Abstract
The total synthesis of the complex pyrrole–imidazole alkaloids (±)–massadine and (±)–massadine chloride is described using a carefully orchestrated sequence of manipulations on highly polar and structurally complex intermediates. Key to the completion of this synthetic endeavor was the exploration of a unique and chemoselective method to oxidize unprotected guanidines under aqueous conditions in air. This oxidation has been optimized and applied to a selection of spirocyclic guanidines of varying complexity. Additionally, the 3,7–epi analogues of these interesting natural products have been synthesized and fully characterized.
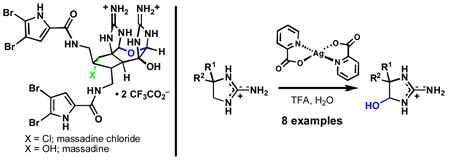
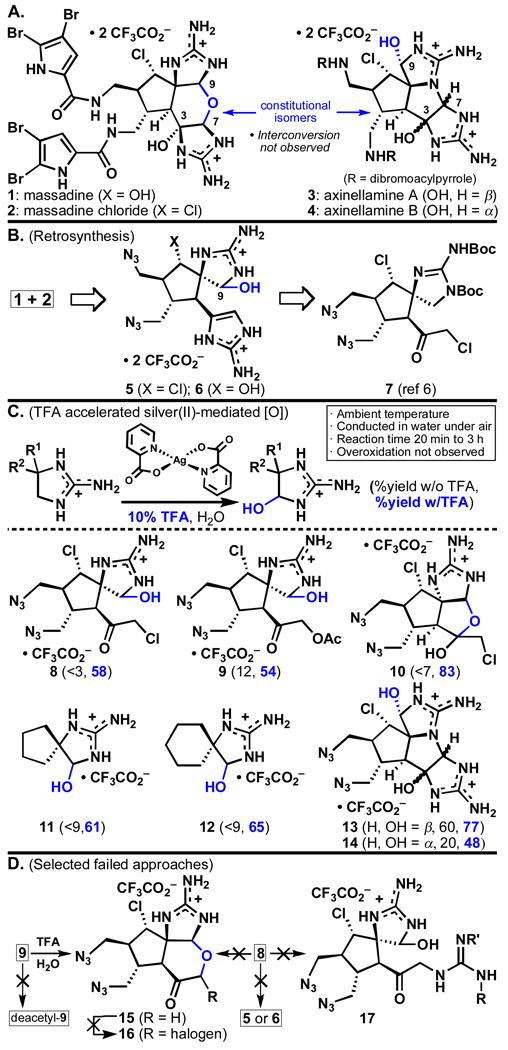
The palau’amines,1 massadines (1–2, Figure 1A),2 axinellamines (3–4),3 and the tetrameric stylissadines4,1c are dimeric pyrrole–imidazole alkaloids that express a high degree of complexity among small marine natural products. Their highly polar structures, varying modes of halogenation, dense arrangement of functionalities, and stereochemical complexity render them appealing targets for total synthesis. Additionally, many of these alkaloids possess notable biological activities, including immunosuppressant,1b cytotoxic,1b anti–inflammatory,1c and anti-fungal.2a As such, many elegant approaches to their synthesis have been published and reviewed.5 Earlier this year we reported a synthesis of axinellamines A and B (3–4) enabled by a unique late–stage chemoselective oxidation.6 This communication reports a greatly improved version of this oxidation protocol and its application to the total syntheses of massadine (1) and massadine chloride (2).
Figure 1.
(A) Structures of the massadines (1–2) and axinellamines (3–4). (B) Retrosynthetic analysis of the massadines (1–2). (C) Chemoselective silver(II) oxidation. (D) Selected failed approaches.
Due to their constitutionally isomeric nature, one could envision massadine chloride (2) arising from the axinellamines (3–4) via aminal opening and hemiaminal closure at C7 (Figure 1A). However, such a rearrangement was not detected under a variety of thermal, acidic, and basic conditions. A distinct approach to the massadine core was therefore required. Advanced intermediates 5 and 6 (Figure 1B) were identified as plausible precursors to 1 and 2, assuming that subsequent cyclization would occur at oxygen (massadine–type cyclization) rather than nitrogen (axinellamine–type cyclization) while the guanidine moiety was protonated. Spirocycles 5 and 6 might arise from α–chloroketone 7, an entity available in multi-gram quantities.6a Two main factors complicate the preparation of intermediates such as 5 and 6. First, the silver(II)–mediated oxidation6b reported earlier was not general and many routes failed simply due to an inability to obtain useful quantities of hemiaminal intermediates. Secondly, the dense functionality of these systems combined with a deliberate avoidance of complex protecting group schemes led to several dead-ends (vide infra).
The first problem was addressed by optimization of the silver(II)–mediated oxidation (Figure 1C). Since our initial report,6b it has been found that conducting the reaction in the presence of TFA significantly accelerates it, leads to higher conversion, and in some cases is entirely enabling (i.e. 8, 10–12). The axinellamine precursors 13 and 14, that previously required heating and extended reaction times,6b can now be prepared in 77% and 48% yield in under 3 hours at ambient temperature. This reaction appears to be general for these types of systems, and five additional examples are shown in Figure 1C. With a versatile, chemoselective, operationally simple, and direct oxidation of such systems in hand, rapid simultaneous exploration of routes to the massadines could commence.
The second problem is summarized briefly in Figure 1D with a sampling of failed routes to intermediates 5 and 6. Despite repeated attempts, it was found that oxidation of C9 needed to occur prior to 2–aminoimidazole formation. While pre–oxidized acetate 9 could be closed to the tetrahydropyran core 15, further α–oxidation failed (15 → 16); formation of deacetyl- 9 also proved problematic. The α–chloro ketone 8 was also a dead-end (due to the sensitivity of the hemiaminal moiety): closure to 15 or elaboration of the 2–aminoimidazole with guanidine equivalents failed to afford 5, 6, or 17.
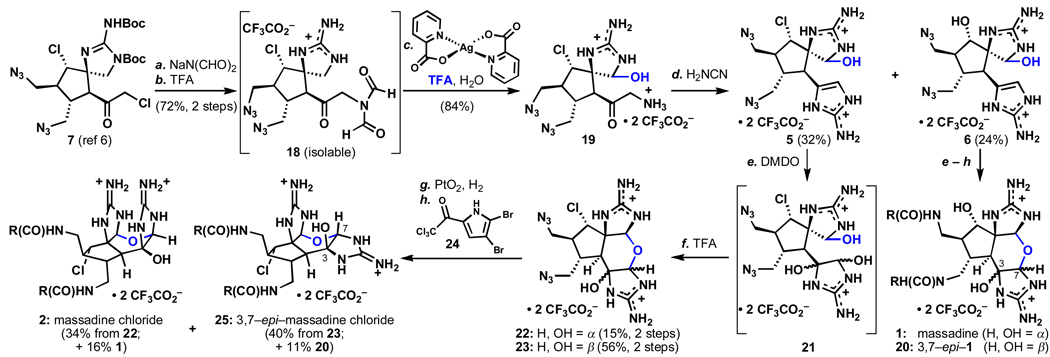
Ultimately, an iterative formation7 of the 2–aminoimidazole allowed access to 5 and 6, which could be converted into massadine chloride (2) and massadine (1) as outlined in Scheme 1. Thus, displacement of the α-chlorine in 7 with sodium diformylamide under carefully controlled pH followed by Boc–removal with trifluoroacetic acid provided 18 in 72% yield (2 steps). Oxidation with silver(II)picolinate in 10 % TFA/H2O installed the C9 hemiaminal in 84% isolated yield after deformylation (100 mg scale). Treatment with cyanamide under buffered conditions constructed the desired 2–aminoimidazole 5 (32%) along with its hydroxy analogue 6 (24%), resulting from the displacement of the α–chloride with retention of configuration in a manner similar to the known conversion of massadine chloride (2) to massadine (1).2b
Scheme 1.
Total syntheses of the massadines.a
aReagents and conditions: (a) Sodium diformylamide (1.2 equiv), TBAI (0.1 equiv), THF, 23 °C, 2 h, 72%; (b) 2:1 TFA:DCM, 23 °C, 2 h, quant; (c) silver(II)picolinate (2.5 eq), 9:1 H2O:TFA, 23 °C, 35 min, then TFA (to 1:1 v:v), 38 °C, 18 h, 84%; (d) cyanamide (excess), 0.2 M NaOH (to pH 5.0), 78 °C, 2 h, 24% 5, 32% 6; (e) DMDO (1.3 equiv), 9:1 H2O:TFA, 0 °C, 1h (f) TFA, 23 °C, 3 h, 71% over two steps, 1:3.7 22:23, (65% for −OH series, 1:1.9); (f) PtO2 (0.3 equiv), H2 (1 atm), 19:1 H2O:TFA, 1 h, 23 °C; (g) 24 (16 equiv), iPr2EtN (16 equiv) DMF, 14 h, 23 °C, 34% (22→2), 44% (23→25) (40% for 1 and 40% for 20, −OH series). TBAI = tetrabutylammonium iodide, TFA = trifluoroacetic acid, DMDO = dimethyldioxirane, R = 5–(2,3–dibromopyrrole).
Unlike the axinellamines (3–4), the massadines (1–2) were not isolated in Nature with their C3,C7 epimers. Thus, we had postulated that the natural configuration was favored thermodynamically, perhaps due to a π–stacking or hydrogen bonding effect between the two guanidine moieties (compare 2 and 27, Scheme 1). To our surprise, when 2–aminoimidazole 5 was exposed to a variety of oxidative conditions followed by acid–mediated closure, the C3,C7–epi products were favored. After extensive optimization, it was found that a 1:3.7 (22:23) ratio of diastereomers could be achieved with dimethyldioxirane in water followed by ring closure in neat TFA. Careful control of the pH during the oxidation was required to suppress N–cyclization (axinellamine mode).8 Reduction of the azide moieties in 22 and 23 with PtO2 under H2 followed by exposure to bromopyrrole 24 provided massadine chloride (2, xx%) and 3,7–epi–massadine chloride (25, xx%).8 The identical sequence with the hydroxy-analogue 6 provided access to massadine (1) and 3,7–epi–massadine (20) in a 1:2 ratio.8 Notably, 25 could be converted into 20 in warm water,8 analogous to the known conversion of 2 to 1.2b Equilibration from 2 and 1 to 25 and 20, respectively (or vice versa), was not observed under acidic conditions.
In summary, the discovery and optimization of a robust method to chemoselectively oxidize a variety (8–14, 19) of unprotected guanidines has allowed a synthetic entry to the massadines (1–2) without recourse to a complicated orthogonal protecting group scheme. As a side note, the diastereoselectivity of oxidative closure using using 9–deoxy–5 in the total synthesis of the axinellamines, is 1:1 and both isomers are found in Nature.6 The stereochemistry expressed in 1 and 2 at C3 and C7, correlates with that of axinellamine B (4), yet no epi–series has been isolated for the massadines (1–2). The stability and ease of formation of the C3,C7–epi–massadines begs the question: could there be a natural epi–series of the massadines (e.g. 20, 25), analogous to axinellamine A (3)?
Supplementary Material
Acknowledgement
Mr. Derek Barbas is gratefully acknowledged for his technical contributions. Financial support for this work was provided by the NIH (GM-073949), the NSF (predoctoral fellowship for I.B.S.), NSERC (postdoctoral fellowship for I.S.Y.), and Bristol-Myers-Squibb (postdoctoral funding for S.S.). Special thanks to Prof. Matthias Köck for providing natural samples of 1 and 2.
Footnotes
Supporting Information Available: Detailed experimental procedures, copies of all spectral data and full characterization. This material is available via the Internet at http://pubs.acs.org.
References
- 1.For original isolation, see: Kinnel RB, Gehrken H-P, Scheuer PJ. J. Am. Chem. Soc. 1993;115:3376. Kinnel RB, Gehrken H-P, Swali R, Skoropowski G, Scheuer PJ. J. Org. Chem. 1998;63:3281.. For reassignment of the original structure, see: Grube A, Köck M. Angew. Chem. Int. Ed. 2007;46:2320. doi: 10.1002/anie.200604076. Buchanan MS, Carroll AR, Addepalli R, Avery VM, Hooper JNA, Quinn RJ. J. Org. Chem. 2007;72:2309. doi: 10.1021/jo062007q. Kobayashi H, Kitamura K, Nagai K, Nakao Y, Fusetani N, van Soest RWM, Matsunaga S. Tetrahedron Lett. 2007;48:2127. Buchanan MS, Carroll AR, Quinn RJ. Tetrahedron Lett. 2007;48:4573.
- 2.(a) Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, van Soest RWM, Fusetani N. Org. Lett. 2003;5:2255. doi: 10.1021/ol034564u. [DOI] [PubMed] [Google Scholar]; (b) Grube A, Immel S, Baran PS, Köck M. Angew. Chem. Int. Ed. 2007;46:6721. doi: 10.1002/anie.200701935. [DOI] [PubMed] [Google Scholar]
- 3.Urban S, Leone P, de A, Carroll AR, Fechner GA, Smith J, Hooper JNA, Quinn RJ. J. Org. Chem. 1999;64:731. doi: 10.1021/jo981034g. [DOI] [PubMed] [Google Scholar]
- 4.Grube A, Köck M. Org. Lett. 2006;8:4675. doi: 10.1021/ol061317s. [DOI] [PubMed] [Google Scholar]
- 5.For work done through early 2007, see the following reviews and references therein: Hoffmann H, Lindel T. Synthesis. 2003:1753. Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551. Köck M, Grube A, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2007;46:6586. doi: 10.1002/anie.200701798.. Reports late 2007 to present: Lanman BA, Overman LE, Paulini R, White NS. J. Am. Chem. Soc. 2007;129:12896. doi: 10.1021/ja074939x. Sivappa R, Hernandez NM, He Y, Lovely CJ. Org. Lett. 2007;9:3861. doi: 10.1021/ol0711568. Tang L, Romo D. Heterocycles. 2007;74:999. Cernak TA, Gleason JL. J. Org. Chem. 2008;73:102. doi: 10.1021/jo701866g. Wang S, Romo D. Angew. Chem. Int. Ed. 2008;47:1284. doi: 10.1002/anie.200703998. Bultman MS, Ma J, Gin DY. Angew. Chem. Int. Ed. 2008;47:6821. doi: 10.1002/anie.200801969. Zancanella MA, Romo D. Org. Lett. 2008;10:3685. doi: 10.1021/ol801289b.
- 6.(a) Yamaguchi J, Seiple IB, Young IS, O’Malley DP, Maue M, Baran PS. Angew. Chem. Int. Ed. 2008;47:3578. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]; (b) O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2008;47:3581. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]
- 7.(a) Baran PS, Zografos AL, O’Malley DP. J. Am. Chem. Soc. 2004;126:3726. doi: 10.1021/ja049648s. [DOI] [PubMed] [Google Scholar]; (b) O’Malley DP, Li K, Maue M, Zografos AL, Baran PS. J. Am. Chem. Soc. 2007;129:4762. doi: 10.1021/ja069035a. [DOI] [PubMed] [Google Scholar]
- 8.See Supporting Information for details. Synthetic material was spectroscopically identical to natural samples provided by Prof. Matthias Köck.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.