Abstract
Incorporation of the anti-CD20 monoclonal antibody rituximab into front-line regimens for diffuse large B-cell lymphoma (DLBCL) has resulted in improved survival. Despite this progress, many patients develop refractory or recurrent DLBCL and then receive autologous hematopoietic stem cell transplantation (AuHCT). It is unclear to what extent pre-transplant exposure to rituximab affects outcomes following AuHCT. Outcomes of 994 patients receiving AuHCT for DLBCL between 1996 and 2003 were analyzed according to whether rituximab was (n=176, “+R” group) or was not (n=818, “ −R” group) administered with front-line or salvage therapy prior to AuHCT. The +R group had superior progression-free survival (50% versus 38%, p=0.008) and overall survival (57% versus 45%, p=0.006) at 3 years. Platelet and neutrophil engraftment were not affected by exposure to rituximab. Non-relapse mortality (NRM) did not differ significantly between the +R and −R groups. In multivariate analysis, the +R group had improved progression-free survival (relative risk of relapse/progression or death 0.64, p<0.001) and improved overall survival (relative risk of death of 0.74, p=0.039). We conclude that pre-transplant rituximab is associated with a lower rate of progression and improved survival following AuHCT for DLBCL, with no evidence of impaired engraftment or increased NRM.
Key words or short phrases: autologous hematopoietic stem cell transplantation, lymphoma, rituximab
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin lymphoma (NHL). The probability of being cured by the initial treatment is predicted by the International Prognostic Index (IPI), which accounts for age, performance status, tumor stage, lactate dehydrogenase level (LDH) and the number of sites of extranodal disease as prognostic variables.(1) Prior to the introduction of rituximab, the probability of long-term survival ranged between 26 and 73%, depending on the IPI.(1) With the addition of rituximab to standard frontline chemotherapy, outcomes have improved across all IPI groups.(2–5)
For patients with relapsed, chemosensitive DLBCL, the Parma trial established high-dose chemotherapy and AuHCT as superior to conventional salvage chemotherapy alone.(6) However, this study was carried out in the pre-rituximab era, making its relevance in DLBCL patients treated with rituximab-containing frontline or salvage regimens uncertain. It has been reported that pre-transplant rituximab exposure may affect outcomes following high-dose therapy and AuHCT. For example, in one single-center retrospective study, inclusion of rituximab in pre-transplant salvage therapy was associated with improved survival and delayed platelet engraftment, in patients with intermediate-grade B-cell non-Hodgkin lymphoma undergoing AuHCT.(7)
We hypothesized that pre-transplant exposure to rituximab may affect outcomes following AuHCT for DLBCL, including relapse/progression, survival, toxicity, and engraftment. Utilizing the Center for International Blood and Transplantation (CIBMTR) database, we retrospectively compared outcomes for adult patients undergoing autotransplant for large cell lymphoma, in rituximab naïve (−R) and rituximab exposed (+R) patients.
PATIENTS AND METHODS
Data Sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee or the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all consecutive transplants; compliance is monitored by on-site audits. Subjects are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
The CIBMTR collects data at two levels: registration and research. Registration data include disease type, age, sex, pretransplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. Requests for data on progression or death for registered patients are at six-month intervals. All CIBMTR teams contribute Registration data. Research data are collected on a subset of registered patients selected using a weighted randomization scheme and include detailed disease, and pre- and post-transplant clinical information.
Patients
A total of 1155 patients who underwent autologous transplantation for DLBCL between 1996 and 2003 were reported to the CIBMTR database. Eight patients with age at transplant <18 years and 11 patients with post-transplant rituximab for maintenance were excluded. Seventy-four patients who relapsed >10 years after initial diagnosis and 56 patients with bone marrow grafts were excluded. Twelve patients who received rituximab with the conditioning regimen were also excluded. A total of 994 patients were then analyzed. 176 received rituximab prior to transplant, either during first-line therapy and/or during salvage therapy, while 818 patients were rituximab naïve at the time of transplant.
Study Endpoints
Outcomes analyzed included engraftment, non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS). NRM was defined as death within 28 days post-transplant or death without lymphoma progression. Subjects with lymphoma progression were censored at the time of progression and a cumulative incidence estimate was derived with progression or relapse as the competing risk. Progression/relapse was defined as progressive lymphoma post-transplant (≥28 d) or lymphoma recurrence. It could follow a period of “stable” disease post-transplant, or a partial or complete remission. Progression/relapse represents new or larger areas of lymphoma (≥25% increase in largest diameter) compared to the best post-transplant lymphoma state. Relapse/progression was summarized by the cumulative incidence estimate with NRM as the competing risk. For PFS, subjects were considered treatment-failures at the time of lymphoma progression or death from any cause. Subjects alive without evidence of lymphoma-progression were censored at last follow-up and the PFS event was summarized by a survival curve. The OS interval variable was defined as time from the date of transplant to the date of death or last contact and summarized by a survival curve.
Statistical Analysis
Subject-, disease-, and transplant-related variables for subjects receiving rituximab and no rituximab were compared using the chi-square statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Univariate probabilities of neutrophil and platelet recovery and NRM were calculated using cumulative incidence curves to accommodate corresponding competing risks.(8) Probabilities of OS and PFS were calculated using Kaplan-Meier estimator.(9) Confidence intervals (CI) were calculated with a log-transformation.
To compare the outcomes of NRM, progression/relapse, PFS and OS, a Cox proportional hazards model was used to adjust for potential imbalance in baseline characteristics between treatment cohorts. A stepwise forward method was used to identify covariates which influenced outcomes. Each model contained the main effect (rituximab vs no rituximab). Any covariate with a p-value ≤0.05 was considered significant. The proportionality assumption for Cox-regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Tests indicated that all variables met the proportional-hazards assumption except Karnofsky performance score. Cox regression models stratified on Karnofsky score were used for each outcome event. Final results were expressed as relative risks (RR) of the event and its 95% confidence intervals. The following variables were considered in model building: rituximab vs. no rituximab (main effect), age at transplant, Karnofsky performance status at transplant, number of lines of chemotherapy, bone marrow involvement at transplant, disease status at transplant, size of largest lymphoma mass prior to transplant, time from diagnosis to transplant, conditioning regimen, year of transplant and G-CSF or GM-CSF given within 7-days post transplant. Rituximab vs. no rituximab was retained in all steps of model-building since it was the main effect of interest. Potential interactions between the main effect and all significant risk factors were tested, with no interactions were detected for all significant risk factors. Analyses were performed using SAS software, version 8.2 (SAS Institute).
RESULTS
Patient characteristics
Patient, disease and transplant related characteristics are summarized in Table 1. One hundred seventy-six patients (+R cohort) received rituximab prior to transplant, either during first-line therapy and/or during salvage therapy, and 818 patients (−R cohort) were rituximab naïve at the time of transplant. The median follow-up of survivors was 44 months and 62 months respectively for the +R and −R cohorts. Completeness of follow up was 90%.
Table 1.
Patient-, disease-, and transplant characteristics.
| Rituximab (+R) | No Rituximab (−R) | ||||
|---|---|---|---|---|---|
| Variable | N eval | N (%) | N eval | N (%) | P-valuea |
| Number of patients | 176 | 818 | |||
| Age, median (range), years | 176 | 58 (20 – 76) | 818 | 52 (18 – 75) | <0.001 |
| Age at transplant, years | 176 | 818 | <0.001 | ||
| 18–30 | 16 ( 9) | 76 ( 9) | |||
| 31–40 | 17 (10) | 105 (13) | |||
| 41–50 | 28 (16) | 204 (25) | |||
| 51–60 | 43 (24) | 244 (30) | |||
| ≥61 | 72 (41) | 189 (23) | |||
| Age at transplant, years | 176 | 818 | <0.001 | ||
| <55 | 79 (45) | 489 (60) | |||
| ≥55 | 97 (55) | 329 (40) | |||
| Male sex | 176 | 89 (51) | 817 | 473 (58) | 0.08 |
| Karnofsky score pre-transplant <90 | 166 | 64 (39) | 795 | 292 (37) | 0.66 |
| Second line age-adjusted International Prognostic Index (IPI) at transplant |
176 | 818 | 0.27 | ||
| Low | 40 (23) | 201 (25) | |||
| Low-Intermediate | 45 (25) | 216 (26) | |||
| High-Intermediate | 17 (10) | 113 (14) | |||
| High | 2 ( 1) | 16 ( 2) | |||
| Missing | 72 (41) | 272 (33) | |||
| Disease status at transplant | 176 | 761 | 0.45 | ||
| CR1 | 38 (22) | 130 (17) | |||
| CR2+ | 35 (20) | 124 (16) | |||
| PIF-sensitive | 32 (18) | 152 (20) | |||
| PIF-resistant | 12 ( 7) | 43 ( 6) | |||
| REL-sensitive | 45 (25) | 256 (34) | |||
| REL-resistant | 14 ( 8) | 56 ( 7) | |||
| Number of prior lines of therapy | 176 | 815 | <0.001 | ||
| 1 | 13 ( 8) | 119 (15) | |||
| 2 | 62 (35) | 370 (45) | |||
| 3 | 65 (37) | 234 (29) | |||
| 4 | 34 (19) | 67 ( 8) | |||
| 5 | 2 ( 1) | 20 ( 2) | |||
| Timing of rituximab treatment: | 176 | NA | --- | ||
| With 1st line chemotherapy only | 66 (38) | ||||
| With salvage therapy only | 110 (62) | ||||
| Chemosensitive disease at transplant | 173 | 784 | 0.75 | ||
| Sensitive | 146 (84) | 653 (83) | |||
| Marrow involvement at transplant | 172 | 3 ( 2) | 752 | 37 ( 5) | 0.06 |
| Size of largest mass of any kind prior to transplant, cm |
60 | 274 | 0.23 | ||
| <5 | 44 (73) | 179 (65) | |||
| ≥5 | 16 (27) | 95 (35) | |||
| Disease stage at diagnosis | 168 | 802 | 0.10 | ||
| I | 21 (13) | 88 (11) | |||
| II | 27 (16) | 190 (24) | |||
| III | 54 (32) | 195 (24) | |||
| IV | 66 (39) | 326 (41) | |||
| Interval from diagnosis to transplant, median (range), months |
176 | 14 (3 – 200) | 818 | 13 (2 – 277) | 0.46 |
| Interval from diagnosis to transplant, months |
176 | 818 | 0.75 | ||
| <12 | 76 (43) | 364 (45) | |||
| ≥ 12 | 100 (57) | 454 (55) | |||
| Radiation therapy post-transplant | 176 | 810 | 0.57 | ||
| Yes | 24 (14) | 124 (15) | |||
| No | 152 (86) | 686 (85) | |||
| Conditioning regimen | 176 | 818 | 0.18 | ||
| TBI-based | 27 (15) | 114 (14) | |||
| BEAM and similar | 102 (58) | 522 (64) | |||
| CBV or similar | 27 (15) | 77 ( 9) | |||
| BuMEL/BuCy | 10 ( 6) | 48 ( 6) | |||
| Others | 10 ( 6) | 57 ( 7) | |||
| Interval from last rituximab given to transplant, median (range), months |
170 | 5 (1 – 34) | NA | --- | |
| Graft type | 176 | 818 | 0.47 | ||
| Peripheral Blood | 165 (94) | 754 (92) | |||
| BM+PBSC | 11 ( 6) | 64 ( 8) | |||
| Year of transplant | 176 | 818 | <0.001 | ||
| 1996–1998 | 7 ( 4) | 481 (59) | |||
| 1999–2001 | 80 (45) | 281 (34) | |||
| 2002–2003 | 89 (51) | 56 ( 7) | |||
| G-CSF or GM-CSF given within 7-days post transplant |
176 | 159 (90) | 818 | 710 (87) | 0.20 |
| Median follow-up of survivors, months | 102 | 42 (2 – 83) | 336 | 62 (1 – 116) | --- |
Abbreviations: DLCL = diffuse large cell lymphoma; CR = complete remission; PIF = primary induction failure; REL = relapse; TBI = total body irradiation; BEAM = BCNU+etoposite+Ara-C+melphalan; CBV = cyclosphamide+BCNU+VP16 = etoposide; BM = bone marrow; PBSC = peripheral blood stem cell; EVAL = evaluable.
The chi-square test was used for discrete covariates; the Kruskal-Wallis test was used for continuous covariates.
The +R group had a higher median age (58 vs. 52 years old, p<0.001) and a higher proportion of patients aged 55 or older (55% vs. 40%, p<0.001). The +R group was also more heavily pre-treated prior to transplant, with a higher proportion of patients having received greater than 2 lines of chemotherapy (57% vs. 40%, p<0.001). However, this latter difference may be accounted to some extent by the fact that rituximab alone was counted as a regimen. Rituximab was administered with first-line chemotherapy in 38% of patients, and with salvage therapy only in 62% of patients, with no patients receiving rituximab with both first-line and salvage therapy. The cohorts were well matched for disease status at transplant, bulky disease, second line age adjusted IPI scores and bone marrow involvement. In addition, pre-transplant chemosensitivity and Ann Arbor stage at transplant did not differ between the groups.
The +R and the −R groups received AuHCT at a similar interval from diagnosis and received similar conditioning regimens. As expected, transplant occurred between 1999–2003 in 96% of patients in the +R group, and between 1996–2001 in 93% of the −R patients (p<0.001). A similar proportion of patients in each group received myeloid growth factors post-transplant. Use of post-transplant radiation therapy was similar in the two groups.
Outcomes
Engraftment
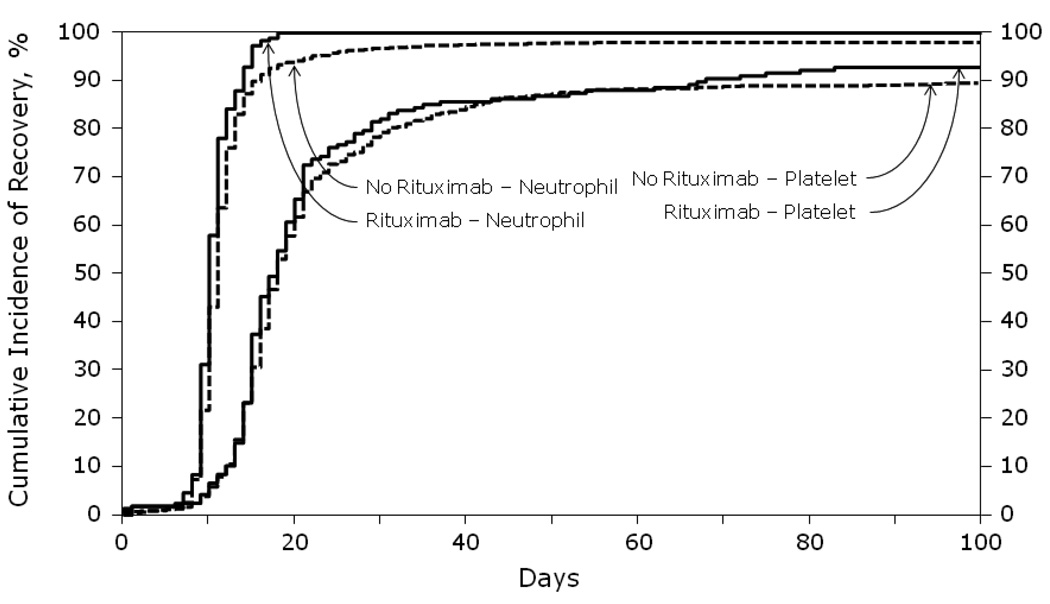
The cumulative incidence of platelet and neutrophil engraftment at 28 and 100 days was similar in the two groups (Figure 1). There was no clinically significant difference in the rates of neutrophil engraftment (defined as ANC>0.5 × 109/L) or platelet engraftment (defined as a platelet count of 20,000/µL with no transfusion requirement) between the 2 cohorts. The patients who received rituximab within 3 months of transplant (n=60) were analyzed separately for engraftment delay. These patients also achieved neutrophil engraftment by day 17 and had platelet recovery at a median of 17 days with no difference compared to the −R patients (p=0.23) (data not shown).
Figure 1.
Cumulative incidence of neutrophil and platelet recovery after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given prior to transplant.
Non-relapse mortality / causes of death
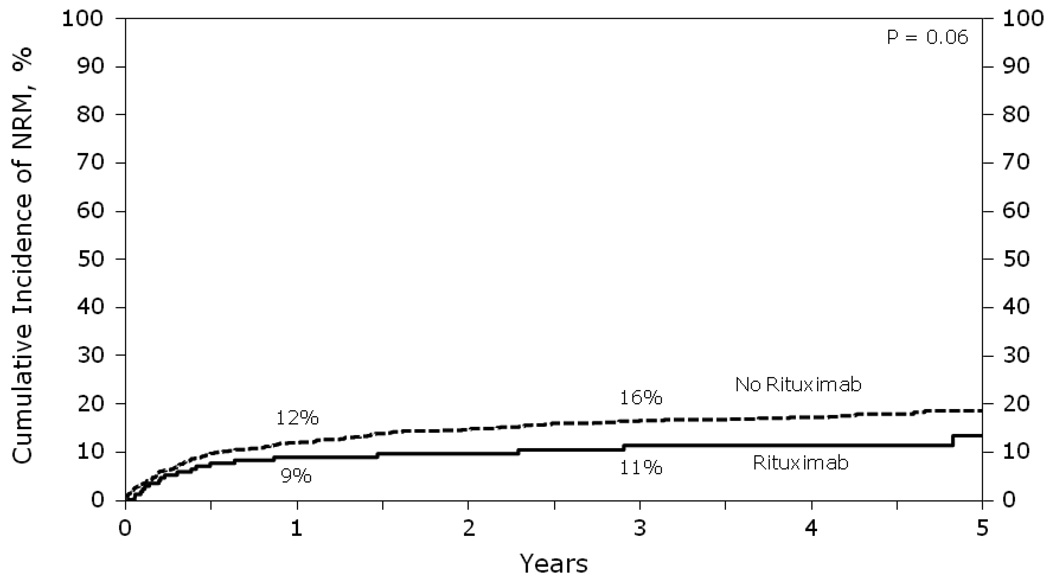
The cumulative incidence of non-relapse mortality (NRM) at 1, 3, or 5 years did not differ significantly between the two groups (p = 0.06, Figure 2). In multivariate analysis, higher age (≥ 55 years; RR=1.79, p<0.001) and transplant >1 year from diagnosis (RR=1.68, p=0.002) were associated with higher risk of NRM. Pre-transplant rituximab did not impact NRM (p=0.18) (Table 2). Causes of death were similar between the +R and −R groups, with 58–60% of deaths from lymphoma and 40–42% of deaths from causes other than relapse (Table 5).
Figure 2.
Cumulative incidence of NRM after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given prior to transplant.
Table 2.
Multivariate analyses for NRM*
| Variables: | N | Relative Risk of NRM (95% CI) |
P-value |
|---|---|---|---|
| Main effect: | |||
| No rituximab | 812 | 1.00 | |
| Rituximab | 174 | 0.70 (0.41 – 1.18) | 0.18 |
| Other significant covariates: | |||
| Age at transplant, years | |||
| <55 | 562 | 1.00 | |
| ≥ 55 | 424 | 1.79 (1.31 – 2.45) | <0.001 |
| Time from diagnosis to transplant, years | |||
| ≤1 | 435 | 1.00 | |
| >1 | 551 | 1.68 (1.21 – 2.34) | 0.002 |
| Year of transplant | |||
| 1996–1999 | 728 | 1.00 | |
| 2000–2003 | 258 | 0.63 (0.40 – 1.00) | 0.05 |
Abbreviations: CI = confidence interval.
Cox models stratified on Karnofsky performance score
Table 5.
Causes of death before day 100
| Rituximab | No Rituximab | |||
|---|---|---|---|---|
| Causes of death | N eval | N (%) | N eval | N (%) |
| Number of patients | 24 | 114 | ||
| Relapse / progression | 14 (58) | 68 (60) | ||
| Other causes | 10 (42) | 46 (40) | ||
| Pulmonary syndrome | 2 ( 8) | 10 ( 9) | ||
| Infection | 2 ( 8) | 11 ( 9) | ||
| Organ failure | 3 (14) | 19 (17) | ||
| Hemorrhage | 1 ( 4) | 4 ( 3) | ||
| New malignancy | 1 ( 4) | 1 ( 1) | ||
| Unknown | 1 ( 4) | 1 ( 1) | ||
Relapse/Progression & Progression Free Survival
The risk of relapse/progression was lower in the +R group compared to the −R group (RR=0.67, p=0.004). Other significant covariates associated with higher risk of relapse/progression were older age (≥55 years, RR=1.36, p=0.002), the lack of a complete remission or chemosensitive status at transplant (p<0.001) and 3 or more lines of prior chemotherapy (RR=1.71, p<0.001).
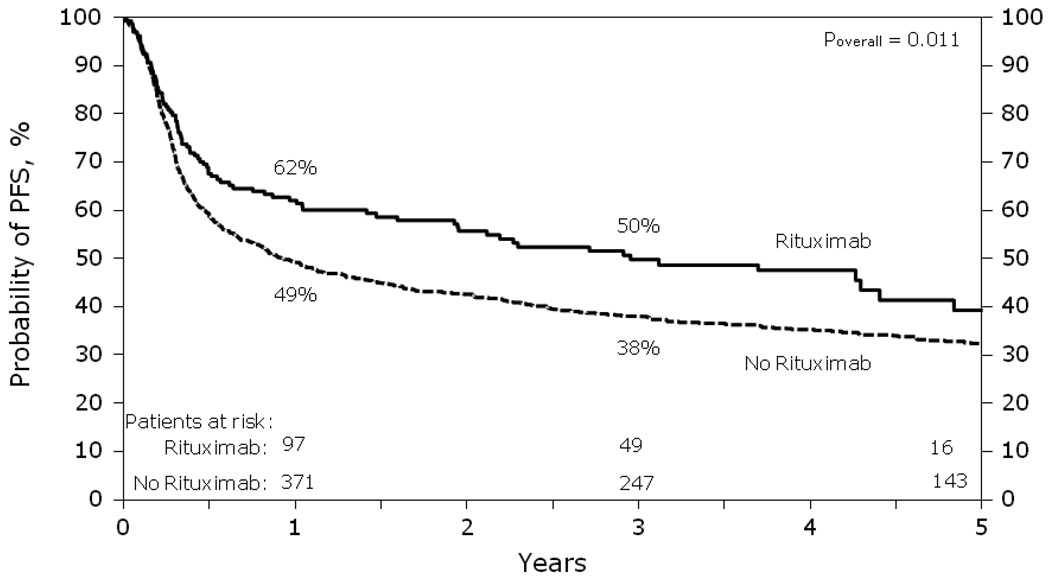
Progression-free survival was superior in the +R group compared to the −R group resulting in a lower risk of treatment failure from relapse/progression or death in the +R cohort compared to the −R cohort (RR= 0.64, p<0.001) (Table 3 & Figure 3). Point-wise estimates of PFS at the 1 and 3 year time points for the +R cohort vs. the −R cohort were 62% vs. 49% (p=0.002) and 50% vs. 38% (p=0.008) respectively. Other significant covariates associated with improved progression-free survival and lower risk of treatment failure were age <55, first complete remission at the time of AuHCT and less than three lines of preceding chemotherapy (Table 3).
Table 3.
Multivariate analyses for PFS*
| Variables: | N | Relative Risk of relapse/progression or death (95% CI) |
P-value |
|---|---|---|---|
| Main effect: | |||
| No rituximab | 812 | 1.00 | |
| Rituximab | 174 | 0.64 (0.50 – 0.81) | <0.001 |
| Other significant covariates: | |||
| Age at transplant, years | |||
| <55 | 562 | 1.00 | |
| ≥ 55 | 424 | 1.45 (1.23 – 1.71) | <0.001 |
| Disease status at transplant | |||
| (1) CR1 | 167 | 1.00 | <0.001a |
| (2) PIF-sensitive | 182 | 1.24 (0.91 – 1.69) | 0.18 |
| (3) PIF-resistant | 54 | 3.38 (2.30 – 4.96) | <0.001 |
| (4) REL-sensitive | 298 | 2.02 (1.53 – 2.67) | <0.001 |
| (5) REL-resistant | 69 | 2.65 (1.83 – 3.82) | <0.001 |
| (6) CR2+ | 158 | 1.57 (1.14 – 2.14) | 0.010 |
| (7) Unknown | 58 | 2.13 (1.45 – 3.15) | 0.001 |
| Number of lines of chemotherapy | |||
| ≤2 | 561 | 1.00 | <0.001b |
| >2 | 418 | 1.61 (1.36 – 1.91) | <0.001 |
Abbreviations: CI = confidence interval.
Cox models stratified on karnofsky performance score
Six degrees of freedom
Two degrees of freedom
Figure 3.
Probability of PFS after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given prior to transplant.
Survival
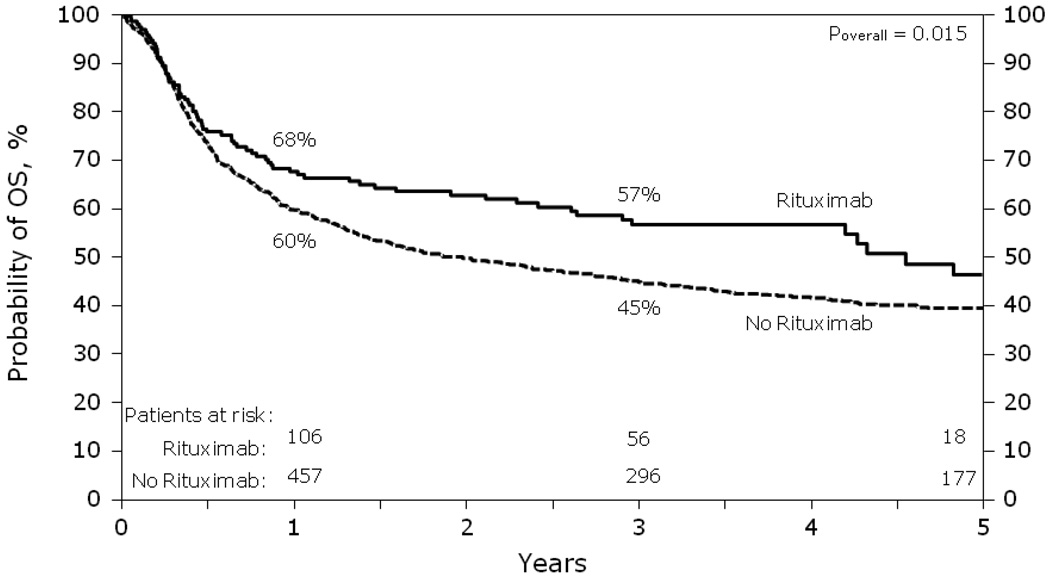
Survival was superior in the +R group with a lower relative risk of mortality (RR= 0.74, p=0.039) (Table 4 & Figure 4). Point-wise estimates of OS at the 1 and 3 year time points for the +R cohort vs. the −R cohort were 68% vs. 60% (p=0.049) and 57% vs. 45% (p=0.006) respectively. In multivariate analysis, age <55, first complete remission at the time of AuHCT, less than 3 lines of chemotherapy and later year of transplant were all associated with lower mortality and improved survival (Table 4).
Table 4.
Multivariate analyses for OS*
| Variables: | N | Relative Risk of death (95% CI) |
P-value |
|---|---|---|---|
| Main effect: | |||
| No rituximab | 818 | 1.00 | |
| Rituximab | 176 | 0.74 (0.56 – 0.99) | 0.039 |
| Other significant covariates: | |||
| Age at transplant, years | |||
| <55 | 568 | 1.00 | |
| ≥ 55 | 426 | 1.53 (1.29 – 1.83) | <0.001 |
| Disease status at transplant | |||
| (1) CR1 | 168 | 1.00 | <0.001a |
| (2) PIF-sensitive | 184 | 1.29 (0.91 – 1.82) | 0.15 |
| (3) PIF-resistant | 54 | 3.23 (2.14 – 4.87) | <0.001 |
| (4) REL-sensitive | 301 | 2.06 (1.51 – 2.81) | <0.001 |
| (5) REL-resistant | 69 | 2.57 (1.73 – 3.83) | <0.001 |
| (6) CR2+ | 159 | 1.58 (1.12 – 2.24) | 0.010 |
| (7) Unknown | 59 | 2.27 (1.50 – 3.44) | <0.001 |
| Number of lines of chemotherapy | |||
| ≤2 | 564 | 1.00 | <0.001b |
| >2 | 422 | 1.53 (1.28 – 1.82) | <0.001 |
| Year of transplant | |||
| 1996–1999 | 735 | 1.00 | |
| 2000–2003 | 259 | 0.73 (0.57 – 0.94) | 0.013 |
Abbreviations: CI = confidence interval.
Cox models stratified on karnofsky performance score
Six degrees of freedom
Two degrees of freedom
Figure 4.
Probability of OS after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given prior to transplant.
Timing of Rituximab
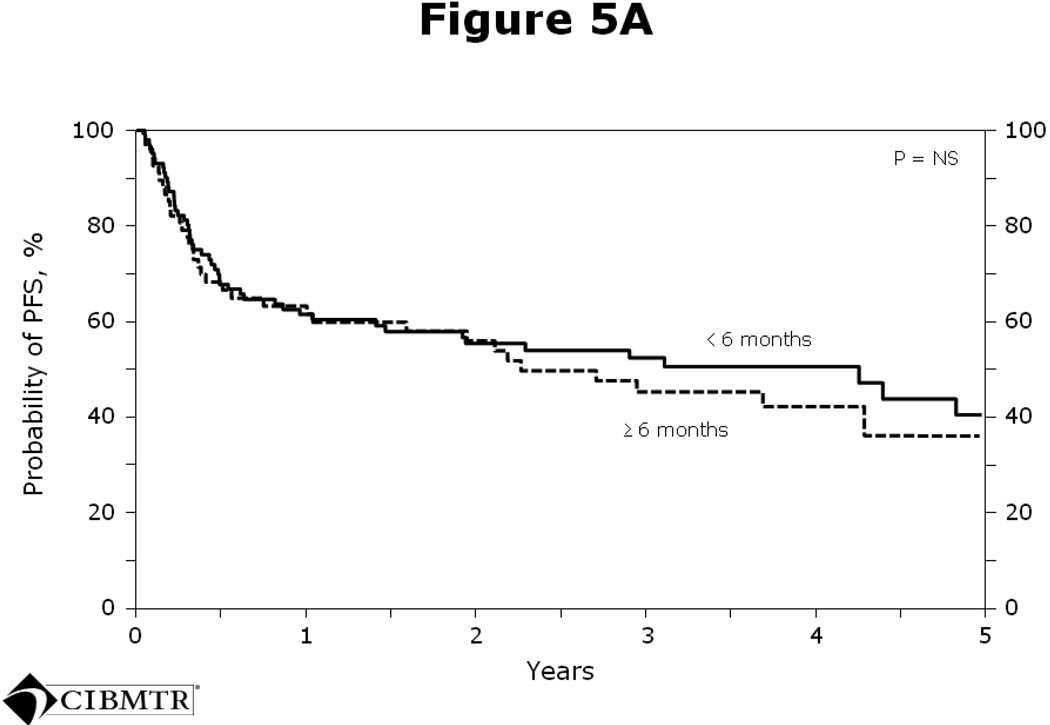
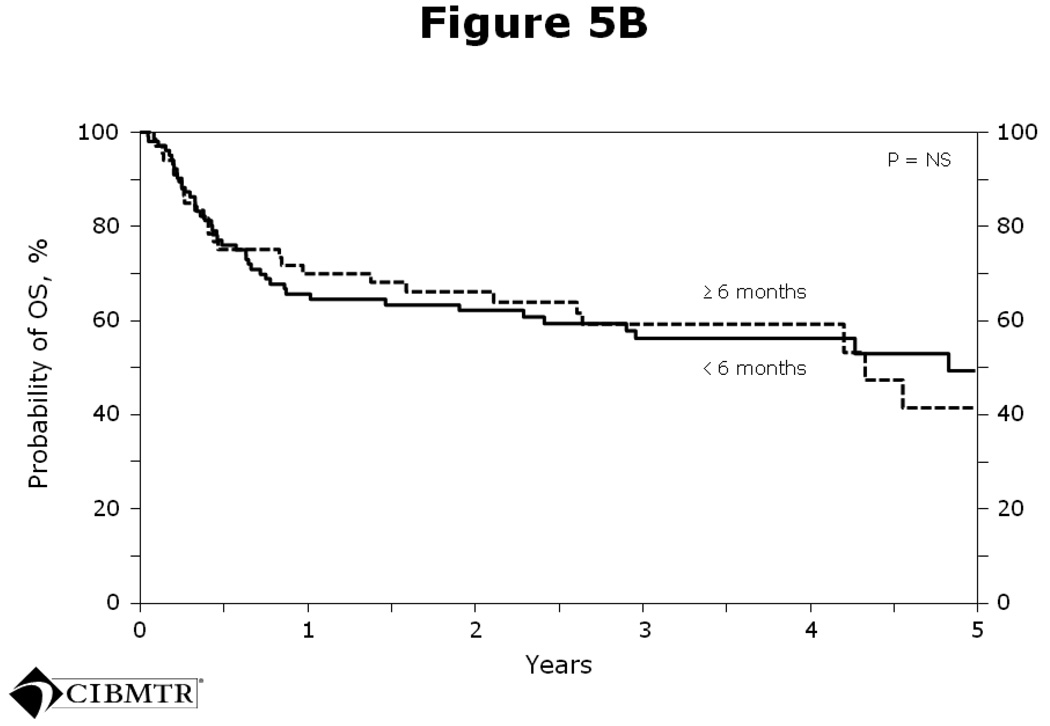
The average interval from last rituximab dose to transplant was 5 months. On further analysis, no significant differences in PFS (Figure 5A) or OS (Figure 5B) were seen in patients who received rituximab within 6 months of transplant versus > 6 months prior to transplant.
Figure 5.
Figure 5A Probability of PFS after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given < 6 months or ≥ 6 months prior to transplant.
Figure 5B Probability of OS after autologous HCT for diffuse large B-cell lymphoma analyzed by whether rituximab was given < 6 months or ≥ 6 months prior to transplant.
DISCUSSION
The Parma trial remains the only published prospective, randomized trial comparing salvage chemotherapy alone to autologous hematopoietic stem cell transplantation (AuHCT) for relapsed DLBCL. Based on this study, AuHCT remains the standard of care for patients with chemosensitive relapsed and refractory DLBCL.(6) The Parma trial predates the introduction of rituximab into clinical practice; in contrast, patients with DLBCL are now routinely treated with rituximab as part of their frontline and/or subsequent therapies. As a result, the outcomes following AuHCT for DLBCL in the rituximab era are not fully known. Our results indicate that pre-transplant rituximab is not associated with impaired engraftment or increased non-relapse mortality. In addition, improved progression-free and overall survival was seen in patients exposed to rituximab prior to transplant.
There are several possible explanations for the observation of enhanced PFS and OS following AuHCT in the rituximab exposed patients. (1) It is possible that the +R group was a more favorable group based on baseline patient characteristics. However, this seems unlikely since the +R group was actually older. In addition, the two groups were very similar in terms of IPI score at transplant, disease status at transplant, performance status, stage, chemosensitivity and bulky disease. (2) The fact that the +R patients underwent transplant in later years than the −R group could also account for improved outcomes in the +R group. However this would only be expected to influence survival by decreasing NRM (due to improvements in supportive care over time) and would not be expected to affect PFS (since the conditioning regimens used in both groups were similar). There was no significant difference in NRM between the two groups. (3) In theory, improved PFS and OS in the +R group could be due to delayed activity of rituximab that was received during salvage therapy. However, the average interval from last rituximab dose to transplant was 5 months, and no significant differences were seen in 1-, 3-, or 5- year PFS or OS in patients who received rituximab within 6 months of transplant versus > 6 months prior to transplant. (4) It is possible that pre-transplant rituximab sensitizes or alters specific effector cell populations, or affects immune reconstitution in ways that lead to enhanced anti-lymphoma effects. Unfortunately, post-transplant immune reconstitution data was not uniformly collected from the patients in this study, precluding further testing this hypothesis. (5) Lastly, it is known that inclusion of rituximab in first-line therapy has improved the outcome of specific subsets of DLBCL, such as those which are bcl6-negative, bcl2-positive, or of non-germinal center origin.(10–12) As a result, there could be important biological differences in the +R and −R patients in our study, which may account for the improved outcome of the +R patients following AuHCT.
Although several cases of delayed neutropenia associated with rituximab have been described,(13, 14) the stem cell yield following rituximab therapy appears unaffected.(7, 15) An additional concern is that pre-transplant rituximab may affect engraftment kinetics.(7) Our study supports the concept that pre-transplant exposure to rituximab does not compromise peripheral blood stem cell product quality or engraftment.
One might expect a priori that relapsed or refractory DLBCL patients already exposed to rituximab will be more likely to have rituximab-refractory disease, and will therefore also be inherently more difficult to rescue with rituximab-containing salvage therapy followed by AuHCT. However, our data appear to contradict this notion, since patients previously exposed to rituximab actually had improved PFS and OS.
It is possible that, depending on the exact timing of exposure to rituximab (as part of first-line therapy and/or with salvage therapy), the outcomes following AuHCT may differ. The number of patients in the +R group was not sufficient to allow for meaningful subgroup analysis based on rituximab exposure during first-line therapy or salvage therapy, so our study does not shed light on this issue. In a recently published study from the GITIL (Gruppo Italiano Terapie Innnovative nei Linfomi), the benefit of rituximab prior to AuHCT was most apparent in follicular and diffuse large B-cell lymphoma patients who received rituximab with salvage therapy but not with first-line therapy.(16) In a much smaller study from Germany, an improved outcome after AuHCT for aggressive NHL was associated with addition of rituximab to salvage therapy. In that study, patients were largely (87%) rituximab-naïve prior to salvage therapy.(17) A recent abstract by Ashraf et al reported single-center outcomes of 63 DLBCL patients who underwent AuHCT between 1991 and 2008. Similar to our findings, significantly better disease control after AuHCT was seen in patients who had rituximab as part of their front-line therapy.(18) In the ongoing CORAL (Collaborative Trial in Relapsed Aggressive Lymphoma) study, relapsed and refractory CD20-positive DLBCL patients are randomized between 2 different rituximab based salvage chemotherapy regimens, followed by AuHCT and further second randomization of observation versus maintenance rituximab.(19) The CORAL study enrolls both patients with and without rituximab in first-line therapy and upon completion will hopefully further clarify the impact of rituximab exposure at different time points prior to transplant.
The patient cohorts in this study are representative of a period of transition in practice when the use of rituximab was increasingly being adopted for DLBCL. Therefore a contemporary cohort of patients who were rituximab naïve at AuHCT was available for comparison to the +R cohort. In the context of current clinical practice in the United States, rituximab is generally used in both first line and subsequent therapies for DLBCL. Thus it is very unlikely that current AuHCT recipients for DLBCL will be rituximab naive. However, our study provides post hoc validation for this practice and confirms the safety of prior rituximab in the AuHCT setting.
In this study, with a median of 42 months of follow up in the +R group, there were only a small number of patients with 5 or more years of follow up. It was therefore not possible to perform statistically significant analyses of longer term survival outcomes beyond those reported above. The magnitude of benefit of pre-transplant rituximab beyond 5 years after AuHCT remains uncertain. Longer follow up would clarify whether rituximab only serves to delay DLBCL relapse, or whether it leads to a higher rate of long-term disease-free survival. The question of whether post-AuHCT “maintenance” therapy (using rituximab and/or other agents) may offer benefit for DLBCL patients also remains unanswered. Long-term data from randomized trials, such as the ongoing CORAL study, will be required to further address these questions.
ACKNOWLEDGEMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 5.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 6.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 7.Hoerr AL, Gao F, Hidalgo J, et al. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:4561–4566. doi: 10.1200/JCO.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Springer; 2003. [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53 [Google Scholar]
- 10.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 11.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109:4930–4935. doi: 10.1182/blood-2006-09-047068. [DOI] [PubMed] [Google Scholar]
- 13.Chaiwatanatorn K, Lee N, Grigg A, Filshie R, Firkin F. Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol. 2003;121:913–918. doi: 10.1046/j.1365-2141.2003.04385.x. [DOI] [PubMed] [Google Scholar]
- 14.Motl SE, Baskin RC. Delayed-onset grade 4 neutropenia associated with rituximab therapy in a patient with lymphoma: case report and literature review. Pharmacotherapy. 2005;25:1151–1155. doi: 10.1592/phco.2005.25.8.1151. [DOI] [PubMed] [Google Scholar]
- 15.Benekli M, Hahn T, Shafi F, et al. Effect of rituximab on peripheral blood stem cell mobilization and engraftment kinetics in non-Hodgkin's lymphoma patients. Bone Marrow Transplant. 2003;32:139–143. doi: 10.1038/sj.bmt.1704106. [DOI] [PubMed] [Google Scholar]
- 16.Tarella C, Zanni M, Magni M, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multicenter Gruppo Italiano Terapie Innnovative nei linfomi survey. J Clin Oncol. 2008;26:3166–3175. doi: 10.1200/JCO.2007.14.4204. [DOI] [PubMed] [Google Scholar]
- 17.Sieniawski M, Staak O, Glossmann JP, et al. Rituximab added to an intensified salvage chemotherapy program followed by autologous stem cell transplantation improved the outcome in relapsed and refractory aggressive non-Hodgkin lymphoma. Ann Hematol. 2007;86:107–115. doi: 10.1007/s00277-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 18.Ashraf U, Mahajan R, Hahn R, et al. The effects of rituximab added to front-line or salvage chemotherapy in diffuse large B-cell lymphoma (DLBCL) undergoing high-dose chemotherapy (HDC) and autologous stem cell transplant (ASCT) Blood. 2008;112 Abstract 1138. [Google Scholar]
- 19.Gisselbrecht C, Schmitz N, Mounier N, et al. R-ICE Versus R-DHAP in Relapsed Patients with CD20 Diffuse Large B-Cell Lymphoma (DLBCL) Followed by Stem Cell Transplantation and Maintenance Treatment with Rituximab or Not: First Interim Analysis on 200 Patients CORAL Study. Blood. 2007;110 Abstract 517. [Google Scholar]