Abstract
Objectives
To assess the importance of lipids and lipoproteins on longitudinal cognitive performance and cognitive health in late life and to consider moderating factors such as age and sex which may clarify conflicting prior evidence.
Design
A 16-year prospective cohort study of health and cognitive aging.
Participants
819 adults from the Swedish Adoption Twin Study of Aging (SATSA), 50 years and older at the first cognitive testing, including 21 twin pairs discordant for dementia.
Measurements
Up to five occasions of cognitive measurements encompassing verbal, spatial, memory and perceptual speed domains across a 16-year span. Baseline serum lipids and lipoproteins including HDL, apoA1, apoB, total serum cholesterol, and triglycerides.
Results
The effect of lipids on cognitive change was most evident prior to age 65. In women higher HDL and lower apoB and triglycerides predicted better maintenance of cognitive abilities over age, particularly verbal ability and perceptual speed. Lipid values were less predictive of cognitive trajectories in men and, where observed, were in the contrary direction: i.e., higher total cholesterol and apoB values predicted better perceptual speed performance though faster rates of decline. In twin pairs discordant for dementia, higher total cholesterol and apoB levels were observed in the twin who subsequently developed dementia.
Conclusions
Elevated lipids may constitute a more important risk factor for cognitive health before age 65 than after. Findings for women are consistent with clinical recommendations, while for men the findings correspond with earlier age-associated shifts in lipid profiles and the importance of lipid homeostasis to cognitive health.
Keywords: Lipids, lipoproteins, cognitive change, gender differences
Introduction
Cognitive health in old age may be related to cardiovascular risk factors such as serum lipid levels; however, how lipids relate to cognitive change is unclear. Despite the likely involvement of cholesterol metabolism in Alzheimer's disease (AD) and dementia, results have been inconsistent across epidemiological studies, particularly for late life dementias (1, 2), while very few studies have considered cognitive performance declines beyond mental status test scores or mild cognitive impairment. If serum cholesterol or other lipids alter dementia risk, we would expect to see notable impacts on longitudinal cognitive performance and change that predates clinically detectable impairments.
Reviews of existing literature suggest that high midlife cholesterol, or decline in cholesterol from mid to late life, may be important to later risk of cognitive impairments, AD, and dementia (1), while serum cholesterol measured in late life is less clearly related (1, 2). Findings for total serum cholesterol and cognitive decline, typically measured with cognitive status exams, are mixed and at best suggest a weak association (1). The scant number of studies predicting longitudinal cognitive performance from serum lipids report equivocal findings for change in verbal ability, memory, or perceptual speed traits over a 5 to 10 year period (3-5). Consideration of APOE genotype has not clarified the associations between serum lipids and cognitive change (1, 3, 5). The suggestion of a potential age-moderation of the effect of serum total cholesterol on cognitive health and the insufficient examinations of other serum lipids warrant further longitudinal studies (1, 2).
In particular, apoplipoproteins B (apoB) and A1 (apoA1) have not been examined in studies of cognitive change despite their role in lipid metabolism and associations with relevant health outcomes. ApoB forms the primary protein component of atherogenic lipoprotein particles (e.g. LDL) while apoA1 forms the primary anti-atherogenic lipoprotein particles (HDL) (6). ApoB acts as a receptor ligand for LDL receptors (7) while apoA1 promotes the effusion of cholesterol from tissues (6). ApoB, apoA1, and their ratio (apoB /apoA1) may be more reliable predictors of cardiovascular events and coronary heart disease mortality (7-9) compared to routine lipid measurements. Ratios of apoB to apoA1 of 1.0 or higher may constitute a high cardiovascular risk and between 0.7-0.9 a medium cardiovascular risk (7).
The possibility of nonlinear serum cholesterol-cognition relationships (10) or age-period effects affecting the magnitude of the association between serum lipids and cognitive health (1, 2) may explain the ambiguous relationship between serum lipids and cognitive change in the existing longitudinal studies of cognitive performance. Furthermore, studies have not considered differential lipid-cognition associations by gender, but have typically controlled for gender rather than testing for moderation of effects, despite evidence for differing lipid profiles between older men and women (11-16).
The primary purpose of the current study is to examine the prediction of cognitive change across verbal, spatial, memory and perceptual speed domains from baseline serum lipid values in Swedish adults 50 years and older. In addition to a longer follow-up time of up to 16 years compared to earlier published longitudinal studies, we consider: multiple lipid parameters, multiple cognitive domains, whether lipids have different effects on cognitive trajectories before and after age 65 years, and sex differences. In addition, we consider baseline lipid values in twin pairs discordant for dementia.
Method
Participants
The Swedish Adoption/Twin Study of Aging (SASTA) is a population based-study drawn from the Swedish Twin Registry (17, 18) that includes pairs of twins separated before the age of 11 and reared apart and a sample of twins reared together matched on gender, birth date and county of birth (19). Participants in the in-person testing (IPT) assessments were aged 50 to 96 years of age (59.7% female). The sample for the current analyses includes 819 twins with available lipid and cognitive assessments, representing 96.7% of those tested at IPT sessions1. IPT participants who did not provide blood samples (N = 28) tended to be female (78.6%) and older at baseline (M = 5.11 years older).
Sample sizes at each IPT reflects a combination of loss of participants due to dropout or death and entry of new participants who became age 50. For the current analysis, the sample size at each IPT was 606, 590, 566, 539, and 443, respectively. Those with 1, 2, 3, 4 or 5 occasions of measurement were 79 (9.6%), 169 (20.6%), 191 (23.3%), 146 (17.8%), and 234 (28.6%), respectively. There was no sex difference in the number of IPT measurements (p = .490).
Measures and Procedures
Cognitive performance
Five in-person testing sessions (IPT1, IPT2, IPT3, IPT5 and IPT62) included a cognitive battery assessment: IPT1 took place between 1986 and 1988. The second and third IPT sessions occurred at three-year intervals, IPT5 occurred seven years later and IPT6 at a three-year interval (20). In total, the span of observations is up to 16 years. Cognitive traits include principal component scores formed from the following tests, standardized to IPT1 means and standard deviations to preserve a metric to assess change at subsequent waves: Verbal abilities (Information subtest, Analogies, Synonyms), Spatial abilities (Koh's Block Design, Figure Logic, Card Rotations), Memory (Digit Span, Thurstone's Picture Memory, Names and Faces immediate and delayed recall task), and Perceptual Speed (Symbol Digit, Figure Identification). In addition, the first principal component of all cognitive tests, ‘g’, was formed. All component scores were rescaled as t-scores by adding a constant of 50 and multiplying by 10. Additional information on the cognitive tests is provided elsewhere (21, 22).
Dementia
Individuals aged 60 years and older were screened for cognitive dysfunction at each wave of SATSA data collection. Screening used in-person cognitive performance including the MMSE (23, 24) or, for anyone not visited, the TELE interview (25). Those who screened positive were assessed clinically, following the CERAD standard workup protocol (26). Diagnoses were made by consensus conference, using DSM-IIIR or DSM-IV criteria for dementia, NINCDS/ADRDA criteria for AD, and other standard criteria for other dementias.
As of May 2007, 74 individuals who had been tested with the psychometric battery and who had lipid values available were determined to have developed incident dementia. For these individuals, all data measured concurrent with or subsequent to dementia onset were set to missing. Thus, any cognitive and lipid data prior to dementia onset are included in the longitudinal analyses. We believe that this approach is most consonant with the recognition that some in the study who have not developed dementia may still do so in the future.
Serum lipids
Serum samples extracted from whole blood taken at IPT sessions were frozen at -70°C prior to lipid analysis. Serum triglycerides and cholesterol were measured using calorimetric enzymatic assay procedures at all IPTs (IPT1-IPT3, IPT5-IPT6) (27-30). HDL was measured at IPT1 using a precipitation procedure (27) while later measurements (IPT5, IPT6) used a homogenous method (Abbott Scandinavia, AB). Apolipoprotein A1 and B concentrations were determined by radioimmunoassay at IPT1 (27) and immunoturbidimetric tests (31, 32) at subsequent IPTs (IPT2-IPT3, IPT5-IPT6). ApoA1 and apoB values obtained prior to the establishment of international reference standards (IPT1-IPT3) were transformed according to relevant calibration studies (31, 33). All lipid values are reported in mmol/L units with the exception of apoA1 and apoB which are reported in g/L units.
Lipid values from the first available IPT session were used in the current analyses. Outliers were excluded according to previous work (28). For 96.8% of the sample, lipids were assessed at their first IPT session while for 3.2% of the sample lipids were available from subsequent IPTs.3 Altogether, 67% of the sample had baseline lipid measurements from IPT1, and 33% from later IPTs. There was no significant sex difference in baseline wave (p > .512). No significant differences were found in baseline HDL, apoA1 or apoB levels before and after changes to assay technique (p > .539), adjusting for age, fasting, medication use, and sex. Prior to analyses, triglycerides, HDL, apoB, and the apoB /apoA1 ratio values were log transformed to reduce skew and kurtosis.
Covariates
Covariates were taken from the same IPT session as basline lipids. Covariates controlled for in the analyses included: fasting status (no, yes), smoking status (never, past, current), alcohol use (no, yes), use of prescription or non-prescription medications (none, any medication), body mass index (BMI), and mean arterial pressure (MAP) (see Table 1). Among those who fasted, the average fast time reported was 8.50 hours (SD = 3.70). For analysis, quantitative covariates were mean-centered while non-use was coded as zero for dichotomous or ordinal variables.
Table 1.
Descriptive statistics for baseline lipids and covariates.
| Males | Females | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| HDL | 319 | 1.32 | 0.35 | 455 | 1.61 | 0.43 | 774 | 1.49 | 0.42 |
| HDL (log) | 319 | 0.24 | 0.26 | 455 | 0.44 | 0.26 | 774 | 0.36 | 0.28 |
| ApoA1 | 335 | 1.33 | 0.24 | 484 | 1.54 | 0.27 | 819 | 1.45 | 0.28 |
| ApoB | 335 | 1.40 | 0.29 | 484 | 1.47 | 0.37 | 819 | 1.44 | 0.34 |
| ApoB (log) | 335 | 0.32 | 0.21 | 484 | 0.35 | 0.25 | 819 | 0.34 | 0.24 |
| ApoB/ApoA1 | 335 | 1.10 | 0.30 | 484 | 0.99 | 0.33 | 819 | 1.03 | 0.33 |
| ApoB/ApoA1 (log) | 335 | 0.05 | 0.29 | 484 | -0.06 | 0.33 | 819 | -0.02 | 0.32 |
| Total cholesterol | 334 | 6.22 | 1.07 | 481 | 6.89 | 1.41 | 815 | 6.62 | 1.33 |
| Triglycerides | 334 | 1.67 | 0.97 | 484 | 1.49 | 0.79 | 818 | 1.56 | 0.88 |
| Triglycerides (log) | 334 | 0.38 | 0.51 | 484 | 0.28 | 0.47 | 818 | 0.32 | 0.49 |
| BMI | 335 | 25.85 | 3.37 | 484 | 25.67 | 4.27 | 819 | 25.96 | 3.93 |
| Mean Arterial Pressure (MAP) | 334 | 110.26 | 12.96 | 478 | 108.57 | 13.45 | 812 | 109.78 | 13.22 |
| Variable | N | % | N | % | N | % | |||
| Fasting Status | 335 | 41.2 | 484 | 37.8 | 819 | 39.2 | |||
| Smoking Statusa | 324 | 64.8 | 468 | 28.2 | 792 | 43.1 | |||
| Alcohol Use | 316 | 85.8 | 464 | 66.8 | 780 | 74.4 | |||
| Medication Use | 333 | 59.5 | 479 | 73.3 | 812 | 67.6 | |||
ever plus current smoker shown. Note. log = natural logarithm; BMI = body mass index
Statistics
We applied latent growth models to cognitive data using SAS PROC Mixed with full maximum likelihood estimation (22, 34). The growth model included three latent variables: performance level at age 65 years (i.e., intercept, I), linear slope prior to age 65 years (S1), and linear slope after age 65 years (S2). Observed variables included cognitive scores and corresponding ages at testing. Variances and covariances were estimated for the intercept (I) and the slope components (S1, S2) thereby allowing individual differences in growth. Pair dependencies were accounted for by estimating between- and within-pair variances and covariances.
An unconditional growth model was fit as a comparison to a subsequent model where a baseline lipid parameter was entered as a predictor of the growth components (I, S1, S2). The difference chi-square test with 3 df indicated the extent of improvement in fit by adding the baseline lipid parameter as a predictor of growth processes. Given a significant omnibus chi-square test, individual significance of the lipid parameter on I, S1, and S2 was examined. A follow-up series of models were fit with covariates to determine if the effect of the lipid predictor remained significant. Models were fit separately for males and females.
For descriptive purposes, we present expected cognitive trajectories for significant associations, depicting those at the mean and those one standard deviation above or below the mean on the respective lipid parameter. As the cognitive trait scores are scaled as t-scores with standard deviations of 10 (see above), 8-, 5-, and 3-point differences constitute large (0.8), medium (0.5), and small (0.3) effect sizes, respectively. For comparability with known limits, figures depicting cognitive trajectories predicted from log-transformed lipid and lipoproteins are presented based on back-transformed (exponentiated) units.
Discordant pair analysis
A total of 21 pairs discordant for dementia were identified (6 MZ, 15 DZ; 6 male, 15 female). Pairs were included if the affected twin had lipid values prior to dementia onset and the unaffected cotwin had been followed at least one year after the age of onset of the case. Average onset age was 77.29 years (SD = 7.08 years). Types of dementia included: probable AD (N = 7), possible AD (N = 6), vascular dementia (N = 2), mixed AD and vascular dementia (N = 2), and not otherwise specified (N = 4). Lipid values were obtained on average 6.45 years (SD = 3.81) prior to age of onset in those with dementia.
Results
The average age at which baseline lipids were assessed was 63.76 years (SD = 8.49 years). Median length of longitudinal follow-up was 10.02 years. Sample statistics for serum lipids and covariates are presented in Table 1. Person by occasion samples sizes and descriptive statistics of the cognitive measures are presented in Table 2 including cross-wave correlations.
Table 2.
Descriptive statistics for cognitive trait scores scaled in t-score units.
| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | NPerson* Occasion | M | SD | rocc | NPerson* Occasion | M | SD | rocc | |
| Verbal | 1010 | 53.38 | 9.76 | 0.88 | 1424 | 50.26 | 9.45 | 0.86 | |
| Spatial | 982 | 53.63 | 10.74 | 0.85 | 1380 | 49.22 | 9.92 | 0.79 | |
| Memory | 975 | 50.70 | 10.93 | 0.77 | 1425 | 52.83 | 10.96 | 0.72 | |
| Perceptual Speed | 1031 | 49.19 | 10.52 | 0.84 | 1467 | 49.73 | 10.56 | 0.78 | |
| General cognitive ability (g) | 877 | 53.14 | 10.74 | 0.91 | 1248 | 51.74 | 10.46 | 0.88 | |
Note. rocc = Average Pearson r between testing occasions
HDL
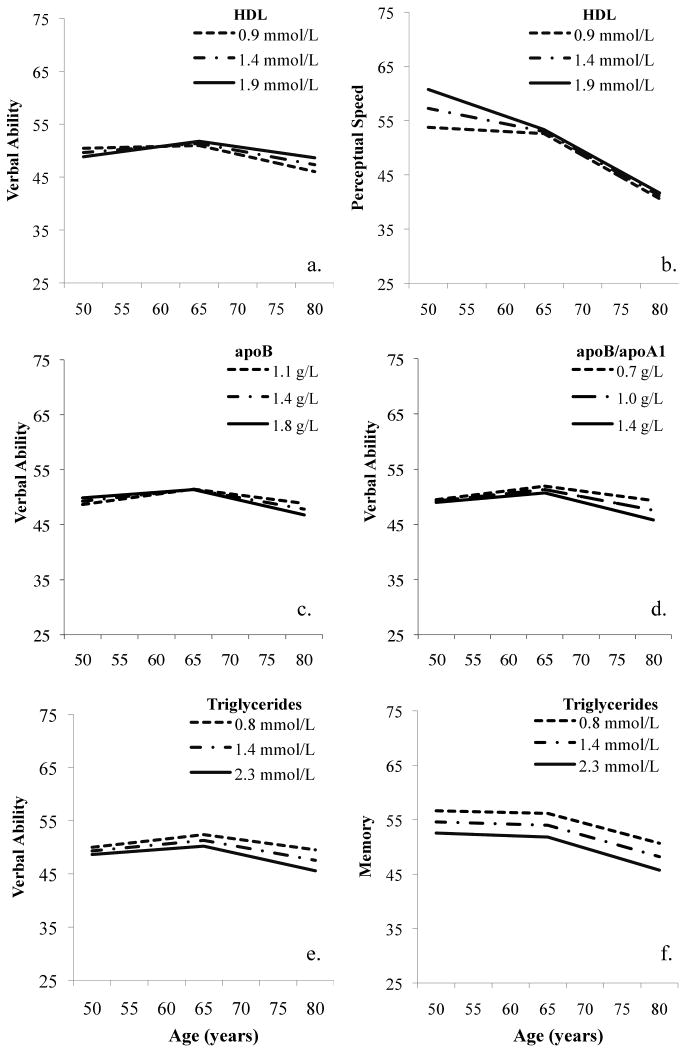
HDL did not predict cognitive change for males in any domain. In females, greater levels of HDL were associated with increasing verbal ability prior to age 65 and greater maintenance of ability after age 65 [Δχ2(3) = 12.0, p = 0.007] (see Figure 1a). HDL predicted change in verbal ability before 65 years (S1) (p = 0.046), and achieved trend significance for change after age 65 (S2) (p = 0.062). The increase in verbal ability from 50 to 65 years was 2.41 points greater in those with a HDL level of 1.9 mmol/L, considered desirable, than those with HDL values of 0.9 mmol/L, considered low. Similarly, decline in verbal ability from 65 to 80 years was 1.81 points smaller in those with a more desirable HDL level at 1.9 mmol/L than those with low HDL at 0.9 mmol/L. HDL remained a significant predictor of verbal ability trajectories when controlling for covariates (p = 0.014).
Figure 1.
Serum lipids and cognitive trajectories in females: (a, b) HDL; (c) ApoB; (d) ApoB/ApoA1 ratio, (e,f) Triglycerides.
In addition, increasing values of HDL predicted better perceptual speed performance but faster rates of change prior to age 65 in women, suggesting that performance benefits wane over age [Δχ2(3) = 15.7, p = 0.002] (see Figure 1b). The prediction of decline before age 65 (S1) was individually significant (p = 0.003). Decline in perceptual speed from 50 to 65 years was 6.23 points greater in those with low versus desirable HDL values i.e., 0.9 mmol/L versus 1.9 mmol/L. Omnibus significance remained when controlling for covariates (p = 0.027).
Results controlling for occasion of HDL measurement lead to virtually identical findings (available upon request).
ApoA1
No significant effects of apoA1 were found for cognitive change in men across any domain. For women, perceptual speed achieved significance [Δχ2(3) = 10.1, p = 0.018] with a pattern similar to HDL where increasing values of apoA1 were associated with better performance but faster rates of change before age 65 (S1, p = .016) (no figure shown). However, significance fell after controlling for covariates (p = 0.117).
ApoB
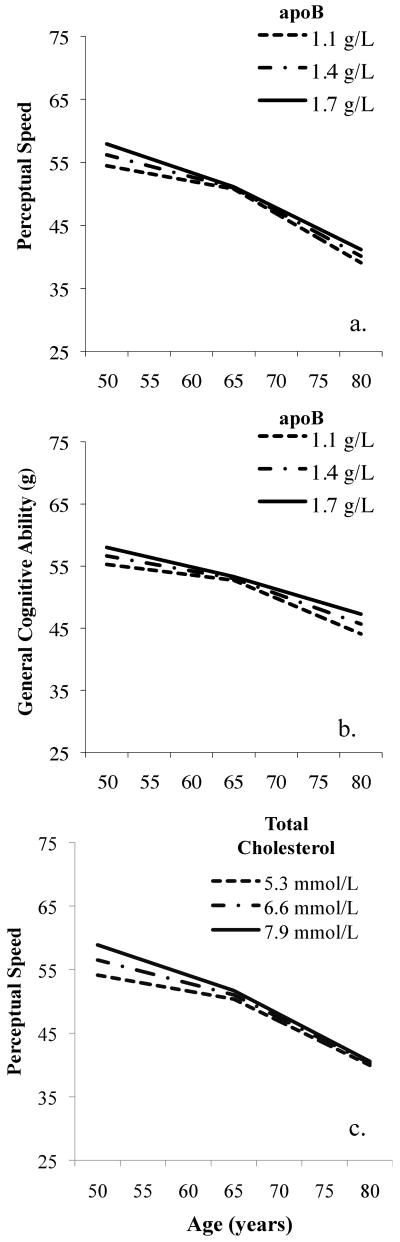
ApoB values predicted perceptual speed in men [Δχ2(3) = 8.5, p = 0.037] where higher apoB values were associated with better performance but steeper rates of decline prior to age 65 (S1) (p = 0.030), suggesting diminishing benefits of higher apoB values (see Figure 2a). From age 50 to 65 years, the loss in perceptual speed was 3.13 points greater at 1.7 g/L compared to 1.1 g/L. Similar trajectory patterns were observed with respect to general cognitive performance (g) [Δχ2(3) = 9.2, p = 0.027] where higher values of apoB predicted steeper rates of decline before age 65 years (S1) at trend significance (p = 0.077) and less steep decline after age 65 (S2) (p = 0.029) (see Figure 2b). When controlling for covariates, omnibus significance increased for perceptual speed (p = .016) but decreased for g (p = .053).
Figure 2.
Serum lipids and cognitive trajectories in males: (a,b) ApoB, (c) Total cholesterol.
ApoB values predicted verbal ability in women [Δχ2(3) = 10.4, p = 0.015] where higher values of apoB predicted faster rates of decline after 65 years (S2) (p = 0.039). From age 65 to 80 years, loss in verbal ability was 2.04 points greater for those at 1.8 g/L compared to those at 1.1 g/L (see Figure 1c). When controlling for covariates, omnibus significance increased (p = 0.003).
ApoB/ApoA1 ratio
No significant effects of the apoB/apoA1 ratio were found for cognitive change in men across any domain. For women, the apoB/apoA1 ratio significantly predicted verbal ability performance [Δχ2(3) = 11.3, p = 0.010] that was maintained after inclusion of covariates (p = 0.015). Trajectory patterns mirrored that observed for apoB alone where higher apoB/apoA1 ratios predicted a faster rate of decline after 65 years (S2) (p = 0.029). From age 65 to 80 years, those with a ratio of 1.4, considered at high risk for cardiovascular events, lost 2.28 points more than those at 0.7, considered at medium risk (see Figure 1d). In addition, perceptual speed achieved significance [Δχ2(3) = 9.3, p = 0.026] with a pattern complementing HDL and apoA1 findings (no figure shown), though significance fell with covariates (p = 0.090).
Total Cholesterol
Total serum cholesterol was associated with perceptual speed in men [Δχ2(3) = 7.9, p = 0.048] where higher values of total cholesterol were associated with better performance though faster rates of decline prior to age 65 (see Figure 2c). Total cholesterol predicted change prior to 65 years (S1) at trend significance (p = 0.058) while performance level at age 65 (I) or change after age 65 (S2) were not individually significant. Decline in perceptual speed between 50 and 65 years is 3.41 points greater at cholesterol values of 7.9 mmol/L, considered high, versus those at 5.3 mmol/L, considered borderline high (see Figure 2c). With covariates, the omnibus significance level remained (p = 0.042).
In women, no significant effects of total serum cholesterol were found across any domain.
Nonlinear effects of total cholesterol on cognitive change were explored in both sexes but no significant findings emerged.
Triglycerides
Higher triglycerides predicted worse performance and faster decline in general cognitive ability (g) prior to age 65 in males [Δχ2(3) = 7.9, p = 0.048] although significance fell after controlling for covariates (p = 0.204) (no figure shown).
Lower values of triglycerides were associated with increased verbal ability in women up to age 65 and greater maintained ability after age 65 [Δχ2(3) = 14.6, p = 0.002] (see Figure 1e). The impact of triglycerides on verbal ability at age 65 years (I) was significant (p = 0.027), with trend significance for change after age 65 (S2) (p = 0.0715). At age 65 years there was a 2.17 point advantage in verbal ability with a triglyceride level of 0.8 mmol/L, considered in the normal range, compared to those at 2.3 mmol/L, considered in the high range. The verbal ability advantage of those with lower versus higher triglycerides nearly doubled by age 80 years (3.94 points). Omnibus significance remained when including covariates (p = 0.0244).
Lower values of triglycerides predicted better memory ability across age for women [Δχ2(3) = 26.4, p < 0.0001] (see Figure 1f). Memory performance at age 65 (I) was better at lower levels of triglycerides (p < 0.0003). At age 65 years there was a 4.20 point advantage with triglycerides at 0.8 mmol/L compared to 2.3 mmol/L. This advantage was maintained from 50 to 80 years. Omnibus significance remained when including covariates (p = 0.0030).
In addition, triglyceride levels predicted spatial ability [Δχ2(3) = 13.2, p = 0.004], perceptual speed [Δχ2(3) = 15.3, p = 0.002], and general cognitive ability (g) in women [Δχ2(3) = 18.6, p < 0.001], with similar patterns to that described for verbal ability and memory (figures not shown). However, when controlling for covariates omnibus significance fell to trend levels (spatial ability, p = .0858; perceptual speed, p = .0858; g, p = .0526).
Lipids in discordant pairs
We next considered 21 pairs discordant for incident dementia, for whom baseline lipid values were assayed for both members of the pair at either the first or second in-person testing occasion (IPT1 or IPT2). The affected twin had significantly higher values on total cholesterol and apoB than their unaffected cotwin (see Table 3). Sex, fasting status, medication use, age at onset in the demented twin, and number of years between the lipid assay and dementia onset in the demented twin were not significant predictors of pair differences (all p > .09). The covariate-adjusted pair differences remained significant for total cholesterol (p = .012) and at trend significance for apoB (p = .067).
Table 3.
Paired t-test analyses of lipid values for twin pairs discordant for dementia.
| Variable | N pairs | Mean Difference untransformed | Mean Difference log transformed | t | p |
|---|---|---|---|---|---|
| HDL | 17 | 0.024 | 0.009 | 0.176 | 0.863 |
| ApoA1 | 21 | 0.030 | --- | 0.566 | 0.578 |
| ApoB | 21 | 0.103 | 0.078 | 2.362 | 0.028 |
| ApoB/ApoA1 | 21 | 0.069 | 0.069 | 1.692 | 0.106 |
| Triglycerides | 21 | 0.067 | 0.017 | 0.164 | 0.871 |
| Total cholesterol | 21 | 0.395 | --- | 2.363 | 0.028 |
Note. log = natural log of values. T-tests for differences between log transformed variables shown where transformation applied. The lipids values for the unaffected twin were subtracted from their affected cotwin where positive differences indicate higher lipid values associated with dementia status.
Discussion
The goal of the current study was to examine predictive relationships of serum lipid and lipoprotein parameters for cognitive change in a population-based sample of twins. Our findings suggest that the impact of lipids and lipoproteins on cognitive trajectories is most prominent prior to age 65. Moreover, the results emphasize the importance of lipids for later life cognition in women: low triglycerides, low apoB and high HDL values were beneficial to maintaining cognitive abilities over age, particularly for verbal ability and perceptual speed. Lipid values were less predictive of cognitive trajectories in men and, where associations were found, were in the contrary direction: higher apoB and total cholesterol levels were advantageous to cognitive performance though effects diminished by age 65. Finally, the importance of lipid and lipoprotein levels was extended from cognitive trajectories to dementia risk: higher apoB and total serum cholesterol levels, measured more than 6 years on average before dementia onset, were found in the affected than in the unaffected twin.
The current study clarified prior equivocal findings by considering whether lipids have distinctive effects on cognitive trajectories before and after age 65. We observed greater predictive relationships between lipids and cognition prior to age 65. This observation is consistent with a recent review and meta-analysis of prospective studies of lipids and dementia suggesting that high midlife cholesterol may be particularly important to later cognitive impairments and dementia as compared to late-life cholesterol values (1). None of the prior studies of lipids and longitudinal cognitive performance considered nonlinear models of cognitive change or that the period of effect may differ (3-5). In two studies where significant lipid-cognitive change effects were reported (3, 4), some or all participants were younger than 65 years at baseline. However, findings for triglycerides and total cholesterol were not consistent between the studies, perhaps because of differing cognitive domains considered or gender composition, i.e., males only (4) versus males and females considered together (3).
Patterns of lipid-cognition relationships differed for males and females, a point not directly considered in recent studies (3, 5). We observed that the direction of effects for women were generally expected from clinical recommendations, i.e., higher levels of HDL or lower levels of triglycerides and apoB values were predictive of better maintained cognitive performance. In contrast, higher serum cholesterol or apoB values in men led to better perceptual speed and general cognitive performance, although that advantage diminished by age 65. The effects of apoB and total cholesterol for men are consistent with a cross-sectional study of young to middle-aged adults up to 59 years of age that reported that higher serum non-HDL- and total cholesterol were associated with faster visuomotor speed performance in men but not women (35). Moreover, Swan and colleagues (4) compared total cholesterol in six male MZ twin pairs discordant for decline on a perceptual speed task (average age 55.2 years) reporting that higher total serum cholesterol was observed for the non-declining twin. Finally, in men with incident dementia from the Honolulu-Asia Aging Study, total serum cholesterol declines occurred 15 years prior to diagnosis, resulting in lower levels than for those who remained intact (36). This observation is further supportive of the current study findings that men with higher cholesterol may better maintain cognitive capabilities.
Broader support that lipid-cognition relationships could differ between men and women can be taken from studies of gender differences in serum lipid patterns. For example, movement of fat in the blood stream is nearly double the rate in women than in men, and greater change in serum lipids are observed in women than men with intake of elevated carbohydrates or fat while reduced benefits accrue with dietary restrictions of fat (37). Furthermore, earlier age-graded changes in serum lipids occur in men than in women (11-16). Finally, lipid profiles differ in men and women in terms of predicting cardiovascular disease (CVD) or coronary heart disease (CHD) where HDL and triglycerides emerge as a stronger or unique predictor, respectively, of CVD or CHD in women compared to men (13, 14, 38).
Prior findings of gender differences in lipid profiles over age are mirrored by gender differences in lipid-cognitive change relationships found in the current study. For men, effects were essentially concentrated before age 65 while for women effects were noted before and after age 65. As the period of effect may extend beyond age 65 for women, gender should be considered in future studies of the effect of lipids on cognitive performance and cognitive health in late life.
The lipoproteins apoA1, apoB, and the apoB/apoA1 ratio have not been examined in prior longitudinal studies of cognition. Recently these markers have been considered to be more reliable than routine lipid measurements to predict relevant outcomes including cardiovascular events (7-9). In the current study, findings for apoA1 were weaker compared to HDL. Meanwhile, ApoB showed stronger findings of association with cognitive trajectories compared to total cholesterol, though both were associated with risk of dementia in the analysis of twin pairs discordant for dementia. While total serum cholesterol was unrelated to cognitive trajectories in women, higher apoB values predicted verbal ability declines after age 65. Higher apoB values in males appeared to confer early advantages on perceptual speed and general cognitive performance but faster rates of decline before age 65, similar to total cholesterol but only observed for perceptual speed. ApoB levels tend to peak and then subsequently decline 10 years earlier in men (∼60 years) than women (∼70 years) (31). Thus, in the current study, higher apoB values in males may reflect better lipid homeostasis and thereby better-maintained cognitive performance until age 65, whereas higher apoB levels in women may confer a particular risk to declining performance after age 65. The apoB/apoA1 ratio showed few relationships with cognitive change and none were unique to that observed for apoB or apoA1. The comparability of apoB to the apoB/apoA1 ratio is consistent with a recent US study of CHD mortality (8).
Limitations
A greater number of significant findings were observed in women than in men, although power may have been reduced in men given a smaller sample size. Power to detect potential sex or age-period effects in the discordant pair analyses was untenable with 21 pairs.
To reduce the number of tests and the chance of type 1 error, we considered omnibus three degree-of-freedom tests of the effect of each lipid on growth trajectories. With that, 30 analyses were performed each for males and for females (six lipid markers, five cognitive traits). Further adjustment of p-values in light of a-priori hypotheses is likely to be overly stringent. Nonetheless, Sidak–adjusted p-values, accounting for correlation among the cognitive traits, would have suggested values less than .0143 to be significant (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm), resulting in 13% of analyses being significant rather than 25%, which is still well above 5% chance.
Conclusions
Cholesterol control is often included in advice about how to maintain cognitive health (e.g., the Alzheimer's Association/CDC Healthy Brain Initiative booklet (39)). Our results suggest that cholesterol control may be most salient for cognitive health in the young-old age period generally, and for women may extend into old-old age periods. Additional research is needed to confirm the noted gender differences in lipid-cognition relationships in the present study. Lastly, temporal relationships between longitudinal lipid and longitudinal cognitive profiles must be examined in future research to verify whether changes in serum lipids lead to changes in cognitive performance.
Footnotes
Ten individuals were determined to have dementia at baseline and were excluded.
Neither the cognitive battery nor lipids were assessed at IPT4; only a brief telephone cognitive screening was completed.
For HDL, assessed at fewer IPTs, 82% had values concurrent with their first IPT session.
References
- 1.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008 May;16(5):343–54. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Solomon A. Cholesterol as a risk factor for Alzheimer's disease -epidemiological evidence. Acta Neurologica Scandinavica. 2006;185:50–7. doi: 10.1111/j.1600-0404.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 3.de Frias CM, Bunce D, Wahlin A, Adolfsson R, Sleegers K, Cruts M, Van Broeckhoven C, Nilsson LG. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. The Journals of Gerontology. 2007 Mar;62(2):P112–8. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- 4.Swan GE, LaRue A, Carmelli D, Reed TE, Fabsitz RR. Decline in cognitive performance in aging twins. Heritability and biobehavioral predictors from the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology. 1992 May;49(5):476–81. doi: 10.1001/archneur.1992.00530290058012. [DOI] [PubMed] [Google Scholar]
- 5.Reitz C, Luchsinger J, Tang MX, Manly J, Mayeux R. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology. 2005 Apr 26;64(8):1378–83. doi: 10.1212/01.WNL.0000158274.31318.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. FASEB J. 2008 December 1;22(12):4044–54. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walldius G, Jungner I. Is there a better marker of cardiovascular risk than LDL cholesterol? Apolipoproteins B and A-I - new risk factors and targets for therapy. Nutrition, Metabolism and Cardiovascular Diseases. 2007;17(8):565–71. doi: 10.1016/j.numecd.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J, Walldius G, Hellenius ML, Hamsten A. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. European Heart journal. 2009 Mar;30(6):710–7. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. 2006 May;259(5):481–92. doi: 10.1111/j.1365-2796.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, McKeown RE, Hajjar I. Serum cholesterol levels are associated with impaired recall memory among older people. Age and ageing. 2005 Mar;34(2):178–82. doi: 10.1093/ageing/afi027. [DOI] [PubMed] [Google Scholar]
- 11.Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, Saugo M, Giacomazzo M, Martini B, Mazza A, D'Este D, Pessina AC. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. Journal of Hypertension. 2008 Oct;26(10):1983–92. doi: 10.1097/HJH.0b013e32830bfdd9. [DOI] [PubMed] [Google Scholar]
- 12.Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA, Borhani NO, Wong ND, O'Leary DH. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation. 1992 Sep;86(3):858–69. doi: 10.1161/01.cir.86.3.858. [DOI] [PubMed] [Google Scholar]
- 13.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999 Mar 9;99(9):1165–72. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 14.LaRosa JC. Lipids and cardiovascular disease: do the findings and therapy apply equally to men and women? Womens Health Issues. 1992 Summer;2(2):102–11. doi: 10.1016/s1049-3867(05)80278-6. discussion 11-3. [DOI] [PubMed] [Google Scholar]
- 15.Schubert CM, Rogers NL, Remsberg KE, Sun SS, Chumlea WC, Demerath EW, Czerwinski SA, Towne B, Siervogel RM. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2006;30(2):251–60. doi: 10.1038/sj.ijo.0803129. [DOI] [PubMed] [Google Scholar]
- 16.Torng PL, Su TC, Sung FC, Chien KL, Huang SC, Chow SN, Lee YT. Effects of menopause on intraindividual changes in serum lipids, blood pressure, and body weight--the Chin-Shan Community Cardiovascular Cohort study. Atherosclerosis. 2002 Apr;161(2):409–15. doi: 10.1016/s0021-9150(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein P, deFaire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen NL, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the Third Millenium. Twin Research. 2002;5(5):427–32. doi: 10.1375/136905202320906219. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen NL, Friberg L, Floderus-Myrhed B, McClearn GE, Plomin R. Swedish early separated twins: Identification and characterization. Acta Geneticae Medicae et Gemellologiae (Roma) 1984;33:243–50. doi: 10.1017/s0001566000007285. [DOI] [PubMed] [Google Scholar]
- 20.Finkel D, Pedersen NL. Processing Speed and Longitudinal Trajectories of Change for Cognitive Abilities: The Swedish Adoption / Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11(2-3):325–45. [Google Scholar]
- 21.Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3(6):346–52. [Google Scholar]
- 22.Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative Genetic Analysis of Latent Growth Curve Models of Cognitive Abilities in Adulthood. Developmental Psychology. 2005;41(1):3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Posner SF, Viitanen M, Winblad B, Ahlbom A. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. Journal of Gerontology: Medical Sciences. 1997 Mar;52(2):M117–25. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen NL, Gatz M, Berg S, Johansson B. How heritable is Alzheimer's disease late in life? Findings from Swedish twins. Ann Neurol. 2004 Feb;55(2):180–5. doi: 10.1002/ana.10999. [DOI] [PubMed] [Google Scholar]
- 25.Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995 Fall;7(3):429–38. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989 Sep;39(9):1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993 Apr 22;328(16):1150–6. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 28.Iliadou A, Lichtenstein P, de Faire U, Pedersen NL. Variation in genetic and environmental influences in serum lipid and apolipoprotein levels across the lifespan in Swedish male and female twins. American Journal of Medical Genetics. 2001 Jul 22;102(1):48–58. doi: 10.1002/1096-8628(20010722)102:1<48::aid-ajmg1388>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clinical Chemistry. 1974 Apr;20(4):470–5. [PubMed] [Google Scholar]
- 30.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry. 1982 Oct;28(10):2077–80. [PubMed] [Google Scholar]
- 31.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clinical Chemistry. 1998 Aug;44(8 Pt 1):1641–9. [PubMed] [Google Scholar]
- 32.Riepponen P, Marniemi J, Rautaoja T. Immunoturbidimetric determination of apolipoproteins A-1 and B in serum. Scandinavian Journal of Clinical and Laboratory Investigation. 1987 Nov;47(7):739–44. [PubMed] [Google Scholar]
- 33.Marcovina SM, Albers JJ, Dati F, Ledue TB, Ritchie RF. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. Clinical Chemistry. 1991 Oct;37(10 Pt 1):1676–82. [PubMed] [Google Scholar]
- 34.Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003 May;39(3):535–50. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Muldoon MF, McKeown RE. Serum cholesterol concentrations are associated with visuomotor speed in men: findings from the third National Health and Nutrition Examination Survey, 1988-1994. The American Journal of Clinical Nutrition. 2004 Aug;80(2):291–8. doi: 10.1093/ajcn/80.2.291. [DOI] [PubMed] [Google Scholar]
- 36.Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Archives of Neurology. 2007 Jan;64(1):103–7. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- 37.Knopp RH, Paramsothy P, Retzlaff BM, Fish B, Walden C, Dowdy A, Tsunehara C, Aikawa K, Cheung MC. Gender differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Current Atherosclerosis Reports. 2005 Nov;7(6):472–9. doi: 10.1007/s11883-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 38.Durstine JL. Gender differences in lipids and lipoproteins after cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2004 Jul-Aug;24(4):257–8. doi: 10.1097/00008483-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention and the Alzheimer's Association. Chicago, IL: 2007. The Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health. Available at http://www.cdc.gov/aging and http://www.alz.org. [Google Scholar]