DR, TDT +), 10 to 20% are T-cell (CD2, CD3, CD5, and/or CD7 +), and <5% are mature B-cell or Burkitt-type (CD20, surface-IgM+).
Cytogenetic studies are subsequently used to further define the risk of relapse. The t(12;21) translocation, the most frequent recurrent chromosomal translocation associated with childhood ALL, is identified in approximately 25% of cases and this is associated with a favorable prognosis [3–6]. Gene rearrangements of the mixed-lineage leukemia (MLL) gene located at 11q23 is the most common cytogenetic finding in infants with ALL, which has an extremely poor prognosis [7–10]. The so called Philadelphia chromosome (Ph+), which results from a translocation between chromosomes 9 and 22, t(9;22), also confers adverse risk [11]. The t(1;19) translocation is also associated with an increased risk of relapse, but this can be offset by therapy intensification [12,13]. Hyperdiploidy, which most often includes trisomies of chromosomes 4, 7, and/or 10, carries a favorable prognosis [14–18]. Hypodiploid cases are at higher risk of relapse [19–22]. Recently, gene expression analysis has been shown to allow further discrimination in regard to risk classification and treatment response prediction [23].
The initial response to therapy has important prognostic utility. A rapid early response (RER), defined as a marrow blast count below 5% within 7 to 14 days, or clearance of peripheral blasts within 7 to 10 days, has a better outcome than those whose response is slower (SER) [24–30]. Response to therapy can be further quantified by flow cytometric or molecular analysis of minimal residual disease (MRD), which has been shown to correlate with outcome [31,32]
Non-Transplant Therapy
Approximately 80% of children with ALL are cured with chemotherapy, the intensity of which is determined by risk-group assignment and treatment stratification. The majority of patients fall into the standard risk category characterized by age of 1 to 9 years, WBC <50,000/µL, B-precursor phenotype, and absence of high-risk chromosomal abnormalities. Therapy for B-precursor and T-cell ALL consists of induction, consolidation/intensification/re-induction, CNS sterilization, and maintenance for a total of 2 to 3 years [33] [34–40]. Individuals with mature B-cell phenotype are treated as per Burkitt lymphoma regimens, which most commonly employ dose and sequence intensive, short course combination chemotherapy [41–43].
The prognosis after relapsed ALL depends on the duration of the first remission (CR1) and the site of relapse [44–47]. Outcome after short CR1 duration (<12–18 months) is very poor, as is the prognosis for individuals who are unable to achieve a second remission. Those with isolated extramedullary relapse fair better than those with marrow relapse [48,49].
Transplantation
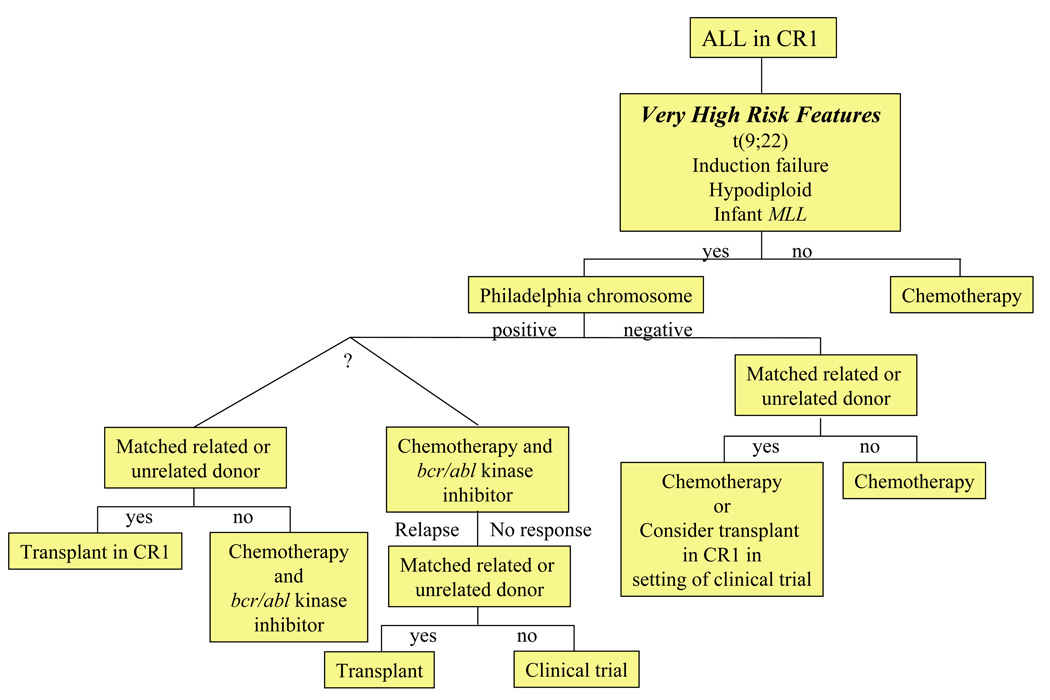
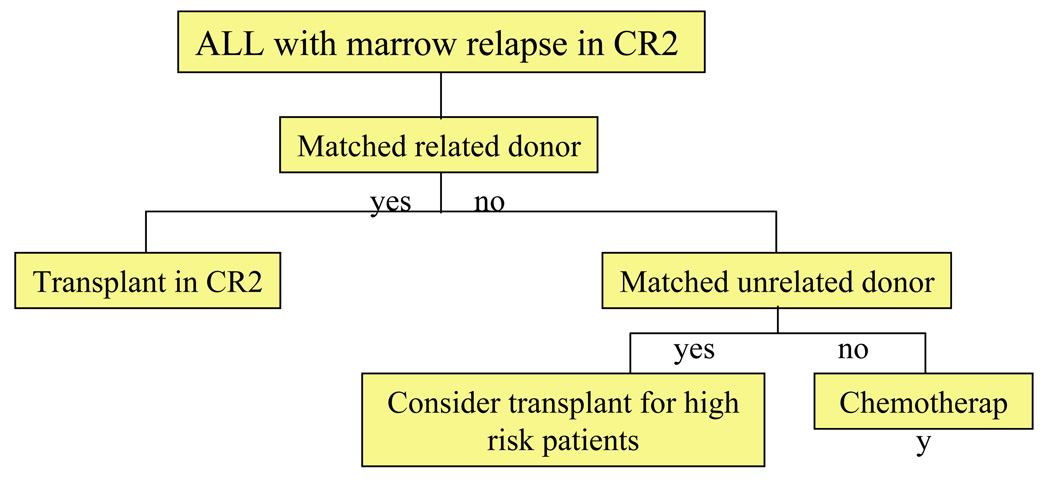
There have been no large prospective controlled clinical trials to evaluate the relative efficacy of allogeneic HSCT in comparison to chemotherapy for childhood ALL. However, multiple comparative studies suggest that relapse rates are lower after HSCT [50]. Some of the benefits in regard to relapse-free survival are offset by transplant-associated morbidity and mortality [51]. Consequently, HSCT is usually reserved for the management of relapse and it is rarely employed for children in CR1 except for those with extremely high-risk features (Table 1;]Figure 1). Results of recent trials of HSCT for children and adolescents with ALL in second remission (CR2) are presented in Table 2. For those with HLA-matched sibling donors, allogeneic HSCT in second remission is considered standard. Unrelated donor HSCT is usually reserved for those at high risk of relapse with chemotherapy (Figure 1, Figure 2). Importantly, the approach in individual cases will vary based on risk/benefit analysis, donor options, and access to transplantation. The American Society for Blood and Marrow Transplantation (ASBMT) has published consensus guidelines for the use of HSCT in childhood ALL (Table 3) [50]. Suggested algorithms for HSCT in pediatric ALL are presented in Figure 1 and Figure 2.
Table 1.
Results of SCT for Pediatric Patients with ALL in First Remission
| Study Group |
Dates of Study |
High risk indicator | Patients (n) | Outcome (years) |
Reference |
|---|---|---|---|---|---|
| Toronto | 1985–2001 | t(9;22) | 11 MRD, MUD | 53% EFS (4) | Sharathkumar 2004 [154] |
| 10 chemo | |||||
| UKALL -X | 1985–90 | WBC >100,000/µL +/− t(9;22), Near-haploid, Induction failure |
76 MRD, 25 MUD | 45% EFS (10) | Wheeler 2000 [155] |
| UKALL -XI | 1990–97 | 351 chemo | 39% EFS (10) | ||
| AIEOP/ GITMO |
1986–94 | WBC >100,000/µL BFM risk index >1.7 t(9;22), t(8;11) Steroid resistance T-cell disease Induction failure |
30 MRD | 58% DFS (4) | Uderzo 1997 [156] |
| 130 chemo | 48% DFS (4) | ||||
| NOPHO | 1981–91 | WBC >100,000/µL | 22 MRD | 73% DFS (10) | Saarinen 1996 [157] |
| 44 chemo* | 50% DFS (10) | ||||
| 405chemo† | 59% DFS (10) | ||||
| IBMTR | 1978–90 | t(9;22) | 33 MRD | 38% DFS (2) | Barrett 1992 [158] |
| Groupe d’Etude de la Greffe de Moelle Osseuse |
1980–87 | t(9;22) WBC >100,000/µL Induction failure |
32 MRD | 84% DFS (2.5) | Bordigoni 1989 [159] |
chemo = chemotherapy, EFS = event free survival, DFS = disease free survival
matched control patients
unmatched patients
Figure 1.
ALL: Algorithm for transplantation in first remission.
Table 2.
Results of SCT for Pediatric Patients with ALL in Second Remission
| Study Group | Dates of Study |
Patients (n) | Outcome (years) |
Reference |
|---|---|---|---|---|
| BFM | 1985–91 | 51 MRD | 52% EFS (5) | Dopfer 1991 [160] |
| IBMTR/POG | 1983–91 | 255 MRD | 40% DFS (5) | Barrett 1994 [51] |
| 255 chemo | 17% DFS (5) | |||
| Leiden | 1982–91 | 25 MRD | 44% DFS (4) | Hoogerbrugge 1995 [161] |
| 97 chemo | 24% DFS (4) | |||
| AIEOP/GITMO | 1980–90 | 57 MRD | 41% DFS (5) | Uderzo 1995 [162] |
| 230 chemo | 21% DFS (5) | |||
| Paris | 1983–93 | 42 MRD | 53% (4) | Moussalem 1995 [163] |
| UKALL-X | 1985–90 | 83 MRD, 27 MUD | 40% EFS(5) | Wheeler 1998 [164] |
| 61 ABMT | 34% EFS | |||
| 261 chemo | (5) 26% EFS (5) | |||
| UKALL-R1 | 1991–95 | 63 MRD | 46% EFS (5) | Harrison 2000 [165] |
| 41 MUD | 54% EFS (5) | |||
| 15 ABMT, 89 chemo | 43% EFS (5) | |||
| IBMTR/COG | 1991–97 | CR1 < 36 months | Eapen 2006 [52] | |
| 92 MRD + TBI | 32% OS (8) | |||
| 19 MRD no TBI | 44% OS (8) | |||
| 110 Chemo | (8)18% OS | |||
| CR1 ≥ 36 months | ||||
| 61 MRD + TBI | 66% OS (8) | |||
| 14 MRD no TBI | 63% OS (8) | |||
| 78 Chemo | 32% OS (8) | |||
| COG | 1995–98 | 32 MRD | 42% DFS (3) | Gaynon 2006 [45] |
| 19 MUD | 29% DFS (3) | |||
| 23 chemo | 30% DFS (3) | |||
chemo = chemotherapy, MUD = matched unrelated donor, MRD = matched related donor, EFS = event free survival, DFS = disease free survival, OS = overall survival, ABMT = autologous bone marrow transplant, TBI = total body irradiation
Figure 2.
ALL: Algorithm for transplantation in second remission.
Table 3.
Stem Cell Transplantation for Pediatric ALL - 2005 American Society for Blood and Marrow Transplantation Expert Panel Consensus [Hahn BBMT 2005]
| Recommendation | Indication | References |
|---|---|---|
| SCT in CR1 | Benefit demonstrated for matched related donor SCT for Philadelphia chromosome + only. |
Wheeler 2000 [155] |
| Chessells 1992 [166] | ||
| Not recommended for other high-risk patients, except in the setting of a clinical trial. |
Arico 2000 [11] | |
| Uderzo 1997 [156] | ||
| SCT in CR2 with prior bone marrow relapse |
Recommended for those with matched related donors. |
Barrett 1994 [51] |
| Wheeler 1998 [164] | ||
| Evidence insufficient to recommend unrelated donor SCT. |
Uderzo 1995 [162] | |
| Harrison 2000 [165] | ||
A retrospective matched cohort analysis performed by the Children’s Oncology Group (COG) and International Bone Marrow Transplant Registry (IBMTR) compared matched related HSCT to chemotherapy for children with ALL in CR2. Leukemia-free survival and relapse rates were better after HSCT in all patient groups regardless of the CR1 duration [51]. In a more recent study from the COG and IBMTR, overall survival, leukemia-free survival and treatment-related mortality were superior for patients with a short CR1 duration ( < 36 months) who underwent HSCT with total body irradiation (TBI) based conditioning regimens (vs. chemotherapy and non-TBI transplant regimens). For those with a late relapse (≥36 months CR1 duration), outcomes were equivalent in the chemotherapy and TBI transplant group. Those treated with HSCT without TBI had inferior relapse and disease-free survival (DFS) rates regardless of CR1 duration [52].
Despite the lower relapse rates after HSCT, this approach carries the risk of transplant-associated mortality and morbidity (e.g., graft-versus-host disease [GVHD]). Further, chemotherapy alone can be effective. Approximately 30 – 40% of children who sustain a late relapse (> 36 months CR1 duration) may achieve long-term DFS with aggressive chemotherapy alone [45–47,51,53,54].
Decisions about the role for and timing of HSCT for children with relapsed ALL are commonly individualized based on biologic, clinical, treatment, and donor factors (Figure 2). Transplant is usually recommended for children with relapse who have HLA-matched sibling donors irrespective of other prognostic factors. An alternative approach for those with a long CR1 duration is to reserve HSCT in the event of another relapse. For individuals who sustain bone marrow relapse during front-line therapy or within 6 months of completion of therapy, the prognosis is poor with chemotherapy alone and HSCT with an alternative (i.e., unrelated or HLA-mismatched related) donor should be considered. HSCT is also often considered for T-cell ALL with marrow relapse. Additional factors that place an individual at high risk of subsequent relapse or that limit the ability to administer chemotherapy (e.g., allergy, organ toxicity) also warrant consideration of HSCT.
HSCT in first remission has no proven benefits for patients defined as high-risk by WBC count, gender, and age. However, transplantation is commonly considered for those at very high risk of relapse with standard therapy (e.g., hypodiploidy, induction failure) (Table 1; Figure 1). Although historically HSCT has been considered for children with Ph+ ALL [11,55], the addition of the tyrosine kinase inhibitor imatinib mesylate to chemotherapy appears to have improved non-transplant outcome [56], diminishing the role of HSCT as upfront therapy. The role of HSCT for other very high-risk groups should be considered in the setting of a clinical trial [50]. HSCT in infants <18 months old, especially those with MLL-rearrangements remains controversial due to the high risk of adverse effects of transplant conditioning in such young patients [57]. Some series report outcomes following HSCT in CR1 that may be superior to chemotherapy [38,58–60]. However, others reveal no definitive benefit in comparison to intensive chemotherapy [61–63]. Results of an IBMTR database review indicate three-year probabilities of DFS of approximately 50% after HLA-matched sibling and unrelated donor transplantation in CR1 for infants with ALL [52].
Conditioning Regimens
Multiple studies indicate that TBI-based transplant conditioning regimens are associated with lower risk of relapse in comparison to chemotherapy-only regimens for children with ALL [50,52,64,65]. Notably, second HSCT using TBI has been successful for children who have relapsed after a busulfan-based preparative regimen [66].
Disease Status
Individuals with ALL should be transplanted in complete remission and there is little to no role for HSCT in patients with ALL who are not in CR [67]. Further, recent data indicate that the MRD level at the time of HSCT correlates with outcome. Importantly, children with no detectable MRD (<1 leukemia cell in 10,000 bone marrow cells) have excellent post-transplant outcomes [68]. In addition, many series report that patients transplanted in earlier remissions fare better than those with a history of multiple relapses, although such studies are subject to significant selection bias.
Donor Selection
Outcomes of alternative donor transplantation have improved in recent years and a number of groups report equivalent outcomes to HLA-identical sibling donors using matched unrelated, partially matched related, partially mismatched unrelated cord blood, and haploidentical donors [69–76]. Donor T cell depletion and improvements in supportive care of infection and GVHD have improved the outcomes of such alternative donor transplants. However, T cell depletion is associated with increased risk of graft rejection, mixed chimerism, delayed immune reconstitution and infectious complications. Treatment-associated mortality remains high, exceeding 20% in published series of alternative donor transplants for ALL, due in part to the high-risk nature of patients treated with that approach. Rates of extensive chronic GVHD also remain high after alternative donor transplants [72–74].
Second Transplantation
For patients who relapse following allogeneic HSCT for ALL, a second transplant may be possible, although the outlook is very poor [77]. This approach carries a high risk of mortality due to progressive disease and/or treatment-associated toxicity. Although remission can be obtained in as many as 50% to 70% of patients, the duration is typically short and only 10% to 30% achieve long-term event-free survival. The prognosis is better for those with longer remission duration after the first HSCT [71,78,79]. Donor leukocyte infusion (DLI) has a limited role in the setting of ALL and post-transplant relapse, although successful remission induction with withdrawal of immunosuppression and/or DLI has been reported in a small percentage of cases (discussed elsewhere in this edition) [80–84].
Acute Myelogenous Leukemia (AML)
Prognostic Variables and Risk Stratification at Diagnosis
The French-American-British (FAB) classification system categorizes AML into seven distinct subtypes based on morphology and phenotype. AML subtype and other clinical and biologic features that influence outcome have recently been used to stratify treatment [85].
Non-Transplant Therapy
In most cases AML treatment consists of intensive induction, consolidation, and CNS-directed chemotherapy. Approximately 75 to 90% of children with AML will achieve a CR and increasing the treatment intensity of induction improves DFS rates [86–88]. Post-remission consolidation chemotherapy is essential and can be delivered in standard doses or as high-dose therapy with autologous stem cell rescue with similar DFS rates [89–96] [97]. Despite treatment intensification the outcome is guarded for most children with AML, and only about 50% are cured with chemotherapy alone [98]. Individuals with acute promyelocytic leukemia (FAB M3) have a better prognosis, with 80% DFS rates observed when all-trans-retinoic acid is added during induction and a maintenance phase [99–102]. Young children with trisomy 21 who develop AML also have excellent outcomes and require less intensive therapy [103–105].
Transplantation
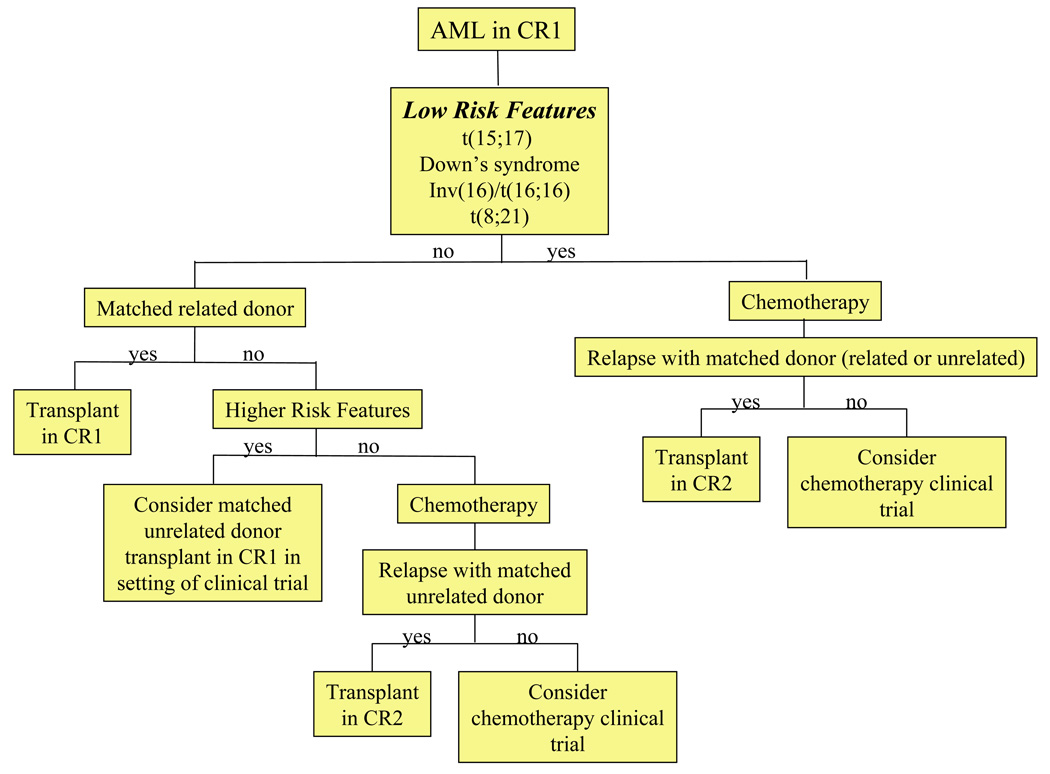
Given the relative poor outcome for pediatric patients with AML, allogeneic HSCT has commonly been used as consolidation in CR1. There have been multiple “genetic randomization” studies of matched related allogeneic HSCT in which individuals who have matched sibling donors are assigned to transplantation. Allogeneic HSCT confers a lower risk of relapse and improves DFS in comparison to chemotherapy with or without autologous rescue (Table 4) [86–88,91,93–96,106–109]. However, clinical benefits can be offset by transplant-related morbidity and mortality, which may eliminate any overall survival advantage in low risk groups [87,110–113]. Consequently, there is some debate as to whether allogeneic HSCT should be employed in CR1 or CR2 for AML in childhood [113–115]. The ASBMT has published consensus guidelines for the use of HSCT in pediatric AML (Table 5) [91] and a suggested approach is presented in Figure 3.
Table 4.
Results of SCT as Post-Remission Therapy for Childhood AML in First Remission
| Study Group | Disease Free Survival | Median Follow- Up |
Reference | ||
|---|---|---|---|---|---|
| Matched Related Donor SCT |
Chemotherapy | Autologous SCT |
|||
| AML-80 | 43% | 31% | - | 6 years | Dahl 1990 [106] |
| AIEOP LAM-87 | 51%* | 27% | 21% | 5 years | Amadori 1993 [94] |
| CCG-213 | 54%* | 37% | - | 5 years | Wells 1994 [108] |
| CCG-251 | 45%* | 32% | - | 8 years | Nesbit 1994 [107] |
| POG-8821 | 52%* | 36% | 38% | 3 years | Ravindranath 1996 [93] |
| MRC AML-10 | 61%* | 46% | 68%ˆ | 7 years | Stevens 1998 [87] |
| AML BFM-93 | 64% | 61% | - | 5 years | Creutzig 2001 [86] |
| CCG-2891 | 55%* | 47% | 42% | 8 years | Woods 2001 [95] |
| LAME-89/91 | 72%* | 48% | - | 6 years | Perel 2002 [88] Aladjidi 2003 [109] |
Key: * p≤0.05 allogeneic vs. others;
p≤0.05 autologous vs. chemotherapy
Table 5.
Stem Cell Transplantation for Pediatric AML 2007 American Society for Blood and Marrow Transplantation Expert Panel Consensus [Oliansky BBMT 2007]
| Recommendation | Indication | References |
|---|---|---|
| SCT in CR1 | Benefit demonstrated for matched related donor SCT. |
Alonzo 2005 [167] |
| Woods 2001 [95] | ||
| Ravindranath 1996 [93] | ||
| Wells 1994 [108] | ||
| Nesbit 1994 [107] | ||
| Amadori 1993 [94] | ||
| SCT in CR2 | Recommended for those with matched related donors. |
Aladjidi 2003 [109] Pession 2000 [168] Gorin 1996 [169] |
| Evidence insufficient to recommend unrelated donor SCT, except in the setting of a clinical trial. | ||
Figure 3.
AML: Algorithm for transplantation.
In the U.S., matched related sibling donor HSCT is the most common consolidation therapy employed for children with AML in CR1 outside of specific low risk groups [91]. This approach is based largely on clinical trials conducted by the Pediatric Oncology Group (POG) and the Children’s Cancer Group (CCG) [116,117]. Both groups reported superior outcomes for high and intermediate risk patients treated with HSCT in CR1. The 5-year overall survival for patients transplanted with a matched sibling donor in CR1 ranges from 52% to 72% (Table 4) [91] [117] [116] [86–88,95,109].
Long-term DFS can be achieved in approximately 30% of children with AML who are transplanted in CR2 with either matched unrelated or mismatched related donors [109]. Consequently, HSCT is sometimes reserved for management of patients who relapse after chemotherapy, especially for low risk groups [115] or those without sibling donors. ASBMT consensus guidelines recommend HSCT in CR2 only for patients with matched related donors, as evidence supporting unrelated donor HSCT is lacking [91].
Conditioning Regimens
Comparative clinical trials in adults reveal similar results with busulfan/cyclophosphamide in comparison to TBI/cyclophosphamide [118]. Pediatric studies are limited, although no obvious differences are apparent [119]. In general, busulfan/cyclophosphamide is the most commonly employed pre-transplant preparative regimen employed in pediatric AML.
Disease Status
In most of the published series of HSCT for pediatric AML, transplantation is performed for patients in remission [91]. Although some individuals with AML who undergo HSCT in relapse can achieve long-term DFS [67], small pediatric studies suggest that the percentage of pre-transplant blasts correlates with post-transplant relapse and that outcomes are improved when HSCT is performed in remission [120,121].
Donor Selection
Donors mismatched for natural killer cell killer (NK) immunoglobulin-like receptor (NK KIR) may improve post-transplant outcome in AML due to allogeneic NK-cell mediated anti-leukemic effects [122], an approach that is currently under study in pediatric AML (discussed elsewhere in this edition).
Second Transplants
As in ALL, the outcome of second transplants for children with AML varies based on the interval from prior transplantation [123].
Chronic Myelogenous Leukemia (CML)
Prognostic Variables and Risk Stratification at Diagnosis
CML is characterized by the presence of the Philadelphia chromosome and the associated translocation product bcr/abl. CML has three defined clinical phases: chronic, accelerated, and blast crisis, with most patients presenting with chronic phase. Response to treatment and survival correlate with phase of disease. Blast crisis is clinically indistinguishable from acute leukemia and treatment responses are short lived.
Non-Transplant Therapy
The kinase activity of the bcr/abl fusion protein is inhibited by imatinib mesylate (Gleevec) and related kinase inhibitors, and these agents have transformed the approach to treatment with CML [124]. Imatinib induces complete remissions in most patients with chronic phase CML, although continuous treatment appears to be required and resistance may develop [125,126]. Thus, there is as of yet no evidence that this new class of kinase inhibitors will be curative and they cannot be recommended as a replacement for allogeneic HSCT in children who have an HLA-matched donor [127]. A number of criteria have been proposed for deciding when to proceed from kinase inhibitor therapy to HSCT, including loss of therapeutic response or failure to achieve a complete hematologic response by 3 months or a substantial cytogenetic response by 3 to 6 months of treatment [128].
Transplantation
Allogeneic HSCT is the only proven cure for CML and donor availability should be considered soon after diagnosis for all children with this disorder. Post-transplant DFS rates are inversely related to age and exceed 80% for young children with matched sibling donors in first chronic phase.
Conditioning Regimens
Busulfan/cyclophosphamide is the most common preparative regimen used for pediatric patients with CML undergoing SCT.
Disease Status
Results are best when HSCT is performed in first chronic phase and with a shorter diagnosis-to-transplant interval. Success is substantially diminished for the accelerated phase or blast crisis and attempts should be made to induce a second chronic phase prior to transplant [129,130].
Donor Selection
In general, pediatric patients have relatively low risk of transplant-related mortality and results are similar with matched unrelated and related donors. Thus, unrelated donor HSCT is usually recommended for those who lack sibling donors [124,127,130–133].
Second Transplants
DLIs have been well demonstrated in adult studies and in a small pediatric series to be effective in the management of post-transplant relapse of chronic phase CML [82]. When DLI is unsuccessful, second transplants should be considered, especially in cases where there was no prior development of GVHD [84].
Juvenile Myelomonocytic Leukemia (JMML) and Myelodysplastic Syndromes (MDS)
Prognostic Variables and Risk Stratification at Diagnosis
The myelodysplastic syndromes represent a heterogeneous group of disorders characterized by ineffective hematopoiesis, impaired maturation of myeloid progenitors, cytopenias, dysplastic changes, and a propensity for the development of AML [134,135]. The major diagnostic groups within MDS encountered in pediatric patients include JMML, myeloid leukemia of Down syndrome, and MDS occurring de novo and secondary to previous therapy or pre-existing disorders [136–138]. In general, pediatric MDS carries a poor prognosis and clinical variables have little practical utility in guiding therapy [139–142].
Non-Transplant Therapy
In general, therapeutic options are limited in MDS and outcome is guarded. Some patients with MDS initially have an indolent course without therapy [141]. AML-type chemotherapy is associated with low response and high relapse rates [135,142]. JMML is resistant to therapy. Although chemotherapy may reduce disease burden, responses are usually short lived and the disease rapidly progresses with a median survival of approximately 1 year [143]. The European Working Group of MDS (EWOG-MDS) in Childhood reported a retrospective analysis of 110 cases of JMML. The probability of survival at 10 years was 6% for the non-transplant group vs. 39% after transplantation [140].
Transplantation
HSCT is considered the only curative treatment for childhood MDS and JMML.
Given the low response rates to non-transplant therapies, and because failure rates after HSCT appear lower when HSCT is performed soon after diagnosis, strong consideration should be given for early transplantation, especially when a matched sibling donor is available. DFS rates of 50 to 64% are reported with HSCT. Results of the largest published transplant series for children with MDS and JMML are summarized in Table 6 [144–150]. Individuals with JMML who develop GVHD have a lower incidence of relapse [134,151].
Table 6.
Results of SCT for Pediatric Patients with MDS and JMML
| Patients (n) | Survival (years) | Reference | ||||
|---|---|---|---|---|---|---|
| RA/RARS | RAEB | RAEB/T | MDS/AML | JMML | ||
| 48 MRD 52 MUD |
55% EFS (5) 49% EFS (5) |
Locatelli 2005 [145] |
||||
| 30 MRD, 27 MMRD, 30 MUD, 7 MMUD |
59% EFS (3) 74% OS (3) |
58%EFS (3) 68%OS (3) |
18% EFS (3) 18% OS (3) |
27% EFS (3) 33% OS (3) |
Yusuf 2004 [144] |
|
| 46 MUD | 24% DFS (2) | Smith 2002 [150] |
||||
| 9 MUD 3 MMRD |
64% EFS (3) | Bunin 1999 [149] |
||||
| 131 MRD | 52% DFS (5) 57% OS (5) |
34% DFS (5) 42% OS (5) |
19% DFS (5) 24% OS (5) |
26% DFS (5) 28% OS (5) |
Runde 1998 [148] |
|
| 60 MRD 19 MUD |
36% OS (4) 31% OS (4) |
Arico* 1997 [146] |
||||
| 14 MRD, 1 MMRD, 7 MUD, 2 MMUD (2nd SCT) |
32% DFS (5) | Yoshimi 2007 [147] |
||||
MRD = matched related donor, MMRD = mismatched related donor, MUD = matched unrelated donor, MMUD = mismatched unrelated donor
review article
Conditioning Regimens
Busulfan- and TBI- based pre-transplant preparative regimens have both been employed for pediatric MDS and JMML, and neither has been shown to be superior. Results with second transplants suggest that radiation may be advantageous (see below). Given the risks of radiation in young children, however, busulfan-based regimens are most commonly employed for children with JMML.
Disease Status
Outcome may be improved for individuals transplanted with lower blast percentage and induction chemotherapy is commonly employed for patients with elevated bone marrow blasts to induce a CR prior to HSCT [142] [152]. However, definitive recommendations cannot be made given the paucity of data.
Donor Selection
Given the poor prognosis without transplant and the favorable results of matched unrelated donor HSCT in pediatrics, transplantation is usually recommended for children with JMML and MDS without regard to the donor type (Table 6) [135,145,146,149,150].
Second Transplants
Initial management of post-transplant relapse should include withdrawal of immunosuppression and/or DLI, although this is frequently ineffective. In contrast to other types of leukemia, children with JMML have outcomes after second HSCT that are comparable to results of first transplant [147]. The EWOG-MDS observed a number of important factors in regard to second transplants for JMML. Most patients received transplants from the same donor, but with reduced GVHD prophylaxis and using TBI-based regimens (vs. busulfan-based conditioning) in comparison to the first transplant. Chronic GVHD was significantly associated with improved DFS after second HSCT. Notably, there was no apparent impact of the interval between transplants [145].
General Considerations in the Use of HSCT for Childhood Leukemia
Graft-vs.-Host Disease and the Graft-vs.-Leukemia Effect
As noted above, an allogeneic graft versus leukemia (GVL) effect is an important component of the curative potential of HSCT for certain leukemias. Thus, interventions designed to decrease the incidence and severity of GVHD must be balanced against the risk of leukemic relapse.
Late Effects
Leukemia remains the leading indication for HSCT in pediatrics and with improvements in post-transplant DFS rates, acute and long-term toxicities have assumed an increasing impact on organ function, quality of life, and overall survival. The risks of conditioning regimens on the developing child should be closely considered during pre-transplant planning.
Reduced Intensity Conditioning Regimens
Reduced intensity pre-transplant regimens have been developed in order to decrease the toxicity associated with myeloablative conditioning. Based on positive results in adults with hematologic malignancies, pilot studies of reduced intensity conditioning regimens have been conducted in pediatric populations [153]. Due to the potency of the GVL effect in CML, this approach is particularly appealing in that disorder. However, safety and efficacy in the more common acute leukemias of childhood have yet to be demonstrated.
The Future of HSCT for Pediatric Leukemias
Allogeneic HSCT will likely continue to play a part in the curative treatment of childhood leukemias well into the future. Scientific discovery and technological advances in transplant immunology, cancer biology, and supportive care will continue to transform the approach to HSCT. As is discussed elsewhere in this edition, novel approaches to donor selection, graft source and manipulation, immunotherapy, and tumor-directed targeted treatment with applications in hematologic malignancies are being developed and advanced in the pediatric setting. Alternative donors have assumed an increasing role in HSCT for pediatric leukemias, especially umbilical cord blood in the U.S. and haplo-identical donors in Europe. It is hoped that such strategies will lead to continued decreases in transplant-associated toxicities as well as improvements in relapse-free and overall survival for children with leukemia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alan S. Wayne, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health Building 10, Room 1-3750, 9000 Rockville Pike, MSC 1104, Bethesda, MD 20892-1104, Tel: 301-496-4256, waynea@mail.nih.gov.
Kristin Baird, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health Building 10, Room 1-3750, 9000 Rockville Pike, MSC 1104, Bethesda, MD 20892-1104, Tel: 301-496-4256 kbaird@mail.nih.gov.
R. Maarten Egeler, Department of Pediatrics/BMT Unit, Leiden University Medical Center, Postbus 9600, 2300 RC, Leiden, The Netherlands, Tel: +31-71-526-2166, R.M.Egeler@lumc.nl.
References
- 1.Gloeckler Ries LAPC, Bunin GR, Reis LAG SM, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. vol 99. Edited by National Cancer Institute SP: NIH Publication; 1999. pp. 1–16. [Google Scholar]
- 2.Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 3.McLean TW, Ringold S, Neuberg D, Stegmaier K, Tantravahi R, Ritz J, Koeffler HP, Takeuchi S, Janssen JW, Seriu T, et al. TEL/AML-1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood. 1996;88:4252–4258. [PubMed] [Google Scholar]
- 4.Borkhardt A, Cazzaniga G, Viehmann S, Valsecchi MG, Ludwig WD, Burci L, Mangioni S, Schrappe M, Riehm H, Lampert F, et al. Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials. Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Munster Study Group. Blood. 1997;90:571–577. [PubMed] [Google Scholar]
- 5.Uckun FM, Pallisgaard N, Hokland P, Navara C, Narla R, Gaynon PS, Sather H, Heerema N. Expression of TEL-AML1 fusion transcripts and response to induction therapy in standard risk acute lymphoblastic leukemia. Leuk Lymphoma. 2001;42:41–56. doi: 10.3109/10428190109097675. [DOI] [PubMed] [Google Scholar]
- 6.Kanerva J, Saarinen-Pihkala UM, Niini T, Riikonen P, Mottonen M, Makipernaa A, Salmi TT, Vettenranta K, Knuutila S. Favorable outcome in 20-year follow-up of children with very-low-risk ALL and minimal standard therapy, with special reference to TEL-AML1 fusion. Pediatr Blood Cancer. 2004;42:30–35. doi: 10.1002/pbc.10417. [DOI] [PubMed] [Google Scholar]
- 7.Johansson B, Moorman AV, Haas OA, Watmore AE, Cheung KL, Swanton S, Secker-Walker LM. Hematologic malignancies with t(4;11)(q21;q23)--a cytogenetic, morphologic, immunophenotypic and clinical study of 183 cases. European 11q23 Workshop participants. Leukemia. 1998;12:779–787. doi: 10.1038/sj.leu.2401012. [DOI] [PubMed] [Google Scholar]
- 8.Pui CH, Frankel LS, Carroll AJ, Raimondi SC, Shuster JJ, Head DR, Crist WM, Land VJ, Pullen DJ, Steuber CP, et al. Clinical characteristics and treatment outcome of childhood acute lymphoblastic leukemia with the t(4;11)(q21;q23): a collaborative study of 40 cases. Blood. 1991;77:440–447. [PubMed] [Google Scholar]
- 9.Crist W, Boyett J, Pullen J, van Eys J, Vietti T. Clinical and biologic features predict poor prognosis in acute lymphoid leukemias in children and adolescents: a Pediatric Oncology Group review. Med Pediatr Oncol. 1986;14:135–139. [PubMed] [Google Scholar]
- 10.Reaman G, Zeltzer P, Bleyer WA, Amendola B, Level C, Sather H, Hammond D. Acute lymphoblastic leukemia in infants less than one year of age: a cumulative experience of the Children's Cancer Study Group. J Clin Oncol. 1985;3:1513–1521. doi: 10.1200/JCO.1985.3.11.1513. [DOI] [PubMed] [Google Scholar]
- 11.Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G, Kamps W, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 12.Crist WM, Carroll AJ, Shuster JJ, Behm FG, Whitehead M, Vietti TJ, Look AT, Mahoney D, Ragab A, Pullen DJ, et al. Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): a Pediatric Oncology Group study. Blood. 1990;76:117–122. [PubMed] [Google Scholar]
- 13.Uckun FM, Sensel MG, Sather HN, Gaynon PS, Arthur DC, Lange BJ, Steinherz PG, Kraft P, Hutchinson R, Nachman JB, et al. Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children's Cancer Group. J Clin Oncol. 1998;16:527–535. doi: 10.1200/JCO.1998.16.2.527. [DOI] [PubMed] [Google Scholar]
- 14.Harris MB, Shuster JJ, Carroll A, Look AT, Borowitz MJ, Crist WM, Nitschke R, Pullen J, Steuber CP, Land VJ. Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood. 1992;79:3316–3324. [PubMed] [Google Scholar]
- 15.Moorman AV, Richards SM, Martineau M, Cheung KL, Robinson HM, Jalali GR, Broadfield ZJ, Harris RL, Taylor KE, Gibson BE, et al. Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood. 2003;102:2756–2762. doi: 10.1182/blood-2003-04-1128. [DOI] [PubMed] [Google Scholar]
- 16.Charrin C, Thomas X, Ffrench M, Le QH, Andrieux J, Mozziconacci MJ, Lai JL, Bilhou-Nabera C, Michaux L, Bernheim A, et al. A report from the LALA-94 and LALA-SA groups on hypodiploidy with 30 to 39 chromosomes and near-triploidy: 2 possible expressions of a sole entity conferring poor prognosis in adult acute lymphoblastic leukemia (ALL) Blood. 2004;104:2444–2451. doi: 10.1182/blood-2003-04-1299. [DOI] [PubMed] [Google Scholar]
- 17.Heerema NA, Sather HN, Sensel MG, Zhang T, Hutchinson RJ, Nachman JB, Lange BJ, Steinherz PG, Bostrom BC, Reaman GH, et al. Prognostic impact of trisomies of chromosomes 10, 17, and 5 among children with acute lymphoblastic leukemia and high hyperdiploidy (> 50 chromosomes) J Clin Oncol. 2000;18:1876–1887. doi: 10.1200/JCO.2000.18.9.1876. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe MJ, Shuster JJ, Sather HN, Camitta BM, Pullen J, Schultz KR, Borowitz MJ, Gaynon PS, Carroll AJ, Heerema NA. High concordance from independent studies by the Children's Cancer Group (CCG), Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10 and 17 in children with NCI Standard-Risk B-precursor Acute Lymphoblastic Leukemia: a Children's Oncology Group (COG) initiative. Leukemia. 2005;19:734–740. doi: 10.1038/sj.leu.2403673. [DOI] [PubMed] [Google Scholar]
- 19.Harrison CJ, Moorman AV, Broadfield ZJ, Cheung KL, Harris RL, Reza Jalali G, Robinson HM, Barber KE, Richards SM, Mitchell CD, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol. 2004;125:552–559. doi: 10.1111/j.1365-2141.2004.04948.x. [DOI] [PubMed] [Google Scholar]
- 20.Heerema NA, Nachman JB, Sather HN, Sensel MG, Lee MK, Hutchinson R, Lange BJ, Steinherz PG, Bostrom B, Gaynon PS, et al. Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: a report from the children's cancer group. Blood. 1999;94:4036–4045. [PubMed] [Google Scholar]
- 21.Raimondi SC, Zhou Y, Mathew S, Shurtleff SA, Sandlund JT, Rivera GK, Behm FG, Pui CH. Reassessment of the prognostic significance of hypodiploidy in pediatric patients with acute lymphoblastic leukemia. Cancer. 2003;98:2715–2722. doi: 10.1002/cncr.11841. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Carroll AJ, Raimondi SC, Land VJ, Crist WM, Shuster JJ, Williams DL, Pullen DJ, Borowitz MJ, Behm FG, et al. Clinical presentation karyotypic characterization, and treatment outcome of childhood acute lymphoblastic leukemia with a near-haploid or hypodiploid less than 45 line. Blood. 1990;75:1170–1177. [PubMed] [Google Scholar]
- 23.Mullighan CG, Downing JR. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: recent insights and future directions. Leukemia. 2009;23:1209–1218. doi: 10.1038/leu.2009.18. [DOI] [PubMed] [Google Scholar]
- 24.Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, Reaman GH, Sather HN, Steinherz PG, Trigg ME, et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer. 1997;80:1717–1726. doi: 10.1002/(sici)1097-0142(19971101)80:9<1717::aid-cncr4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, Johnstone HS, Sather HN, Trigg ME, Chappell R, et al. Cytoreduction and prognosis in acute lymphoblastic leukemia--the importance of early marrow response: report from the Childrens Cancer Group. J Clin Oncol. 1996;14:389–398. doi: 10.1200/JCO.1996.14.2.389. [DOI] [PubMed] [Google Scholar]
- 26.Arico M, Basso G, Mandelli F, Rizzari C, Colella R, Barisone E, Zanesco L, Rondelli R, Pession A, Masera G. Good steroid response in vivo predicts a favorable outcome in children with T-cell acute lymphoblastic leukemia. The Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) Cancer. 1995;75:1684–1693. doi: 10.1002/1097-0142(19950401)75:7<1684::aid-cncr2820750720>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995;86:1292–1295. [PubMed] [Google Scholar]
- 28.Rautonen J, Hovi L, Siimes MA. Slow disappearance of peripheral blast cells: an independent risk factor indicating poor prognosis in children with acute lymphoblastic leukemia. Blood. 1988;71:989–991. [PubMed] [Google Scholar]
- 29.Griffin TC, Shuster JJ, Buchanan GR, Murphy SB, Camitta BM, Amylon MD. Slow disappearance of peripheral blood blasts is an adverse prognostic factor in childhood T cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Leukemia. 2000;14:792–795. doi: 10.1038/sj.leu.2401768. [DOI] [PubMed] [Google Scholar]
- 30.Panzer-Grumayer ER, Schneider M, Panzer S, Fasching K, Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000;95:790–794. [PubMed] [Google Scholar]
- 31.van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, Stolz F, Schrappe M, Masera G, Kamps WA, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 32.Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, Sandlund JT, Rivera GK, Rubnitz JE, Ribeiro RC, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- 33.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 34.Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W, Niemeyer C, Henze G, Feldges A, Zintl F, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 35.Richards S, Burrett J, Hann I, Chessells J, Hill F, Bailey C. Improved survival with early intensification: combined results from the Medical Research Council childhood ALL randomised trials, UKALL X and UKALL XI. Medical Research Council Working Party on Childhood Leukaemia. Leukemia. 1998;12:1031–1036. doi: 10.1038/sj.leu.2401065. [DOI] [PubMed] [Google Scholar]
- 36.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 37.Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, Katz J, Yu A, Laver J, Ravindranath Y, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 38.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk BI, Howard SC, Hudson MM, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE, Asselin BL. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 40.Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, Schirg E, Henze G, Schellong G, Gadner H, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95:416–421. [PubMed] [Google Scholar]
- 41.Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 42.Atra A, Imeson JD, Hobson R, Gerrard M, Hann IM, Eden OB, Carter RL, Pinkerton CR. Improved outcome in children with advanced stage B-cell non-Hodgkin's lymphoma (B-NHL): results of the United Kingdom Children Cancer Study Group (UKCCSG) 9002 protocol. Br J Cancer. 2000;82:1396–1402. doi: 10.1054/bjoc.1999.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowman WP, Shuster JJ, Cook B, Griffin T, Behm F, Pullen J, Link M, Head D, Carroll A, Berard C, et al. Improved survival for children with B-cell acute lymphoblastic leukemia and stage IV small noncleaved-cell lymphoma: a pediatric oncology group study. J Clin Oncol. 1996;14:1252–1261. doi: 10.1200/JCO.1996.14.4.1252. [DOI] [PubMed] [Google Scholar]
- 44.Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998;102:423–438. doi: 10.1046/j.1365-2141.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 45.Gaynon PS, Harris RE, Altman AJ, Bostrom BC, Breneman JC, Hawks R, Steele D, Zipf T, Stram DO, Villaluna D, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 46.Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, Winick NJ, Hunger SP, Gaynon PS, Loh ML. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchey AK, Pollock BH, Lauer SJ, Andejeski Y, Barredo J, Buchanan GR. Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a pediatric oncology group study. J Clin Oncol. 1999;17:3745–3752. doi: 10.1200/JCO.1999.17.12.3745. [DOI] [PubMed] [Google Scholar]
- 49.Buchanan GR, Boyett JM, Pollock BH, Smith SD, Yanofsky RA, Ghim T, Wharam MD, Crist WM, Vietti TJ, Johnson W, et al. Improved treatment results in boys with overt testicular relapse during or shortly after initial therapy for acute lymphoblastic leukemia. A Pediatric Oncology group study. Cancer. 1991;68:48–55. doi: 10.1002/1097-0142(19910701)68:1<48::aid-cncr2820680110>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 50.Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, Larson RA, Parsons S, Seidenfeld J, Weisdorf D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2005;11:823–861. doi: 10.1016/j.bbmt.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Barrett AJ, Horowitz MM, Pollock BH, Zhang MJ, Bortin MM, Buchanan GR, Camitta BM, Ochs J, Graham-Pole J, Rowlings PA, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994;331:1253–1258. doi: 10.1056/NEJM199411103311902. [DOI] [PubMed] [Google Scholar]
- 52.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, Billett A, Homans A, Camitta B, Carroll WL, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchanan GR, Rivera GK, Pollock BH, Boyett JM, Chauvenet AR, Wagner H, Maybee DA, Crist WM, Pinkel D. Alternating drug pairs with or without periodic reinduction in children with acute lymphoblastic leukemia in second bone marrow remission: a Pediatric Oncology Group Study. Cancer. 2000;88:1166–1174. doi: 10.1002/(sici)1097-0142(20000301)88:5<1166::aid-cncr29>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Sadowitz PD, Smith SD, Shuster J, Wharam MD, Buchanan GR, Rivera GK. Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1993;81:602–609. [PubMed] [Google Scholar]
- 55.Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C, Stary J, Felice MS, Magyarosy E, Conter V, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005;366:635–642. doi: 10.1016/S0140-6736(05)66998-X. [DOI] [PubMed] [Google Scholar]
- 56.Schultz KRBW, Slayton W, Aledo A, Devidas M, Sather H, Borowitz MJ, Davies SM, Trigg M, Pasut B, Jorstad D, Eslinger T, Burden LE, Wang C, Rutledge R, Gaynon PS, Carroll AJ, Heerema NA, Winick N, Hunger S, Carroll WL, Camitta B. Improved early event free survival in children with Philadelphia chromosome-positive acute lymphoblastic leukemia with intensive imatinib in combination with high dose chemotherapy: Children's Oncology Group (COG) Study AALL0031. Blood. 2007;110:4a. (abstact) [Google Scholar]
- 57.Eapen M, Rubinstein P, Zhang MJ, Camitta BM, Stevens C, Cairo MS, Davies SM, Doyle JJ, Kurtzberg J, Pulsipher MA, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 58.Jacobsohn DA, Hewlett B, Morgan E, Tse W, Duerst RE, Kletzel M. Favorable outcome for infant acute lymphoblastic leukemia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:999–1005. doi: 10.1016/j.bbmt.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 59.Kosaka Y, Koh K, Kinukawa N, Wakazono Y, Isoyama K, Oda T, Hayashi Y, Ohta S, Moritake H, Oda M, et al. Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood. 2004;104:3527–3534. doi: 10.1182/blood-2004-04-1390. [DOI] [PubMed] [Google Scholar]
- 60.Silverman LB, Weinstein HJ. Treatment of childhood leukemia. Curr Opin Oncol. 1997;9:26–33. doi: 10.1097/00001622-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM, Carroll A, Eden OB, Evans WE, Gadner H, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17:700–706. doi: 10.1038/sj.leu.2402883. [DOI] [PubMed] [Google Scholar]
- 62.Luciani M, Rana I, Pansini V, Caniglia M, Coletti V, Maraschini A, Lombardi A, De Rossi G. Infant leukaemia: clinical, biological and therapeutic advances. Acta Paediatr Suppl. 2006;95:47–51. doi: 10.1111/j.1651-2227.2006.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 63.Nagayama J, Tomizawa D, Koh K, Nagatoshi Y, Hotta N, Kishimoto T, Takahashi Y, Kuno T, Sugita K, Sato T, et al. Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group. Blood. 2006;107:4663–4665. doi: 10.1182/blood-2005-11-4728. [DOI] [PubMed] [Google Scholar]
- 64.Davies SM, Ramsay NK, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY, Camitta BM, Gale RP, Giralt S, Heilmann C, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18:340–347. doi: 10.1200/JCO.2000.18.2.340. [DOI] [PubMed] [Google Scholar]
- 65.Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32:543–548. doi: 10.1038/sj.bmt.1704198. [DOI] [PubMed] [Google Scholar]
- 66.Shah AJ, Lenarsky C, Kapoor N, Crooks GM, Kohn DB, Parkman R, Epport K, Wilson K, Weinberg K. Busulfan and cyclophosphamide as a conditioning regimen for pediatric acute lymphoblastic leukemia patients undergoing bone marrow transplantation. J Pediatr Hematol Oncol. 2004;26:91–97. doi: 10.1097/00043426-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, Buckner CD, Anasetti C, Appelbaum FR, Badger C, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 68.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, Barth A, Borkhardt A, Peters C, Handgretinger R, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 69.Dini G, Valsecchi MG, Micalizzi C, Busca A, Balduzzi A, Arcese W, Cesaro S, Prete A, Rabusin M, Mazzolari E, et al. Impact of marrow unrelated donor search duration on outcome of children with acute lymphoblastic leukemia in second remission. Bone Marrow Transplant. 2003;32:325–331. doi: 10.1038/sj.bmt.1704132. [DOI] [PubMed] [Google Scholar]
- 70.Bunin N, Carston M, Wall D, Adams R, Casper J, Kamani N, King R. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- 71.Saarinen-Pihkala UM, Gustafsson G, Ringden O, Heilmann C, Glomstein A, Lonnerholm G, Abrahamsson J, Bekassy AN, Schroeder H, Mellander L. No disadvantage in outcome of using matched unrelated donors as compared with matched sibling donors for bone marrow transplantation in children with acute lymphoblastic leukemia in second remission. J Clin Oncol. 2001;19:3406–3414. doi: 10.1200/JCO.2001.19.14.3406. [DOI] [PubMed] [Google Scholar]
- 72.Green A, Clarke E, Hunt L, Canterbury A, Lankester A, Hale G, Waldmann H, Goodman S, Cornish JM, Marks DI, et al. Children with acute lymphoblastic leukemia who receive T-cell-depleted HLA mismatched marrow allografts from unrelated donors have an increased incidence of primary graft failure but a similar overall transplant outcome. Blood. 1999;94:2236–2246. [PubMed] [Google Scholar]
- 73.Oakhill A, Pamphilon DH, Potter MN, Steward CG, Goodman S, Green A, Goulden P, Goulden NJ, Hale G, Waldmann H, et al. Unrelated donor bone marrow transplantation for children with relapsed acute lymphoblastic leukaemia in second complete remission. Br J Haematol. 1996;94:574–578. doi: 10.1046/j.1365-2141.1996.d01-1834.x. [DOI] [PubMed] [Google Scholar]
- 74.Fleming DR, Henslee-Downey PJ, Romond EH, Harder EJ, Marciniak E, Munn RK, Messino MJ, Macdonald JS, Bishop M, Rayens MK, et al. Allogeneic bone marrow transplantation with T cell-depleted partially matched related donors for advanced acute lymphoblastic leukemia in children and adults: a comparative matched cohort study. Bone Marrow Transplant. 1996;17:917–922. [PubMed] [Google Scholar]
- 75.Ball LM, Lankester AC, Bredius RG, Fibbe WE, van Tol MJ, Egeler RM. Graft dysfunction and delayed immune reconstitution following haploidentical peripheral blood hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35 Suppl 1:S35–S38. doi: 10.1038/sj.bmt.1704842. [DOI] [PubMed] [Google Scholar]
- 76.Lang P, Greil J, Bader P, Handgretinger R, Klingebiel T, Schumm M, Schlegel PG, Feuchtinger T, Pfeiffer M, Scheel-Walter H, et al. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis. 2004;33:281–287. doi: 10.1016/j.bcmd.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Bosi A, Laszlo D, Labopin M, Reffeirs J, Michallet M, Gluckman E, Alessandrino PE, Locatelli F, Vernant JP, Sierra J, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 78.Schroeder H, Gustafsson G, Saarinen-Pihkala UM, Glomstein A, Jonmundsson G, Nysom K, Ringden O, Mellander L. Allogeneic bone marrow transplantation in second remission of childhood acute lymphoblastic leukemia: a population-based case control study from the Nordic countries. Bone Marrow Transplant. 1999;23:555–560. doi: 10.1038/sj.bmt.1701617. [DOI] [PubMed] [Google Scholar]
- 79.Sanders JE, Thomas ED, Buckner CD, Doney K. Marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 1987;70:324–326. [PubMed] [Google Scholar]
- 80.Lawson SE, Darbyshire PJ. Use of donor lymphocytes in extramedullary relapse of childhood acute lymphoblastic leukaemia following bone marrow transplantation. Bone Marrow Transplant. 1998;22:829–830. doi: 10.1038/sj.bmt.1701428. [DOI] [PubMed] [Google Scholar]
- 81.Atra A, Millar B, Shepherd V, Shankar A, Wilson K, Treleaven J, Pritchard-Jones K, Meller ST, Pinkerton CR. Donor lymphocyte infusion for childhood acute lymphoblastic leukaemia relapsing after bone marrow transplantation. Br J Haematol. 1997;97:165–168. doi: 10.1046/j.1365-2141.1997.62650.x. [DOI] [PubMed] [Google Scholar]
- 82.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 83.Helg C, Starobinski M, Jeannet M, Chapuis B. Donor lymphocyte infusion for the treatment of relapse after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 1998;29:301–313. doi: 10.3109/10428199809068567. [DOI] [PubMed] [Google Scholar]
- 84.Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, Flowers ME, Casper J, Leahey A, Parker P, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214–1221. [PubMed] [Google Scholar]
- 85.Absalon MJ, Smith FO. Treatment strategies for pediatric acute myeloid leukemia. Expert Opin Pharmacother. 2009;10:57–79. doi: 10.1517/14656560802627929. [DOI] [PubMed] [Google Scholar]
- 86.Creutzig U, Ritter J, Zimmermann M, Reinhardt D, Hermann J, Berthold F, Henze G, Jurgens H, Kabisch H, Havers W, et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of Study Acute Myeloid Leukemia-Berlin-Frankfurt-Munster 93. J Clin Oncol. 2001;19:2705–2713. doi: 10.1200/JCO.2001.19.10.2705. [DOI] [PubMed] [Google Scholar]
- 87.Stevens RF, Hann IM, Wheatley K, Gray RG. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council's 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 88.Perel Y, Auvrignon A, Leblanc T, Vannier JP, Michel G, Nelken B, Gandemer V, Schmitt C, Lamagnere JP, De Lumley L, et al. Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. Leucamie Aique Myeloide Enfant. J Clin Oncol. 2002;20:2774–2782. doi: 10.1200/JCO.2002.07.300. [DOI] [PubMed] [Google Scholar]
- 89.Capizzi RL, Poole M, Cooper MR, Richards F, 2nd, Stuart JJ, Jackson DV, Jr, White DR, Spurr CL, Hopkins JO, Muss HB, et al. Treatment of poor risk acute leukemia with sequential high-dose ARA-C and asparaginase. Blood. 1984;63:694–700. [PubMed] [Google Scholar]
- 90.Woods WG, Ruymann FB, Lampkin BC, Buckley JD, Bernstein ID, Srivastava AK, Smithson WA, Benjamin DR, Feig SA, Kim TH, et al. The role of timing of high-dose cytosine arabinoside intensification and of maintenance therapy in the treatment of children with acute nonlymphocytic leukemia. Cancer. 1990;66:1106–1113. doi: 10.1002/1097-0142(19900915)66:6<1106::aid-cncr2820660605>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 91.Oliansky DM, Rizzo JD, Aplan PD, Arceci RJ, Leone L, Ravindranath Y, Sanders JE, Smith FO, 3rd, Wilmot F, McCarthy PL, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2007;13:1–25. doi: 10.1016/j.bbmt.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 92.Bleakley M, Lau L, Shaw PJ, Kaufman A. Bone marrow transplantation for paediatric AML in first remission: a systematic review and meta-analysis. Bone Marrow Transplant. 2002;29:843–852. doi: 10.1038/sj.bmt.1703528. [DOI] [PubMed] [Google Scholar]
- 93.Ravindranath Y, Yeager AM, Chang MN, Steuber CP, Krischer J, Graham-Pole J, Carroll A, Inoue S, Camitta B, Weinstein HJ. Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. Pediatric Oncology Group. N Engl J Med. 1996;334:1428–1434. doi: 10.1056/NEJM199605303342203. [DOI] [PubMed] [Google Scholar]
- 94.Amadori S, Testi AM, Arico M, Comelli A, Giuliano M, Madon E, Masera G, Rondelli R, Zanesco L, Mandelli F. Prospective comparative study of bone marrow transplantation and postremission chemotherapy for childhood acute myelogenous leukemia. The Associazione Italiana Ematologia ed Oncologia Pediatrica Cooperative Group. J Clin Oncol. 1993;11:1046–1054. doi: 10.1200/JCO.1993.11.6.1046. [DOI] [PubMed] [Google Scholar]
- 95.Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, Barnard DR, Dusenbery K, DeSwarte J, Arthur DC, Lange BJ, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 96.Feig SA, Lampkin B, Nesbit ME, Woods WG, Versteeg CM, Buckley JD, Kim T, Hammond GD. Outcome of BMT during first complete remission of AML: a comparison of two sequential studies by the Children's Cancer Group. Bone Marrow Transplant. 1993;12:65–71. [PubMed] [Google Scholar]
- 97.Burnett AK, Goldstone AH, Stevens RM, Hann IM, Rees JK, Gray RG, Wheatley K. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: results of MRC AML 10 trial. UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351:700–708. doi: 10.1016/s0140-6736(97)09214-3. [DOI] [PubMed] [Google Scholar]
- 98.Razzouk BI, Estey E, Pounds S, Lensing S, Pierce S, Brandt M, Rubnitz JE, Ribeiro RC, Rytting M, Pui CH, et al. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106:2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- 99.Ortega JJ, Madero L, Martin G, Verdeguer A, Garcia P, Parody R, Fuster J, Molines A, Novo A, Deben G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23:7632–7640. doi: 10.1200/JCO.2005.01.3359. [DOI] [PubMed] [Google Scholar]
- 100.Testi AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, Menna G, Locatelli F, Pession A, Barisone E, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–453. doi: 10.1182/blood-2004-05-1971. [DOI] [PubMed] [Google Scholar]
- 101.De Botton S, Chevret S, Sanz M, Dombret H, Thomas X, Guerci A, Fey M, Rayon C, Huguet F, Sotto JJ, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: results of APL 93 trial. Br J Haematol. 2000;111:801–806. doi: 10.1046/j.1365-2141.2000.02442.x. [DOI] [PubMed] [Google Scholar]
- 102.Fenaux P, Chevret S, Guerci A, Fegueux N, Dombret H, Thomas X, Sanz M, Link H, Maloisel F, Gardin C, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. European APL group. Leukemia. 2000;14:1371–1377. doi: 10.1038/sj.leu.2401859. [DOI] [PubMed] [Google Scholar]
- 103.Ravindranath Y, Abella E, Krischer JP, Wiley J, Inoue S, Harris M, Chauvenet A, Alvarado CS, Dubowy R, Ritchey AK, et al. Acute myeloid leukemia (AML) in Down's syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80:2210–2214. [PubMed] [Google Scholar]
- 104.Gamis AS, Woods WG, Alonzo TA, Buxton A, Lange B, Barnard DR, Gold S, Smith FO. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children's Cancer Group Study 2891. J Clin Oncol. 2003;21:3415–3422. doi: 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 105.Massey GV. Transient leukemia in newborns with Down syndrome. Pediatr Blood Cancer. 2005;44:29–32. doi: 10.1002/pbc.20141. [DOI] [PubMed] [Google Scholar]
- 106.Dahl GV, Kalwinsky DK, Mirro J, Jr, Look AT, Pui CH, Murphy SB, Mason C, Ruggiero M, Schell M, Johnson FL, et al. Allogeneic bone marrow transplantation in a program of intensive sequential chemotherapy for children and young adults with acute nonlymphocytic leukemia in first remission. J Clin Oncol. 1990;8:295–303. doi: 10.1200/JCO.1990.8.2.295. [DOI] [PubMed] [Google Scholar]
- 107.Nesbit ME, Jr, Buckley JD, Feig SA, Anderson JR, Lampkin B, Bernstein ID, Kim TH, Piomelli S, Kersey JH, Coccia PF, et al. Chemotherapy for induction of remission of childhood acute myeloid leukemia followed by marrow transplantation or multiagent chemotherapy: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12:127–135. doi: 10.1200/JCO.1994.12.1.127. [DOI] [PubMed] [Google Scholar]
- 108.Wells RJ, Woods WG, Buckley JD, Odom LF, Benjamin D, Bernstein I, Betcher D, Feig S, Kim T, Ruymann F, et al. Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Childrens Cancer Group study. J Clin Oncol. 1994;12:2367–2377. doi: 10.1200/JCO.1994.12.11.2367. [DOI] [PubMed] [Google Scholar]
- 109.Aladjidi N, Auvrignon A, Leblanc T, Perel Y, Benard A, Bordigoni P, Gandemer V, Thuret I, Dalle JH, Piguet C, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21:4377–4385. doi: 10.1200/JCO.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 110.Watson M, Buck G, Wheatley K, Homewood JR, Goldstone AH, Rees JK, Burnett AK. Adverse impact of bone marrow transplantation on quality of life in acute myeloid leukaemia patients; analysis of the UK Medical Research Council AML 10 Trial. Eur J Cancer. 2004;40:971–978. doi: 10.1016/S0959-8049(03)00628-2. [DOI] [PubMed] [Google Scholar]
- 111.Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, Willman C, Hurd DD, Bennett JM, Blume KG, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 112.Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, Leoni F, Damasio E, Visani G, Papa G, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 113.Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JH, Harrison G. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 114.Chen AR, Alonzo TA, Woods WG, Arceci RJ. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?--an American view. Br J Haematol. 2002;118:378–384. doi: 10.1046/j.1365-2141.2002.03701.x. [DOI] [PubMed] [Google Scholar]
- 115.Creutzig U, Reinhardt D. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?--a European view. Br J Haematol. 2002;118:365–377. doi: 10.1046/j.1365-2141.2002.03697.x. [DOI] [PubMed] [Google Scholar]
- 116.Ravindranath Y, Chang M, Steuber CP, Becton D, Dahl G, Civin C, Camitta B, Carroll A, Raimondi SC, Weinstein HJ. Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): a review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia. 2005;19:2101–2116. doi: 10.1038/sj.leu.2403927. [DOI] [PubMed] [Google Scholar]
- 117.Smith FO, Alonzo TA, Gerbing RB, Woods WG, Arceci RJ. Long-term results of children with acute myeloid leukemia: a report of three consecutive Phase III trials by the Children's Cancer Group: CCG 251 CCG 213 and CCG 2891. Leukemia. 2005;19:2054–2062. doi: 10.1038/sj.leu.2403925. [DOI] [PubMed] [Google Scholar]
- 118.Ferry C, Socie G. Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp Hematol. 2003;31:1182–1186. doi: 10.1016/j.exphem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 119.Michel G, Gluckman E, Esperou-Bourdeau H, Reiffers J, Pico JL, Bordigoni P, Thuret I, Blaise D, Bernaudin F, Jouet JP, et al. Allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: impact of conditioning regimen without total-body irradiation--a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 1994;12:1217–1222. doi: 10.1200/JCO.1994.12.6.1217. [DOI] [PubMed] [Google Scholar]
- 120.Woodard P, Barfield R, Hale G, Horwitz E, Leung W, Ribeiro R, Rubnitz J, Srivistava DK, Tong X, Yusuf U, et al. Outcome of hematopoietic stem cell transplantation for pediatric patients with therapy-related acute myeloid leukemia or myelodysplastic syndrome. Pediatr Blood Cancer. 2006;47:931–935. doi: 10.1002/pbc.20596. [DOI] [PubMed] [Google Scholar]
- 121.Woolfrey AE, Gooley TA, Sievers EL, Milner LA, Andrews RG, Walters M, Hoffmeister P, Hansen JA, Anasetti C, Bryant E, et al. Bone marrow transplantation for children less than 2 years of age with acute myelogenous leukemia or myelodysplastic syndrome. Blood. 1998;92:3546–3556. [PubMed] [Google Scholar]
- 122.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O'Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meshinchi S, Leisenring WM, Carpenter PA, Woolfrey AE, Sievers EL, Radich JP, Sanders JE. Survival after second hematopoietic stem cell transplantation for recurrent pediatric acute myeloid leukemia. Biol Blood Marrow Transplant. 2003;9:706–713. doi: 10.1016/j.bbmt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Pulsipher MA. Treatment of CML in pediatric patients: should imatinib mesylate (STI-571, Gleevec) or allogeneic hematopoietic cell transplant be front-line therapy? Pediatr Blood Cancer. 2004;43:523–533. doi: 10.1002/pbc.20062. [DOI] [PubMed] [Google Scholar]
- 125.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 126.Shah NP. Loss of response to imatinib: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2005:183–187. doi: 10.1182/asheducation-2005.1.183. [DOI] [PubMed] [Google Scholar]
- 127.Kolb EA, Pan Q, Ladanyi M, Steinherz PG. Imatinib mesylate in Philadelphia chromosome-positive leukemia of childhood. Cancer. 2003;98:2643–2650. doi: 10.1002/cncr.11895. [DOI] [PubMed] [Google Scholar]
- 128.Goldman JM, Marin D. Management decisions in chronic myeloid leukemia. Semin Hematol. 2003;40:97–103. doi: 10.1053/shem.2003.50009. [DOI] [PubMed] [Google Scholar]
- 129.Wassmann B, Pfeifer H, Scheuring U, Klein SA, Gokbuget N, Binckebanck A, Martin H, Gschaidmeier H, Hoelzer D, Ottmann OG. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (SCT) in relapsed or refractory Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL) Leukemia. 2002;16:2358–2365. doi: 10.1038/sj.leu.2402770. [DOI] [PubMed] [Google Scholar]
- 130.Weisdorf DJ, Anasetti C, Antin JH, Kernan NA, Kollman C, Snyder D, Petersdorf E, Nelson G, McGlave P. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 131.Goldman JM, Druker BJ. Chronic myeloid leukemia: current treatment options. Blood. 2001;98:2039–2042. doi: 10.1182/blood.v98.7.2039. [DOI] [PubMed] [Google Scholar]
- 132.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, Frassoni F, Gahrton G, Kolb HJ, Niederwieser D, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 133.Silver RT, Woolf SH, Hehlmann R, Appelbaum FR, Anderson J, Bennett C, Goldman JM, Guilhot F, Kantarjian HM, Lichtin AE, et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood. 1999;94:1517–1536. [PubMed] [Google Scholar]
- 134.Stary J, Locatelli F, Niemeyer CM. Stem cell transplantation for aplastic anemia and myelodysplastic syndrome. Bone Marrow Transplant. 2005;35 Suppl 1:S13–S16. doi: 10.1038/sj.bmt.1704836. [DOI] [PubMed] [Google Scholar]
- 135.Niemeyer CM, Kratz CP, Hasle H. Pediatric myelodysplastic syndromes. Curr Treat Options Oncol. 2005;6:209–214. doi: 10.1007/s11864-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 136.Hasle H, Niemeyer CM, Chessells JM, Baumann I, Bennett JM, Kerndrup G, Head DR. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17:277–282. doi: 10.1038/sj.leu.2402765. [DOI] [PubMed] [Google Scholar]
- 137.Mandel K, Dror Y, Poon A, Freedman MH. A practical, comprehensive classification for pediatric myelodysplastic syndromes: the CCC system. J Pediatr Hematol Oncol. 2002;24:596–605. doi: 10.1097/00043426-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 138.Occhipinti E, Correa H, Yu L, Craver R. Comparison of two new classifications for pediatric myelodysplastic and myeloproliferative disorders. Pediatr Blood Cancer. 2005;44:240–244. doi: 10.1002/pbc.20174. [DOI] [PubMed] [Google Scholar]
- 139.Hasle H, Baumann I, Bergstrasser E, Fenu S, Fischer A, Kardos G, Kerndrup G, Locatelli F, Rogge T, Schultz KR, et al. The International Prognostic Scoring System (IPSS) for childhood myelodysplastic syndrome (MDS) and juvenile myelomonocytic leukemia (JMML) Leukemia. 2004;18:2008–2014. doi: 10.1038/sj.leu.2403489. [DOI] [PubMed] [Google Scholar]
- 140.Niemeyer CM, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Haas O, Harbott J, Hasle H, Kerndrup G, et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89:3534–3543. [PubMed] [Google Scholar]
- 141.Hasle H, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Fenu S, Fonatsch C, Haas OA, Harbott J, et al. Myelodysplastic syndrome, juvenile myelomonocytic leukemia and acute myeloid leukemia, associated with complete or partial monosomy 7. European Working Group on MDS in Childhood (EWOG-MDS) Leukemia. 1999;13:376–385. doi: 10.1038/sj.leu.2401342. [DOI] [PubMed] [Google Scholar]
- 142.Woods WG, Barnard DR, Alonzo TA, Buckley JD, Kobrinsky N, Arthur DC, Sanders J, Neudorf S, Gold S, Lange BJ. Prospective study of 90 children requiring treatment for juvenile myelomonocytic leukemia or myelodysplastic syndrome: a report from the Children's Cancer Group. J Clin Oncol. 2002;20:434–440. doi: 10.1200/JCO.2002.20.2.434. [DOI] [PubMed] [Google Scholar]
- 143.Freedman MH, Estrov Z, Chan HS. Juvenile chronic myelogenous leukemia. Am J Pediatr Hematol Oncol. 1988;10:261–267. doi: 10.1097/00043426-198823000-00016. [DOI] [PubMed] [Google Scholar]
- 144.Yusuf U, Frangoul HA, Gooley TA, Woolfrey AE, Carpenter PA, Andrews RG, Deeg HJ, Appelbaum FR, Anasetti C, Storb R, et al. Allogeneic bone marrow transplantation in children with myelodysplastic syndrome or juvenile myelomonocytic leukemia: the Seattle experience. Bone Marrow Transplant. 2004;33:805–814. doi: 10.1038/sj.bmt.1704438. [DOI] [PubMed] [Google Scholar]
- 145.Locatelli F, Nollke P, Zecca M, Korthof E, Lanino E, Peters C, Pession A, Kabisch H, Uderzo C, Bonfim CS, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105:410–419. doi: 10.1182/blood-2004-05-1944. [DOI] [PubMed] [Google Scholar]
- 146.Arico M, Biondi A, Pui CH. Juvenile myelomonocytic leukemia. Blood. 1997;90:479–488. [PubMed] [Google Scholar]
- 147.Yoshimi A, Mohamed M, Bierings M, Urban C, Korthof E, Zecca M, Sykora KW, Duffner U, Trebo M, Matthes-Martin S, et al. Second allogeneic hematopoietic stem cell transplantation (HSCT) results in outcome similar to that of first HSCT for patients with juvenile myelomonocytic leukemia. Leukemia. 2007;21:556–560. doi: 10.1038/sj.leu.2404537. [DOI] [PubMed] [Google Scholar]
- 148.Runde V, de Witte T, Arnold R, Gratwohl A, Hermans J, van Biezen A, Niederwieser D, Labopin M, Walter-Noel MP, Bacigalupo A, et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:255–261. doi: 10.1038/sj.bmt.1701084. [DOI] [PubMed] [Google Scholar]
- 149.Bunin N, Saunders F, Leahey A, Doyle J, Calderwood S, Freedman MH. Alternative donor bone marrow transplantation for children with juvenile myelomonocytic leukemia. J Pediatr Hematol Oncol. 1999;21:479–485. [PubMed] [Google Scholar]
- 150.Smith FO, King R, Nelson G, Wagner JE, Robertson KA, Sanders JE, Bunin N, Emaunel PD, Davies SM. Unrelated donor bone marrow transplantation for children with juvenile myelomonocytic leukaemia. Br J Haematol. 2002;116:716–724. doi: 10.1046/j.0007-1048.2001.03333.x. [DOI] [PubMed] [Google Scholar]
- 151.Yoshimi A, Bader P, Matthes-Martin S, Stary J, Sedlacek P, Duffner U, Klingebiel T, Dilloo D, Holter W, Zintl F, et al. Donor leukocyte infusion after hematopoietic stem cell transplantation in patients with juvenile myelomonocytic leukemia. Leukemia. 2005;19:971–977. doi: 10.1038/sj.leu.2403721. [DOI] [PubMed] [Google Scholar]
- 152.Creutzig U, Bender-Gotze C, Ritter J, Zimmermann M, Stollmann-Gibbels B, Korholz D, Niemeyer C. The role of intensive AML-specific therapy in treatment of children with RAEB and RAEB-t. Leukemia. 1998;12:652–659. doi: 10.1038/sj.leu.2400987. [DOI] [PubMed] [Google Scholar]
- 153.Kletzel M, Jacobsohn D, Tse W, Duerst R. Reduced intensity transplants (RIT) in pediatrics: a review. Pediatr Transplant. 2005;9 Suppl 7:63–70. doi: 10.1111/j.1399-3046.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 154.Sharathkumar A, Saunders EF, Dror Y, Grant R, Greenberg M, Weitzman S, Chan H, Calderwood S, Freedman MH, Doyle J. Allogeneic bone marrow transplantation vs chemotherapy for children with Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2004;33:39–45. doi: 10.1038/sj.bmt.1704319. [DOI] [PubMed] [Google Scholar]
- 155.Wheeler KA, Richards SM, Bailey CC, Gibson B, Hann IM, Hill FG, Chessells JM. Bone marrow transplantation versus chemotherapy in the treatment of very high-risk childhood acute lymphoblastic leukemia in first remission: results from Medical Research Council UKALL X and XI. Blood. 2000;96:2412–2418. [PubMed] [Google Scholar]
- 156.Uderzo C, Valsecchi MG, Balduzzi A, Dini G, Miniero R, Locatelli F, Rondelli R, Pession A, Arcese W, Bacigalupo A, et al. Allogeneic bone marrow transplantation versus chemotherapy in high-risk childhood acute lymphoblastic leukaemia in first remission. Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP) and the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Br J Haematol. 1997;96:387–394. doi: 10.1046/j.1365-2141.1997.d01-2033.x. [DOI] [PubMed] [Google Scholar]
- 157.Saarinen UM, Mellander L, Nysom K, Ringden O, Schroeder H, Glomstein A, Gustafsson G. Allogeneic bone marrow transplantation in first remission for children with very high-risk acute lymphoblastic leukemia: a retrospective case-control study in the Nordic countries. Nordic Society for Pediatric Hematology and Oncology (NOPHO) Bone Marrow Transplant. 1996;17:357–363. [PubMed] [Google Scholar]
- 158.Barrett AJ, Horowitz MM, Ash RC, Atkinson K, Gale RP, Goldman JM, Henslee-Downey PJ, Herzig RH, Speck B, Zwaan FE, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79:3067–3070. [PubMed] [Google Scholar]
- 159.Bordigoni P, Vernant JP, Souillet G, Gluckman E, Marininchi D, Milpied N, Fischer A, Benz Lemoine E, Jouet JP, Reiffers J. Allogeneic bone marrow transplantation for children with acute lymphoblastic leukemia in first remission: a cooperative study of the Groupe d'Etude de la Greffe de Moelle Osseuse. J Clin Oncol. 1989;7:747–753. doi: 10.1200/JCO.1989.7.6.747. [DOI] [PubMed] [Google Scholar]
- 160.Dopfer R, Henze G, Bender-Gotze C, Ebell W, Ehninger G, Friedrich W, Gadner H, Klingebiel T, Peters C, Riehm H, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission after intensive primary and relapse therapy according to the BFM- and CoALL-protocols: results of the German Cooperative Study. Blood. 1991;78:2780–2784. [PubMed] [Google Scholar]
- 161.Hoogerbrugge PM, Gerritsen EJ, vd Does-van den Berg A, vd Berg H, Zwinderman AH, Hermans J, Vossen JM. Case-control analysis of allogeneic bone marrow transplantation versus maintenance chemotherapy for relapsed ALL in children. Bone Marrow Transplant. 1995;15:255–259. [PubMed] [Google Scholar]
- 162.Uderzo C, Valsecchi MG, Bacigalupo A, Meloni G, Messina C, Polchi P, Di Girolamo G, Dini G, Miniero R, Locatelli F, et al. Treatment of childhood acute lymphoblastic leukemia in second remission with allogeneic bone marrow transplantation and chemotherapy: ten-year experience of the Italian Bone Marrow Transplantation Group and the Italian Pediatric Hematology Oncology Association. J Clin Oncol. 1995;13:352–358. doi: 10.1200/JCO.1995.13.2.352. [DOI] [PubMed] [Google Scholar]
- 163.Moussalem M, Esperou Bourdeau H, Devergie A, Baruchel A, Ribaud P, Socie G, Parquet N, Traineau R, Hirsch I, Schaison G, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission: factors predictive of survival, relapse and graft-versus-host disease. Bone Marrow Transplant. 1995;15:943–947. [PubMed] [Google Scholar]
- 164.Wheeler K, Richards S, Bailey C, Chessells J. Comparison of bone marrow transplant and chemotherapy for relapsed childhood acute lymphoblastic leukaemia: the MRC UKALL X experience. Medical Research Council Working Party on Childhood Leukaemia. Br J Haematol. 1998;101:94–103. doi: 10.1046/j.1365-2141.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 165.Harrison G, Richards S, Lawson S, Darbyshire P, Pinkerton R, Stevens R, Oakhill A, Eden OB. Comparison of allogeneic transplant versus chemotherapy for relapsed childhood acute lymphoblastic leukaemia in the MRC UKALL R1 trial. MRC Childhood Leukaemia Working Party. Ann Oncol. 2000;11:999–1006. doi: 10.1023/a:1008381801403. [DOI] [PubMed] [Google Scholar]
- 166.Chessells JM, Bailey C, Wheeler K, Richards SM. Bone marrow transplantation for high-risk childhood lymphoblastic leukaemia in first remission: experience in MRC UKALL X. Lancet. 1992;340:565–568. doi: 10.1016/0140-6736(92)92103-m. [DOI] [PubMed] [Google Scholar]
- 167.Alonzo TA, Wells RJ, Woods WG, Lange B, Gerbing RB, Buxton AB, Neudorf S, Sanders J, Smith FO, Feig SA. Postremission therapy for children with acute myeloid leukemia: the children's cancer group experience in the transplant era. Leukemia. 2005;19:965–970. doi: 10.1038/sj.leu.2403763. [DOI] [PubMed] [Google Scholar]
- 168.Pession A, Rondelli R, Paolucci P, Pastore G, Dini G, Bonetti F, Madon E, Mandelli F, Zanesco L, Uderzo C, et al. Hematopoietic stem cell transplantation in childhood: report from the bone marrow transplantation group of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) Haematologica. 2000;85:638–646. [PubMed] [Google Scholar]
- 169.Gorin NC, Labopin M, Fouillard L, Meloni G, Frassoni F, Iriondo A, Brunet Mauri S, Goldstone AH, Harousseau JL, Reiffers J, et al. Retrospective evaluation of autologous bone marrow transplantation vs allogeneic bone marrow transplantation from an HLA identical related donor in acute myelocytic leukemia. A study of the European Cooperative Group for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 1996;18:111–117. [PubMed] [Google Scholar]