Abstract
Disinhibition in the dorsal horn accompanies peripheral nerve injury and causes the development of hypersensitivity to mild stimuli. This demonstrates the critical importance of inhibition in the dorsal horn for maintaining normal sensory signaling. Here we show that disinhibition induces a novel polysynaptic low-threshold input onto lamina I output neurons, suggesting that inhibition normally suppresses a preexisting pathway that probably contribute to abnormal pain sensations such as allodynia. In addition, we show that a significant proportion of superficial dorsal horn inhibitory neurons are activated by low-threshold input. These neurons are well situated to contribute to suppressing low-threshold activation of pain output neurons in lamina I. We further discuss several aspects of inhibition in the dorsal horn that might contribute to suppressing pathological signaling.
Keywords: inhibition, pain, tonic inhibition, dorsal horn
Introduction
The spinal cord dorsal horn is a key region in the central nervous system (CNS) in which sensory information is received, integrated, and relayed to higher brain structures. In fact, the dorsal horn receives inputs from a wide variety of primary afferent fibers including nociceptors, chemoreceptors, and thermoreceptors that respond to stimuli from the skin, muscles, joints, and viscera. The patterns of termination of primary afferents within the spinal cord are related to axonal diameter, receptive field, and sensory modality. Most nociceptive primary afferents are small-diameter thinly myelinated Aδ or unmyelinated C fibers. These terminate primarily in the superficial part of the dorsal horn, specifically in lamina I and II. Dorsal horn neurons in lamina III/IV receive low-threshold inputs from large-diameter, more heavily myelinated Aβ fibers of mechanoreceptors. Thus roughly speaking, touch sensitive fibers terminate deeper and nociceptive fibers terminate more superficially within the dorsal horn.
The dorsal horn neurons themselves are a mixture of neuron types. Nociceptive projection neurons are found in lamina I. Excitatory and inhibitory interneurons are found throughout lamina I, II, and III/IV. Some indications are beginning to emerge that there are detectable activity patterns in the dorsal horn indicating a dorsally directed flow of information. For example, a recent study used laser-scanning photostimulation to uncage glutamate to activate individual presynaptic dorsal horn neurons while recording postsynaptic responses. Excitatory synaptic drive was observed to move ventral to dorsal across lamina II toward lamina I through neurons with long ventral dendrites called vertical neurons.1 This pattern suggested the idea that there are polysynaptic circuits that drive up toward the output neurons in lamina I.1 The presence of such polysynaptic, ventral to dorsal circuits has been demonstrated under conditions of disinhibition2–4 and is believed to be associated with behavioral allodynia.3,5 Details of actual circuitries within lamina I and II, however, mostly remain obscure. Similarly, the general issue of how sensory modalities are processed properly within dorsal horn is still unclear. In this paper, we discuss the role of inhibition in dorsal horn for maintaining separation of sensory modalities such as touch and pain.
Results and discussion
Disinhibition unmasks low-threshold input to pain output neurons
Chronic neuropathic pain often develops following peripheral nerve injury. One manifestation of that pain is allodynia, a painful response to a normally nonpainful stimulus. Behavioral studies have demonstrated that pharmacological disruption of dorsal horn inhibition in vivo using intrathecal antagonists for GABAA or glycine receptors transiently causes allodynia.6–8 This suggests that inhibition is a critical element in maintaining separation between touch sensitive afferent input and projection neurons in lamina I that normally transmits information about noxious stimuli to higher centers in the CNS. Evidence that peripheral nerve injury causes a disruption of inhibition was subsequently demonstrated and shown to be, at least in part, due to a loss of Cl− gradient in lamina I neurons associated with accumulation of activated microglia.9,10 Effective inhibition mediated by both GABAA and glycine receptors depends on a strong Cl− gradient and thus loss of the gradient diminishes inhibition.
Similarly, in vitro experiments have shown a strong impact of dorsal horn inhibition on excitatory synaptic drive from low-threshold peripheral fibers onto neurons in lamina I and II, a region where high-threshold fibers normally dominate. Disinhibition in vitro strongly enhances low-threshold activation of lamina II neurons,11 demonstrating the importance of local inhibition for suppressing low-threshold drive. More recently, we have specifically tested whether pharmacological disinhibition is able to allow strong, low-threshold drive of lamina I output neurons.4
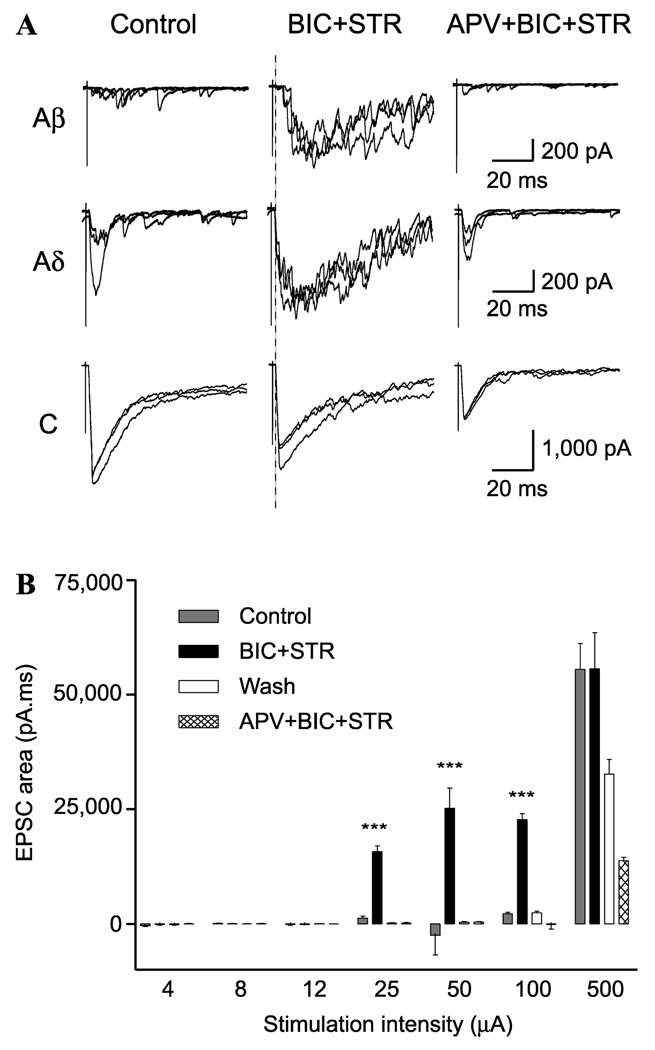
We took advantage of the fact that many projection neurons in lamina I express receptors for substance P, NK1 receptors.12 Spinal cord slices were incubated with tetramethylrhodamine-conjugated substance P (TMR-SP) to label NK1 receptor positive (NK1R+) neurons before recording dorsal-root evoked excitatory postsynaptic currents (EPSCs). Under control conditions, lamina I NK1R+ neurons were shown to receive predominantly high-threshold (Aδ/C fiber) monosynaptic input. In the example shown in Figure 1A (left column), the NK1R+ neuron received polysynaptic Aδ fiber and monosynaptic C fiber input.4 However, blockade of local GABAergic and glycinergic inhibition with bicuculline (10 µM) and strychnine (300 nM) revealed significant A fiber input to the lamina I NK1R+ neuron that was predominantly Aβ fiber mediated (Fig. 1A, middle column). The total integrated current of EPSCs evoked using Aβ (25 µA) and Aδ (100 µA), but not C fiber (500 µA) stimulation intensities were significantly increased after disinhibition (Fig. 1B). This novel Aβ fiber input was identified as polysynaptic in nature because the input showed both failures and substantial variability of latency when recorded at high-stimulation frequencies (not shown). These results suggest the presence of an excitatory, polysynaptic pathway between low-threshold afferents and nociceptive NK1R+ projection neurons that is normally suppressed by inhibition.
Figure 1.
Disinhibition reveals polysynaptic Aβ fiber input to lamina I NK1R+ neurons.4 (A, B) Show data from a neuron with C fiber monosynaptic input that has polysynaptic Aβ fiber input revealed during disinhibition. (A, left column) Example of EPSCs evoked by stimulation (0.1 ms) using Aβ (25 µA),Aδ (100 µA), and C fiber (500 µA) stimulation intensities at low frequency under control conditions. Each trace comprises three superimposed traces evoked at 0.05 Hz. (Middle column) EPSCs evoked by the same stimulation protocol but in the presence of bicuculline (BIC; 10 µM) and strychnine (STR; 300 nM). (Right column) In the presence of APV (30 µM), BIC, and STR. B, the synaptic response stimulus-intensity profile generated by calculating the total EPSC area under the curve from the artifact to the end of the recording (900 ms) for each of the three EPSCs at each intensity tested and for all conditions.
The polysynaptic excitatory pathway revealed by disinhibition was critically dependent upon NMDA receptor activation as shown in Figure 1A (right column). All of the novel polysynaptic activity that was activated in the presence of bicuculline and strychnine was inhibited by the NMDA selective receptor antagonist, APV (30 µM). In the presence of APV, the synaptic activity observed in the absence of bicuculline and strychnine are still apparent even though the newly revealed polysynaptic activity is blocked. These data indicate that disinhibition enhances the contribution of NMDA receptor activation to the polysynaptic low-threshold drive.
Subset of inhibitory neurons in dorsal horn receive low-threshold input
Pharmacological disinhibition, mimicking disinhibition associated with nerve injury, allowed us to observe an underlying polysynaptic excitatory pathway in the dorsal horn between low-threshold afferents and lamina I output neurons. This raises the question of how inhibition suppresses this pathway under normal, nonpathological, or nonpharmacologically altered conditions. It is known that about 30% of the neurons in the superficial dorsal horn are immunoreactive for GABA, and glycine coexists in a subpopulation of these neurons, supporting the idea that inhibitory neurons have an important role of in local network activity.13 Normal inhibition of the polysynaptic excitatory pathway acts as a gate, preventing the painful consequences. One way to control such a gate would be to simultaneously have low-threshold synaptic drive of inhibitory neurons that could suppress the existing polysynaptic excitatory pathway between low threshold afferents and NK1R+ projection neurons.
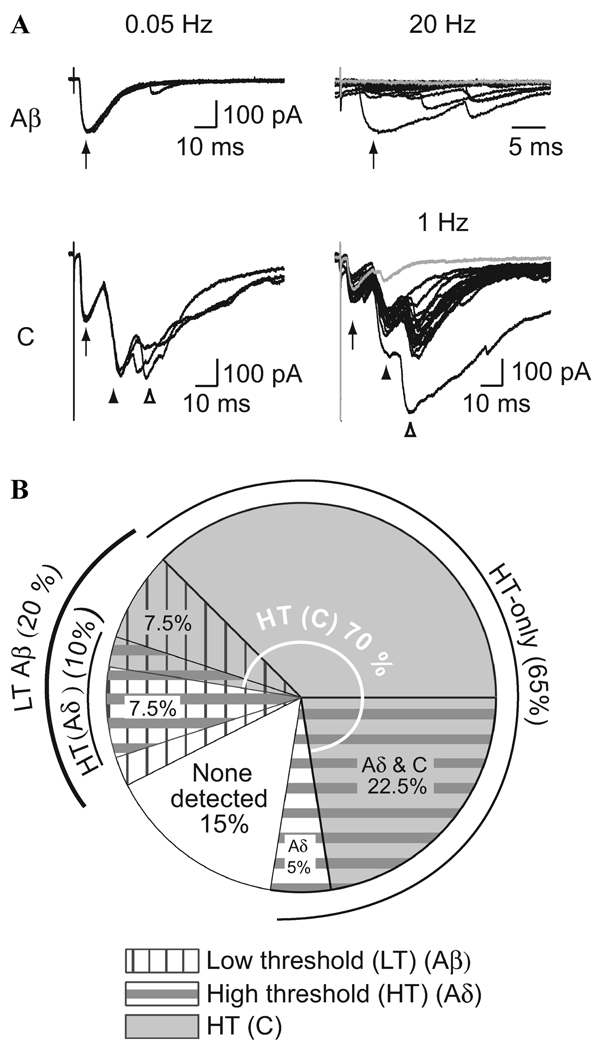
To test for low-threshold synaptic excitation of lamina I and II inhibitory neurons, we used homozygotic transgenic mice that express EGFP under control of the gad1 gene promoter to identify glutamic acid decarboxylase 67 (GAD67) GABAergic neurons. This results in fluorescent labeling of 30–70% of the GABAergic neurons in the dorsal horn.14–16 Recording from these neurons and stimulating the dorsal root to activate low- and high-threshold sensory afferent fibers allowed us to investigate the excitatory synaptic inputs onto GABAergic inhibitory neurons.17 A subclass of EGFP positive GABAergic neurons had low threshold, Aβ fiber input as well as input from high-threshold fibers (Aδ and/or C). For example, the data shown in Figure 2A were recorded from a neuron that received Aβ fiber input identified as polysynaptic due to synaptic failures seen at high-frequency (20 Hz) stimulation (arrows). In addition, this neuron received both monosynaptic (arrowheads) and polysynaptic (open arrowheads) C fiber input.17 Indeed, Aβ fibers activate a significant proportion (~20%) of lamina I and II GABAergic neurons (Fig. 2B). This occurs with similar excitatory synaptic drive throughout postnatal maturation, but with a greater prevalence at younger ages.17
Figure 2.
Low-threshold (Aβ) fiber input as well as input from high-threshold fibers (Aδ and/or C) to GABAergic neurons.17 (A) Example recordings from a P35 GABAergic neuron with input from Aβ and C fibers. (Upper panel) Three consecutive traces show responses to low-frequency Aβ fiber stimulation (left, 0.05 Hz, 25 µA, arrow). Twenty consecutive Aβ fiber responses to high-frequency stimulation (right, 20 Hz, arrow, expanded timescale). (Lower panel) C fiber input was observed when the stimulation intensities were increased (left, 0.05 Hz, 500 µA, arrowheads). The C fiber response had a monosynaptic component with no failures (but note the small amplitude in the gray trace) when tested at high frequency (right, 1 Hz, filled arrowhead). There was also a later, polysynaptic C fiber component with failures (open arrowhead, illustrated by the gray trace). (B) The proportion of GABAergic neurons with input from different afferent fiber classes is summarized. For simplicity, this representation does not distinguish between monosynaptic and polysynaptic responses. There is considerable overlap between the subsets of GABAergic neurons receiving input from each class of afferent fiber type.
These GABAergic neurons receiving low-threshold, primary afferent, synaptic drive are well suited to contribute to suppressing low-threshold activation of output projection neurons. Low-threshold activation of inhibitory neurons is also consistent with the classical idea that nociceptive projection neurons are activated mainly by noxious afferent input that “opens” the gate while low-threshold, non-noxious fibers inhibit this signal and “close” the gate.18 However, nearly all of the inhibitory neurons tested in our studies that received low-threshold input also received high-threshold excitatory drive (Fig. 2B), a combination that is not predicted by the gate theory of pain. It may be that more extensive information about local circuitry considered together with sensory modality will be required to understand the impact these inhibitory neurons have on pain detection.
Glycinergic inhibition at the gate of polysynaptic pathway causing mechanical allodynia
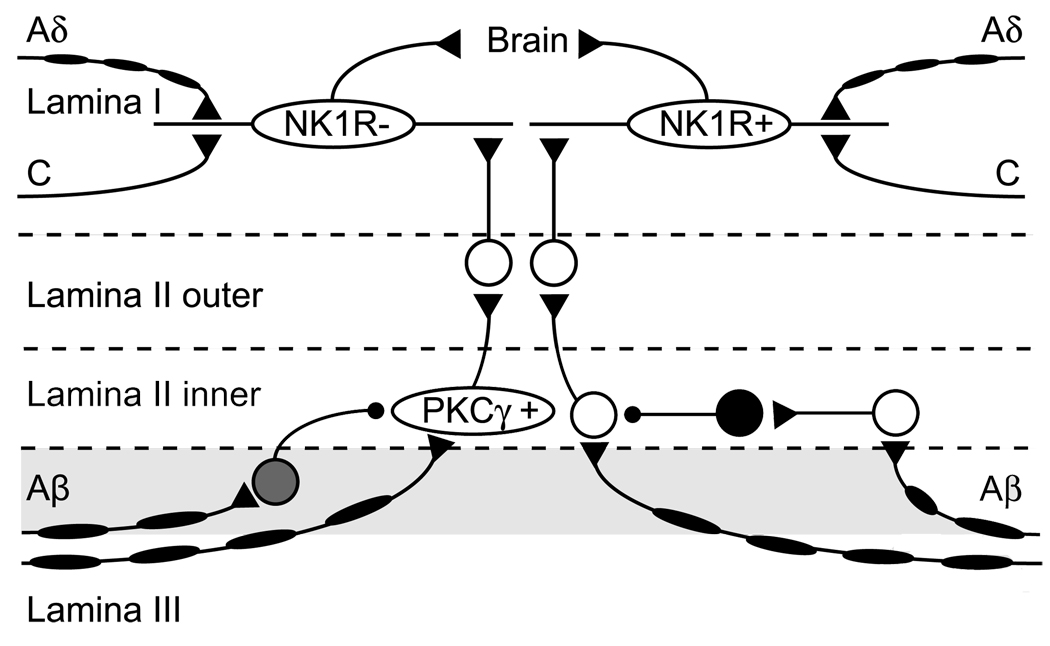
Recent evidence has been accumulating regarding a similar but different polysynaptic pathway that subserves a specific type of allodynia, dynamic mechanical allodynia. This pathway begins in inner lamina II (lamina IIi). In this region of the dorsal horn, there is a population of excitatory interneurons that express the enzyme, PKCγ and that receive low-threshold afferent input.19 These PKCγ+ positive (PKCγ+) neurons are a key element for activated circuits after disinhibition by intrathecal application of glycine receptor antagonist, unmasking normally blocked local excitatory circuits onto nociceptive output neurons.2,3 However, this circuit does not involve lamina I NK1R+ neurons.3 This evidence suggests that dynamic mechanical allodynia is transmitted through a specific pathway that involves nonnociceptive myelinated primary afferents, PKCγ+ neurons as a gateway, and NK1R negative (NK1R−) lamina I projection neurons, as shown in Figure 3. Thus, glycinergic inhibition appears to be a key player to prevent dynamic mechanical allodynia.
Figure 3.
Schematic diagram illustrating two putative dorsal horn neural networks involved in mechanical allodynia. The schematic diagram was drawn based on evidence reported by several groups.1–4,17,19 NK1R+ neurons that receive innocuous input through polysynaptic pathways seem to be part of a local excitatory circuit that mediates mechanical allodynia after loss of GABAergic and glycinergic inhibitory control.4 Inhibitory interneurons in lamina II (black) receiving polysynaptic Aβ fiber input may inhibit neurons that relay non-nociceptive information to NK1R+ neurons.17 Moreover, PKC γ+ neurons that receive innocuous input via Aβ fibers at the lamina II/III border (shaded) are part of a local excitatory circuit that mediates dynamic mechanical allodynia after loss of glycinergic inhibitory control.2 We tentatively put a glycinergic inhibitory interneuron (gray) at the lamina II/III border. Given local inhibitory synaptic connection within same lamina,1 glycinergic inhibitory interneurons may be at lamina II/III border, although their exact location is still unknown. The target of the PKC γ+ neuron appears to be lamina I neurons lacking NK1 receptors.3
What is the source of this glycinergic inhibition? A significant proportion of superficial dorsal horn neurons have immunoreactivity for glycine. In addition, a recent study reported that presynaptic inhibitory neurons, which send inhibitory output onto postsynaptic lamina I–III neurons, are located relatively close to postsynaptic neurons along the dorso-ventral axis, suggesting local inhibitory synaptic connections are mostly made within the same lamina.1 Therefore, we predict that glycinergic inhibitory neurons located near the lamina II/III border may directly inhibit PKCγ+ neurons to close the gate by blocking the pathway activated for dynamic mechanical allodynia. Figure 3 shows a schematic diagram for putative neuron networks causing mechanical allodynia and suppressing it under normal condition (Fig. 3).
Region- and cell type-specific inhibitory control of superficial dorsal horn neurons
Given that the distinct physiological roles of different dorsal horn neurons depend on their location and their neurochemical properties, it is important to determine whether, in fact, dorsal horn neurons receive synaptic inhibition in a region- and/or cell type-specific manner. It has been shown that adult lamina I neurons receive only glycinergic synaptic inhibition, whereas about a half of lamina II neurons received pure GABAergic and the rest received mixed GABAergic and glycinergic inhibition.20 In addition, other studies have focused on cell type-specific inhibition in the dorsal horn. For example, central cells, which are commonly situated in the mid-zone of the lamina II, receive GABAergic inhibition fromislet cells.21 Moreover, inhibitory synaptic connections have been demonstrated between lamina II inhibitory interneurons identified by endogenous EGFP under the control of the GAD65 promoter.22 However, what drives the activity of identified neurons with specific physiological roles in the dorsal horn has still not been well studied. We expect that genetic and/or neurochemical approaches to neuron identification will facilitate investigations of cell type-specific inhibitory control of dorsal horn neurons. This in turn would provide insight into sensory information processing within spinal cord dorsal horn. Interestingly, some lamina II neurons receive tonic GABAergic and glycinergic inhibition, suggesting that superficial dorsal horn neurons are inhibited through two different modes, phasic, and tonic, similar to other CNS regions.23–25 We predict that tonic inhibition may be important in regulating the inhibitory tone in the dorsal horn.
Conclusions
There are several polysynaptic excitatory circuits in the dorsal horn that carry low threshold signals to lamina I output neurons. The inhibition of these pathways is critically important to avoid pathological pain. This focuses attention on the mechanisms by which inhibition functions within the dorsal horn.
Acknowledgment
This study was supported by NIH NS 029797.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kato G, et al. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J. Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miraucourt LS, et al. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor- independent mechanical allodynia. J. Neurosci. 2009;29:2519–2527. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J. Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller AF, et al. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer C, Roberts LA, Komisaruk BR. Hyperalgesia induced by altered glycinergic activity at the spinal cord. Life Sci. 1985;37:875–882. doi: 10.1016/0024-3205(85)90523-5. [DOI] [PubMed] [Google Scholar]
- 7.Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- 8.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 9.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 10.Coull JA, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 11.Baba H, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 12.Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur. J. Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 13.Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J. Comp. Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty KJ, Sawchuk MA, Hochman S. Properties of mouse spinal lamina IGABAergic interneurons. J. Neurophysiol. 2005;94:3221–3227. doi: 10.1152/jn.00184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty KJ, Sawchuk MA, Hochman S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience. 2009;163:909–919. doi: 10.1016/j.neuroscience.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinke B, et al. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J. Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J. Neurosci. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzack R, Wall PD. Pain mechanisms: a new theory. Science (New York, N.Y.) 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 19.Neumann S, et al. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J.Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller AF, et al. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I–II of the rat spinal dorsal horn. J. Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J. Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrakakis C, et al. Inhibitory coupling between inhibitory interneurons in the spinal cord dorsal horn. Mol. Pain. 2009;5:24. doi: 10.1186/1744-8069-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol. Pain. 2006;2:36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi A, Mashimo T, Uchida I. GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport. 2006;17:1331–1335. doi: 10.1097/01.wnr.0000230515.86090.bc. [DOI] [PubMed] [Google Scholar]
- 25.Takazawa T, MacDermott AB. Disinhibition mediated by tonic activation of GABAergic and glycinergic receptors on inhibitory interneurons in mouse superficial dorsal horn. 2008 SFN Abstracts771.778. [Google Scholar]