Abstract
The enlargement and species-specific elaboration of the cerebral neocortex during evolution holds the secret of humans’ mental abilities; however, the genetic origin and cellular mechanisms generating the distinct evolutionary advancements are not well understood. This article describes how novelties that make us human may have been introduced during evolution, based on findings in the embryonic cerebral cortex in different mammalian species. The data on the differences in gene expression, new molecular pathways and novel cellular interactions that have led to these evolutionary advances may also provide insight into the pathogenesis and therapies for human-specific neuropsychiatric disorders.
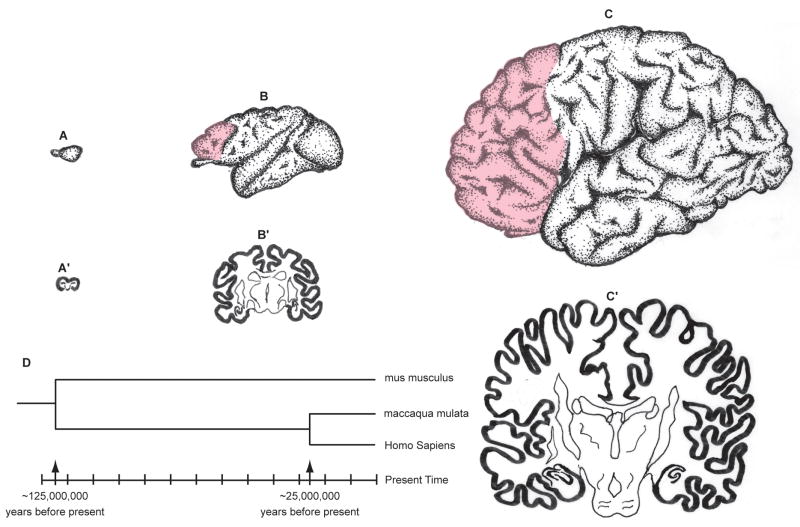
The neocortex, as the name implies, is the newest addition to our brain and is considered to be the crowning achievement of evolution and the biological substrate of human mental prowess. Although its origin can be traced to reptiles1,2, that have emerged during the Carboniferous Period, it first appears as a uniform, six-layered sheet consisting of radially deployed neurons in the early small mammals that emerged from their reptilian ancestors during the transition of the Triassic/Jurassic periods. Increases in size and complexity of the cerebral cortex has culminated in the modern human that had separated from the mouse line between 90 and 100 million years ago and from the Old World monkeys, such as macaque, 25 million years before the present time (FIG. 1D and REFS. 3,4). If any organ of our body should be substantially different from any other species, it is the cerebral neocortex, the center of extraordinary human cognitive abilities. It is, therefore, surprising how little modern research has been done to elucidate how this human difference emerged. It appears that we are sometimes so seduced by similarities between species that we neglect the differences.
Figure 1.
Cerebral hemispheres of the mouse (A), macaque monkey (B) and human (C) drawn at approximately the same scale to convey the overall difference in size and elaboration. The pink overlay indicates the prefrontal cortex that has no counterpart in mouse. The sections across cerebra of the same species (A′, B′, C′) illustrate the relative small increase in the thickness of the cortex compared to a large difference in surface of 1: 100: 1000 X in mouse, macaque monkey and human, respectively. D. The time-scale of phylogenetic divergence of mus musculus, maccaca mulata and homo sapiens based on the DNA sequencing data (Reviewed in REFS. 3, 4)
There are several possible explanations for this tendency. First, most contemporary researchers of the cerebral cortex, including myself, work on the mouse as the best and most economical experimental model system5. However, although the basic principles of cortical development may be similar in all mammals, the modifications of developmental events during the millennia of primate evolution produced not only quantitative, but also qualitative changes in its cellular composition and pattern of synaptic circuitry. Second, we all have accepted the concept, advocated aptly by Charles Darwin, that the biological world is unified, and that “there is no fundamental difference between man and the higher mammals in their mental faculties”6. As a result, we hope that all basic human traits can be deduced from this commonality. Thirdly, there is a widespread perception that research on the development of the human brain is basically descriptive. However, the unprecedented possibilities for applying the most advanced methods of molecular and cell biology to the developing human brain is rapidly changing this perception.
There are numerous examples of quantitative and qualitative differences between the rodent and primate neocortex. However, these examples are based on comparative anatomical studies done on the adult brains, and surprisingly little is understood about how these differences have emerged during evolution. Since the excavated skulls of our common predecessors are empty, the best, and perhaps the only way to understand cortical evolution at the cellular level is by comparison of the differential gene expression and developmental events during embryonic development in living mammals7. This approach, known as Evo-Devo (BOX 1 and REF. 8) has now replaced the old and controversial, now generally discredited Haeckel’s law that “Ontogeny is the brief and rapid recapitulation of phylogeny”. Evo-Devo reflects the realization that modern developmental neurobiology can explain how the genetic information contained within progenitor cells regulates the number, phenotype, migration and allocation of neurons in the developing brain where they establish species-specific patterns of synaptic connections. Ideally, the Evo-Devo approach when applied to human embryos could provide sufficient information, but for obvious ethical and logistic reasons, experimental manipulation is not possible in human; and, thus, comparative analysis of the developmental events in other mammalian species has to be used to reveal the origin of evolutionary novelties in the cerebral cortex. The hope is that understanding these events will help to unravel the secret of how these events have emerged during evolution.
Box 1. Evo-Devo approach to reconstruct neocortical evolution.
The use of modern approaches and advanced methods to study embryonic brain development in living species provides insights into possible cellular and molecular mechanisms that have enabled the evolutionary expansion and elaboration of organs in extinct common ancestors. The new subfield of developmental biology called Evo-Devo, was created by a desire to elucidate how novel features may have been created by gene mutation and, if inherited, propagated during evolution by natural selection7,8. However, the contemporary Evo-Devo approach should be distinguished from the 19th century Heckle’s law of ontogenetic recapitulation of phylogeny (for historical review in REFS. 1,2,7). The Evo-Devo approach has gained strength in recent years from the modern research of gene expression and experimental manipulation of embryonic development in model organisms142. It assumes that developmental events altered by gene mutations within gene clusters, which change the coding for proteins that create new developmental mechanisms and new structures can give us insight into how they were established in extinct ancestors. The Evo-Devo approach embraces the concept of modularity or functional cellular units, which range from body segments, somites, or limb buds to the cortical columns.. Alterations and elaboration of such modules, if inheritable, often result in lethality; but, if useful enhance the survival and propagation of species.
Evo-Devo is an accepted and rapidly growing research field. Thus, the present article is, by necessity, based on only selected data focused on cortical development in the human, non-human primate and rodent embryonic forebrain that are relevant for understanding neocortical evolution. For example, experimental inhibition or overexpression of specific genes in the embryo allows to test their affect on development of the cerebral cortex35,38,75. In addition, it is possible to explore the timing of expression of a compendium of new genes and their modification directly in the embryonic human cortex128. Based on the available data from rodents, human and non-human primates, we can now propose models of how gene expression within the neural stem cells and progenitors in the proliferative embryonic zones control the number of columnar units, regulate neuronal migration and allocation into proper laminar and radial locations in the cortical plate, where they promote differentiation into species-specific phenotypes, establish new patterns of synaptic connections and determine the expression of particular neurotransmitters and receptors within the cytoarchitectonic areas subserving specific functions139. Thus, the Evo-Devo approach represents a scientific way to imagine how this may have happened in our long extinct common ancestors which we will never be able to verify in real time.
In this review, I will provide selective examples of what evolutionary concepts can be deduced from the developmental studies (Evo-Devo) carried out in embryonic brains of rodents (mostly mus musculus), non-human primates (mostly maccaca mulata) and in human (homo sapiens) (FIG. 1). Although there is a large body of data on other mammalian species, I have selected only these three because of the space limitations and because they were the subjects of research in my laboratory. I made this decision with a full understanding that the only thing they share is a common, long extinct, ancestor. Although comparative anatomical and DNA sequencing data indicate that these three, presently living species, belong to different branches on the phylogenetic tree, they effectively illustrate different levels of neocortical expansion and elaboration (FIG. 1), which is often taken, implicitly and erroneously, as an indication of the lineage continuity between them, with human at the top.
Peculiarities of the Cerebral Cortex
The evolution of the cerebral neocortex cannot be fully appreciated without understanding the unusual way that it is built in each individual. There are several features of cortical development that differ from the development of other organs of the body and even from the rest of the brain.
First, the cerebral cortex in all mammalian species, including human, is a cellular sheet composed of projection (or pyramidal) and local circuit neurons (or interneurons) deployed in horizontal layers, intersected by vertical (or radial) columns that are stereotypically interconnected in the vertical dimension and share extrinsic connectivity9–10. However, this apparent cytoarchitectural uniformity exhibits different degrees of variability in each area depending on their function. This compartmentalization is particularly pronounced in the large and convoluted human neocortex, with more that 50 distinct cytoarchitectonic areas that were described more than a century ago11.
Second, a remarkable feature of cortical development in all mammalian species is that none of its constituent neurons is generated within the cortex itself. Rather, they are generated in the transient proliferative embryonic zones (VZ, SVZ) situated near the surface of the cerebral lateral ventricles; and, after their last cell division, then acquire their proper areal and laminar positions through long-distance radial and tangential migration across intermediate zone (IZ) (reviewed in REFS. 12–17). Neurons migrating radially to the increasingly distant cortex follow the shafts of a transient population of radial glial (RG) cells 18–20. A detailed description of the complex migratory process and its underlying complex and multiple molecular mechanisms cannot be described in this short essay. Figure 2, conveys the basic concepts, accompanied by two animated movies on the website to illustrate the dynamic nature of cortical development (#1: http://rakiclab.med.yale.edu/MigratingCorticalNeuron.html and #2 http://rakiclab.med.yale.edu/RadialMigration.html.
Figure 2.
Figure 2A. Radial unit lineage model of cortical neurogenesis. The model illustrates how changes in the mode and the rates of cell proliferation and/or programmed cell death within the neural stem cell pool (red circles) in the ventricular zone (VZ) that divide symmetrically (SD) at early embryonic stages causes an exponential increase in the number of radial columns. That, in turn, results in surface expansion of the cerebral cortex (CC) without changes in its thickness. In contrast, similar changes in proliferation kinetics occurring in the founder cells (blue circles), which divide asymmetrically (AsD), cause a linear increase in the number of neurons within radial columns without a change in the cortical surface area. Based on the radial unit hypothesis12,32. Reproduced from REF. 140).
Figure 2B. The model radial neuronal migration which underlies columnar organization based on REF. 12,32, reproduced from REF.139. The cohorts of neurons generated in the ventricular zone (VZ) traverse the intermediate (IZ) and subplate zones (SP) containing “waiting” afferents from several sources (CC, TR, MB, MA) and finally pass through the earlier generated deep layers before settling in at the interface between cortical plate (CP) and marginal zone (MZ). The positional information of the neurons in the VZ and corresponding protomap within the SP and CP is preserved during cortical expansion by transient radial glial scaffolding. You can access the animated version (Rakic/Leydon model) by clicking at: http://rakiclab.med.yale.edu/RadialMigration.html
Third, not only do the postmitotic neurons migrate to the overlaying cortex, but also each generation bypasses the previous one, a phenomenon known as the “inside-out gradient of neurogenesis”21, a phenomenon that is particularly pronounced in primates, in which the cohorts of isochronously generated neurons eventually occupy a relatively narrow strata within the cortex (REF 22 and Animated Movie #2: http://rakiclab.med.yale.edu/RadialMigration.html). The sole exception to the inside-out sequence of neurogenesis in primates has been observed in layer I, where, contrary to rodents, neurogenesis continues throughout the entire period of corticogenesis23. The biological significance of the inside-to-outside sequence of settling of neurons in the cerebral cortex is not clear24, but if disturbed, by either genetic or environmental factors, individual neurons display abnormal cortical function25. Only recently have methodological advances in molecular, cellular and developmental neurobiology given us an opportunity to study mechanisms involved in neuronal migration at a level that was not possible just a few years ago (Reviewed in REFS. 26–28). In most mammals, including human, corticogenesis is completed before or around the time of birth29, and, cortical neurons, once they reach their final position, last throughout adult life without replacement29.
Human ascent through cortical expansion
The first step in the evolutionary ascent of the human cerebral cortex is its enlargement, which occurs mainly by expansion of the surface area without a comparable increase in thickness (FIG. 1A′, B′, C′). It is counterintuitive that such a large expansion of the cortex is achieved by the substantial prolongation, rather than reduction in duration of the cell cycle30–31. Genetic dissection of basic cellular events and unraveling the kinetics of neural stem cell proliferation in mice, as well as the analysis of corticogenesis in human and non-human primates, provides some insight into the possible cellular mechanisms of cortical surface expansion during development and evolution32. The modest, twofold increase in cortical thickness is in part due to the enlargement of neurons and neuropile in the extracellular space.
An explanation of the cellular mechanism for the enormous cortical expansion in surface without a comparable increase in thickness has been offered by the radial unit hypothesis (FIG. 2 and REF. 12). According to this model, an increase in the number of neural stem cells by symmetrical divisions before the onset of neurogenesis would result in an exponential increase in the number of founder cells that give rise to radial cortical columns (FIG. 3). At later stages the number of neurons increases linearly within each column mostly by asymmetrical divisions of neural progenitors32. For example, fewer than 7 extra rounds of cell divisions in the VZ at the early embryonic stages can account for the 1,000-fold difference in total cortical surface area between mouse and human (FIG. 1A′, B′, C′ and FIG. 3). The fact that the human forebrain primordium is much bigger than in the mouse and monkey, even at embryonic stages before the onset of cortical neurogenesis19,33,34, supports this model. Furthermore, this model predicts that additional neurons will follow adjacent shafts of RG cells and form more columns, resulting in an expanded laminar rather than nuclear shaped structure. However, radial unit hypothesis does not implies homogeneity of units across the species. On the contrary, there is a large body of evidence that radial columns differ across cerebral hemisphere and in change considerable in size and composition during cortical evolution (BOX. 2).
Figure 3.
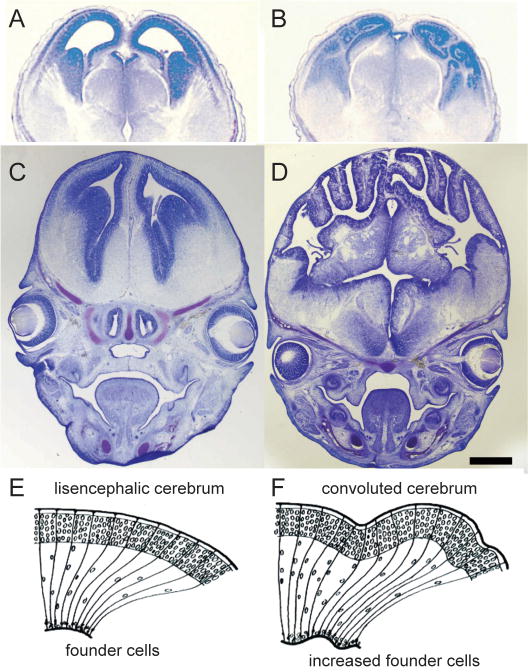
Enlargement of cortical surface by decrease in programmed cell death or increase in proliferation. Example of wildtype mouse (A) and a littermate in which both copies of Caspase 9 were deleted, later leading to expansion of the progenitor pool, increased number of radial columns, and convoluted cortical surface (B). (C, D), Enlarged brains and heads of β-catenin transgenic mice with horizontal expansion of precursor population. Mid-coronal section through the forebrain stained with cresyl violet of an embryonic day 15.5 wild-type littermate control (C) and comparable section of a transgenic animal (D) expressing a Δ90β-catenin–GFP fusion protein in neural precursors. The forebrain of transgenic animals is enlarged overall, with increased surface area and folding of the epithelial surface. Drawings in E and F are the models of how an increased number of progenitors can be distributed via radial glial scaffolding to form a sheet, rather than a lump of postmitotic neurons. A and B. reproduced from REF. 35; C and D, from REF.38, with permission.
Box 2.
During evolution, the neocortical surface expands by the addition of radial columns23,30 (FIG. 2), but the composition of the columns also undergoes changes. The notion of the homogeneity of the columns141 has been abandoned in favour of their heterogeneity, both in different functional areas of an individual, as well as across species142,143. In all mammals, functional columns consist of an intermix of projection (excitatory, glutaminergic) neurons and inhibitory (GABAergic) interneurons that are stereotypically interconnected in the vertical dimension144 but receive diverse extrinsic input9,10,140. To achieve proper cellular composition, several neuronal clones move between their parental radial glial cells within the subventricular zone (SVZ) and the intermediate zone (IZ) of the embryonic cerebral wall145. The disturbance of this intermixing, essential for proper cortical operations, may underlie disorders of selected cognitive functions143. However, although this lateral shift of migrating neurons in the SVZ–IZ was recognized 35 years ago based on reconstruction from serial electron microscopic sections145, the molecular mechanisms that regulate this intermix of neuronal clones has been elusive. A recent study using a combination of the most advanced molecular technology showed that, during radial neuronal migration, the lateral intermixing of neurons that belong to different clones within a given functional column depends on the expression levels of A-type Eph receptor tyrosine kinase and their ligands, ephrin-As146. Eph/Ephrin-dependent intermixing in the SVZ and IZ may be only one of several mechanisms for allocating proper composition of neuronal phenotypes that have been operating during mammalian evolution. However, it can serve as an example of how developmental studies in mice may provide an insight into mechanisms of evolutionary elaboration of columnar composition that may have happened in our extinct ancestors.
Experimental tests of the radial unit hypothesis
The radial unit model has been put to the test by the manipulation of cell production in the VZ by either diminishing the rate of programmed cell death (apoptosis), or by increasing the rate of neural stem cell proliferation. Mice deficient in Caspase 3 and 9 proteases, which are essential for the normal process of programmed cell death (apoptosis), an essential process during development35,36, have a larger number of neural stem (founder) cells and radial glial cells (RG). These cells, in turn, produce more than the normal number of radial columns and, hence, have a larger cortical surface, which then begins to form incipient convolutions (FIG. 4AB and REF. 37).
Figure 4.
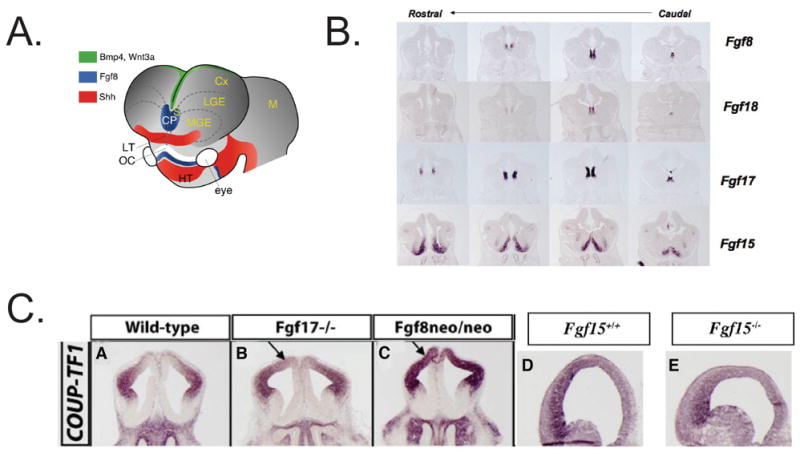
Control of arealization of the frontal cortex by Fgf expression. A. Patterning centers in the forebrain from REF 141. Fgf expression is in the rostral patterning center (also called commissural plate, CP). B. Fgf expression in the rostral patterning center in coronal sections of an E12.5 mouse telencephalon from REF. 76 C. Transcription factors expressed in the E12.5 rostral telencephalon; arrows show approximate boundaries of their expression in the anlage of the frontal cortex CX: Cortex; LGE: lateral ganglionic eminence; SE: septum. Dotted lines: cortical/subcortical boundary. (Faedo and Rubenstein, unpublished). D. Repression of COUP-TF1 expression by Fgf17 (Fgf17−/− null mutant) (B), Fgf8 (hypomorphic neo/neo mutant) (C). Promotion of COUP-TF1 expression by Fgf15 (Fgf15−/− null mutant) (E). Coronal sections of E12.5 telencephalon from REFS. 76,77.
Similarly, an even more dramatic increase in cortical surface occurs with an experimentally induced increase in neuronal production. For example, the β-catenin-mediated signaling pathway acts on the decision of the neuronal precursor cells to exit the cell cycle38 and hence, influences the number of postmitotic neurons. Thus, transgenic mice expressing a stabilized form of β-catenin transgene in neural precursors develop enlarged brains with an increased number of precursor (founder) cells in the VZ that lead to a larger number of radial columns and the formation of elaborate cerebral convolutions in the mouse, that normally has a smooth (lissencephalic) hemispheric surface (FIG. 4B, C and REFS. 38, 39). Despite severe additional brain abnormalities in these mice, many of the overproduced neurons migrate radially, and settle into apparently normal laminar and radial positions. Thus, it is both impressive and instructive that the expansion of the precursor pool can generate a much larger surface of the neocortex of relative uniform thickness, rather than just lumps of the ectopic neurons. In contrast, over-expression of cyclin-dependent kinase (cdk) inhibitor p27, which acts on neuronal progenitors at later developmental stages, decreases the production of neurons destined for the more superficial layers within the radial columns without increasing its surface40.
Mice that are deficient for caspase 9 or carry mutations in β-catenins or cdk are not viable, and thus, it is unlikely that mutation of these particular genes were involved in neocortical evolution. However, these experiments can serve as an example of the Evo-Devo approach to theorize how a single gene mutation, that may have occurred in our common, long extinct ancestors, could increase the number of proliferative founder cells in the VZ, which triggers a cascade of events that lead to an increase in the number of radial units and expanded cortical surface without an increase in its thickness. It also shows that these types of genetic and cellular mechanisms, by which the neocortex may have expanded during evolution, are research goals that can be achieved using methods of modern developmental neurobiology. It is indeed remarkable that by a single gene mutation, a lissencephalic (smooth) neocortex typical of the rodent brain can be transformed into a gyrencephalic (convoluted) cortex that is characteristic of the primate cerebral hemispheres.
Development of convolutions
While the cerebral cortex in rodents is smooth, as the cortical surface expands during evolution in many species with a larger cerebrum, it began to form convolutions, which are particularly prominent in primates (FIG. 1). It has been recognized as common sense that the increase in cortical surface has to have lead to its folding. However, formation of convolutions involves rather complicated logistical problems: one of them being that the pattern of convolutions is not random, but highly reproducible within each species with relatively small individual variations. To this account, several hypotheses for a role of the long cortico-cortical axonal tracts in this process have been proposed41–43. Indeed, the formation of the convolutions in the human cerebrum is associated with the formation of the voluminous intermediate zone that eventually transforms into subcortical white matter. This zone is, in primates, crisscrossed by the large number of distinct axonal fascicles forming cortico-cortical connections that contribute to the shape of the convolution. It has been hypothesized that the tension created by these fascicles is responsible for the stereotyped shape and orientation of the convolutions in gyrencephalic brains43.
Evolutionary adaptation of radial glial cells
The transient population of radial glial (RG) cells in the developing cerebral cortex serves both as neural stem cells as well as guides for migration of their offspring and adjacent neuronal progenitors44–46. However, formation of convolutions requires longer, curvilinear migratory pathways and more durable RG shafts, as they continue to serve as guides for neuronal migration from the VZ to the distant cerebral cortex at later fetal stages. For example, in the primate forebrain, many RG cells transiently stop dividing while their fibers span the entire cerebral wall, retaining their endfeet at the ventricular and pial surfaces47. A single RG fiber in primates may serve as a guide for cohorts of up to 30 simultaneously migrating neurons, including some of the interneurons that at late stages of corticogenesis, originate from the expanded subventricular (SVZ) zone48,49 and from the larger pool of progenitors with short radial processes46,50,51. It was suggested that greater stability and longevity of the RG scaffolding, which consists in part of the elongated fibers of non-dividing RG cells, may be an essential evolutionary adaptation to enable proper allocation of neurons to the expanded and convoluted cerebral cortex32,49. Indeed, the RG in both human and non-human primates differentiate precociously and, for example, express GFAP and Vimentin at the onset of corticogenesis, which is much earlier than in rodents, where these proteins are expressed only after birth48, 50–55. Thus, even basic cell types in the embryonic forebrain, such as the RG, have diversified during evolution to accommodate the needs for cortical expansion and the development of cerebral convolutions.
Multiplication and elaboration of cortical maps
During evolution, functionally distinct cortical areas expand at individual rates and new areas are added, forming an elaborate mosaic56. For example, Broca and Wernicke areas or the prefrontal granular cortex cannot be either anatomically or functionally identified in rodents (FIG. 1 and REFS. 4,10,57). The history of the ideas about the developmental mechanisms that could explain how this elaborate parcellation has occurred in evolution, and the early debates about whether structural differences between areas are induced in the initially homogeneous population of cortical neurons by the patterned activity of the inputs arriving from the periphery via the thalamus (tabula rasa hypothesis); or, whether the cortical progenitors themselves are targets of evolution (protomap hypothesis), has been recently reviewed28,58,59 and will not be repeated here. In brief, the consensus that has emerged from the experimental manipulations and neurogentic analyses in both rodents and primates is that the species-specific diversity of the neocortical areas originates from differential gene expression in the neural stem cells in the embryonic VZ, and this information is transferred radially via migrating postmitotic neurons to the overlying cortical plate. Thus, each area actually attracts appropriate inputs, rather than being specified by them. However, activity-dependent mechanisms have a significant role at subsequent stages of synaptic refinements of the already formed connections12,60–62. Recent experimental studies performed in mice shed some light on how the cortical areas expand at a species-specific differential rate and how new cytoarchitectonic areas might have been introduced during evolution.
Duplication through morphogenetic factors
The evolution of the cerebral cortex involves both an increase in the number of new cytoarchitectonic areas, as well as duplication of sensory representations63. It is generally accepted that the regionalization of the neocortex is initiated by patterning centers that secrete signaling molecules such as the fibroblast growth factors (FGFs), wingless (Wnts) and bone morphogenetic protein (BMP), which regulate the position and size of cortical areas (Reviewed in REFS. 28,64,65). For example, the mouse forebrain has an anterior patterning center at the commissural plate that secretes Fgf8 and Fgf17. Fukuchi-Shimogori and Grove64 have shown that perturbations of the site and expression level Fgf8 can profoundly change the somatosensory map of the mouse cerebral cortex: overexpression of Fgf8 level in the embryonic forebrain induced by electroporation results in formation of an additional barrel field, with a mirror image representation of mouse whiskers64. The mechanism of this remarkable duplication of the well defined cortical area is unknown, but it is instructive that Fgf8 expression is coordinated by distinct sets of transcription factors, namely the zinc-finger and sonic hedgehog signaling systems that are involved in neuronal production and specification65–69. Similar duplication of the sensory areas with a reverse representation of the periphery has occurred during evolution; and, these examples show how it could occur rapidly by a small genetic change70.
Decisions in the proliferative centers
Numerous recent studies demonstrated that the subdivisions and area enlargements of the cortical areas in embryonic mice could be traced back to the proliferative VZ and SVZ zones (Reviewed in REFS. 28,58,65). For example, the expression of Fgf8 in the anterior parts of the telencephalon suppresses the posteriorly expressed chick ovalbumin upstream transcription factor I (COUP-TFI) and homeobox (EMX2) in the telencephalic neuroepithelial cells65, 71–74, decreasing their anterior but increasing their posterior expression. The development of frontal and sensory subdivisions is dependent on the graded action of Fgf874 and Fgf1775,76 and provides an elegant example of how one area, such as the frontal cortex, can expand differentially and independently of the growth rate of other areas (FIG. 4C and REFS. 76,77). Furthermore, the size of an area can be regulated by the change in Fgf17 expression at early embryonic stages independent of the synaptic input from the periphery via the thalamus (FIG. 4 and REF. 75). The fact that it is now possible to enlarge and/or duplicate distinct cytoarchitectonic areas in the neocortex by genetic manipulation opens an unprecedented opportunity to explore how these maps develop in each individual, as well as how they may have been introduced during neocortical evolution. As a next step, it is important to search for the additional genes and morphoregulatory molecules that may be involved in cortical specification, and to develop rodent, and possibly non-human primate models, of cortical dysgenesis that mimic specific genetic or acquired cortical disorders in human78. Thus, the creation of transgenic mice, in which the number of neurons and radial units is increased, serves as an example of how a larger-than-normal number of founder cells can create a cortex with an increased surface area and number of neurons that eventually form new connections.
In the spirit of Evo-Devo reasoning, the above examples show that it is possible to experimentally duplicate a cortical area in embryos with a proper topographical representation by changing the expression of various morphoregulatory factors. Again, as in the case of cortical expansion, we do not know whether genes that produce these particular molecules have been involved in areal enlargement and/or duplication during evolution. However, these studies illustrate how a single mutation could have a sudden and profound effect during evolution on the pattern of cortical parcellation and gives us some insights into how it could have occurred at the genetic and cellular level70.
More neurons more connections
Obviously, an increase in the number of neurons, radial columns and cytoarchitectonic areas is not sufficient to explain the multitude of functional advances made in the human cerebral cortex, which must also involve the elaboration of neuronal connections (e.g., REFS. 2,62,79). There is a large amount of comparative anatomical data showing that, in spite of enormous variety and inter-individual variability, the basic pattern of neuronal connection is species-specific and highly reproducible. It is generally agreed that functional usefulness of the evolutionarily new cortical connection generated initially by random mutation must be validated through the process of natural selection and, if proven advantageous, may contribute to the survival and propagation of the species. However, since neurons are generated before they form axonal connections, an increase in neuronal number, diversification of their types and attainment of their positions is the first steps in cortical evolution. As reviewed below, the new classes of neurons form new pattern of connections. In this complex process, innate neuronal activity is essential for the validation and refinement of synaptic connections that leads to their selective stabilization and/or elimination via competitive interactions80–86. This is a large research field that deserve separate review article and cannot be considered here in sufficient and deserving detail. However, within the subject of cortical evolution, it is important to mention that there should be made a clear distinction between the formation of stereotypic, species-specific connections, that are established by surface mediated attractions and innate activity-dependent refinements, from the experience-dependent synaptic plasticity that shapes connections in cooperation with the outside environment in each individual. These later, experience-dependent changes, are not inheritable, and thus, cannot contribute to evolutionary advances, which requires gene mutation in germinal cells.
Introduction of new types of cells and migratory pathways
Apart from an increase in the number and allocation of neurons into distinct cytoarchitectonic areas, the evolution of the cerebral cortex has also been accompanied by an introduction of evolutionarily new cell types that migrate via old or new routes to the appropriate, often distant locations. During 100 million years of separation from a common ancestor, the mouse and human cerebral cortex has acquired a number of different features, only some of which are briefly described below.
The Subpial Granular Layer (SGL)
This prominent cellular layer situated below the pial surface of the fetal human neocortex was described by O. Ranke more than a century ago, but was examined in a more systematic way by Brun87 and Gadisseux et al.88. It is considered to be another transient embryonic layer, but so far, it has not been observed in rodents17. Although very prominent and voluminous in the developing neocortex of human and non-human primates23, because of its absence or negligent size in mice, it has not been sufficiently explored. There are only a handful of studies on its developmental history and function of the SGL in human and non-human primates. It appears around the 11th gestational week in the human Marginal Zone (MZ), peaks in size during mid-gestation and disappears by the time of birth17,33,89. Analysis of proliferative activity in the developing cat and primate cerebrum indicate that the SGL produces neurons that may descend to the underlying cortical plate 87,23. Based on the uneven size and high incorporation of tritiated thymidine in the SGL overlaying the developing cortex in monkey embryos, it has been hypothesized that it contributes to a wealth of interneurons in certain cortical areas, such as the primary visual cortex, which has an extraordinary large layer IV23. Thus, better characterization of the subpopulations of interneurons that originate in the SGL may help in elucidating their function as well as their possible involvement in cortical disorders that do not occur or can not be mimicked in mice.
Migration from the Ganglionic Eminence to the Thalamus
Another human-specific migratory pathway formed during midgestation by neurons streaming from the Ganglionic Eminence (GE) of the ventral telencephalon to the dorsal thalamus of the diencephalon, called the corpus gangliothalamicum (CGT), was discovered four decades ago90. This voluminous, easily identifiable stream of migrating bipolar neurons in human could not be found in any other species that have been examined33, 91. More recently, cell labeling in organotypic slice cultures of human embryonic brain tissue enabled tracing the migration of these cells directly from the GE to the dorsal thalamic association nuclei92. These migratory cells, which rely on homotypic-neurophilic guidance and express Dlx1/2 homeodomain-containing proteins that guide their tangential migration, eventually form GABAergic neurons in the dorsal thalamus, in particular, the pulvinar and mediodorsal nuclei, that are anatomically related to the association neocortex involved in higher cognitive functions, including symbolic reasoning or language92. Thus, it appears that the human thalamus, in order to accommodate the increased axonal input from the expanding association neocortex, recruits an additional complement of neurons from the nearby, still mitotically active GE in the ventral telencephalon after the diencephalic proliferative VZ becomes exhausted92. This large, easily identifiable migratory stream in the human fetus can not be analyzed experimentally, as it is not present in any other species so far examined, including rodents, carnivores and New and Old World primates.
Two origins of interneurons - a working hypothesis
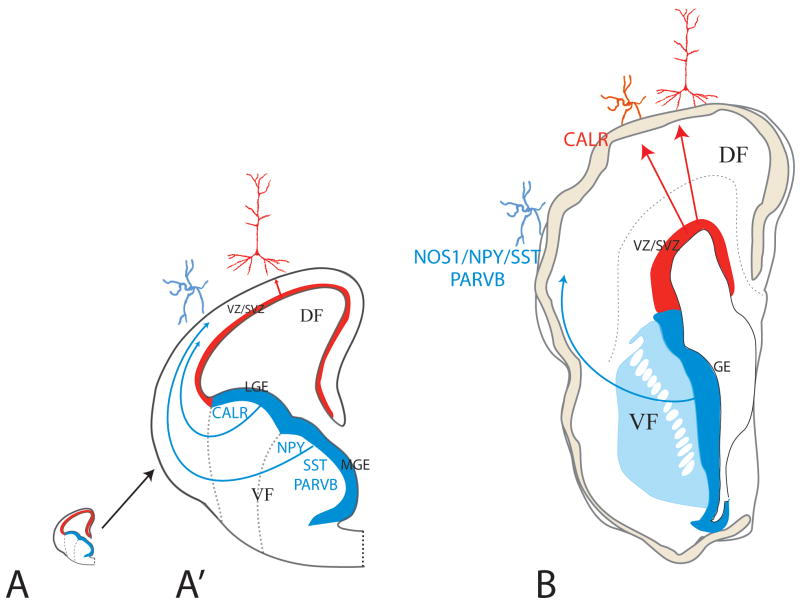
It was Ramon y Cajal93 who suggested that extraordinary human mental abilities are closely related to the increase in the number and diversity of cortical interneurons that he discovered using the Golgi silver impregnation method. However, since that time, many subclasses of primate-specific interneurons have been characterized94,95. In spite of their larger number and higher diversity in primates, our knowledge of the origin and development of the interneurons has been derived almost exclusively from experiments in rodents where we can use more advanced methods. Thus, tracing cell lineages by the use of retroviral vectors in mice showed that interneurons originate from different progenitors than the projection neurons96. In addition, the concept that the great majority, if not all, of the interneurons originate from the GE in the ventral pallium and then migrate tangentially to the dorsal telencephalon, comes from ingenious experiments performed in mouse (FIG. 6A,A′ and REFS. 14,97–100).
Figure 6.
A. Neocortical areas of the human fetal cerebral hemispheres examined by Johnson et al.(2009) included four distinct subdivisions of the prefrontal cortex (PFC) (blue): orbital, medial, dorsolateral and ventrolateral (Brodman’s areas: 9, 13, 32,44, 45) and perisylvian areas (1–3,4,40,41,42) (yellow). In addition to the ventrolateral PFC, which encompasses the prospective Broca’s area, two other speech and language-related areas were assayed: parietal and temporal association cortices, which encompass the prospective Geschwind’s and Wernicke’s areas, respectively. B-C. Examples of different clusters of correlated gene expression patterns in the PFC and perisylvian (C) cortical areas (Adapted from REF. 124).
We know little about origin of GABAergic interneurons in other species. Experimental studies in primates, particularly human, are by their nature limited or impossible; and, the suggestion that the enlarged number of interneurons in human originate from the voluminous subventricular zone can be traced to classical histological observation33. However, more recently, the few studies that have succeeded to explore the origin of interneurons in these species with more advanced methods, uniformly suggest that cortical GABAergic neurons seem to originate from both the GE as well as the VZ/SVZ in these species (Fig. 6B and REFS. 101–106). This stands in contrast to the exclusively GE origin in rodents. Importantly, the Letinic et al., fate-mapping experiments in human mid-fetal slice cultures suggest that the percentage of cortical GABAergic neurons arising from the dorsal pallium VZ/SVZ is higher than those arising from ventral progenitors101. Furthermore, it has been shown that proliferative (BrdU+) cells in slices of the dorsal human proliferative VZ/SVZ during mid gestation express ventral transcription factors (Dlx1,2 and Nkx2.1)105 and that bipolar morphology and orientation of Dlx+ cells also indicate local origin and radial migration89. These studies collectively support the idea that in primates, there are two types of interneurons with respect to their origin: those that are born in the GE of the ventral telencephalon such as in rodents; and, in addition, those that are born in the VZ/SVZ of the dorsal telencephalon (FIG. 6B). Both GE- and VZ/SVZ-derived interneurons use GABA as a neurotransmitter, but their morphology as well as their molecular characteristics indicate that their function may be different and related to the human-specific higher cortical functions.
Recently, Fertuzinhos et al.106 took advantage of the severe ventral midline defects present in the human congenital disorder named holoprosencephaly (HPE). In HPE, the development of the ventral forebrain structures, including the medial GE, is incomplete. Examination of the distribution of various classes of cortical GABAergic interneurons showed that the neocortex and hippocampus of HPE specimens with severe ventral forebrain hypoplasia lack specific subtypes of cortical GABAergic neurons that in mice are known to derive from the GE, while other subtypes seem to be little or not affected by ventral midline defects, suggesting that they could possibly originate in the preserved dorsal pallium VZ/SVZ. These findings, based on the detailed analysis of expression of various cell-specific markers in the fetal and infant brains of HPE specimens with mild and severe ventral forebrain defects, as well as in age-matched controls, revealed that NOS1/NPY/SST- and parvalbumin-positive cells are consistently absent in both fetal and infant stages while calretinin-positive GABAergic cell numbers seem to be little or not affected. Furthermore, the absence of NOS1/NPY/SST- and PVALB-positive cells correlated with the dramatic depletion of TITF1-positive medial GE progenitors, which in mice are the main source of these subtypes of interneurons. Although this type of study on human genetic malformations cannot be conclusive, the results suggest that NOS1/NPY/SST- and PVALB-positive cells originate in the GE and migrate tangentially into the cortex, while the calretinin-positive cells could have arisen from the dorsal neuroepithelium of the lateral ventricular wall and migrated to the cortex radially together with glutamatergic projection neurons. The study cannot eliminate the possibility that the calretinin-positive cells could have arisen from less affected GE progenitors. It is noteworthy that the neurons expressing NOS1/NPY/SST are most numerous in the derivatives of the MZ and subplate zone (SPZ) in monkeys107. In contrast, the calretinin expressing interneurons populate cortical layers II-VI and include the double bouquet cells that are absent from the rodent cortex (108,109, 110; reviewed in REF. 95).
The finding of evolutionarily novel origins that produce different subtypes of interneurons in human and non-human primates does not negate the significance of the tangential migration of cortical GABAergic cells from the GE that were discovered in the mouse. In fact, studies in human101,102,106 and cynemogoulus monkey103 indicate that the GE produces a significant portion of interneurons that migrate to the neocortex via the tangential route. However, human and non-human primates possess a greater diversity of inhibitory interneurons that have to be recognized and better understood to uncover a possible role in human psychiatric disorders95 as the VZ/SVZ generated interneurons may be involved in a variety of disorders of higher brain function111.
Predecessor Neurons
The most recently discovered new type of neuron in human, the Predecessor cell, has so far not been observed in any other species34. These large, bipolar cells are the earliest generated neurons in the human forebrain that emerge under the pial surface of the ventro-lateral cerebral wall at the end of the 1st gestational month, before completion of the neural tube closure and before onset of neurogenesis in the VZ/SVZ of the dorsal telencephalon. They are TU20 and Tbr1 positive, have long horizontal processes and form an extensive network over the forebrain primordium34. We have speculated that predecessors may be a transient population, involved in determining the number of functional radial units in the cerebral cortex, but experimental proof is missing. As a next step, it is essential to examine where they exist in non-human primates in order to determine their lineage, pattern of gene expression, function and eventual fate.
The selected examples of human-specific developmental features and cellular events described above are by no means exclusive. Such evolutionary novelties are likely the result of gene mutations and/or the introduction of new genes and/or the addition of the developmental mechanisms and cell-cell interactions that act during the progenitor’s exit from the mitotic cycle. They generate a different outcome depending on new evolutionary contexts. Recent studies indicate that even subtypes of the pyramidal cells destined for layer V, that are the main efferent system connecting the cortex with subcortical structures, are determined at the time of their last division in the VZ112–114. These experimental studies in mice indicate that evolutionary novelties that have emerged during evolution, including introduction of new neuronal types, may start with a mutation of the neuronal stem cells in the proliferative zones.
Small is big: Genetic differences
One of the most frequently repeated statements, which has almost become a cliché, is the emphasis on how small the genetic differences are between humans and the rest of the animal kingdom. Indeed, only about 1% of the genes are human specific; and yet, their effect on timing, sequence and level of gene expression have obviously great functional significance. For example, the human brain is most notably characterized by expansion in size and complexity of the association areas of the neocortex, most notably in the prefrontal cortex (PFC) but also in the perisylvian areas related to speech and language processing115. During development these areas in human have a several fold larger subplate zone that consists of multipolar neurons and incoming “waiting” thalamic axons116 and they mature at a slower pace more than the other areas117. Furthermore, some, if not most, of the specific functional PFC subdivisions do not exist in lower mammals and their development, therefore, cannot be modeled easily in mice4,10. Yet, it is precisely these areas that are probably most significant for the uniquely human mental abilities, as well as neuropsychiatric disorders such as dyslexia, autism or schizophrenia.
Although microarray and other techniques have been available for more than a decade and have been performed in mice118,119, genome-wide expression studies to date of the human brain have been few. The most comprehensive studies in terms of brain regions or developmental time-points surveyed were done on aged brains120, 121, or on comparisons between disordered or diseased and healthy brains123–124.
Region specific differential gene expression
A recent study from Johnson et al.125 has provided a major step forward, examining 13 brain regions from both the left and right sides of the mid-fetal human brain. Using the most advanced generation of microarrays, these researchers profiled over one million known and predicted exons in nine distinct neocortical areas, including four subdivisions of the prefrontal cortex, as well as the temporal and parietal association areas of the perisylvian cortex (Figure 7A), thus enabling researchers to look for the first time on a genome-wide scale at the genetic and molecular mechanisms that distinguish these areas from each other and from the primary sensory cortical areas during development. The data revealed a huge number of transcriptional differences, including genes both differentially expressed and alternatively spliced. For example, the authors found that while the greatest distinction within the neocortex is between lobes – most notably between the prefrontal and non-frontal areas – there are also expression patterns common to the perisylvian areas (VLPFC, prospective Broca’s area; PAS, prospective Geschwind’s area; and TAS, prospective Wernicke’s area), which, although distributed across three lobes, are functionally linked by their involvement in speech and language. The functions of these genes commonly enriched in perisylvian areas might therefore hold some clues to the evolution of structure and connectivity that allowed for the development of language in our species. In addition, Johnson et al. have noted that the prospective Broca’s area (and its right hemisphere homolog) is, on the basis of gene expression patterns, more similar to the neighboring motor cortex than to the PFC125. Interestingly, these authors did not identify any statistically significant left-right transcriptional asymmetry at the population level, concluding that such asymmetries most likely occur earlier during fetal gestation as previously suggested126; but, also noting that more sensitive technologies, as well as greater sample sizes, may yet uncover such asymmetry at the late mid-fetal stage.
The application of weighted gene co-expression network analysis to microarray-derived expression data has previously provided unique insights into the genetic mechanisms of human brain evolution and disease127. Applying this analysis to their data from the developing human brain led to identification of networks of genes linked both to brain regions, including the prefrontal cortex and to biological processes such as alternative splicing125. Genes with the highest degree of interaction within such networks, called “hubs”, may represent critical transcription or other regulatory factors, and are prime candidates for functional characterization.
Several recent studies indicate that tissue- and cell type-specific enhancers play critical roles in neuronal specification and development128; and, other work has identified subsets of these enhancers that, through recent evolutionary modification, may have led to the emergence of novel gene expression patterns and neocortical areal phenotypes129. By generating such spatially comprehensive expression data in the fetal neocortex, Johnson et al. have opened the door to the systematic investigation of the relationship between such recently evolved enhancers and transcriptional specificity. They identified specific examples of genes differentially expressed in the developing human neocortex associated with putative recently-evolved enhancers, providing a short list of candidate genes and regulatory elements for functional characterization. They further found that the number of such associations was statistically disproportionate relative to the genome-wide frequency of such putative enhancers, providing further evidence that regulatory evolution may have played an important role in the evolution of the human cortex.
The recent data in human124 have uncovered an order of magnitude of greater transcriptional differences between neocortical areas than has been obtained in comparable studies in rodent128,129. For example, the gene CNTNAP2, previously studied for its role in autism and specific language impairment132–135 is specifically and highly enriched in the orbital prefrontal cortex, an area involved in regulation of social behavior in humans and which has no comparable analog in rodents124. The mouse homolog Cntnap2 has not been found to be expressed in any areal pattern or gradient in the mouse brain at any stage of development (REF. 136, and M.B. Johnson and N. Sestan, personal communication). Thus, this gene exemplifies the importance of studying the human cortex, wherein structures and gene expression patterns not found in rodents give rise to abilities, such as language, but also disorders, such as autism, for which there is also no currently accepted mouse model 137. Importantly, the type of data promises to provide new insights into the complex transcriptional and molecular underpinnings of cortical evolution and development of human specific neuronal and synaptic organization.
Future analyses contrasting these fetal human brain gene networks with comparable data from mouse (as has been done for human and chimpanzee adult brain138) should aid in identifying those genes whose importance in cortical development has increased in the course of human brain evolution. Although the importance of spatial and temporal specificity of gene expression during development and evolution has long been recognized138), it is only recently that the molecular mechanisms of this specificity have begun to be investigated due to availability of the complete genome sequences of human, mouse and other mammalian species.
Conclusion
This type of research gives us confidence that the advent of the human in the universe, achieved through expansion and elaboration of the cerebral cortex, can and will eventually be elucidated using the Evo-Devo approach. Based on the recent findings from the descriptive and experimental embryonic studies of various mammalian species, we can develop realistic models of how gene mutations within the cell progenitors during evolution affect neuron number, regulate their migration into proper regions, promote formation of new phenotypes that establish new areas with new connections which express particular sets of neurotransmitters and receptors. Neglecting or minimizing evolutionary differences between the species may be among the reasons for the failures of many clinical trials that were based exclusively on the highly promising findings in rodents. However, the Evo-Devo approach to studying corticoneurogenesis is not only important to the compelling problems of congenital disorders of higher cortical functions in humans, but also provides a hint about how we may have evolved to be masters of our destiny.
Figure 5.
The schematic illustration of half of a cross-section through fetal rodent (A) and human (B) forebrains at the peak of corticogenesis at approximately same scale (A′ is an enlargement of the mouse forebrain to render the drawing legible). In rodents (A′), the main source of interneurons is the ganglionic eminence (GE) of the ventral telencephalon, which then migrate tangentially to the neocortex in the dorsal telencephalon. In contrast, interneurons in the human forebrain originate both in the GE as well as locally in the ventricular zone/subventricular zone (VZ/SVZ) of the dorsal telencephalon subjacent to the neocortex. Interneurons originating from the cortical VZ/SVZ and GE express different set of markers. (Figure based on REF. 142 (mouse) and REFS. 101,106 (human).
Acknowledgments
I am grateful to the former and present members of my laboratory whose skills, wisdom, hard work and insightful discussions made this article possible. I am also grateful to the U.S. Public Health Service and private philanthropic organizations that provided funding over the past four decades including NINDS, NEI, NIMH, NIDA, March of Dimes, NARSAD, NAAR and MOD Foundations and the Kavli Institute for Neuroscience at Yale.
Glossary
- Transient embryonic layers
Layers identified in the embryonic brain, such as proliferative ventricular (VZ) and subventricular (SVZ) zones or migratory intermediate (IZ) zone that lack direct counterparts in the adult brain, as defined by the Boulder Committee (see REF 17)
- Patterning center
Group of cells in the embryonic brain that secretes molecules (morphogens) which initiate differential expression of transcription factors that specify formation of the cortical areas
- Cortical parcellation
Regionalization of the cerebral neocortex into areas with distinct structural and functional attributes
- Homotypic–neurophilic guidance
Mode of neuronal migration along the surface of other neurons that depends on membrane bound adhesion molecules present on both migrating and guiding neurons as opposed to heterotypic gliophilic migration that is guided by the shafts of radial glial cells
- Association areas
Areas of the neocortex that are particularly large in the human cortex (e.g. prefrontal granular cortex (PFC) or language related Broca and Wernicke areas) are considered as analyzers for integration of information from various sensory and motor areas
- Network of genes
A collection of genes which are co-regulated or interact with each other
- Enhancers
A short region of DNA that can be bound with the proteins including transcription factors to enhance transcription levels of genes in gene clusters
References
- 1.Striedter GF. Principles of Brain Evolution. Sinauer; Sunderland, MA: 2005. [Google Scholar]
- 2.Northcutt RG. Evolution of the telencephalon in non-mammals. Ann Rev Neurosci. 1981;4:301–350. doi: 10.1146/annurev.ne.04.030181.001505. [DOI] [PubMed] [Google Scholar]
- 3.Murphy WJ, Pevzner PA, O’Brian SJ. Mammalian phylogenomic comes of age. Trends Genet. 2004;20:631–9. doi: 10.1016/j.tig.2004.09.005. A concise and informative review of the DNA sequencing-based time-scale of phylogenetic divergence of various mammalian species. [DOI] [PubMed] [Google Scholar]
- 4.Preuss TM. The cognitive neuroscience of human uniqueness. In: Gazzaniga MS, editor. The Cognitive Neuroscience IV. The MIT Press; Cambridge, MA: 2009. in the press. [Google Scholar]
- 5.Goffinet AM, Rakic P, editors. Mouse Brain Development. Springer-Verlag; Berlin; New York: 2000. [Google Scholar]
- 6.Darwin C. Descent of Man. J. Murray; London, Great Britain: 1871. [Google Scholar]
- 7.Gould SJ. Ontogeny and Phylogeny. Harvard University Press; Cambridge, MA: 1977. [Google Scholar]
- 8.Carroll SB. Evo-Devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Mountcastle VB. The evolution of ideas concerning the function of the neocortex. Cereb Cortex. 1995;5:289–295. doi: 10.1093/cercor/5.4.289. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory in Handbook of Physiology. In: Plum F, editor. The Nervous System, Higher Functions of the Brain. Section I. Part 1. V. Chapter 9. Bethesda, Md: Am. Physiol. Soc; 1987. pp. 373–417. [Google Scholar]
- 11.Brodmann K. Beitrage zur histologischen localization der grosshirnrinde dritte mitteilung: Die rinderfelder niederen affen. J Psychol Neurol. 1905;9:177–226. [Google Scholar]
- 12.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. A review the initial evidence that phenotype, as well as the laminar and areal position of cortical neurons are specified at the proliferative zones and are only later influenced by the incoming axonal input (Protomap Hypothesis). The article also proposes the Radial Unit Hypothesis of cortical development and evolution that has recently been supported by genetic and cell biological methods (see also references 38, 39, 65, 75, 76) [DOI] [PubMed] [Google Scholar]
- 13.Rakic P. Principles of neuronal cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 14.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. A comprehensive and highly informative review on the pattern of neuronal migration to the cerebral cortex with a particular emphasis on the tangential migration of GABAergic interneurons from the ganglionic eminence of the ventral telencephalon (see references 98, 99) [DOI] [PubMed] [Google Scholar]
- 15.Molnár Z, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–34. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Bystron I, Blakemore C, Rakic P. Development of human cerebral cortex.. Boulder Committee revisited. Nature Review Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. A historical review of the discoveries of the transient embryonic zones and with an update of their nomenclature. [DOI] [PubMed] [Google Scholar]
- 18.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. This paper presents evidence from a combination of the Golgi method and serial electron microscopy that postmitotic neurons in the large and convoluted primate cerebrum follow the increasingly elongated and curvilinear shafts of the radial glial cells, some of which do not divide while serving transiently as migratory guides (see reference 47) [DOI] [PubMed] [Google Scholar]
- 19.Sidman RL, Rakic P. Neuronal migration with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. This is the first and the most comprehensive review of the modes and patterns of neuronal migration in the embryonic and fetal human brain with a special emphasis on the cerebral and cerebellar cortices (see reference 33) [DOI] [PubMed] [Google Scholar]
- 20.Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 22.Rakic P. Neurons in the monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;l83:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 23.Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboitiz F, Montiela J, Lópeza J. An hypothesis on the early evolution of the development of the isocortex. Brain Research Bulletin. 2002;57:481–483. doi: 10.1016/s0361-9230(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 25.Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Ann Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. The review of the early evidence of the data from spontaneous mutation in mice showing that the basic neuronal phenotype reflects the time of neuron origin irrespective of their subsequent laminar positions. This finding lead to the conclusion that neurons attract appropriate thalamic input rather than being initially equipotent and specified by the type of input as previously assumed (see references 75, 137) [DOI] [PubMed] [Google Scholar]
- 26.Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 2000;23:352–9. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- 27.Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–3. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: Making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakic P. Pre and post-developmental neurogenesis in primates. Clinical Neurosci Res. 2002;2:29–39. [Google Scholar]
- 30.Kornack DR, Rakic P. Changes in cell cycle kinetics during the development and evolution of primate neocortex. Proc Nat Acad Sci USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–50. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 32.Rakic P. A small step for the cell - a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. Review of the mechanisms of neocortical expansion with a suggestion on how a single or only few genes can shift the timing from symmetric to asymmetric mode of cell division in the embryonic ventricular zone and can suddenly and exponentially change the surface area of the neocortex. [DOI] [PubMed] [Google Scholar]
- 33.Sidman RL, Rakic P. Development of the human central nervous system. In: Haymaker W, Adams RD, editors. Histology and Histopathology of the Nervous System. C.C. Thomas; 1982. pp. 3–145. [Google Scholar]
- 34.Bystron I, Rakic P, Molnar Z, Blakemore C. The first neurons of the human cerebral cortex. Nature Neurosci. 2006;9:880–885. doi: 10.1038/nn1726. The authors use the newest immunocytochemical methods on fresh tissues from the early stages of the embryonic human telencephalon to discover a previously unrecognized cell class, termed “predecessor neuron”. Both the evolutionary implication and medical significance of this finding in the human cortical primordium are discussed. [DOI] [PubMed] [Google Scholar]
- 35.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking Caspase-9. Cell. 1998;94:325–33. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 36.Kuida K, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 37.Haydar TF, Kuan CY, Flavell RA, Rakic P. The Role of cell death in regulating the size and shape of the mammalian forebrain. Cereb Cortex. 1999;9:621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- 38.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 39.Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- 40.Tarui T, et al. Overexpression of p27(Kip1), probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb Cortex. 2005;15:1343–1355. doi: 10.1093/cercor/bhi017. [DOI] [PubMed] [Google Scholar]
- 41.Richman DP, Steward RM, Hutchinson JW, Caviness VS., Jr Mechanical model of brain convolutional development. Science. 1975;189:18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- 42.Goldman-Rakic PS, Rakic P. Experimental modification of gyral patterns. In: Geschwind N, Galaburda AM, editors. Cerebral Dominance, The Biological Foundation. Harvard University Press; Cambridge, MA: 1984. pp. 179–192. [Google Scholar]
- 43.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 44.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. The experimental evidence, obtained from the live images of retrovirally labeled cells in the slices of the embryonic mouse cerebral wall, that radial glial cells can produce neurons that directly or after additional mitotic divisions, migrate and form radial columns in the overlaying cortical plate. [DOI] [PubMed] [Google Scholar]
- 45.Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. Review of the evidence that radial glial cells can produce neurons (see reference 44) [DOI] [PubMed] [Google Scholar]
- 46.Gal JS, et al. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmechel DE, Rakic P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature. 1979;227:303–305. doi: 10.1038/277303a0. [DOI] [PubMed] [Google Scholar]
- 48.Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: Morphogenesis and transformation into astrocytes. Anat Embryol. 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- 49.Rakic P. Elusive radial glial cells: Historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- 50.Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: An ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. The first evidence that neuronal and glial cell lines can be distinguished at the initial stages of corticoneurogenesis in primates, the finding that has been confirmed in human post-mortem material (see references 54, 55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levitt P, Cooper ML, Rakic P. Early divergence and changing proportions of neuronal and glial precursor cells in the primate cerebral ventricular zone. Dev Biology. 1983;96:472–484. doi: 10.1016/0012-1606(83)90184-7. [DOI] [PubMed] [Google Scholar]
- 52.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acid protein in radial glial cells and astrocytes of the developing rhesus monkey brain. Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 53.Kadhim HJ, Gadisseux JF, Evrard P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: Histochemical and electron microscopic study. J Neuropath Exp Neurol. 1988;47:166–188. doi: 10.1097/00005072-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- 55.Howard BM, et al. Radial glia cells in the developing human brain. Neuroscientist. 2008;14:459–73. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 57.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 58.O’Leary DDM, Borngasser D. Cortical ventricular zone progenitors and their progeny maintain spatial relationships and radial patterning during preplate development indicating an early protomap. Cereb Cortex. 2006;16(Suppl 1):i46–i56. doi: 10.1093/cercor/bhk019. This review article reviews evidence that the initial neuronal phenotypes for the prospective species-specific pattern and size of cytoarchitectonic areas are indicated early in the proliferative zones (see Protomap Hypotheses references 13, 65) as well as recent experimental evidence (see references 75, 76) [DOI] [PubMed] [Google Scholar]
- 59.Lukaszewicz AC, et al. The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb Cortex. 2006;16(Suppl 1):i26–34. doi: 10.1093/cercor/bhk011. [DOI] [PubMed] [Google Scholar]
- 60.Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- 61.Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981;214:928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- 62.Shatz CJ. Impulse activity and the patterning of connections during cns development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 63.Kaas JH, Preuss TM, editors. The Evolution of Primate Nervous Systems. Vol. 4. Elsevier; Oxford, U.K: 2007. Evolution of Nervous Systems. [Google Scholar]
- 64.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. The authors use in utero microelectroporation-mediated gene transfer to introduce an extra source of FGF8 into the occipital pole which results in an extra somato-sensory (barrel) cytoarchitectonic field with a nearly perfect duplication of the topographic map. [DOI] [PubMed] [Google Scholar]
- 65.Mallamaci A, Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur J Neurosci. 2006;23:847–56. doi: 10.1111/j.1460-9568.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- 66.Sahara S, Kawakami Y, Izpisua Belmonte JC, O’Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rash G, Grove EG. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 69.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Nat Acad Sci(USA) 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rakic P. Neurocreationalism: making new cortical maps. Science. 2001;294:1011–1012. doi: 10.1126/science.294.5544.1011. [DOI] [PubMed] [Google Scholar]
- 71.Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 72.Garel S, et al. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 73.Storm EE, et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–44. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 74.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 75.Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci (USA) 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. The experimental evidence that prospective cytoarchitectonic areas are specified in the proliferative zones, as predicted by the protomap hypothesis (see reference 12). The authors also show that the manipulation of cell proliferation rate in the VZ can independently change the size of a selected cortical area (see reference 64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol. 2008;509:144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borello U, Cobos I, Long JE, Murre C, Rubenstein JLR. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Development. 2008;3:17. doi: 10.1186/1749-8104-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubenstein JLR, Rakic P. Genetic control of cortical development. Cereb Cortex. 1999;9:521–52. doi: 10.1093/cercor/9.6.521. [DOI] [PubMed] [Google Scholar]
- 79.Krubitzer L, Kaas J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr Opin Neurobiol. 2005;15:444–53. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–12. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 81.Rakic P, Riley KP. Overproduction and elimination of retinal axons in the fetal rhesus monkey. Science. 1983;209:1441–1444. doi: 10.1126/science.6828871. [DOI] [PubMed] [Google Scholar]
- 82.Rakic P, Riley KP. 1983 Regulation of axon numbers in the primate optic nerve by prenatal binocular competition. Nature. 1983;305:135–137. doi: 10.1038/305135a0. [DOI] [PubMed] [Google Scholar]
- 83.Huttenlocher PR, de Courten C, Gare LJ, Van der Loos H. Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247–52. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 84.Rakic P, Bourgeois JP, Eckenhoff ME, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 85.LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shatz CJ. Form from function in visual system development. Harvey Lect. 1997–1998;93:17–34. [PubMed] [Google Scholar]
- 87.Brun A. The subpial granular layer of the foetal cerebral cortex in man. Acta Pathol Microbiol Scand Suppl. 1965;179:1–98. [PubMed] [Google Scholar]
- 88.Gadisseux J-F, Goffinet AM, Lyon G, Evrard P. The human transient subpial granular layer: An optical, immunohistochemical, and ultrastructural analysis. J Comp Neurol. 2004;324:94–114. doi: 10.1002/cne.903240108. The detailed study of the subpial granular layers in the developing human cerebral cortex, that, in spite of its large size in human (see reference 87) is neglected in the literature because of its absence in rodents. [DOI] [PubMed] [Google Scholar]
- 89.Rakic P, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003;13:1072–83. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- 90.Rakic P, Sidman RL. Telencephalic origin of pulvinar neurons in fetal human brain. Z Anat Entwickl-Gersch. 1969;129:53–82. doi: 10.1007/BF00521955. [DOI] [PubMed] [Google Scholar]
- 91.Letinic K, Kostovic I. Transient fetal structure, the gangliothalamic body, connects telencephalic germinal zone with all thalamic regions in the developing human brain. J Comp Neurol. 1997;384:373–395. doi: 10.1002/(sici)1096-9861(19970804)384:3<373::aid-cne5>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 92.Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nature Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. The use of contemporary methods, including retroviral labelling in the slices of embryonic human brain tissue, to follow migratory pathway of a class of thalamic internerons from their origin in the telencephalic ganglionic eminence to the diencephalons, to settle in the thalamic association nuclei. This uniquely-human migratory stream was initially observed by classical histological methods (see reference 90) [DOI] [PubMed] [Google Scholar]
- 93.Ramón y Cajal S. Textura del sistema nervioso del hombre y vertebrados. Vol. 2. Moya; Madrid, Spain: 1899. [Google Scholar]
- 94.DeFelipe J. Cortical interneurons: from Cajal to 2001. Progr Brain Res. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- 95.Jones EG. The origins of cortical interneurons: Mouse versus monkey and human cerebral cortex. doi: 10.1093/cercor/bhp088. Advance Access published May 8, 2009. [DOI] [PubMed] [Google Scholar]
- 96.Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb Cortex. 1991;1:463–491. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- 97.DeCarlos JA, LopezMascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson S, Mione M, Yun K, Rubenstein JLR. Differential origins of neocortical projection and local circuit neurons: Role of Dlx genes in neocortical interneurogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 99.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. A study of the origin and tangential migration of early generated GABAergic interneurons from the medial portion of the ganglionic eminence of the ventral telencephalon to the cerebral cortex (See also comprehensive review on this subject in references 14, 98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–17. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Letinic K, Zoncu R, Rakic P. Origin of GABAegic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. The evidence obtained by using retroviral labeling in the supravital slices of the embryonic human forebrain, showing that a large proportion of interneurons in the primates are generated in the local SVZ and migrate radially to the suprajacent cortex (see references 102,103,106). The evolutionary and medical implication for human-specific psychiatric disorders is discussed (see reference 111) [DOI] [PubMed] [Google Scholar]
- 102.Petanjek Z, Dujmović A, Kostović I, Esclapez M. Distinct origin of GABA-ergic neurons in forebrain of man, nonhuman primates and lower mammals. Coll Antropol. 2008;32(Suppl 1):9–17. [PubMed] [Google Scholar]
- 103.Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rakic S, Zecevic N. Emerging complexity of cortical layer I in humans. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- 105.Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fertuzinhos S, et al. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp009. Advance Access published on February 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hendry SHC, Jones EG, Emson PC. Morphology, distribution and synaptic relations of somatostatin and neuropeptide y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramon y Cajal S. In: Histologie du Système Nerveux de l’Homme et des Vertébrés. Azoulay L, editor. II. Maloine; Paris, France: 1911. [Google Scholar]
- 109.DeFelipe J, Gonzalez-Albo MC, Del Rio MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol. 1999;412:515–526. doi: 10.1002/(sici)1096-9861(19990927)412:3<515::aid-cne10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 110.Hendry SHC, et al. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76:467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- 111.Lewis DA, Levitt P. Schizophrenia as disorder of neurodevelopment. Ann Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 112.Chen JG, et al. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–7. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen B, Schaevitz R, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molyneaux BJ, et al. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–31. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 115.Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- 116.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. The detailed temporal and regional description of the Subplate Zone in developing human and non-human primate cerebrum which shows its enlargements subjacent to the prospective association areas (see reference 117) [DOI] [PubMed] [Google Scholar]
- 117.Kostovic I. Structural and histochemical reorganization of the human prefrontal neocortex during perinatal and postnatal life. Prog Brain Res. 1990;85:223–239. doi: 10.1016/s0079-6123(08)62682-5. [DOI] [PubMed] [Google Scholar]
- 118.Zapala A, et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc Natl Acad Sci USA. 2005;102:10357–62. doi: 10.1073/pnas.0503357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Semeralul MO, et al. Microarray analysis of the developing cortex. J Neurobiol. 2006;66:1646–58. doi: 10.1002/neu.20302. [DOI] [PubMed] [Google Scholar]
- 120.Roth RB, et al. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics. 2006;7:67–80. doi: 10.1007/s10048-006-0032-6. [DOI] [PubMed] [Google Scholar]
- 121.Haroutunian V, Katsel P, Dracheva S, Davis KL. The human homolog of the QKI gene affected in the severe dysmyelination “quaking” mouse phenotype: downregulated in multiple brain regions in schizophrenia. Am J Psychiatry. 2006;163:1834–7. doi: 10.1176/ajp.2006.163.10.1834. [DOI] [PubMed] [Google Scholar]
- 122.Mirnics K, et al. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 123.Ryan MM, et al. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry. 2006;11:965–978. doi: 10.1038/sj.mp.4001875. [DOI] [PubMed] [Google Scholar]
- 124.Mao R, et al. Primary and secondary transcriptional effects in the developing human Down syndrome brain and heart. Genome Biol. 2005;6:R107. doi: 10.1186/gb-2005-6-13-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson MB, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. A whole-genome, exon-level expression analysis of fetal human cortex that reveals a large number of human specific gene expression, alternative splicing patterns and coexpression networks in the prefrontal and parietal association neocortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun T, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–98. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oldham MC, Horvath S, Geschwind DH. Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc Natl Acad Sci USA. 2006;103:17973–8. doi: 10.1073/pnas.0605938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kwan KY, et al. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Nat Acad Sci USA. 2008;105:16021–26. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prabhakar S, et al. Human-specific gain of function in a developmental enhancer. Science. 2008;321:1346–1350. doi: 10.1126/science.1159974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kudo LC, et al. Genetic analysis of anterior posterior expression gradients in the developing mammalian forebrain. Cereb Cortex. 2007;17:2108–22. doi: 10.1093/cercor/bhl118. [DOI] [PubMed] [Google Scholar]
- 131.Muhlfriedel S, et al. Novel genes differentially expressed in cortical regions during late neurogenesis. Europ Jrnl Neurosci. 2007;26:33–50. doi: 10.1111/j.1460-9568.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 132.Arking DE, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of Autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alarcón M, et al. Linkage, association and gene-expression analyses identify CNTNAP2 as an Autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bakkaloglu B, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in Autism Spectrum Disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vernes SC, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abrahams BS, et al. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci(USA) 2007;104:17849–54. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levitt P. Developmental neurobiology and clinical disorders: lost in translation? Neuron. 2005;46:407–412. doi: 10.1016/j.neuron.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 138.King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–16. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- 139.Rakic P. The radial edifice of cortical architecture: From neuronal silhouettes to genetic engineering. Special Issue on: Centenery of Neuroscience Discovery: Reflecting on the Nobel Prize to Golgi and Cajal in 1906. Brain Res Rev. 2007;55:204–19. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rakic P. Less is more: progenitor death and cortical size. Nature Neurosci. 2005;8:981–2. doi: 10.1038/nn0805-981. [DOI] [PubMed] [Google Scholar]
- 141.Sur M, Rubenstein JLR. Patterning and plasticity of the cerebral cortex. Science. 1973;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 142.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 143.Carroll SB. Evolution at two levels: On genes and form. PLoS Biol. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rockel AJ, Hiorns RW, Powell TPS. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- 145.Herculano-Housel S, Collins CE, Wang P, Kaas J. The basic non-uniformity of the cerebral cortex. Proc Nat Acad Sci (USA) 2008;105:12593–598. doi: 10.1073/pnas.0805417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rakic P. Confusing cortical columns. Proc Nat Acad Sci (USA) 2008;105:12099–12100. doi: 10.1073/pnas.0807271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–4. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rakic P, Stensaas LJ, Sayre EP, Sidman RL. Computer-aided three-dimensional reconstruction and quantitative analysis of cells from serial electronmicroscopic montages of fetal monkey brain. Nature. 1974;250:31–34. doi: 10.1038/250031a0. [DOI] [PubMed] [Google Scholar]
- 149.Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA/ephrin-A signaling. Nature. 2009 doi: 10.1038/nature08362. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]