Abstract
The HIV-1 pandemic is a complex mix of diverse epidemics within and between countries and regions of the world, and is undoubtedly the defining public-health crisis of our time. Research has deepened our understanding of how the virus replicates, manipulates, and hides in an infected person. Although our understanding of pathogenesis and transmission dynamics has become more nuanced and prevention options have expanded, a cure or protective vaccine remains elusive. Antiretroviral treatment has transformed AIDS from an inevitably fatal condition to a chronic, manageable disease in some settings. This transformation has yet to be realised in those parts of the world that continue to bear a disproportionate burden of new HIV-1 infections and are most a% ected by increasing morbidity and mortality. This Seminar provides an update on epidemiology, pathogenesis, treatment, and prevention interventions pertinent to HIV-1.
HIV pandemic
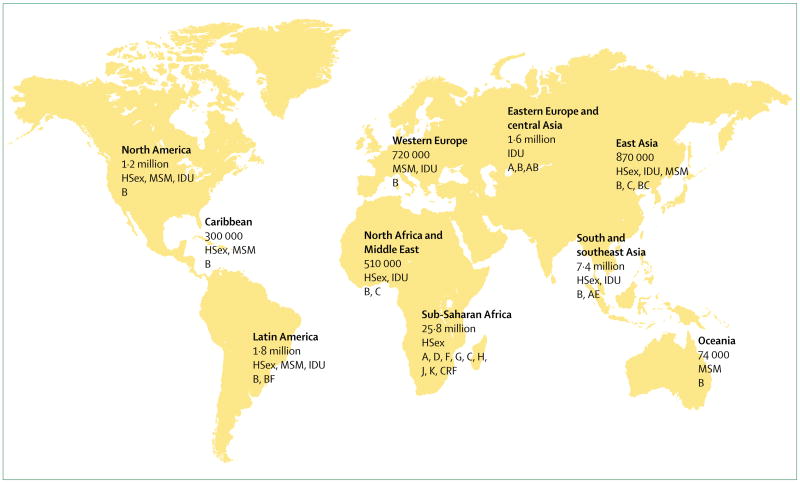
An estimated 38·6 (33·4–46·0) million people live with HIV-1 worldwide, while about 25 million have died already.1 In 2005 alone, there were 4·1 million new HIV-1 infections and 2·8 million AIDS deaths.1 These estimates mask the dynamic nature of this evolving epidemic in relation to temporal changes, geographic distribution, magnitude, viral diversity, and mode of transmission. Today, there is no region of the world untouched by this pandemic (figure 1).2
Figure 1. Worldwide distribution of HIV-1 infections, modes of transmission, and HIV-1 subtypes.
HSex=heterosexual. MSM=Men who have sex with men. IDU=injection drug users. Based on Joint UNAIDS and WHO AIDS epidemic update December, 2005.
Heterosexual transmission remains the dominant mode of transmission and accounts for about 85% of all HIV-1 infections. Southern Africa remains the epicentre of the pandemic and continues to have high rates of new HIV-1 infections.3 Although overall HIV-1 prevalence remains low in the emerging epidemics in China and India, the absolute numbers, which are fast approaching those seen in southern Africa, are of concern.1 Outside of sub-Saharan Africa, a third of all HIV-1 infections are acquired through injecting drug use, most (an estimated 8·8 million) of which are in eastern Europe and central and southeast Asia.1 The rapid spread of HIV-1 in these regions through injecting drug use is of importance, since it is a bridge for rapid establishment of more generalised epidemics.
A defining feature of the pandemic in the current decade is the increasing burden of HIV-1 infections in women,4 which has additional implications for mother-to-child transmission. Women now make up about 42% of those infected worldwide; over 70% of whom live in sub-Saharan Africa.1 Overall, a quarter of all new HIV-1 infections are in adults aged younger than 25 years.1 HIV-1 infection rates are three to six times higher in female adolescents than in their male counterparts,1,5–7 and this difference is attributed to sexual coupling patterns of young women with older men. Population prevalence of HIV-1 infection, concurrent sexual relationships, partner change, sexual practices, the presence of other sexually transmitted diseases,8–11 and population mobility patterns12–14 for economic and other reasons (eg, natural disasters and wars) further increase the probability of HIV-1 acquisition.3,15 Emerging data accord with strong links between risk of sexual HIV-1 acquisition and episodic recreational drug or alcohol use.16
Although sub-Saharan Africa continues to bear a disproportionate burden of HIV-1 infections, there is now an increasing number of countries reporting stabilisation or declines in prevalence (eg, Zambia, Tanzania, Kenya, Ghana, Rwanda, Burkina Faso, and Zimbabwe).1 There is some evidence to attribute these reductions to effective changes in sexual behaviour, such as postponement of sexual debut, reduction in casual relationships, and more consistent condom use in casual relationships.17,18 However, increasing morbidity and mortality rates associated with a maturing HIV-1 epidemic need to be considered when interpreting these data.19 For example, the death of a few high-risk individuals who are key to transmission chains could exert a major effect on sexual networks and result in major reductions in infection rates.20 Additionally, since most HIV-1 estimates are based on surveys in antenatal populations, increasing morbidity and mortality could cause the numbers of women in this group to decrease, and thus lead to underestimates of the true prevalence in these countries.19
Although the relative contribution of cell-free virus compared with cell-associated virus in HIV-1 transmission remains unclear, there is growing evidence that viral load is predictive of transmission risk.21,22 The highest levels of viraemia are seen during acute infection and advanced HIV-1 disease.22 Further, co-infections with other sexually transmitted diseases in asymptomatic HIV-1 infected people can increase viral shedding to levels similar to those seen during acute infection.23 Thus, sexually transmitted diseases could enhance HIV-1 transmission to rates similar to those seen during primary infection.24 This observation could help to explain why the efficiency of HIV-1 transmission exceeds, in some settings, the earlier mathematical projections.25 Thus, identification and treatment of recently infected people is an important means to reduce transmission. However, most people are unaware of their HIV-1 status during these crucial first months of infection. Several screening strategies based on laboratory testing and clinical algorithms are being developed and tested26 for efficient identification of early infection before antibody development.27 Additionally, a more aggressive management of sexually transmitted infections in settings with generalised epidemics has the potential to affect current epidemic trajectories.24
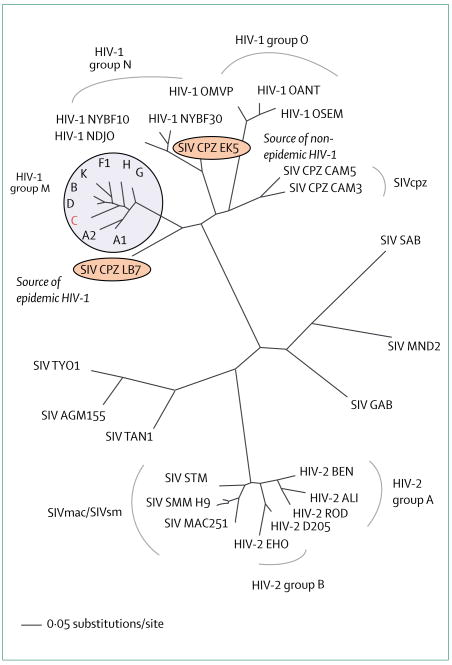
Based on their genetic make-up, HIV-1 viruses are divided into three groups (eg, M [main], N, and O group, figure 2). These HIV-1 groups and HIV-2 probably result from distinct cross-species transmission events.28 Pandemic HIV-1 has diversified into at least nine subtypes (figures 1 and 2) and many circulating recombinant forms,29,30 which encode genetic structures from two or more subtypes (eg, A/E=CRF01; A/G=CRF02). The continuously evolving HIV-1 viral diversity poses an immense challenge to the development of any preventive or therapeutic intervention.29
Figure 2. Phylogenetic relation of lentiviruses in man and non-human primates.
The HIV-1 pandemic is largely due to viral isolates belonging to the HIV-1 M-group, with HIV-1 subtype C being the most prevalent (red). Recombinant circulating forms cluster with the M-group but have been omitted for clarity. HIV-1 M group and the contemporary SIV strains identified in wild chimpanzees in Cameroon (SIVcpzLB7/EK528) are highlighted. HIV-1 sequences cluster closely with SIV from chimpanzees (SIVcpz), whereas HIV-2 resembles SIV from macaques and sooty mangabeys (SIVmac/SIVsm).
In terms of viral diversity, subtype C viruses continue to dominate and account for 55–60% of all HIV-1 infections worldwide (figure 1).30 Non-subtype B isolates might differ in their virological characteristics from the subtype B isolates (eg, viral load, chemokine co-receptor usage, transcriptional activation in specific biological compartments).31–33 However, the clinical consequences of subtype variations remain unclear.
Infection with two or more genetically distinct viruses could lead to new recombinant viruses. Recombination takes place at a higher rate than initially predicted,30 and circulating recombinant forms account for as much as 20% of infections in some regions (eg, southeast Asia).31 These findings are in agreement with the occurrence of co-infections with multiple distinct isolates in a close temporal context.34–36 Further, superinfections in which time points of virus acquisition are months to years apart have been described, although at a much lower frequency than co-infections.34,37–39 Collectively, these observations challenge the assumption that HIV-1 acquisition happens only once with a singular viral strain and that, thereafter, the infected individual is protected from subsequent infections.40 This lack of immunisation has substantial implications for vaccine development. Emerging evidence suggests that clinical progression to AIDS might be more rapid in individuals with dual infections,35 and encouraging safer sex practices in viraemic HIV-1-infected people might be appropriate to keep recurrent exposure to new viral strains to a minimum.
Pathogenesis of HIV-1
The worldwide spread of HIV-1 indicates that the virus effectively counteracts innate, adapted, and intrinsic immunity.41,42 Despite its modest genome size (less than 10 kb) and its few genes (figure 3), HIV-1 excels in taking advantage of cellular pathways while neutralising and hiding from the different components of the immune system.43–45 Notably, our understanding of pathogenesis is often derived from studies of subtype B viruses and non-human primate studies.
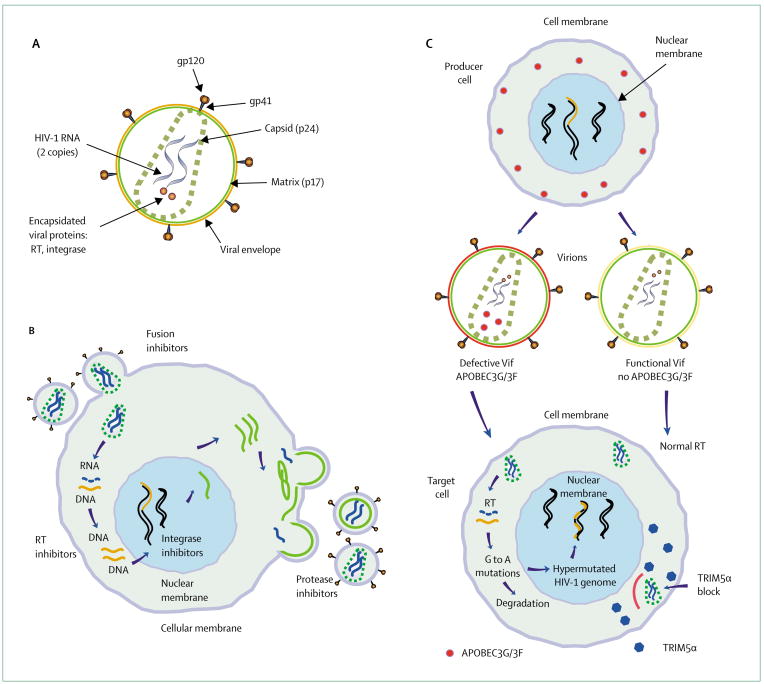
Figure 3. HIV-1 is a retrovirus that encodes three structural genes (Gag, Pol, and Env).
(A) Envelope glycoproteins gp120/41 form the spikes on the virion’s surface. During maturation the gag protein is cleaved and Gag p24 forms the core. The viral genome, viral reverse transcriptase (RT), integrase as well as a number of host proteins are encapsidated. (B) Dir erent steps of the viral life cycle on a cellular level and the potential targets for treatment interventions. (C) HIV-1 has evolved strategies to counteract the restriction factors TRIM5α and APOBEC3G/3F. If left unchecked by HIV-1 Vif, APOBEC3G/3F is encapsidated into the egressing virion, and on infection of a target cell leads to G-to-A hypermutations in the viral genome. Rhesus TRIM5α inhibits HIV-1 replication early after infection of the target cell before the step of reverse transcription.
The HIV-1 life cycle is complex (figure 3) and its duration and outcome is dependent on target cell type and cell activation.46 In the early steps, HIV-1 gains access to cells without causing immediate lethal damages but the entry process can stimulate intracellular signal cascades, which in turn might facilitate viral replication.47,48 The two molecules on the HIV-1 envelope, the external glycoprotein (gp120) and the transmembrane protein (gp41), form the spikes on the virion’s surface.49 During the entry process, gp120 attaches to the cell membrane by first binding to the CD4+ receptor. Subsequent interactions between virus and chemokine co-receptors (eg, CCR5, CXCR4) trigger irreversible conformational changes.49,50 The actual fusion event takes place within minutes by pore formation,50,51 and releases the viral core into the cell cytoplasm. After the core disassembles, the viral genome is reverse transcribed into DNA by the virus’ own reverse transcriptase enzyme.46 Related yet distinct viral variants can be generated during this process since reverse transcriptase is error prone and has no proofreading activity.46 At the midpoint of infection, the viral protein integrase in conjunction with host DNA repair enzymes inserts the viral genome into gene-rich, transcriptionally active domains of the host’s chromosomal DNA.52–54 An integrase binding host factor, LEDGF/p75 (lens epithelium-derived growth factor), facilitates integration,55,56 which marks the turning point by irreversibly transforming the cell into a potential virus producer. In the late steps, production of viral particles needs host driven as well as virus driven transcription.46 Viral proteins are transported to and assemble in proximity to the cell membrane. Virus egress from the cell is not lytic and takes advantage of the vesicular sorting pathway (ESCRT-I, II, III), which normally mediates the budding of endosomes into multivesicular bodies.57,58 HIV-1 accesses this protein-sorting pathway by binding TSG101 via its late domain, a short sequence motif in p6 of Gag.59,60 Cleavage of the Gag-Pol poly-protein by the viral protease produces mature infectious virions.46,61
Since cytoplasmic molecules of the producer cell and components from its cell surface lipid bilayer are incorporated into the new viral particle, virions bear characteristics of the cells in which they were produced.62 Incorporated host molecules can determine the virus’ phenotype in diverse ways (eg, shape the replicative features in the next cycle of infection or mediate immune activation of bystander cells62).
Studies of the early events that happen after HIV-1 breaches the mucosal barrier suggest the existence of a window period in which viral propagation is not yet established and host defences could potentially control viral expansion.63 The important co-receptors for HIV-1 infection are two chemokine receptors—CCR5 and CXCR4. Independently of the transmission route, most new infections are established by viral variants that rely on CCR5 usage.64 CXCR4-tropic viruses generally appear in late stages of infection and have been associated with increased pathogenicity and disease progression.65
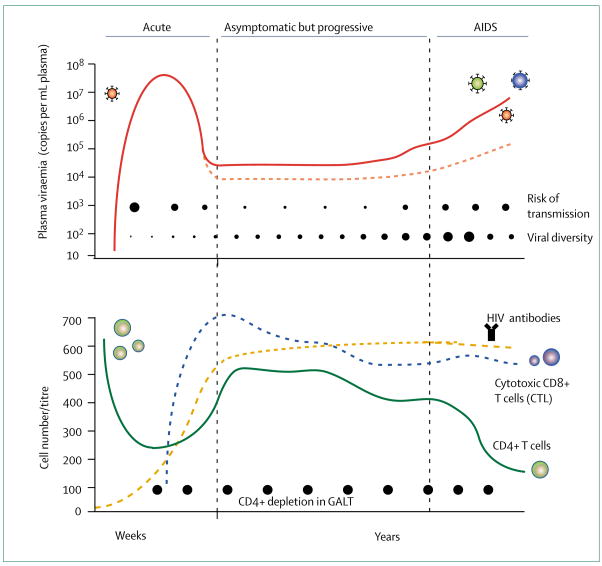
Compelling evidence from non-human primate models (eg, simian immunodeficiency virus [SIV] infection of rhesus macaques) suggest that vaginal transmission results in infection of a small number of CD4+ T lymphocytes, macrophages, and dendritic cells located in the lamina propria.63 Potential pathways for virus transmission involve endocytosis, transcytosis, and virus attachment to mannose C-type lectin receptors (eg, DC-SIGN) located on dendritic cells and macrophages.66 The initial replication takes place in the regional lymph organs (eg, draining lymph nodes) and is composed of few viral variants, and leads to modest primary amplification. With migration of infected T lymphocytes or virions into the bloodstream, secondary amplification in the gastrointestinal tract, spleen, and bone marrow results in massive infection of susceptible cells. In close temporal relation with the resulting peak of viraemia (eg, 106 to 107 copies per mL plasma), clinical symptoms can be manifest during primary HIV-1 infection (figure 4). The level of viraemia characteristic for the chronic phase of infection in an individual (viral set point) differs from the peak viraemia by one or two orders of magnitude. This reduction is largely attributed to HIV-1 specific CD8+ responses but target cell limitation could also play a part. The viral population is most homogeneous early after transmission, but as viral quasi-species diversify in distinct biological compartments, mutant viruses that are resistant to antibody neutralisation, cytotoxic T cells, or antiretroviral drugs are generated and archived in long-lived cells (ie, viral reservoirs).
Figure 4. The course of HIV-1 infection defined by the level of viral replication.
Plasma viraemia (top), and dynamic changes of the CD4+ T-lymphocyte compartments (bottom). Primary infection characterised by high plasma viraemia (red line, top), low CD4 cells (green line, bottom), and absence of HIV-1 specific antibodies (orange line, bottom). Viraemia drops as cytotoxic CD8+ T-lymphocytes (CTL) develop (blue line, bottom) and an individual viral-load set point is reached during chronic infection. Viral set points dir er greatly among individuals (eg, red dotted line, top) and predict disease progression. Viral diversity increases through out the disease (closed circles, top). The risk of transmission is highest in the first weeks when viraemia peaks (closed circles, top). GALT=gut-associated lymphoid tissues.
A pronounced depletion of activated as well as memory CD4+ T cells located in the gut-associated lymphoid tissues has been seen in individuals identified early after infection.67 The preferential depletion of the CD4+ cells in the mucosal lymphoid tissues remains despite years of antiretroviral treatment, a striking observation that contrasts with the fact that the number of CD4+ T lymphocytes in the peripheral blood can return to normal under antiretroviral treatment.
A gradual destruction of the naive and memory CD4+ T-lymphocyte populations is the hallmark of HIV-1 infection, with AIDS being the last disease stage (figure 4).68 Despite the frequent absence of symptoms during early and chronic phase, HIV-1 replication is dynamic throughout the disease. The half-life of a single virion is so short that half the entire plasma virus population is replaced in less than 30 minutes,69 and the total number of virions produced in a chronically infected person can reach more than 10¹P particles per day.69,70 The turnover rates of lymphocyte populations are upregulated many fold during HIV-1 infection, whereas cell proliferation decreases once viral replication is reduced by antiretroviral treatment.71,72 Different depletion mechanisms have been proposed, with an emerging consensus favouring generalised immune activation as cause for constant depletion of the CD4+ cell reservoir.73
Immune activation predicts disease progression74 and, thus, seems to be a central feature of pathogenic HIV-1 infections. Recently, Nef proteins from SIV lineages that are non-pathogenic in their natural hosts (eg, African green monkeys) have proved to down-regulate CD3-T-cell receptors, resulting in reduced cell activation and apoptosis.75 HIV-1 Nef fails to quench T-cell activation, possibly leading to the high degree of immune activation seen in infected people.
Understanding the mechanisms that lead to protection or long-lasting control of infection will guide vaccine development by providing correlates of protection. Natural resistance to HIV-1 infection is rare and varies greatly between individuals. Two groups—long-term non-progressors and highly exposed persistently seronegative individuals—have been studied widely to identify innate and acquired protective determinants (table 1).76 Host resistance factors consist of human leucocyte antigen (HLA) haplotypes, autoantibodies, mutations in the promoter regions, and coding regions of the co-receptors CCR5 and CCR2, as well as the up-regulation of chemokine production (table 1).76,77 Indeed, individuals encoding a truncated CCR5 version (CCR5Δ32) have slower disease progression (heterozygote) or are resistant to CCR5-using viruses (homozygote).78 The CCL3L1 gene encodes MIP1α, a CCR5 co-receptor ligand and chemokine with antiviral activity. Recent findings show that CCL3L1 gene copies vary individually and higher numbers of gene duplications result in reduced susceptibility to infection,77,79 possibly by competitive saturation of CCR5 co-receptor. Cytotoxic T-lymphocyte responses, helper T-cell functions, and humoral responses are some of the acquired factors that modulate the risk of transmission in highly exposed persistently seronegative individuals,76 and could also contribute to spontaneous control of replication in long-term non-progressors. The putative protective role of cytotoxic T-lymphocyte activity has been suggested in seronegative sex workers and in some long-term non-progressors.76,80
Table 1.
Some of the host factors affecting susceptibility to HIV-1 infection
| Innate | Genetic | Acquired | Intrinsic |
|---|---|---|---|
| Autoantibodies | HLA haplotype | Cytotoxic T-cell activity | APOBEC3G/3F |
| Chemokines | CCR5 gene/promoter | Helper T-cell function | TRIM5α |
| Cytokines | CCR2 gene/promoter | Neutralising antibodies | |
| CCL3L1 gene copy number |
HLA=human leucocyte antigen. CCR5=chemokine receptor 5. CCR2=chemokine receptor 2. CCL3L1=CC chemokine ligand like-1, APOBEC3G/3F=apolipoprotein B mRNA editing complex 3.
Mammalian cells are not welcoming micro-environments, but rather deploy a defensive web to curb endogenous and exogenous viruses. HIV-1’s ability to circumvent these defences is as impressive as its efficiency to exploit the cellular machinery. APOBEC3G/3F and TRIM5α are recently described intrinsic restriction factors that are constitutively expressed in many cells.81,82 Both gene loci have been under strong selective pressure throughout primate evolution,83 indicating an ancient need to neutralise foreign DNA and maintain genome stability that precedes the current HIV-1 pandemic.
APOBEC3 enzymes (A3) belong to the superfamily of cytidine deaminases,84 a group of intracellular proteins with DNA/RNA editing activity.84,85 Most representatives of the APOBEC3 group have some mutagenic potential and restrict endogenous retroviruses and mobile genetic elements. The deaminases A3G, A3F, and A3B have potent antiviral activity, with the first two being expressed in cells that are susceptible to HIV-1 infection (T-lymphocytes, macrophages). HIV-1 evades APOBEC3 mutagenesis by expressing Vif, which leads to APOBEC3G/3F but not A3B degradation.42,86–90
We still need to establish how the mechanisms of DNA editing and antiviral activity are interwoven, since some antiviral activity can be maintained despite defective DNA editing.91 The early replication block in non-stimulated CD4+ T cells has been attributed to low molecular mass complexes of APOBEC3G.92 Hypermutated genomes in HIV-1 infected patients93 and mutations in Vif resulting in abrogated or differential APOBEC3 neutralisation capacity have been described.94,95 The degree to which APOBEC3G/3F mRNA expression predicts clinical progression remains an area of intensive investigation.96,97
Several representatives of the heterogeneous family of tripartite motif proteins (TRIM) inhibit retroviruses in a species-specific manner.81,98 TRIM5α from rhesus macaques and African green monkeys inhibit HIV-1 replication, whereas the human homologue is inactive against SIV and HIV-1, leading to the recorded susceptibility of human cells to both viruses.81 Rhesus TRIM5α recognises the capsid domain of HIV-1 Gag and manipulates the kinetics of HIV-1 core disassembly within minutes after cell entry.99,100 Thus, experimental approaches to render HIV-1 resistant to rhesus TRIM5α could lead to immunodeficiency viruses capable of replicating in rhesus macaques. Such a non-primate model would allow testing of antiviral treatment and vaccine interventions with HIV-1 viruses instead of SIV or SIV/HIV chimeric viruses.
Clinical management
Diagnosis
The diagnosis of HIV-1 infection is based on the detection of specific antibodies, antigens, or both, and many commercial kits are available. Serological tests are generally used for screening. A major advance has been the availability of rapid HIV-1 antibody tests. These assays are easy to do and provide results in as little as 20 minutes,101 enabling specimen collection and proper diagnosis at the same visit. Rapid tests are important tools for surveillance, screening, and diagnosis, and can be reliably done on plasma, serum, whole blood, or saliva by health-care providers with little laboratory expertise. The two limitations of these serological tests are detection of infection during primary infection when antibodies are absent, and in infants younger than 18 months who might bear maternal HIV-1 antibodies. In these instances direct virus detection is the only option (eg, quantification of viral RNA [standard] or p24 antigen in heat denatured serum [less expensive]).
For staging purposes, measurement of CD4+ cells and viraemia is required. Plasma viral load is widely used to monitor therapeutic success on antiretroviral treatment. Several commercially available tests provide sensitive quantification of plasma HIV-1 RNA copies. The newer versions of the Amplicor and Quantiplex (Roche, Indianapolis, IN, USA, and Bayer Diagnostics, Walpole, MA, USA, respectively) assays have overcome initial suboptimum performance for non-B subtypes.102 While the viral load determines the rate of destruction of the immune system, the number of CD4+ cells reveals the degree of immunodeficiency and is, therefore, used to assess the stage of infection. CD4+ cell counts together with clinical manifestations (eg, occurrence of opportunistic infections) are key criteria for HIV-1 disease classification. Flow cytometry analysis is the standard method for CD4+ cells quantification.
Standard methods for quantifying viral load and CD4+ cell counts need advanced laboratory infrastructures, and assays require a specimen to be tested within a short time of collection. These requirements pose challenges for resource-constrained settings. The use of dried blood spot specimen has resolved some of the difficulties associated with transportation of samples needed for virological assessments.103 Measurement of reverse transcriptase activity in plasma samples, simplification of gene amplification methods (eg, Taqman technology), and paper-strip quantification (dipstick assays) might provide cost-effective alternatives for the future.104–106 Similarly microcapilliary flow-based systems, CD4+ chips, or total white counts (panleucocyte gating) provide alternatives for establishment of the level of immunodeficiency in resource-limited settings.107–110
Drug treatment
Antiretroviral compounds
Antiretroviral treatment is the best option for longlasting viral suppression and, subsequently, for reduction of morbidity and mortality. However, current drugs do not eradicate HIV-1 infection and lifelong treatment might be needed.
20 of the 21 antiretroviral drugs currently approved by the US Food and Drug Administration target the viral reverse transcriptase or protease (table 2). Eight nucleoside/nucleotide analogues and three non-nucleoside reverse transcriptase inhibitors inhibit viral replication after cell entry but before integration. Fixed-dose combination tablets simplify treatment regimens by reducing the daily pill burden, and drugs with long half-lives allow once or twice daily dosing. Eight protease inhibitors prevent the maturation of virions resulting in production of non-infectious particles. The recently approved darunavir (June, 2006) is the first of its class that retains activity against viruses with reduced susceptibility to protease inhibitors. Enfuvirtide targets a gp41 region of the viral envelope and stops the fusion process before the cell is infected. This drug needs to be injected twice daily and its use is reserved for treatment of heavily drug-experienced patients since it can help overcome existing drug resistance.111,112 Development of new antiretrovirals focuses on molecules that target entry, reverse transcription, integration, or maturation. Compounds that have been designed to inhibit resistant viruses are urgently needed since many patients treated during the past decades harbour viral strains with reduced susceptibilities to many if not all available drugs (table 3).
Table 2.
Antiretroviral drugs currently approved by US Food and Drug Administration
| Entry | Reverse transcriptase |
Protease | |||
|---|---|---|---|---|---|
| Nucleoside | Nucleotide | Non-nucleoside | |||
| Single compound tablets | Enfuvirtide | Abacavir | Tenofovir | Delaviridine | (Fos)-Amprenavir |
| Didanosine | Efavirenz | Atazanavir | |||
| Emtricitabine | Nevirapine | Darunavir | |||
| Lamivudine | Indinavir | ||||
| Stavudine | Nelfinavir | ||||
| Zalcitabine | Ritonavir | ||||
| Zidovudine | Saquinavir | ||||
| Tripanavir | |||||
| Fixed-dose combination tablets | Abacavir/lamivudine (Epzicom) | Lopinavir/ritonavir | |||
| Zidovudine/lamivudine (Combivir) | |||||
| Tenofovir/emtricitabine (Truvada) | |||||
| Abacavir/lamivudine/zidovudine (Trizavir) | |||||
| Tenofovir/emtricitabine/efavirenz (Atripla) | |||||
Drugs belong to five drug classes and target three dir erent viral steps (entry, reverse transcription, or protease). Availability of these drugs in resource-limited countries is subject to country specific licensing agreements.
Table 3.
Antiretrovirals currently in phase II/III of clinical development
| Drug | Mechanism | Activity against PI and RT resistant strains | |
|---|---|---|---|
| Maraviroc | MVC | CCR5 inhibitor | Yes, but not X4 variants |
| Vicriviroc | SCH D | Yes, but not X4 variants | |
| Etravirine | TMC-125 | Non-nucleoside reverse transcriptase inhibitor | Yes, also NNRTI-resistant strains |
| TMC-278 | Yes, also NNRTI-resistant strains | ||
| n/a | MK-0518 | Integrase strand transfer inhibitor | Yes |
| n/a | GS-9137 | Yes |
The goal of antiretroviral treatment is to decrease the morbidity and mortality that is generally associated with HIV-1 infection. A combination of three or more active drugs is needed to achieve this aim in most patients. Effective treatment returns to near normal the turnover rates of both CD4+ and CD8+ T-cell populations.72 Potent but well tolerated drugs with long half-lives and simplified regimens improve the options for first-line and second-line chemotherapeutic interventions.
Combination antiretroviral treatment
High rate of viral replication, low fidelity of reverse transcription, and the ability to recombine are the viral characteristics that lead to the diversity of HIV-1 species (quasi-species) in chronically infected individuals. This high genetic variability provided the rationale for highly active antiretroviral treatments (HAART). By combination of several potent antiretroviral agents, viral replication is suppressed to such low levels that emergence of drug resistant HIV-1 variants was, if not prevented, at least delayed. By doing so, CD4+ T-lymphocyte numbers increase, leading to a degree of immune reconstitution that is sufficient to reverse clinically apparent immunodeficiency. Widespread introduction of HAART in industrialised countries resulted in a striking decrease in morbidity and mortality, putting forward the hope that HIV-1 infection can be transformed into a treatable chronic disease.113–115
A set of criteria composed of plasma viraemia concentration, absolute or relative CD4+ cell counts, and clinical manifestations, is used to recommend initiation of HAART. The benefits of treatment clearly outweigh the potential side-effects in patients with clinical signs of immunodeficiency (eg, AIDS defining illnesses) or with CD4+ numbers less than 200 per μL (recommendation of US Department of Health and Human Services, October, 2005). However, the best time point to begin treatment remains controversial in asymptomatic patients with modest depletion of CD4+ T cells (eg, more than 350 per μL) and modest levels of viraemia (eg, less than 100 000 copies per mL).116 Studies with clinical endpoints supporting the validity of early versus late interventions in asymptomatic patients are difficult to do and insufficient clinical data are currently available. Early depletion of gut CD4+ T lymphocytes,117 increasing viral diversity, and the poor regenerative abilities of key populations of the immune system provide arguments for beginning treatment as early as possible. The wide application of this principle is restricted by long-term drug toxicities that lead to reduction of quality of life, and by treatment costs. Toxicities (eg, renal, hepato, mitochondrial), metabolic changes (eg, lipodystrophy, diabetes mellitus), and immune reconstitution disease are some of the long-term problems that complicate decade-long HAART.118–121
One strategy addressing life-long daily compliance to HAART has been structured treatment interruptions. The rationale for this approach was based on the premise that the body’s own immune system could keep the virus in check if exposed to a very modest level of viral replication. If successful, this strategy could limit drug toxicity and reduce treatment costs.122 Although preliminary findings for this strategy were mixed in terms of benefits,123–125 the recent early closure of the SMART trial was based on increased morbidity and mortality in the treatment interruption arm.126 Thus, in the absence of clinical benefits, most investigators strongly discourage treatment interruptions except as needed to address treatment intolerance.
HAART in resource-constrained settings
The transformation of AIDS into a chronic disease in industrialised countries has yet to be realised in resource-constrained settings. Access to HAART is an absolute humanitarian necessity to avert mortality in people who are central to the future survival of their countries.127 Despite restricted health infrastructures and diverse co-morbidities in these regions, remarkable therapeutic success rates have been shown, with adherence rates at least comparable with those reported in industrialised countries.128–131 WHO and UNAIDS treatment guidelines focusing on resource-limited settings suggest use of standard first-line regimen followed by a set of more expensive second-line options132 and proposes the use of standardised decision-making steps (eg, when to start, to substitute for side-effects, to switch for virological failure).132,133 In many countries, treatment options are limited not only by the costs of HAART but also by restrictive licensing policies, and current estimates suggest that 80% of people infected with HIV-1 with a clinical need for treatment do not yet have access to antiretroviral drugs.1 Thus, efforts and strategies to further scale up treatment access are crucial,134–137 since antiretroviral treatment is also an effective intervention for prevention.138
Drug resistance
Emergence of drug resistance is the most common reason for treatment failure. Insufficient compliance, drug side-effects, or drug-drug interactions can lead to suboptimum drug concentrations, resulting in viral rebound. Viral resistance has been described to every antiretroviral drug and therefore poses a serious clinical as well as public-health problem.139 HIV-1 subtypes differ in the sequence of mutations leading to drug resistance, and some naturally occurring polymorphisms might actually modulate resistance.140,141 Drug-resistant HIV-1 is transmissible and can be detected in up to 20% of newly infected individuals in countries with broad access to antiretrovirals.34 The prevalence of drug resistance in the untreated population remains low in regions with poor access to treatment.142
Short-term antiretroviral-based interventions are effective in prevention of mother-to-child transmission. However, these interventions could result in drug resistant viral variants in the mother, baby, or both.143 Around half the women who received one dose of nevirapine to prevent mother-to-child transmission harbour viruses resistant to non-nucleoside reverse transcriptase inhibitors (NNRTI).144,145 These resistant viruses replicate efficiently and can be transmitted by breast milk,146 and minor resistant populations present long after the intervention can possibly decrease the effectiveness of subsequent NNRTI-based treatment regimens.147 The combination of short-course zidovudine, lamivudine, and nevirapine prevents peripartum transmission while reducing the risk of nevirapine resistant viruses.148
Viral reservoirs
Viral reservoirs consist of anatomical sanctuaries and a small pool of infected long-lived memory T lymphocytes. HIV-1 latency in long-lived cell populations (eg, memory T lymphocytes, macrophages) poses an obstacle to eradication because current antiviral combination treatments fail to eliminate integrated proviruses from resting cells. Different strategies, including immune-modulatory molecules (interleukin 2, anti-CD3 mAb, interleukin 7), have been used to reactivate resting cells in the setting of HAART. Histone deacetylase-1 inhibitors, like valproic acid, release an inherent transcriptional block and by doing so facilitate viral long terminal repeat-driven expression.149 Augmenting standard antiretroviral treatment with enfuviridine and valproic acid reduced the number of latently infected CD4+ T cells (29–84%), but to establish the relative contribution of each drug with respect to the final outcome is difficult.150
Prevention
Mother-to-child transmission
Prevention of mother-to-child transmission has seen advances in both industrialised and resource-constrained settings.151–153 Intrapartum transmission has been reduced by increasing access to interventions such as one dose of nevirapine to mother and newborn baby.154 Concerns about drug-resistant viral strains have led to several trials with combination treatments to reduce transmission during the intrapartum period.148,152,155 In some settings, elective delivery by caesarean section can further reduce HIV-1 transmission during the intrapartum period, but the benefits of the intervention could be countered by post-partum sepsis and increasing maternal mortality.156
Because HIV-1 can be transmitted by breastfeeding, replacement feeding is recommended in many settings. Poor access to clean running water precludes, however, the use of formula feeding under these circumstances,157 and exclusive breastfeeding with abrupt weaning is one option for reducing transmission.158 A potential novel intervention still being tested is the daily use of antiretrovirals during breastfeeding. More attention is starting to focus on the pregnant mother, especially initiation of antiretroviral therapy in mothers with low CD4+ counts during pregnancy and thereafter.159,160 Only limited data are available regarding the health of uninfected children born to HIV-1-positive mothers.161 In a European cohort of exposed-uninfected children, no serious clinical manifestations were apparent, at least in the short term to medium term (median follow-up 2 years).162
Sexual transmission
Reduction of heterosexual transmission is crucial for control of the epidemic in many parts of the world.1,163 Prevention is achieved through reduction in the number of discordant sexual acts or reduction of the probability of HIV-1 transmission in discordant sexual acts. The first can be achieved through abstinence and sex between concordantly seronegative individuals. Abstinence and lifelong monogamous relationships might not be adequate solutions for many people and therefore several interventions aimed at lowering the risk of transmission per discordant sexual act are in the process of clinical testing. Male and female condoms provide a proven and affordable prevention option.164,165 In combination, these options are also more commonly referred to as the ABC (abstinence, be faithful, condom use) approach.
Other biomedical prevention interventions include male circumcision, antiretrovirals for prevention (eg, pre-exposure or post-exposure), chemoprophylactic treatment of herpes simplex virus-2 (HSV-2), microbicides, and vaccines. Results from one of three independent phase III male circumcision trials underway in South Africa, Kenya, and Uganda has helped to allay some of the ambivalence around the protective effect of male circumcision.8,166 The findings from the South African trial show a 60% protective effect of male circumcision.167 The possible mechanism relates to the fact that the foreskin has apocrine glands that secrete lysozymes but also Langerhans cells expressing CD4 and other receptors.168,169 These skin-specific dendritic cells can uptake virus and are believed to play a part in transport of the virus to susceptible T cells. Immunofluorescence studies of foreskin mucosa suggest that these tissues might be more susceptible to HIV-1 infection than cervical mucosa.170 Findings from this proof-of-concept trial need to be compared with evidence from the two trials still underway in Kenya and Uganda, and to acceptability data, behaviour change after circumcision, surgical complication rates, and logistics of undertaking the procedures before policy formulation and wide-scale access as a prevention strategy.171–173
Since high plasma viraemia increases the risk of transmission by as much as an order of magnitude,21 does reducing viral load in the infected partner through, for example, antiretroviral treatment reduce the risk of HIV-1 transmission in the uninfected sexual partner? A trial to explore this question is currently being run jointly by the HIV Prevention Trials Network (www.hptn.org) and the Adult Clinical Trials Group. Mathematical projections estimate up to 80% HIV-1 reduction,174,175 but scarce observational data currently exist.176 Post-exposure prophylaxis is recommended after occupational (eg, needle stick)177 and non-occupational (eg, rape, sexual abuse)178 exposure, although data for efficacy and optimum drug combinations are few.179 Some clinical trials assessing the benefits of once daily pre-exposure chemoprophylaxis with antiretroviral compounds with long biological half-life (eg, tenofovir) have been put on hold or stopped.175,180 Neither the overall idea of pre-exposure prophylaxis nor the drug itself, which is well tolerated, was at the root of the protests. Concerns were centred on clinical trials in resource-poor settings and the perceived scarcity of adequate interventions protecting these vulnerable populations.
HSV-2 might increase both the risk of transmitting and acquiring HIV-1.181,182 Antivirals (eg, aciclovir, valaciclovir) are effective in reducing viral shedding183–185 and HSV-2 transmission in discordant heterosexual couples.182 The future of HSV-2 prevention might reside in the vaccine that is currently under development.186 Whether prophylactic use of aciclovir in populations with high HSV-2 prevalence and incidence rates results in reduced HIV-1 incidence rates remains unresolved but several trials addressing this issue are underway, including HPTN039.
Gender disparities lie at the centre of women’s vulnerability. Prevention options need to be provided that can be used by women independently of their male sexual partner’s knowledge or consent.187 Notwithstanding that redressing these disparities is a long-term challenge, several preventive interventions can be implemented in the interim on the basis of our incomplete understanding at a biological level of HIV-1 risk for women. For example, there seems to be a correlation between levels of sexual hormones (eg, progesterone) and transmission risk.188 Observational studies also highlight the relation between abnormal vaginal flora and increased risk of HIV-1 infection.189,190 The high prevalence of vaginal infections such as bacterial vaginosis (30–50%), vulvovaginal candidosis (10–13%), and trichomonas vaginalis (7–23%) in African women is associated with a substantial risk of HIV-1 acquisition.189 In addition to increasing access to female condoms and treatment of other sexually transmitted infections, trials are underway to assess the use of other barrier methods such as cervical caps, invisible condoms, diaphragms, and diaphragms combined with micro bicides.190 The control of vaginal infections is a potentially important method for decreasing HIV-1 acquisition that has yet to be tested. Periodic presumptive treatment for vaginal infections is being explored as an HIV-1 prevention strategy.191
Microbicides
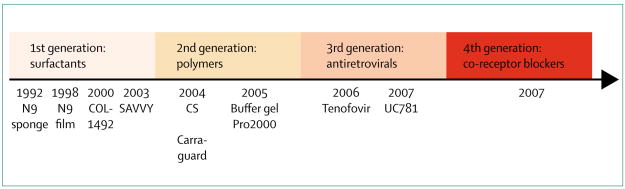
Microbicides are an additional important biomedical intervention technology that is covert and under women’s control.192 These topical products potentially could be used to prevent rectal and vaginal transmission of HIV-1, but proof of concept has been elusive. Although the three phase III trial results of the first microbicidal product (nonoxynol-9) done in the mid-1980s and 1990s did not show protective effects,193,194 they have informed the medical knowledge in terms of product selection, clinical testing, and safety assessments. The past 5 years have seen major advances in investment and product development.66,195,197 Early clinical testing of multiple products including the launch of advanced clinical trials for five different products is continuing (table 4). The development of antiretroviral gels increased the specificity of these third generation microbicides in relation to surfactants, vaginal enhancers, or entry inhibitors that have dominated the product pipeline so far (figure 5). The first antiretroviral gel to undergo early testing is tenofovir gel, and the findings in terms of safety profile, tolerance, low systemic absorption, and slight adverse events are promising.192 As with vaccines, a major obstacle is the absence of a surrogate marker of protection. Additional challenges are adherence to product use and the high rates of pregnancy in trial participants.
Table 4.
Summary of microbicides currently undergoing advanced clinical testing
| Phase | Mode of action | Effective against | |
|---|---|---|---|
| Carraguard | III | Seaweed polymer, entry inhibitor | HIV-1/HIV-2, human papilloma virus, herpes simplex virus |
| Cellulose sulphate | III | Entry inhibitor | Broad range of sexually transmitted diseases; also active as contraceptive |
| PRO2000 | III | Entry inhibitor | HIV-1/HIV-2, human papillomavirus, chlamydia |
| Savvy (C31G) | III | Surfactant | Broad range of sexually transmitted diseases; also active as contraceptive |
| Tenofovir gel | IIb | Nucleotide reverse transcriptase inhibitor | HIV-1/HIV-2 |
| Buffer gel+PRO2000 | IIb/III | Natural vaginal defence/entry inhibitor | HIV-1/HIV-2, chlamydia, gonorrhoea, human papillomavirus, bacterial vaginosis |
| Tenofovir gel+oral | II | Nucleotide reverse transcriptase inhibitor | HIV-1/HIV-2 |
Figure 5. Timeline of microbicide development of different product generation with trial initiation dates as reference.
N9=nonoxonol-9. CS=cellulose sulphate.
Vaccines
A safe, protective, and inexpensive vaccine would be the most efficient and possibly the only way to curb the HIV pandemic.198 Despite intensive research, development of such a candidate vaccine remains elusive. Safety concerns prohibit the use of live-attenuated virus as immunogen.199 Many different approaches with recombinant technologies have been pursued over the past two decades. Initially, efforts were focused on generating neutralising antibodies with recombinant monomeric envelope gp120 (AIDSVAX) as immunogen.200,201 This vaccine did not induce neutralising antibodies and, not unexpectedly, the phase III trials failed to show protection.202,203 Antibody mediated HIV-1 neutralisation is complicated by the high genetic diversity of the variable Env regions, epitopes masked by a carbohydrate shield (glycosylation), and conformational or energetic constraints.204 Since CD8 T-cell responses control to some extent viral replication in vivo, recent vaccine development has focused on eliciting cellular immune responses. Overcoming pre-existing immunity against replication incompetent immunogenic vectors (eg, recombinant adenovirus type 5) is one of the challenges.205 Safety and immunogenicity studies using replication defective vaccine vectors are continuing after preliminary studies in non-human primates showed some protection.204 The immune system generally fails to spontaneously clear HIV-1 and the true correlates of protection continue to be ill defined.198,206 However, the general belief is that approaches aimed at eliciting both humoral and cell mediated immunity are most promising to prevent or at least control retroviral infection.198
Conclusions
An important gateway to both prevention and care is knowledge of HIV-1 status.207 Fear of knowledge of status, including stigma and discrimination, has discouraged many from seeking voluntary counselling and testing services.208 As access to antiretroviral interventions (prevention of mother-to-child transmission, antiretroviral treatment) increases, the opportunities for HIV-1 testing will grow and create opportunities for a prevention-care continuum, with the voluntary counselling and testing services as a point of entry. These changes will result in a shift in prevention efforts from a focus on individuals not infected with HIV-1 to a more effective continuum of prevention that includes uninfected, recently infected, infected, and asymptomatic people, as well as those with advancing HIV disease and on antiretroviral therapy.
HIV/AIDS is an exceptional epidemic that demands an exceptional response. Much progress has been made in a short space of time, despite many scientific and programmatic challenges (figure 6). In the absence of a protective vaccine or a cure, prevention and access to antiretroviral treatments are the best options to slow down the HIV-1 pandemic. Broad implementation of these principles needs improved infrastructures in resource-constrained regions, which have been and will continue to be most affected. The fact that HIV-1 is predominantly sexually transmitted and disproportionately affects populations that are already socially or economically marginalised, or both, poses many ethical, social, economic, and political challenges.
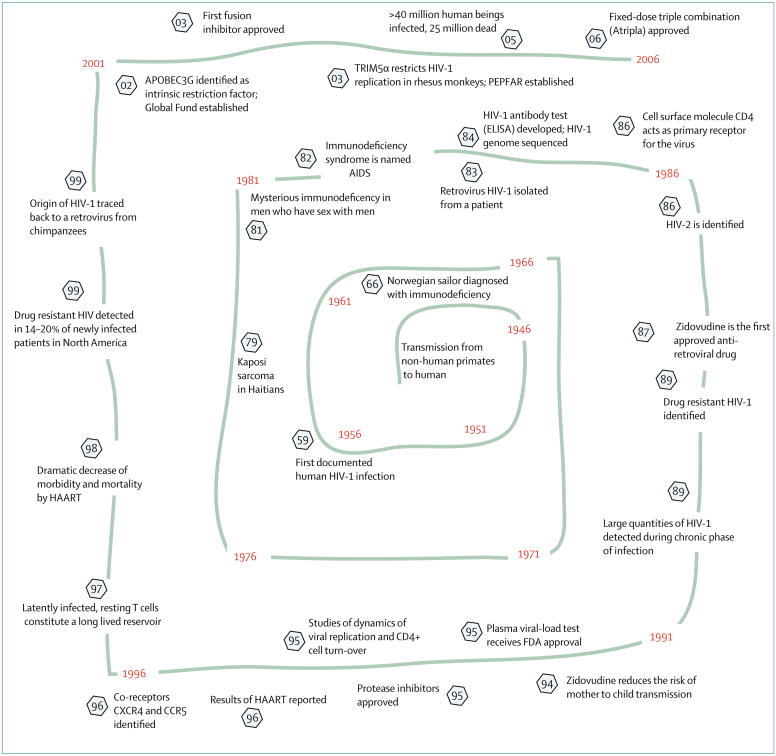
Figure 6. Selected observations, scientific developments, and treatment options pertinent to HIV-1/AIDS.
Estimates place the cross species transmission events leading to the worldwide spread of HIV-1 to the early decades of the 20th century. Numbers circled by a hexagon identify the specific year of an event. PEPFAR=President’s Emergency Plan for AIDS Relief.
In view of the immediacy of the problem, and the fact that both research and programmes are mainly funded by the public sector, there is a greater demand from civil society for co-ownership of research and accountability for use of public funds. On the one hand, this co-ownership defines a changing role and responsibility of science in society, and on the other hand, shows a necessary synergy between activism and science. This partnership has been invaluable for antiretroviral drug development, treatment access in resource-constrained settings, and the scale-up of interventions to reduce mother-to-child transmission.
The increasing number of infected women and the disproportionate burden of infection in resource-constrained settings creates a scientific imperative to ensure research is done for people and in settings who stand to benefit most. The most affected countries face many other economic, political, and development challenges, which have raised issues in undertaking multicentre and multicountry research. Research addressing women-specific topics (such as effect of sexual hormones on transmission and disease progression, viral diversity, and antiretroviral potency) and women-specific prevention interventions including microbicides is crucial. We are probably at one of the most hopeful and optimistic points in our response to the pandemic. There is definitely more attention being directed to HIV-1, more resources (panel), more civil society mobilisation, more governments speaking up, more possibilities for treatment, and more evidence about what prevention and treatment strategies will work than in previous years. The unrelenting growth of the pandemic tells us that current strategies are not enough. Clearly, we need to do some things differently, while also increasing the scale and magnitude of current strategies in keeping with the pandemic.
Panel. Online resources.
Epidemiology
UNAIDS
Treatment recommendations
Centers for Disease Control and Prevention
HIV-1 drug resistance
Stanford University HIV Drug Resistance Database
http://hivdb.stanford.edu/index.html
International AIDS Society–USA
Microbicide
Alliance for Microbicide Development
HIV Prevention Trials Network
Vaccine
International AIDS Vaccine Initiative
Search strategy and selection criteria.
A comprehensive literature review was undertaken by searching the PubMed database online, for English language publications between January, 2000, and June, 2006. The database search terms included keywords such as “HIV/AIDS”, “epidemiology”, “prevention”, “pathogenesis”, “HSV-2”, “male circumcision”, “PMTCT”, “scaling up treatment”, “resource constrained settings”, “antiretroviral pre-exposure prophylaxis”, “HAART”, “restriction”, “host factor”, “HIV pathogenesis”, “resistance”, “latency”. Various combinations of these words were entered. All duplicate articles were removed. A subset of relevant articles was chosen and full-text manuscripts were summarised.
Acknowledgments
We thank P D Bieniasz, W Cates, L Chakrabarti, C Cheng-Mayer, J Coovadia, H Gayle, P A Fryd, R Gray, S Abdool Karim, L Kuhn, K Mayer, P Mane, L C F Mulder, L Myer, and M Wawer for helpful discussions. M Boettiger and C Baxter assisted with literature searches. This work was supported by NIH grant RO1AI064001 (VS), by grant 1 U19AI51794 (QAK) from CAPRISA that forms part of the Comprehensive International Program of Research on AIDS (CIPRA) funded by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) and the US Department of Health and Human Services (DHHS) and grant D43 TW00231 (QAK) from the Columbia University-Southern African Fogarty AIDS International Training and Research Program.
Footnotes
Conflict of interest statement
D D Ho sits on the scientific advisory boards for Monogram, Osel, Achillion, Valiant, Oyagen, Lavipharm, and XTL. Products or work from these companies are not discussed in the review. He holds patents on vaccine candidates. The other authors declare no conflict of interest.
References
- 1.UNAIDS. [(accessed July 20, 2006)];2006 report on the global AIDS epidemic: a UNAIDS 10th anniversary special edition. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
- 2.Inciardi JA, Williams ML. Editor’s introduction: the global epidemiology of HIV and AIDS. AIDS Care. 2005;17 (suppl 1):S1–8. doi: 10.1080/09540120500120435. [DOI] [PubMed] [Google Scholar]
- 3.Hayes R, Weiss H. Epidemiology. Understanding HIV epidemic trends in Africa. Science. 2006;311:620–21. doi: 10.1126/science.1124072. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–83. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 5.Abdool-Karim Q, Abdool-Karim SS. The evolving HIV epidemic in South Africa. Int J Epidemiol. 2002;31:37–40. doi: 10.1093/ije/31.1.37. [DOI] [PubMed] [Google Scholar]
- 6.Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19:1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 7.Shisana O, Davids A. Correcting gender inequalities is central to controlling HIV/AIDS. Bull World Health Organ. 2004;82:812. [PMC free article] [PubMed] [Google Scholar]
- 8.Siegfried N, Muller M, Volmink J, et al. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2003;3:CD003362. doi: 10.1002/14651858.CD003362. [DOI] [PubMed] [Google Scholar]
- 9.Aral SO, Padian NS, Holmes KK. Advances in multilevel approaches to understanding the epidemiology and prevention of sexually transmitted infections and HIV: an overview. J Infect Dis. 2005;191 (suppl 1):S1–6. doi: 10.1086/425290. [DOI] [PubMed] [Google Scholar]
- 10.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28:579–97. doi: 10.1097/00007435-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis. 2005;191 (suppl 1):S168–78. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 12.Bloom SS, Urassa M, Isingo R, Ng’weshemi J, Boerma JT. Community effects on the risk of HIV infection in rural Tanzania. Sex Transm Infect. 2002;78:261–66. doi: 10.1136/sti.78.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunn AJ, Wagner HU, Kamali A, Kengeya-Kayondo JF, Mulder DW. Migration and HIV-1 seroprevalence in a rural Ugandan population. AIDS. 1995;9:503–06. [PubMed] [Google Scholar]
- 14.Lurie MN, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis. 2003;30:149–56. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992;6:1535–39. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2005;39:82–89. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 17.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa’s high HIV prevalence: implications for prevention. Lancet. 2004;364:4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 18.Cates W., Jr Review of non-hormonal contraception (condoms, intrauterine devices, nonoxynol-9 and combos) on HIV acquisition. J Acquir Immune Defic Syndr. 2005;38 (suppl 1):S8–10. doi: 10.1097/01.qai.0000167025.87783.31. [DOI] [PubMed] [Google Scholar]
- 19.Gray RH, Wawer MJ, Serwadda D, et al. Population-based study of fertility in women with HIV-1 infection in Uganda. Lancet. 1998;351:98–103. doi: 10.1016/S0140-6736(97)09381-1. [DOI] [PubMed] [Google Scholar]
- 20.Gregson S, Garnett GP, Nyamukapa CA, et al. HIV decline associated with behavior change in eastern Zimbabwe. Science. 2006;311:664–66. doi: 10.1126/science.1121054. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–29. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 22.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–09. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 23.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391–93. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 25.Aral SO, Peterman TA. Measuring outcomes of behavioural interventions for STD/HIV prevention. Int J STD AIDS. 1996;7(suppl 2):30–38. doi: 10.1258/0956462961917753. [DOI] [PubMed] [Google Scholar]
- 26.Price MA, Miller WC, Kaydos-Daniels SC, et al. Trichomoniasis in men and HIV infection: data from 2 outpatient clinics at Lilongwe Central Hospital, Malawi. J Infect Dis. 2004;190:1448–55. doi: 10.1086/424470. [DOI] [PubMed] [Google Scholar]
- 27.Sharghi N, Bosch RJ, Mayer K, Essex M, Seage GR., 3rd The development and utility of a clinical algorithm to predict early HIV-1 infection. J Acquir Immune Defic Syndr. 2005;40:472–78. doi: 10.1097/01.qai.0000164246.49098.47. [DOI] [PubMed] [Google Scholar]
- 28.Keele BF, Van Heuverswyn F, Li Y, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006 doi: 10.1126/science.1126531. published online May 25, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Thomson MM, Najera R. Molecular epidemiology of HIV-1 variants in the global AIDS pandemic: an update. AIDS Rev. 2005;7:210–24. [PubMed] [Google Scholar]
- 31.Blackard JT, Cohen DE, Mayer KH. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clin Infect Dis. 2002;34:1108–14. doi: 10.1086/339547. [DOI] [PubMed] [Google Scholar]
- 32.Laeyendecker O, Li X, Arroyo M, et al. The effect of HIV subtype on rapid disease progression in Rakai, Uganda. 13th Conference on Retroviruses and Opportunitic Infections; Denver, CO, USA. Feb 6–9, 2006. [Google Scholar]
- 33.Centlivre M, Sommer P, Michel M, et al. The HIV-1 clade C promoter is particularly well adapted to replication in the gut in primary infection. AIDS. 2006;20:657–66. doi: 10.1097/01.aids.0000216365.38572.2f. [DOI] [PubMed] [Google Scholar]
- 34.Steain MC, Wang B, Dwyer DE, Saksena NK. HIV-1 co-infection, superinfection and recombination. Sex Health. 2004;1:239–50. doi: 10.1071/sh04024. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb GS, Nickle DC, Jensen MA, et al. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363:619–22. doi: 10.1016/S0140-6736(04)15596-7. [DOI] [PubMed] [Google Scholar]
- 36.Yerly S, Jost S, Monnat M, et al. HIV-1 co/super-infection in intravenous drug users. AIDS. 2004;18:1413–21. doi: 10.1097/01.aids.0000131330.28762.0c. [DOI] [PubMed] [Google Scholar]
- 37.Chohan B, Lavreys L, Rainwater SM, Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J Virol. 2005;79:10701–08. doi: 10.1128/JVI.79.16.10701-10708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Kuyl AC, Kozaczynska K, van den Burg R, et al. Triple HIV-1 infection. N Engl J Med. 2005;352:2557–59. doi: 10.1056/NEJM200506163522420. [DOI] [PubMed] [Google Scholar]
- 39.Smith DM, Richman DD, Little SJ. HIV superinfection. J Infect Dis. 2005;192:438–44. doi: 10.1086/431682. [DOI] [PubMed] [Google Scholar]
- 40.McCutchan FE, Hoelscher M, Tovanabutra S, et al. In-depth analysis of a heterosexually acquired human immunodeficiency virus type 1 superinfection: evolution, temporal fluctuation, and intercompartment dynamics from the seronegative window period through 30 months postinfection. J Virol. 2005;79:11693–704. doi: 10.1128/JVI.79.18.11693-11704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahalingam S, Meanger J, Foster PS, Lidbury BA. The viral manipulation of the host cellular and immune environments to enhance propagation and survival: a focus on RNA viruses. J Leukoc Biol. 2002;72:429–39. [PubMed] [Google Scholar]
- 42.Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–15. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- 43.Coffin JM. Retroviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields virology. 3. Philadelphia, New York: Lippencott-Raven; 1996. pp. 1767–847. [Google Scholar]
- 44.Barre-Sinoussi F. HIV as the cause of AIDS. Lancet. 1996;348:31–35. doi: 10.1016/s0140-6736(96)09058-7. [DOI] [PubMed] [Google Scholar]
- 45.Emerman M, Malim MH. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–84. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 46.Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview, NY USA: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 47.Balabanian K, Harriague J, Decrion C, et al. CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes. J Immunol. 2004;173:7150–60. doi: 10.4049/jimmunol.173.12.7150. [DOI] [PubMed] [Google Scholar]
- 48.Cicala C, Arthos J, Selig SM, et al. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc Natl Acad Sci USA. 2002;99:9380–85. doi: 10.1073/pnas.142287999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray N, Doms RW. HIV-1 coreceptors and their inhibitors. Curr Top Microbiol Immunol. 2006;303:97–120. doi: 10.1007/978-3-540-33397-5_5. [DOI] [PubMed] [Google Scholar]
- 50.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 51.Platt EJ, Durnin JP, Kabat D. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J Virol. 2005;79:4347–56. doi: 10.1128/JVI.79.7.4347-4356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder A. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–29. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell RS, Beitzel BF, Schroder AR, et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherdin U, Rhodes K, Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990;64:907. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciuffi A, Llano M, Poeschla E, et al. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11:1287–89. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- 56.Turlure F, Maertens G, Rahman S, Cherepanov P, Engelman A. A tripartite DNA-binding element, comprised of the nuclear localization signal and two AT-hook motifs, mediates the association of LEDGF/p75 with chromatin in vivo. Nucleic Acids Res. 2006;34:1663–75. doi: 10.1093/nar/gkl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Serrano J, Zang T, Bieniasz PD. Role of ESCRT-I in retroviral budding. J Virol. 2003;77:4794–804. doi: 10.1128/JVI.77.8.4794-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 59.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–19. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 60.Garrus JE, von Schwedler UK, Pornillos OW, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 61.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 62.Cantin R, Methot S, Tremblay MJ. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J Virol. 2005;79:6577–87. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 64.Briggs JA, Grunewald K, Glass B, Forster F, Krausslich HG, Fuller SD. The mechanism of HIV-1 core assembly: insights from three-dimensional reconstructions of authentic virions. Structure. 2006;14:15–20. doi: 10.1016/j.str.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–28. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 67.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 69.Ramratnam B, Bonhoeffer S, Binley J, et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 1999;354:1782–85. doi: 10.1016/S0140-6736(99)02035-8. [DOI] [PubMed] [Google Scholar]
- 70.Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–90. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- 71.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–41. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohri H, Perelson AS, Tung K, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194:1277–87. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Boer RJ, Mohri H, Ho DD, Perelson AS. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J Immunol. 2003;170:2479–87. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
- 74.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 75.Schindler M, Muench J, Kutsch O, et al. Nef mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–67. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 76.Kulkarni PS, Butera ST, Duerr AC. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003;5:87–103. [PubMed] [Google Scholar]
- 77.Telenti A, Bleiber G. Host genetics of HIV-1 suceptibility. Future Virology. 2006;1:55–70. [Google Scholar]
- 78.Carrington M, Dean M, Martin MP, O’Brien S. Genetics of HIV-1infection: chemokine receptor CCR5 polymorphism and its consequences. Hum Mol Genet. 1999;8:1939–45. doi: 10.1093/hmg/8.10.1939. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez E, Kulkarni H, Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 80.Valdez H, Carlson NL, Post AB, et al. HIV long-term non-progressors maintain brisk CD8 T cell responses to other viral antigens. AIDS. 2002;16:1113–18. doi: 10.1097/00002030-200205240-00004. [DOI] [PubMed] [Google Scholar]
- 81.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 82.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 83.Sawyer SL, Emerman M, Malik HS. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2004;2:E275. doi: 10.1371/journal.pbio.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jarmuz A, Chester A, Bayliss J, et al. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–96. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- 85.Petersen-Mahrt SK, Neuberger MS. In vitro deamination of cytosine to uracil in single-stranded DNA by apolipoprotein B editing complex catalytic subunit 1 (APOBEC1) J Biol Chem. 2003;278:19583–86. doi: 10.1074/jbc.C300114200. [DOI] [PubMed] [Google Scholar]
- 86.Mangeat B, Trono D. Lentiviral vectors and antiretroviral intrinsic immunity. Hum Gene Ther. 2005;16:913–20. doi: 10.1089/hum.2005.16.913. [DOI] [PubMed] [Google Scholar]
- 87.Cullen BR. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J Virol. 2006;80:1067–76. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huthoff H, Malim MH. Cytidine deamination and resistance to retroviral infection: towards a structural understanding of the APOBEC proteins. Virology. 2005;334:147–53. doi: 10.1016/j.virol.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 89.Harris RS, Liddament MT. Retroviral restriction by APOBEC proteins. Nat Rev Immunol. 2004;4:868–77. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- 90.Schrofelbauer B, Yu Q, Landau NR. New insights into the role of Vif in HIV-1 replication. AIDS Rev. 2004;6:34–39. [PubMed] [Google Scholar]
- 91.Navarro F, Bollman B, Chen H, et al. Complementary function of the two catalytic domains of APOBEC3G. Virology. 2005;333:374–86. doi: 10.1016/j.virol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–14. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 93.Kieffer TL, Kulon P, Nettles RE, Han Y, Ray SC, Siliciano RF. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol. 2005;79:1973–80. doi: 10.1128/JVI.79.3.1975-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian C, Yu X, Zhang W, Wang T, Xu R, Yu XF. Differential requirement for conserved tryptophans in human immunodeficiency virus type 1 Vif for the selective suppression of APOBEC3G and APOBEC3F. J Virol. 2006;80:3112–15. doi: 10.1128/JVI.80.6.3112-3115.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon V, Zennou V, Murray D, Huang Y, Ho DD, Bieniasz PD. Natural variation in Vif: differential impact on APOBEC3G/3F and a potential role in HIV-1 diversification. PLoS Pathog. 2005;1:e6. doi: 10.1371/journal.ppat.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin X, Brooks A, Chen H, Bennett R, Reichman R, Smith H. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J Virol. 2005;79:11513–16. doi: 10.1128/JVI.79.17.11513-11516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho SJ, Drechsler H, Burke RC, Arens MQ, Powderly W, Davidson NO. APOBEC3F and APOBEC3G mRNA levels do not correlate with human immunodeficiency virus type 1 plasma viremia or CD4+ T-cell count. J Virol. 2006;80:2069–72. doi: 10.1128/JVI.80.4.2069-2072.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nisole S, Stoye JP, Saib A. Trim family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- 99.Perez-Caballero D, Hatziioannou T, Zhang F, Cowan S, Bieniasz PD. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J Virol. 2005;79:15567–72. doi: 10.1128/JVI.79.24.15567-15572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stremlau M, Perron M, Lee M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5{alpha} restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–19. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–31. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 102.Berger A, Scherzed L, Sturmer M, Preiser W, Doerr HW, Rabenau HF. Comparative evaluation of the Cobas Amplicor HIV-1 Monitor Ultrasensitive test, the new Cobas AmpliPrep/Cobas Amplicor HIV-1 Monitor Ultrasensitive test and the Versant HIV RNA 3.0 assays for quantitation of HIV-1 RNA in plasma samples. J Clin Virol. 2005;33:43–51. doi: 10.1016/j.jcv.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 103.Uttayamakul S, Likanonsakul S, Sunthornkachit R, et al. Usage of dried blood spots for molecular diagnosis and monitoring HIV-1 infection. J Virol Methods. 2005;128:128–34. doi: 10.1016/j.jviromet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Jennings C, Fiscus SA, Crowe SM, et al. Comparison of two human immunodeficiency virus (HIV) RNA surrogate assays to the standard HIV RNA assay. J Clin Microbiol. 2005;43:5950–56. doi: 10.1128/JCM.43.12.5950-5956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lombart JP, Vray M, Kafando A, et al. Plasma virion reverse transcriptase activity and heat dissociation-boosted p24 assay for HIV load in Burkina Faso, West Africa. AIDS. 2005;19:1273–77. doi: 10.1097/01.aids.0000180098.58017.48. [DOI] [PubMed] [Google Scholar]
- 106.Tuaillon E, Gueudin M, Lemee V, et al. Phenotypic susceptibility to nonnucleoside inhibitors of virion-associated reverse transcriptase from different HIV types and groups. J Acquir Immune Defic Syndr. 2004;37:1543–49. doi: 10.1097/00126334-200412150-00001. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez WR, Christodoulides N, Floriano PN, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med. 2005;2:e182. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spacek LA, Shihab HM, Lutwama F, et al. Evaluation of a low-cost method, the Guava EasyCD4 assay, to enumerate CD4-positive lymphocyte counts in HIV-infected patients in the United States and Uganda. J Acquir Immune Defic Syndr. 2006;41:607–10. doi: 10.1097/01.qai.0000214807.98465.a2. [DOI] [PubMed] [Google Scholar]
- 109.Mofenson LM, Harris DR, Moye J, et al. Alternatives to HIV-1 RNA concentration and CD4 count to predict mortality in HIV-1-infected children in resource-poor settings. Lancet. 2003;362:1625–27. doi: 10.1016/s0140-6736(03)14825-8. [DOI] [PubMed] [Google Scholar]
- 110.Ceffa S, Erba F, Assane M, Coelho E, Calgaro M, Brando B. Panleucogating as an accurate and affordable flow cytometric protocol to analyse lymphocyte subsets among HIV-positive patients on HAART treatment in Mozambique. J Biol Regul Homeost Agents. 2005;19:169–75. [PubMed] [Google Scholar]
- 111.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–95. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 112.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–85. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 113.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 114.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–54. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 115.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 116.Mocroft A, Lundgren JD. Starting highly active antiretroviral therapy: why, when and response to HAART. J Antimicrob Chemother. 2004;54:10–03. doi: 10.1093/jac/dkh290. [DOI] [PubMed] [Google Scholar]
- 117.Mehandru S, Tenner-Racz K, Racz P, Markowitz M. The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. J Allergy Clin Immunol. 2005;116:419–22. doi: 10.1016/j.jaci.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 118.Nolan D, Mallal S. Antiretroviral-therapy-associated lipoatrophy: current status and future directions. Sex Health. 2005;2:153–63. doi: 10.1071/sh04058. [DOI] [PubMed] [Google Scholar]
- 119.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–27. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 120.Wyatt CM, Klotman PE. Antiretroviral therapy and the kidney: balancing benefit and risk in patients with HIV infection. Expert Opin Drug Saf. 2006;5:275–87. doi: 10.1517/14740338.5.2.275. [DOI] [PubMed] [Google Scholar]
- 121.Calza L, Manfredi R, Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:10–14. doi: 10.1093/jac/dkh013. [DOI] [PubMed] [Google Scholar]
- 122.Hirschel B. Planned interruptions of anti-HIV treatment. Lancet Infect Dis. 2001;1:53–59. doi: 10.1016/S1473-3099(01)00022-6. [DOI] [PubMed] [Google Scholar]
- 123.Powers AE, Marden SF, McConnell R, et al. Effect of long-cycle structured intermittent versus continuous HAART on quality of life in patients with chronic HIV infection. Aids. 2006;20:837–45. doi: 10.1097/01.aids.0000218547.39339.13. [DOI] [PubMed] [Google Scholar]
- 124.Dybul M, Nies-Kraske E, Daucher M, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–96. doi: 10.1086/376535. [DOI] [PubMed] [Google Scholar]
- 125.Abbas UL, Mellors JW. Interruption of antiretroviral therapy to augment immune control of chronic HIV-1 infection: risk without reward. Proc Natl Acad Sci USA. 2002;99:13377–78. doi: 10.1073/pnas.212518999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.El-Sadr W, Neaton J. Episodic CD4-guided use of ART is inferior to continuous therapy: results of the SMART study. 13th Conference on Retroviruses and Opportunitic Infections; Denver, CO, USA. Feb 6–9, 2006. [Google Scholar]
- 127.Kim JY, Gilks C. Scaling up treatment—why we can’t wait. N Engl J Med. 2005;353:2392–94. doi: 10.1056/NEJMe058261. [DOI] [PubMed] [Google Scholar]
- 128.Orrell C. Antiretroviral adherence in a resource-poor setting. Curr HIV/AIDS Rep. 2005;2:171–76. doi: 10.1007/s11904-005-0012-8. [DOI] [PubMed] [Google Scholar]
- 129.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 130.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 131.Levy NC, Miksad RA, Fein OT. From treatment to prevention: the interplay between HIV/AIDS treatment availability and HIV/AIDS prevention programming in Khayelitsha, South Africa. J Urban Health. 2005;82:498–509. doi: 10.1093/jurban/jti090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.WHO. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach: 2003 revision. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 133.Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS. 2004;18:1159–68. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]
- 134.Calmy A, Klement E, Teck R, Berman D, Pecoul B, Ferradini L. Simplifying and adapting antiretroviral treatment in resource-poor settings: a necessary step to scaling-up. AIDS. 2004;18:2353–60. [PubMed] [Google Scholar]
- 135.Kober K, Van Damme W. Scaling up access to antiretroviral treatment in southern Africa: who will do the job? Lancet. 2004;364:103–07. doi: 10.1016/S0140-6736(04)16597-5. [DOI] [PubMed] [Google Scholar]
- 136.Libamba E, Makombe S, Harries AD, et al. Scaling up antiretroviral therapy in Africa: learning from tuberculosis control programmes—the case of Malawi. Int J Tuberc Lung Dis. 2005;9:1062–71. [PubMed] [Google Scholar]
- 137.Gupta R, Irwin A, Raviglione MC, Kim JY. Scaling-up treatment for HIV/AIDS: lessons learned from multidrug-resistant tuberculosis. Lancet. 2004;363:320–24. doi: 10.1016/S0140-6736(03)15394-9. [DOI] [PubMed] [Google Scholar]
- 138.Bachmann MO. Effectiveness and cost effectiveness of early and late prevention of HIV/AIDS progression with antiretrovirals or antibiotics in Southern African adults. AIDS Care. 2006;18:109–20. doi: 10.1080/09540120500159334. [DOI] [PubMed] [Google Scholar]
- 139.Deeks SG. Treatment of antiretroviral-drug-resistant HIV-1 infection. Lancet. 2003;362:2002–11. doi: 10.1016/S0140-6736(03)15022-2. [DOI] [PubMed] [Google Scholar]
- 140.Kantor R, Katzenstein DA, Efron B, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2:e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parkin NT, Schapiro JM. Antiretroviral drug resistance in non-subtype B HIV-1, HIV-2 and SIV. Antivir Ther. 2004;9:3–12. [PubMed] [Google Scholar]
- 142.Wensing AM, Boucher CA. Worldwide transmission of drug-resistant HIV. AIDS Rev. 2003;5:140–55. [PubMed] [Google Scholar]
- 143.Toni TD, Masquelier B, Lazaro E, et al. Characterization of nevirapine (NVP) resistance mutations and HIV type 1 subtype in women from Abidjan (Cote d’Ivoire) after NVP single-dose prophylaxis of HIV type 1 mother-to-child transmission. AIDS Res Hum Retroviruses. 2005;21:1031–34. doi: 10.1089/aid.2005.21.1031. [DOI] [PubMed] [Google Scholar]
- 144.Eshleman SH, Jackson JB. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 2002;4:59–63. [PubMed] [Google Scholar]
- 145.Flys TS, Chen S, Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006 doi: 10.1097/01.qai.0000221686.67810.20. published online June 12, 2006. [DOI] [PubMed] [Google Scholar]
- 146.Lee EJ, Kantor R, Zijenah L, et al. Breast-milk shedding of drug-resistant HIV-1 subtype C in women exposed to single-dose nevirapine. J Infect Dis. 2005;192:1260–64. doi: 10.1086/444424. [DOI] [PubMed] [Google Scholar]
- 147.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci USA. 2006;103:7094–99. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chaix ML, Ekouevi DK, Rouet F, et al. Low risk of nevirapine resistance mutations in the prevention of mother-to-child transmission of HIV-1: Agence Nationale de Recherches sur le SIDA Ditrame Plus, Abidjan, Cote d’Ivoire. J Infect Dis. 2006;193:482–87. doi: 10.1086/499966. [DOI] [PubMed] [Google Scholar]
- 149.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–08. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 150.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luzuriaga K, Sullivan JL. Prevention of mother-to-child transmission of HIV infection. Clin Infect Dis. 2005;40:466–67. doi: 10.1086/427294. [DOI] [PubMed] [Google Scholar]
- 152.McIntyre J. Strategies to prevent mother-to-child transmission of HIV. Curr Opin Infect Dis. 2006;19:33–38. doi: 10.1097/01.qco.0000200290.99790.72. [DOI] [PubMed] [Google Scholar]
- 153.Newell ML. Current issues in the prevention of mother-to-child transmission of HIV-1 infection. Trans R Soc Trop Med Hyg. 2006;100:1–5. doi: 10.1016/j.trstmh.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 154.Ekouevi DK, Tonwe-Gold B, Dabis F. Advances in the prevention of mother-to-child transmission of HIV-1 infection in resource-limited settings. AIDS Read. 2005;15:479–80. [PubMed] [Google Scholar]
- 155.Cressey TR, Jourdain G, Lallemant MJ, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–88. [PubMed] [Google Scholar]
- 156.Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother-to-child transmission of HIV-1. Cochrane Database Syst Rev. 2005;4:CD005479. doi: 10.1002/14651858.CD005479. [DOI] [PubMed] [Google Scholar]
- 157.Magoni M, Bassani L, Okong P, et al. Mode of infant feeding and HIV infection in children in a program for prevention of mother-to-child transmission in Uganda. AIDS. 2005;19:433–37. doi: 10.1097/01.aids.0000161773.29029.c0. [DOI] [PubMed] [Google Scholar]
- 158.Rollins N, Meda N, Becquet R, et al. Preventing postnatal transmission of HIV-1 through breast-feeding: modifying infant feeding practices. J Acquir Immune Defic Syndr. 2004;35:188–95. doi: 10.1097/00126334-200402010-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duerr A, Hurst S, Kourtis AP, Rutenberg N, Jamieson DJ. Integrating family planning and prevention of mother-to-child HIV transmission in resource-limited settings. Lancet. 2005;366:261–63. doi: 10.1016/S0140-6736(05)66917-6. [DOI] [PubMed] [Google Scholar]
- 160.Welty TK, Bulterys M, Welty ER, et al. Integrating prevention of mother-to-child HIV transmission into routine antenatal care: the key to program expansion in Cameroon. J Acquir Immune Defic Syndr. 2005;40:486–93. doi: 10.1097/01.qai.0000163196.36199.89. [DOI] [PubMed] [Google Scholar]
- 161.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–70. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 162.Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–87. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 163.Chan DJ. Factors affecting sexual transmission of HIV-1: current evidence and implications for prevention. Curr HIV Res. 2005;3:223–41. doi: 10.2174/1570162054368075. [DOI] [PubMed] [Google Scholar]
- 164.de Vincenzi I. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. European Study Group on Heterosexual Transmission of HIV. N Engl J Med. 1994;331:341–46. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 165.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;1:CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 166.Siegfried N, Muller M, Deeks J, et al. HIV and male circumcision—a systematic review with assessment of the quality of studies. Lancet Infect Dis. 2005;5:165–73. doi: 10.1016/S1473-3099(05)01309-5. [DOI] [PubMed] [Google Scholar]
- 167.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Szabo R, Short RV. How does male circumcision protect against HIV infection? BMJ. 2000;320:1592–94. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Soto-Ramirez LE, Renjifo B, McLane MF, et al. HIV-1 Langerhans’ cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–93. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 170.Patterson BK, Landay A, Siegel JN, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–73. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Halperin DT, Fritz K, McFarland W, Woelk G. Acceptability of adult male circumcision for sexually transmitted disease and HIV prevention in Zimbabwe. Sex Transm Dis. 2005;32:238–39. doi: 10.1097/01.olq.0000149782.47456.5b. [DOI] [PubMed] [Google Scholar]
- 172.Garenne M. Male circumcision and HIV control in Africa. PLoS Med. 2006;3:e78. doi: 10.1371/journal.pmed.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bailey RC. Editorial comment: male circumcision to reduce HIV acquisition—not quite yet. AIDS Read. 2005;15:136–37. [PubMed] [Google Scholar]
- 174.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–54. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 175.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 176.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994;5:570–75. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 177.Puro V, Cicalini S, De Carli G, et al. Post-exposure prophylaxis of HIV infection in healthcare workers: recommendations for the European setting. Eur J Epidemiol. 2004;19:577–84. doi: 10.1023/b:ejep.0000032349.57057.8a. [DOI] [PubMed] [Google Scholar]
- 178.Winston A, McAllister J, Amin J, Cooper DA, Carr A. The use of a triple nucleoside-nucleotide regimen for nonoccupational HIV post-exposure prophylaxis. HIV Med. 2005;6:191–97. doi: 10.1111/j.1468-1293.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 179.Bassett IV, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis. 2004;39:395–401. doi: 10.1086/422459. [DOI] [PubMed] [Google Scholar]
- 180.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–54. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 181.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000;355:1981–87. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 182.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 183.Nagot N, Foulongne V, Becquart P, et al. Longitudinal assessment of HIV-1 and HSV-2 shedding in the genital tract of West African women. J Acquir Immune Defic Syndr. 2005;39:632–34. [PubMed] [Google Scholar]
- 184.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 185.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 186.Hosken NA. Development of a therapeutic vaccine for HSV-2. Vaccine. 2005;23:2395–98. doi: 10.1016/j.vaccine.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 187.Duffy L. Culture and context of HIV prevention in rural Zimbabwe: the influence of gender inequality. J Transcult Nurs. 2005;16:23–31. doi: 10.1177/1043659604270962. [DOI] [PubMed] [Google Scholar]
- 188.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–88. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 189.Schwebke JR. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J Infect Dis. 2005;192:1315–17. doi: 10.1086/462430. [DOI] [PubMed] [Google Scholar]
- 190.van der Straten A, Kang MS, Posner SF, Kamba M, Chipato T, Padian NS. Predictors of diaphragm use as a potential sexually transmitted disease/HIV prevention method in Zimbabwe. Sex Transm Dis. 2005;32:64–71. doi: 10.1097/01.olq.0000148301.90343.3a. [DOI] [PubMed] [Google Scholar]
- 191.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 192.Stein ZA. Vaginal microbicides and prevention of HIV infection. Lancet. 1994;343:362–63. doi: 10.1016/s0140-6736(94)91205-x. [DOI] [PubMed] [Google Scholar]
- 193.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–77. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 194.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39:1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 195.Weber J, Desai K, Darbyshire J. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2005;2:e142. doi: 10.1371/journal.pmed.0020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dhawan D, Mayer KH. Microbicides to prevent HIV transmission: overcoming obstacles to chemical barrier protection. J Infect Dis. 2006;193:36–44. doi: 10.1086/499163. [DOI] [PubMed] [Google Scholar]
- 197.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–56. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 198.Ho DD, Huang Y. The HIV-1 vaccine race. Cell. 2002;110:135–38. doi: 10.1016/s0092-8674(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 199.Sheppard HW. Inactivated- or killed-virus HIV/AIDS vaccines. Curr Drug Targets Infect Disord. 2005;5:131–41. doi: 10.2174/1568005054201599. [DOI] [PubMed] [Google Scholar]
- 200.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–88. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 201.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–69. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 202.Cohen J. Public health. AIDS vaccine trial produces disappointment and confusion. Science. 2003;299:1290–91. doi: 10.1126/science.299.5611.1290. [DOI] [PubMed] [Google Scholar]
- 203.Francis DP, Heyward WL, Popovic V, et al. Candidate HIV/AIDS vaccines: lessons learned from the world’s first phase III efficacy trials. AIDS. 2003;17:147–56. doi: 10.1097/01.aids.0000050786.28043.62. [DOI] [PubMed] [Google Scholar]
- 204.Garber DA, Silvestri G, Feinberg MB. Prospects for an AIDS vaccine: three big questions, no easy answers. Lancet Infect Dis. 2004;4:397–413. doi: 10.1016/S1473-3099(04)01056-4. [DOI] [PubMed] [Google Scholar]
- 205.Roberts DM, Nanda A, Havenga MJ, et al. Hexonchimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 206.Jamieson BD, Ibarrondo FJ, Wong JT, et al. Transience of vaccine-induced HIV-1-specific CTL and definition of vaccine “response”. Vaccine. 2006;24:3426–31. doi: 10.1016/j.vaccine.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 207.Rennie S, Behets F. Desperatly seeking targets: the ethics of routine testing in low income countries. Bull World Health Organ. 2006;84:52–57. doi: 10.2471/blt.05.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]