Abstract
OBJECTIVE
To determine whether maternal intrauterine endotoxin administration leads to neurobehavioral deficits in newborn rabbits.
STUDY DESIGN
Pregnant New Zealand white rabbits were injected with 1ml saline (n=8) or 20μg/kg of lipolysaccharide in saline (LPS) (n=8) into the uterine wall on day 28/31 of gestation. On postnatal day 1, kits [saline (n=30) and LPS (n=18) from 4 consecutive litters] underwent neurobehavioral testing. Neonatal brains were stained for microglial cells and myelin.
RESULTS
LPS-group kits were hypertonic and demonstrated significant impairment in posture, righting reflex, locomotion and feeding, along with neuroinflammation indicated by activated microglia, and hypomyelination in the periventricular regions. A greater mortality was noted in the LPS group (16 stillbirths from 3 litters vs. 3 from 1 litter).
CONCLUSION
Maternal intrauterine endotoxin administration leads to white matter injury and motor deficits in the newborn rabbit resulting in a phenotype that resembles those found in periventricular leukomalacia and cerebral palsy.
Keywords: Cerebral palsy, intrauterine inflammation, microglia, perinatal brain injury
INTRODUCTION
Cerebral palsy (CP) describes a group of disorders of the development of movement and posture, that are attributed to non-progressive disturbances in the developing fetal or infant brain.1,2 A number of clinical studies have shown a link between CP, intrauterine infection, perinatal brain injury and specifically periventricular leukomalacia (PVL).3–5 The fetal inflammatory response syndrome induced by intrauterine infection has been implicated in the development of brain injury and CP in the neonate. Elevated concentrations of proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) in amniotic fluid, umbilical cord plasma or neonatal serum have been associated with PVL and long-term neurodevelopmental impairment.6–9 These cytokines may affect the fetal brain directly by a toxic effect on immature oligodendrocytes or indirectly by activation of microglia resulting in white matter injury.10
In recent years, in vitro and in vivo studies have implicated activated microglia in the pathogenesis of PVL and development of white matter injury. Autopsy specimens of patients with PVL have shown an increase in the density of activated microglia diffusely throughout the brain, along with increased expression of reactive oxygen and nitrogen species, that may lead to white matter injury.11 Proinflammatory cytokines have been demonstrated in microglial cells and astrocytes in areas of coagulation necrosis in neonatal brain specimens with PVL.12
Although epidemiological studies have shown a link between chorioamnionitis and CP, a suitable animal model that demonstrates a phenotype of CP with motor deficits following intrauterine inflammation is crucial for understanding the mechanisms involved in brain injury due to inflammation. Animal models of maternal-fetal inflammation using an endotoxin such as lipopolysaccharide (LPS) as the inflammatory stimulus has been shown to induce white matter damage in the offspring; however, its effect on motor or behavioral outcomes in the neonatal period is not clear. 13–15 We have previously shown that intrauterine injection of endotoxin near-term leads to neuroinflammation as indicated by microglial activation in the neonatal brain in a dose dependent manner that can be detected by Positron Emission Tomography (PET) using the tracer 11C-(R)-PK11195.16 In this study, we hypothesized that maternal intrauterine injection of endotoxin near-term, leads to the development of neurobehavioral deficits in newborn rabbits along with neuroinflammation as evidenced by the presence of activated microglial cells that is predominantly localized to the periventricular white matter regions in the neonatal rabbit brain.
MATERIALS AND METHODS
Animal Model
All the animal experimental procedures were approved by the Animal Investigation Committee of Wayne State University. The details of the surgical procedures were described previously by our group.16 Briefly, New Zealand White rabbits (CoVance Research Products Inc., Kalamazoo, MI) with timed pregnancies that were confirmed breeders with a history of delivering 7–11 kits per litter, underwent laparotomy under general anesthesia (2–3% isoflurane by mask) on gestational day 28 (E28, term pregnancy is 31–32 days). One mL of saline for the control group (n=8) or 1mL of saline containing 20μg/kg of LPS (Escherichia Coli serotype O127:B8) (Sigma-Aldrich, St Louis, MO) for the endotoxin group (n=8), was equally divided and injected into the uterine wall using a 27 gauge needle between the fetuses taking care not to enter the amniotic sac. This mode of LPS administration was used in order to limit the inflammation to the uterus and to decrease the variability in response between the kits. The dose of LPS was chosen based on our previous study where comparison of 20, 30 and 40μg/kg dose of LPS demonstrated that substantial microglial activation in the neonatal brain with the least fetal mortality and without any maternal mortality or preterm delivery was noted at a dose of 20μg/kg.16 Heart rate, oxygen saturations, rectal temperature and arterial blood pressure were monitored continuously during the procedure. Maternal serum was collected before laparotomy (0 hours) and at 2, 6, 24 and 48 hours following endotoxin injection. Presence of maternal inflammation was determined by measuring C-reactive protein (CRP) levels in the maternal serum over time using a rabbit-specific CRP sandwich ELISA (ALPCO, Windham, NH). The dams were monitored daily for changes in activity, feeding and fever. The dams were monitored continuously using a surveillance camera to determine the time of delivery. All kits were born spontaneously at 31 days gestational age. The number of live and dead kits, and weight of all live kits was recorded.
Behavioral testing
All live postnatal day 1 (PND1) control and endotoxin kits from four consecutive litters in each group were tested in order to reduce the risk of selection bias. The rabbit kits were assessed and scored for behavioral testing, as described by Derrick et al.17 Briefly, the kits were videotaped and scored on a scale of 0 (worst)-3 (best) by two blinded observers for (1) posture (ability to maintain prone posture), (2) righting reflex (ability to right itself from supine to prone position for ten attempts), (3) Activity and locomotion on a flat surface (assessed by grading the quality, intensity and duration of spontaneous movement of the head, front and back legs), (4) ability to move in a straight line and in circles, (5) co-ordination of suck and swallow assessed by feeding the rabbit kits artificially with formula from a syringe with a dropper and (6) ability to move head during feeding (scored from 0–3 where 0 is no movement of head and 3 is forceful movement of head and body). The tone on passive flexion and extension was assessed using the scoring based on Ashworth scale as described by Derrick et al.17 where 0 indicated no increase in tone and 4 where the limb is rigid in flexion or extension.
Immunohistochemistry
Under deep anesthesia, all PND1 rabbit kits were perfused with 4% paraformaldehyde, and brains fixed, cryoprotected and frozen at −80°C. Seven brains from 5 different control-litters and 6 brains from 5 endotoxin-litters were randomly selected for immunohistochemistry. Thirty μm thick coronal brain sections were cut using a cryostat (Leica Microsystems; Nuchloss, Germany) and mounted on poly-L-lysine coated slides (Sigma-Aldrich, St Louis, MO).
For staining, the slides were covered with biotinylated lycopersicon esculentum tomato lectin (1:100) (Vector Laboratories, Burlingame, CA) for microglia for 1 hour, or with mouse monoclonal anti-myelin basic protein (MBP, 1/250) (Chemicon International, Temecula, CA) overnight followed by biotinylated goat anti-mouse IgG (1:300) (Vector Laboratories, Burlingame, CA) for 2 hours for myelin. Avidin binding was done using Vectastain ABC kit and color developed using 3,3′-diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA). Slides were dehydrated using ethanol, cleared in xylene. and mounted. Images were taken using a Leica DM2500 microscope (Leica Microsystems; Nuchloss, Germany) equipped with a camera.
Statistical Analysis
Prior to the analyses, the distributions of all outcomes were examined and all data were checked for accuracy, and for outliers. The variance of CRP was heterogeneous, increasing across time. A log base 10 transformation was applied to stabilize the variance and the log transformed scores were used for the analyses. Duration of surgery between the groups was determined using T-Test. Due to the lack of independence in the data (kits are nested within litters), Generalized Estimating Equations (GEE) were used to examine differences between the kits in the endotoxin and control groups. For variables that were normally distributed, a normal distribution with identity link was used. Survival, which is a dichotomous variable, was modeled using a binomial distribution and log link. When reported, the means and 95% confidence intervals (CI) for each were obtained from the GEE analysis.
RESULTS
A total of 8 dams in each group underwent laparotomy. There was no significant difference in the duration of surgery between both groups (mean ± SD was 50.5 ± 8.7 min for the control and 53.8 ± 5.4 min for the endotoxin exposed group; p=0.38). There was no maternal mortality noted at this dose of endotoxin injected. No increase in temperature from baseline was noted in the post-surgical period in the dams. An increase in the CRP over time was noted in both groups with a greater increase in the endotoxin dams at 24 and 48 hours when compared to the controls (p=0.04) (Figure 1). All the dams delivered at term. There were 76 kits in the control group and 66 kits in the endotoxin group. Three kits from one litter in the control group and 16 kits from 3 litters in the endotoxin group were stillborn (p=0.08 for mortality between the groups). Even though more endotoxin kits were stillborn, this did not reach statistical significance because they were confined to 3 litters. There was a difference in birth weight between the groups, with the endotoxin kits having a lower birth weight than the saline kits [(mean with (95% CI) was 40.20g (35.86–44.55g) for the endotoxin and 46.15g (42.04–50.26g) for the control kits; p=0.05)].
FIGURE 1. C-reactive protein in maternal serum.
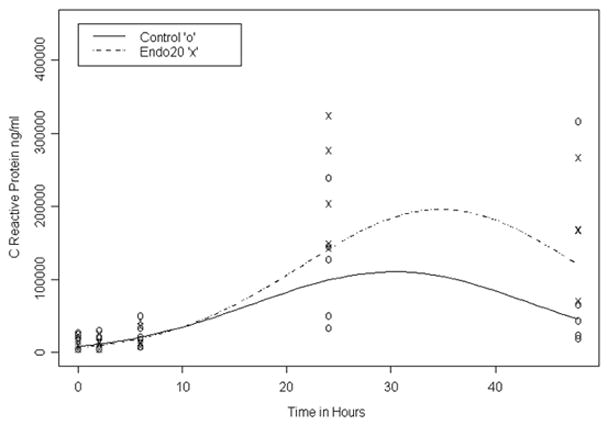
Maternal CRP was measured at baseline (0 hours which is just before start of the laparotomy), and at 2, 6, 24 and 48 hours after administration of endotoxin or saline in utero. An increase in CRP over time was noted at 24 and 48 hours in both groups from baseline values. This increase was slightly higher in the endotoxin dams when compared to saline indicating presence of a systemic inflammation after intrauterine endotoxin administration.
Neurobehavioral Testing
Neurobehavioral testing and scoring was done on all live kits from 4 consecutive litters in each group, resulting in 30 kits in the control group and 18 kits in the endotoxin group. There was a significant difference in the behavioral phenotype between the two groups, with the endotoxin kits showing increased tone with decreased motor activity and locomotion (Videos 1 and 2). The endotoxin kits were unable to consistently maintain a prone position with impaired righting reflex (p<0.005) (Figure 2A). Tone was significantly increased in the endotoxin kits with spasticity and postural abnormalities (p<0.05) (Figures 2B and 3). The intensity, quality and duration of movements of the head, forelimbs and hindlimbs were decreased in the endotoxin kits (p<0.005) (Figure 2C). Newborn rabbits normally demonstrate circular motion and forward movement along with abundant jumpy, jerky movements which were not observed in the endotoxin kits (p<0.005) (Figure 2D). Co-ordination of sucking and swallowing was impaired in the endotoxin kits with decreased head movements while feeding (p<0.005) (Figure 2E).
FIGURE 2. Neurobehavioral scoring of control and endotoxin groups.
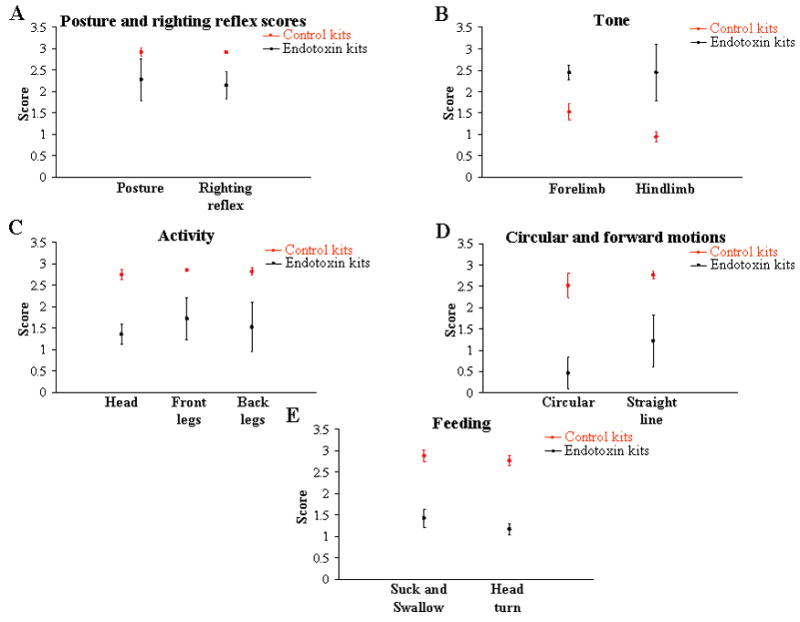
Activity of the control and endotoxin rabbit kits on postnatal day 1 was videotaped and scored by two independent observers. The graphs depict the mean and 95% confidence interval for each score. Compared to the controls, the endotoxin kits (A) were not able to maintain prone position and right themselves rapidly and consistently (B) were hypertonic; (C) showed decreased activity; (D) demonstrated decreased circular and forward motion; and (E) showed a decrease in co-ordination of suck and swallow leading to dribbling of formula from the mouth along with a decrease in normal jerky, reflexive head movement while feeding.
FIGURE 3. Spastic posturing of the extremities in endotoxin when compared to control kits.
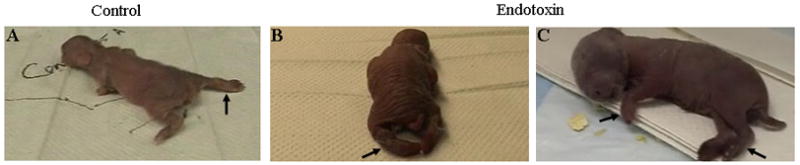
Extremities are normally abducted in the control kit (A), however they are rigid and have fixed abnormal positions in the endotoxin kits (B and C) due to the hypertonia resulting in impairment in locomotion..
Immunohistochemistry
The presence of activated microglial cells was demonstrated on histology by tomato lectin staining (Figure 4). Robust microglial activation was noted in the endotoxin kits when compared to the controls, as evidenced by an increase in density and change in morphology to a less ramified, activated form of the microglial cells. This was observed in the corpus callosum, along the inferior border and angle of the lateral ventricle, in the region of the internal capsule and in the fimbria hippocampus. The presence of microglial cells was specific to these regions with relative sparing of the cortex. Some of the kits were sacrificed at PND 8 and brains stained for the presence of myelin. A decrease in intensity of MBP immunoreactivity was noted in these regions in the 8 day old kits (Figure 5).
FIGURE 4. Microglial cell staining.
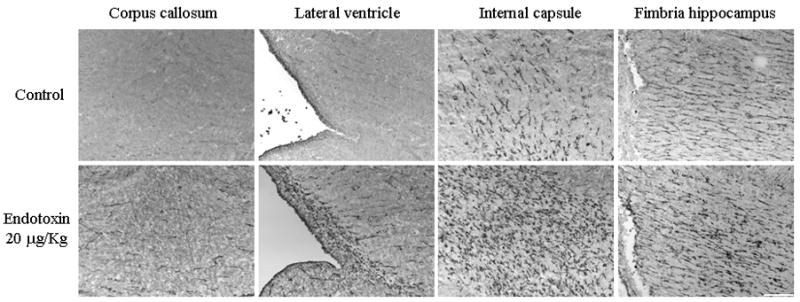
Brain sections from post-natal day 1 control and endotoxin rabbits were stained for microglial cells using tomato lectin. An increase in activated microglial cells is noted in the endotoxin treated kits when compared to the control kits in the regions of the corpus callosum, along the borders of the lateral ventricle, region of the internal capsule and the fimbria hippocampus. Scale bar is 200 μm.
FIGURE 5. Myelin basic protein staining.
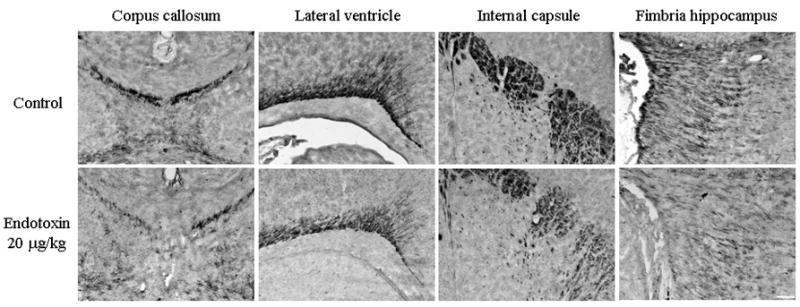
Myelin basic protein staining in a representative postnatal day 8 control and endotoxin kits. Decreased myelination is seen in the kits exposed to maternal inflammation in utero when compared to the saline exposed control kits in the corpus callosum, around the lateral ventricle, internal capsule and the fimbria hippocampus. Scale bar is 200 μm.
COMMENT
The primary findings of this study are: 1) Intrauterine administration of LPS near term gestation results in a phenotype which resembles cerebral palsy; 2) The model described herein is unique because, maternal endotoxin administration resulted in motor deficits in the newborn rabbit, without spontaneous preterm labor and delivery, thus this model has implications for the effect of infection near term gestation; 3) An increased incidence of stillbirth at term that was litter specific (16 deaths from 3 litters for the endotoxin and 3 from 1 litter for the control) with a lower birth weight in the endotoxin kits was noted. We propose that this model will be helpful in determining the pathways involved in perinatal infection/inflammation induced white matter injury and in exploring therapeutic options.
In this study, CRP, an acute phase protein that is produced by hepatocytes in response to proinflammatory cytokines such as IL-6 and IL-1,18,19 was used as a marker of maternal systemic inflammation. Although there are conflicting reports concerning its value, CRP has been proposed as a sensitive clinical test in predicting perinatal infection and PVL.20–23 In our model, a modest increase in the CRP levels indicating the presence of some degree of systemic maternal inflammation was observed following intrauterine endotoxin administration, inspite of the absence of systemic signs such as an increase in temperature, maternal mortality or preterm delivery.
New Zealand white rabbits were chosen for this study due to the similarity in their brain development to that of the human brain. Myelination in rabbits occurs in the perinatal period with rapid changes in immature oligodendrocyte density occurring between E29 to PND5. Myelination begins in the internal capsule at PND5 and in the corpus callosum at PND11.24 (Figure 6). In humans, immature oligodendrocytes increase rapidly in numbers in the third trimester in the white matter,25 myelination begins in late fetal development and early postnatal period, and is more active during first year after term.26,27 In contrast, myelination begins postnatally in rats and mice28 and the maturation of white matter at PND7 is equivalent to that of preterm infants between 30 and 36 weeks. At PND14 the abundance of mature oligodendrocytes seen in the periventricular white matter is similar to the myelination seen in many full-term infants,29 hence in these species testing would be more appropriate at a later date. Since myelination in rabbits starts around term, rabbits may be more appropriate models for demonstrating neonatal brain injury from insults occurring in the perinatal period.30 Immature locomotor function is demonstrated by the newborn rabbit enabling assessment of motor activity as early as PND1 even though myelination is not complete at this age.30
FIGURE 6. Presence of microglial cells in rabbit white matter tracts during development and its relationship to oligodendrocyte maturation.
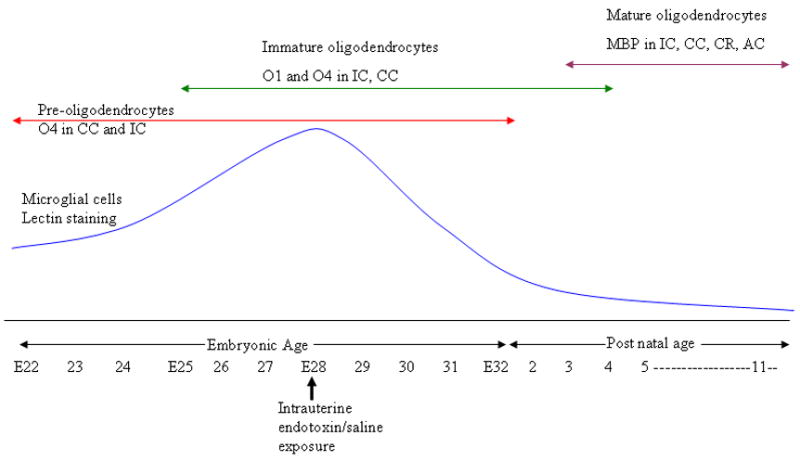
This schematic depicts the time course of microglia and oligodendrocyte density during normal rabbit brain development. Embryonic age 28 was chosen as the time of insult in this study as this corresponds with a normal peak in the density of microglial cells in the periventricular white matter tracts as well as a period of rapid increase in the density of immature oligodendrocytes. Corpus callosum (CC), internal capsule (IC), corona radiata (CR) and anterior commissure (AC).
In rabbits, amoeboid shaped microglial cells are detected in white matter tracts at 22 days gestational age as part of normal development, peaking around 28 days and decreasing in density by PND4.31 This corresponds to the time when there is a rapid increase in immature oligodendrocytes in the rabbit brain. Hence, in this study E28 was chosen as the time of intrauterine endotoxin administration (Figure 6). An ongoing insult to the brain around this time resulting in the proliferation of activated microglial cells may cause injury to the surrounding oligodendrocytes and subsequent hypomyelination.
Animal models of microbial-induced brain injury have shown neuropathological changes in the fetal brain suggestive of PVL and white matter injury secondary to intrauterine inflammation or maternal endotoxin administration. In these models, histologic changes have not consistently correlated with a phenotype of CP in the newborn.13,32,33 Maternal endotoxin administration has been produced using LPS, by instillation in the cervix,13 by intraperitoneal injection32,33 or intrauterine infusion.16,34 In some of these models the incidence of preterm delivery and fetal or maternal mortality have been high.32,34 Other rodent models using lower doses of LPS have demonstrated improved survival with hypomelination, presence of pro-inflammatory cytokines, gliosis and increased programmed cell death in the progeny postnatally13,33 but without a phenotype of CP.14 Some investigators showed that a single exposure to intracervical LPS in pregnant rats did not result in motor deficits; however, multiple exposures later in gestation resulted in a delay in the development of some of the milestones in the surviving pups.14,15 The authors hypothesized that the difference in response between their two models was probably due to the timing of LPS injection in relationship to the fetal brain development. The variable fetal responses among the different animal models appear to be related to the gestational age and fetal brain development at the time of maternal LPS exposure for that specific species. It is possible that our model led to a phenotype of CP because the time period of LPS injection corresponded with the time of maximal microglial cell presence in the white matter tracts in the rabbit brain.31
Using a rabbit model of intra-uterine hypoxic-ischemic injury, Tan et al.30 demonstrated that kits subjected to hypoxia at E22 and E25 were born with hypertonia and a phenotype of CP. This was felt to be due to the relative vulnerability of preoligodendrocytes that are present in large numbers at E25 to hypoxic-ischemia when compared to the immature and mature oligodendrocytes that are more predominant near term.30,35 However in our model, maternal intrauterine endotoxin injection at E28 led to significant hypertonia and white matter injury in the newborn rabbits. We hypothesize that this may be related to the fact that maternal intrauterine inflammation secondary to endotoxin administration causes microglial activation in the fetal brain which in turn may induce injury to the surrounding cells. Since, the microglial cells are localized in the white matter tracts in rabbits at this gestational age (28/31 days), the injury is predominantly localized to the periventricular white matter tracts leading to hypomyelination and neuronal loss in these regions. This hypothesis is supported by other in vitro studies using co-cultures of microglia and oligodendrocytes or neuronal cells, which have shown that the presence of activated microglia is required for oligodendrocyte death and that the activated microglia release agents such as NO, glutamate and MMP-9 that mediate oligodendrocyte and neuronal toxicity.36–38 Hence, it is possible that in the hypoxic-ischemia model there may be primary injury to oligodendrocytes and neuronal cells followed by secondary microglial activation, whereas with endotoxin administration the primary event may be microglial activation followed by secondary injury to surrounding oligodendrocytes and neuronal cells.
Our study had certain limitations. We evaluated myelination at PND8 which may be too early to get an accurate assessment of the degree of permanent white matter injury since myelination is not yet completed. Immunohistochemical techniques to evaluate axonal integrity along with myelin organization and thickness at various time points upto adulthood may be more helpful in accurately evaluating the extent of injury.
In summary, we have developed an animal model of cerebral palsy induced by intrauterine endotoxin administration. It appears that the gestational age at the time of exposure to the inflammatory stimulus in relation to the presence of microglia in the white matter tracts and the development of oligodendrocytes may determine the response in the neonate. In humans, microglial cells are prominent during fetal life and are found in increased density in regions of white matter tracts in late gestation decreasing around term.39 Hence, the presence and degree of white matter injury in fetuses exposed to chorioamnionitis may vary with the timing of the infection. Additional studies are required to demonstrate mechanisms by which activation of microglial cells induced by intrauterine inflammation results in PVL in the neonate.
Supplementary Material
The control kit demonstrates typical jumpy, jerky movements. Multiple vigorous, jerky movements of the head and limbs are also observed. The kit is able to maintain a prone posture and is able to rapidly right itself from supine to prone position. The kit also clearly demonstrates multiple circular movements. While feeding, good co-ordination of sucking and swallowing is noted with vigorous head turns and jerks.
The endotoxin kit 1 is unable to right itself to a prone position due to spasticity of the limbs. The hindlimbs are held rigid in extension and the kit is unable to abduct them. Though Kit 2 appears less affected since it is able to maintain a prone position and appears to have a more rapid righting reflex, the locomotion is severely impaired. This kit does not demonstrate circular movements and is not able to normally abduct the hindlimbs while walking, due to the hypertonia. Feeding is affected due to poor co-ordination of suck and swallow leading to dribbling of formula out of the mouth. The jerky head turns while feeding are also not observed as seen in the control.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS, and the Pediatric Critical Care Scientist Development Program, NICHD.
Footnotes
Presented at the 37th annual meeting of the Society for Neuroscience, San Diego, CA, Nov. 3–7, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 2.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–31. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 3.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–11. [PubMed] [Google Scholar]
- 5.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 7.Goepfert AR, Andrews WW, Carlo W, et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–81. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 8.Heep A, Behrendt D, Nitsch P, Fimmers R, Bartmann P, Dembinski J. Increased serum levels of interleukin 6 are associated with severe intraventricular haemorrhage in extremely premature infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:501–4. doi: 10.1136/fn.88.6.F501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–17. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 10.Redline RW. Infections and other inflammatory conditions. Semin Diagn Pathol. 2007;24:5–13. doi: 10.1053/j.semdp.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventrucular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110:124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 13.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–9. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 14.Poggi SH, Park J, Toso L, et al. No phenotype associated with established lipopolysaccharide model for cerebral palsy. Am J Obstet Gynecol. 2005;192:727–33. doi: 10.1016/j.ajog.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 15.Toso L, Poggi S, Park J, et al. Inflammatory-mediated model of cerebral palsy with developmental sequelae. Am J Obstet Gynecol. 2005;193:933–41. doi: 10.1016/j.ajog.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 16.Kannan S, Saadani-Makki F, Muzik O, et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48:946–54. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- 17.Derrick M, Luo NL, Bregman JC, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–86. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 19.Yap SH, Moshage HJ, Hazenberg BP, et al. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991;1091:405–8. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- 20.Romem Y, Artal R. C-reactive protein as a predictor for chorioamnionitis in cases of premature rupture of the membranes. Am J Obstet Gynecol. 1984;150:546–50. doi: 10.1016/s0002-9378(84)90437-x. [DOI] [PubMed] [Google Scholar]
- 21.Chen SU, Ko TM, Hwa HL, Lu PJ, Ho HN, Yang YS. Maternal serum C-reactive protein level does not change significantly after fetal reduction: it could be used as an indicator of chorioamnionitis. J Assist Reprod Genet. 2001;18:336–40. doi: 10.1023/A:1016684605522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gojnic M, Fazlagic A, Pervulov M, Petkovic S, Mostic T, Jeremic K. The significance of C-reactive protein in the diagnosis of fetal tachycardia and therapy of chorioamnionitis. Clin Exp Obstet Gynecol. 2005;32:114–6. [PubMed] [Google Scholar]
- 23.Skrablin S, Lovric H, Banovic V, Kralik S, Dijakovic A, Kalafatic D. Maternal plasma interleukin-6, interleukin-1beta and C-reactive protein as indicators of tocolysis failure and neonatal outcome after preterm delivery. J Matern Fetal Neonatal Med. 2007;20:335–41. doi: 10.1080/14767050701227877. [DOI] [PubMed] [Google Scholar]
- 24.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–97. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–31. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: What is happening when? Early Hum Dev. 2006;82:257–66. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrand C, Remahl S, Persson H, Bjartman C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40:319–84. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- 29.Craig A, Luo NL, Beardsley DJ, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 30.Tan S, Drobyshevsky A, Jilling T, et al. Model of cerebral palsy in the perinatal rabbit. J Child Neurol. 2005;20:972–9. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- 31.Bass WT, Singer GA, Liuzzi FJ. Transient lectin binding by white matter tract border zone microglia in the foetal rabbit brain. Histochem J. 1998;30:657–66. doi: 10.1023/a:1003597010707. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Rousset CI, Chalon S, Cantagrel S, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–33. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 34.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–11. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reprod Biol. 1990;35:29–33. doi: 10.1016/0028-2243(90)90139-r. [DOI] [PubMed] [Google Scholar]
- 36.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–96. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- 38.Domercq M, Sánchez-Gómez MV, Sherwin C, Etxebarria E, Fern R, Matute C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol. 2007;178:6549–56. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- 39.Billiards SS, Haynes RL, Folkerth RD, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The control kit demonstrates typical jumpy, jerky movements. Multiple vigorous, jerky movements of the head and limbs are also observed. The kit is able to maintain a prone posture and is able to rapidly right itself from supine to prone position. The kit also clearly demonstrates multiple circular movements. While feeding, good co-ordination of sucking and swallowing is noted with vigorous head turns and jerks.
The endotoxin kit 1 is unable to right itself to a prone position due to spasticity of the limbs. The hindlimbs are held rigid in extension and the kit is unable to abduct them. Though Kit 2 appears less affected since it is able to maintain a prone position and appears to have a more rapid righting reflex, the locomotion is severely impaired. This kit does not demonstrate circular movements and is not able to normally abduct the hindlimbs while walking, due to the hypertonia. Feeding is affected due to poor co-ordination of suck and swallow leading to dribbling of formula out of the mouth. The jerky head turns while feeding are also not observed as seen in the control.


