Abstract
In a phase I clinical trial, one hundred healthy young adults were randomized to receive two doses 28 days apart of an inactivated, subvirion vaccine containing 15µg or 45µg of influenza A/H5N1 hemagglutinin (HA) by the intramuscular (IM) route, or 3µg or 9µg of H5 HA by the intradermal(ID) route. Seventy-seven subjects received a third dose. All regimens were safe and well tolerated. Antibody responses after two or three doses were low (≤20% or ≤38%, respectively) and similar in groups given 3µg or 9µg ID or 15µg IM, and were significantly lower than those given 45µg IM. Higher dosages of H5 HA and/or inclusion of adjuvant will be required to enhance immunogenicity by the ID route.
Keywords: Intradermal vaccination, Pandemic influenza, H5N1 vaccine
Introduction
Influenza virus A/H5N1 infections were first recognized to cause infections in humans in Hong Kong in 1997. Since 2003, these viruses have become endemic among the poultry population in many countries. More than 350 laboratory-confirmed human cases of avian influenza A/H5N1 have been reported from 14 countries to the WHO since 2003, and the case fatality rate has exceeded 60%.[1] The emergence of influenza A/H5N1 viruses has raised concerns of a potential influenza pandemic, bolstering efforts to develop immunogenic vaccines against influenza A/H5N1 viruses. Recent clinical trials have shown that standard dosages (15µg of hemagglutinin, or HA) of subunit vaccines are poorly immunogenic.[2–4] Although high dosages of HA (90µg) elicit detectable immune responses in the majority of subjects, there are concerns that current manufacturing capacity will not be sufficient to produce adequate amounts of vaccine in the setting of a pandemic.
Intradermal immunization (ID) is a potential dosage-sparing approach being explored as a possible pandemic influenza vaccine strategy. Several recent studies have evaluated ID administration of interpandemic influenza vaccine with reduced dosages. [5–10] Some of the studies report a potential advantage of ID compared to IM immunization, but it is unknown whether these observations would hold true after ID administration of a potential pandemic influenza A vaccine in unprimed (no preexisting immunity) individuals. In order to address this question, we conducted a Phase I evaluation of ID administration with a monovalent, subvirion, inactivated influenza A/H5N1 virus vaccine.
1 Material and Methods
1.1 Study Design
This study was a single-center, phase I, randomized, open-label, dose-ranging, clinical trial. The study was initiated after the one week safety data of one dose of 15µg and 45µg IM of the same investigational vaccine were evaluated in a larger dose-ranging clinical trial.[4] The primary objectives of this study were to evaluate the dose-related safety, reactogenicity, and immunogenicity after two doses of a monovalent, subvirion, inactivated influenza A/H5N1 virus vaccine administered either by the intradermal (ID) or the intramuscular (IM) route to healthy young adults. The secondary objectives were to evaluate the dose-related immunogenicity of the vaccine at 1 and 7 months after the first dose and to assess the safety, reactogenicity, and immunogenicity after a third dose of vaccine administered approximately 7 months after the second dose.
1.2 Subjects
During July 2005, eligible healthy 18 to 40 year old men and non-pregnant women were enrolled after providing written informed consent. Enrollment criteria for the young adults were similar to the dose-ranging clinical trial of the same vaccine.[4] No screening blood work was performed in this study. The study was approved by the Baylor College of Medicine (BCM) Institutional Review Board in Houston, TX.
1.3 Vaccine
The monovalent, subvirion, inactivated influenza A/H5N1 vaccine was produced through the use of reverse genetics of a seed virus from the human isolate influenza A/Vietnam/1203/2004 (H5N1) virus, as previously described (sanofi pasteur).[4] The formulations used in the study contained 30µg/mL and 90µg/mL H5 HA. Three- and 15µg were administered using 0.1 mL and 0.5 mL, respectively, of the 30µg/mL formulation; and 9- and 45-µg were administered using 0.1 mL and 0.5 mL, respectively of the 90µg/mL formulation.
1.4 Study Procedures
The study included two comparison arms (IM and ID), each with two dosage levels, for a total of 4 vaccine groups. Eligible subjects were randomized 1:1:1:1 (25 subjects in each group) to receive 2 doses of vaccine containing 15µg or 45µg of H5 HA by the IM route; or one-fifth of each IM dose (3µg or 9µg) by the ID route. The first two doses were given 28 days apart. Approximately seven months after the second vaccination eligible subjects received a third dose of vaccine (the same dosage and route assigned at randomization during enrollment). One unblinded vaccinator who was not involved in safety assessments of subjects administered all injections. IM injections were administered using standard techniques and ID injections were administered by the Mantoux technique (needle and syringe) in the deltoid region. Study participants, the study staff performing safety assessments, and laboratory personnel were blinded to vaccine dosage allocation, although the route of administration (IM or ID) was not blinded.
Safety Assessment
After each vaccination, participants were observed in the clinic for approximately 30 minutes. Injection site and systemic symptoms and oral temperatures were recorded in a memory aid for 7 days after each vaccination. Participants returned to the clinic on days 1, 2, and 7 after each vaccination for an arm check, concomitant medication assessment, a targeted physical exam (if indicated), and review of the memory aid and adverse events assessment. Solicited injection site adverse events (AEs) included pain, tenderness, itching, erythema/redness, induration/swelling, and pigmentation. Solicited systemic AEs included temperature, feeling feverish, fatigue/malaise, myalgia/body aches, headache, and nausea. The severity of AEs was scored on a scale from 0 to 3 (0=absence of symptom, 1=mild symptom that did not interfere with activity, 2=moderate symptom that interfered with activity, and 3=severe, incapacitating symptom). Injection site erythema, swelling, and pigmentation were graded on the diameter of measurement [small (<20mm); medium (20–50mm); and large (>50mm)]. All unsolicited adverse events were collected for 28 days after each vaccination. Serious adverse events (SAEs) were defined as life threatening AEs, or AEs that resulted in significant or persistent disability, hospitalization, or death and were collected throughout the study period.
Immunogenicity assessments
Blood samples for antibody assays were collected before and 1 month after each dose of vaccine and about 7 months after the second dose of vaccine. Seroresponse was defined as a 4-fold or greater increase in H5-specific hemagglutination inhibition (HAI) and/or neutralization (Neut) antibody titer after vaccination, compared to baseline. HAI and Neut assays were performed at Southern Research Institute as described previously[4], with the exception that the same starting dilution was defined as 1:10 rather than 1:20, and samples that were negative were assigned a titer of 5.
1.5 Statistical considerations
The sample size of 100 healthy adults (25 in each treatment group) was selected to provide preliminary data regarding the safety, reactogenicity, and immunogenicity of reduced dosage levels of inactivated influenza A/H5N1 vaccine administered by the ID and IM routes. Solicited reactogenicity was analyzed by selecting the most severe response over the 7 day follow-up period, dichotomizing into a binary variable (none/mild versus moderate/severe) and using exact confidence intervals to summarize reactogenicity rates. Analyses were conducted separately for each vaccination and included the distribution of HAI and Neut antibody (including the proportion of subjects achieving a titer of ≥40 and the proportion of subjects achieving a 4-fold increase from baseline) and geometric mean titers (GMTs), with 95% confidence intervals, prior to and after each vaccination.
3 Results
3.1 Subjects
One hundred subjects were enrolled and randomized into the clinical trial from July 11– July 19, 2005. Ninety-seven subjects received two doses of vaccine. Three subjects did not receive the second dose due to intercurrent illnesses unrelated to the vaccine and were excluded from the immunogenicity analysis. Seventy-seven subjects were eligible to receive a third dose. The majority of subjects who did not receive the optional third dose were unable to meet schedule requirements for follow-up. Baseline demographic characteristics of the 100 subjects enrolled are shown in Table 1. An equal number of male and female subjects were enrolled. The mean age of the subjects was 28 years (range 18–40 years) with a median age of 27. The majority of the subjects were non-Hispanic (90%) and white (73%); with similar distributions in each vaccine group.
Table 1.
Demographic Characteristics of the 100 Study Participants Enrolled
| Characteristics | 3µg ID* (N=25) |
9µg ID (N=25) |
15µg IM* (N=25) |
45µg IM (N=25) |
All Groups (N=100) |
|---|---|---|---|---|---|
| Age in years | |||||
| Mean (SD) | 27.2 (4.5) | 28.5 (4.9) | 26.9 (3.8) | 30.2 (4.4) | 28.2 (4.6) |
| Median | 25.8 | 27.1 | 26.9 | 29.9 | 27 |
| Min, Max | (21, 38) | (19, 39) | (18, 35) | (23, 40) | (18, 40) |
| Gender—N (%) | |||||
| Male | 14 (56) | 13 (52) | 11 (44) | 12 (48) | 50 (50) |
| Female | 11 (44) | 12 (48) | 14 (56) | 13 (52) | 50 (50) |
| Ethnic – N (%) | |||||
| Non-Hispanic | 23 (92) | 22 (88) | 23 (92) | 22 (88) | 90 (90) |
| Hispanic | 2 (8) | 3 (12) | 2 (8) | 3 (12) | 10 (10) |
| Race – N (%) | |||||
| American Indian/ Alaskan Native |
0 | 0 | 0 | 1 (4) | 1 (1) |
| Asian | 1 (4) | 5 (20) | 2 (8) | 1 (4) | 9 (9) |
| Black/African American |
2 (8) | 3 (12) | 7 (28) | 4 (16) | 16 (16) |
| Hawaiian/Pacific Islander |
0 | 0 | 0 | 0 | 0 |
| White | 22 (88) | 17 (68) | 16 (64) | 18 (72) | 73 (73) |
| Multi-Racial | 0 | 0 | 0 | 1 (4) | 1 (1) |
3.2 Safety and Reactogenicity
All dosage levels and immunization routes were well tolerated. Four SAEs were reported during the study period and all were considered not associated with vaccine. No deaths occurred.
Injection site reactogenicity
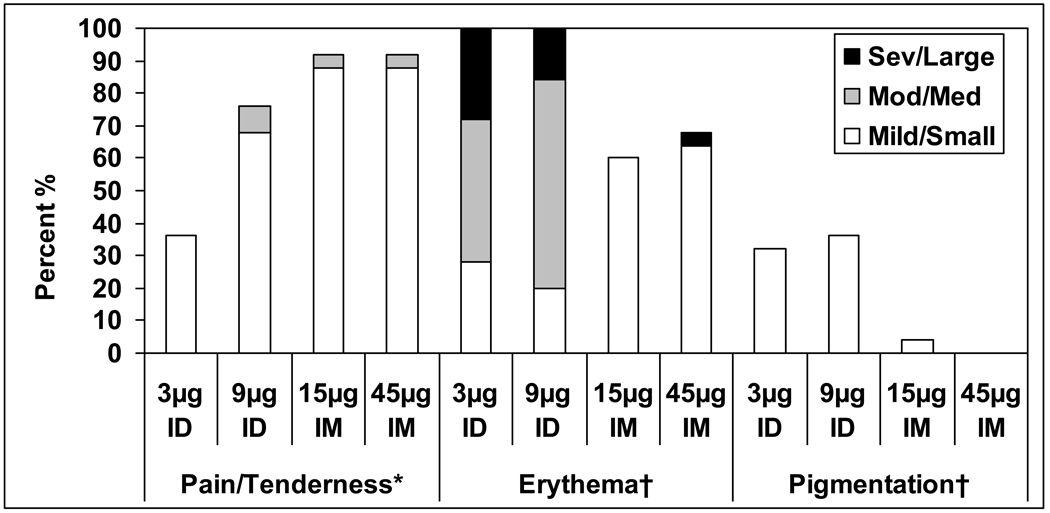
All subjects reported some injection site reactions, but the majority were mild (70%), peaked on Day 0 (68%) or 1 (83%) after vaccination and resolved by day 7 post vaccination (89%). Pain and tenderness were the most frequent injection site reactions among all subjects, 51% (95% CI 40.8, 61.1) and 71% (95% CI 61.1, 79.6), respectively, of which 4% (95% CI 1.1, 9.9) and 5% (95% CI 1.6, 11.3) were reported as moderate/severe, respectively. Among the groups, pain and tenderness were most frequent among the IM groups, with the highest frequency observed in the 45µg IM vaccine group, 76% and 92%, respectively, across all three vaccinations. All the subjects in the ID groups reported erythema at the injection site which typically peaked by Day 2, and decreased in size and resolved during the first 7 days after vaccination. In both the 3 and 9µg ID groups, the majority of the erythema at its peak was medium (20–50mm) in size. No relationship between the maximal size of erythema at the injection site and the degree of immune response was observed (data not shown). The ID groups also experienced a greater frequency of pigmentation at the injection site. These reactions typically consisted of small, hyperpigmented macules that resolved within 4 months of vaccination. Injection site reactions during the 7 days after the first dose of vaccine are shown in Figure 1. Injection site reactions were similar after the second and third doses of vaccine. No severe injection site reactions (pain and tenderness) were reported during the study.
Figure 1.
Percent of subjects with injection site reactions per vaccine group during the week after the first dose of vaccine. Grading for pain/tenderness refers to interference with activity: Grade 1: mild, Grade 2: moderate and Grade 3: severe. Grading for erythema and pigmentation is in terms of size in diameter: Grade 1: Small (<20mm), Grade 2: Medium (20–50mm), and Grade 3: Large (>50mm)
Systemic Reactogenicity
Most of the systemic reactions were mild and peaked on Day 0 or 1 after vaccination. Mild-to-moderate headache (34%) and malaise (19%) were the most frequently reported systemic symptoms following the first vaccination across all vaccine groups. One subject in the 9µg ID group experienced severe systemic symptoms consisting of feeling feverish, malaise, and myalgia associated with an intercurrent illness unrelated to vaccine after the first dose of vaccine. Similar frequencies of systemic reactions were observed among the groups following the second and third vaccine doses. No significant differences in systemic reactions were observed between the ID and IM groups or dosage levels.
3.3 Immunogenicity
Serum antibody responses before and 1 month after each dose of vaccine are shown in Table 2. Preimmunization GMTs of serum HAI and Neut antibodies in each vaccine group were similar (GMTs 5.0–5.9). Two subjects had preexisting HAI and Neut antibody against influenza A/H5N1; all other subjects had no detectable antibody at baseline. Neither of the two subjects with preexisting antibody seroresponded after 1 dose of vaccine. One of the two subjects, who spent her childhood on a poultry farm in southwest Texas, developed a four-fold rise in Neut antibodies after 2 doses of 15µg IM. The second subject reported brief travel to China as an adult; this subject developed a four-fold rise in Neut antibodies after 3 doses of 9µg ID.
Table 2.
Serum HAI and Neutralizing Antibody Responses Before and After Each Dose of Vaccin
| Day 0 | Day 28 | Day 56 | Day 215 | Day 215: Pre dose #3 |
Day 243 | |
|---|---|---|---|---|---|---|
| Pre dose 1 | Post dose 1 | Post Dose 2 | 6 mos Post Dose 2 |
6 mos Post Dose 2 |
Post dose 3 | |
| Vaccine Group | N=100 | N=77 | ||||
| HAI | ||||||
| GMTs (95%CI) | ||||||
| 3 mcg ID | 5.0 | 6.3 (4.8, 8.3) | 7.4 (5.2, 10.5) | 6.0 (4.6, 7.8) | 5.7 (4.3, 7.7) | 7.9 (4.6, 13.7) |
| 9 mcg ID | 5.9 (4.2,8.1) | 5.8 (4.3, 7.7) | 7.3 (4.9, 10.9) | 7.1 (4.2, 11.9) | 6.5 (3.8, 11.0) | 11.4 (5.4, 24.1) |
| 15 mcg IM | 5.7 (4.3, 7.6) | 7.0 (4.7, 10.4) | 9.1 (5.4, 15.2) | 6.1 (4.0, 9.3) | 6.8 (3.5, 12.9) | 10.7 (4.8, 23.9) |
| 45 mcg IM | 5.0 | 11.6 (7.4, 18.4) | 29.9 (17.7, 50.5) | 11.9 (7.1, 20.0) | 11.2 (6.4, 19.4) | 35.8 (17.0, 75.6) |
| % Achieving | ||||||
| titer ≥40 | ||||||
| (95%CI) | ||||||
| 3 mcg ID | 0 (0, 14) | 8 (1, 26) | 4 (<1, 20) | 4 (<1, 20) | 5 (<1, 25) | 15 (3, 38) |
| 9 mcg ID | 5 (<1, 23) | 5 (<1, 23) | 9 (1, 29) | 9 (1, 29) | 5 (<1, 26) | 21 (6, 46) |
| 15 mcg IM | 4 (<1, 20) | 8 (1, 26) | 16 (5, 36) | 4 (<1, 21) | 6 (<1, 30) | 13 (2, 38) |
| 45 mcg IM | 0 (0, 14) | 28 (12, 49) | 56 (35, 76) | 24 (9, 45) | 24 (8, 47) | 62 (38, 82) |
| % ≥4 fold rise | ||||||
| (95%CI) | ||||||
| 3 mcg ID | N/A | 8 (1, 26) | 4 (<1, 20) | 4 (<1, 20) | 5 (<1, 25) | 15 (3, 38) |
| 9 mcg ID | N/A | 0 (0, 15) | 5 (<1, 23) | 9 (1, 29) | 5 (<1, 26) | 21 (6, 46) |
| 15 mcg IM | N/A | 4 (<1, 20) | 12 (3, 31) | 4 (<1, 21) | 6 (<1, 30) | 6 (<1, 30) |
| 45 mcg IM | N/A | 28 (12, 49) | 56 (35, 76) | 24 (9, 45) | 24 (8, 47) | 62 (38, 82) |
| Neut | ||||||
| GMTs (95%CI) | ||||||
| 3 mcg ID | 5.0 | 5.8 (4.9, 7.0) | 9.0 (6.0, 13.5) | 6.7 (5.7, 7.9) | 6.2 (5.4, 7.0) | 11.9 (8.1, 17.5) |
| 9 mcg ID | 5.8 (4.3, 7.7) | 6.1 (4.3, 8.8) | 10.0 (6.3, 16.0) | 10.0 (6.5, 15.4) | 8.8 (5.6, 13.8) | 25.8 (12.8, 52.0) |
| 15 mcg IM | 5.9 (4.3, 8.1) | 7.5 (4.8, 11.7) | 11.6 (6.7, 20.2) | 9.1 (5.8, 14.4) | 9.4 (4.9, 17.9) | 27.5 (12.5, 60.3) |
| 45 mcg IM | 5.4 (4.6, 6.2) | 8.6 (5.7, 12.9) | 21.9 (13.0, 37.0) | 15.4 (10.8, 21.9) | 13.9 (9.9, 19.6) | 65.6 (40.4, 106.6) |
| % Achieving | ||||||
| titer ≥40 | ||||||
| (95%CI) | ||||||
| 3 mcg ID | 0 (0, 14) | 4 (<1, 20) | 12 (3, 31) | 0 (0, 14) | 0 (0, 17) | 10 (1, 32) |
| 9 mcg ID | 5 (<1, 23) | 5 (<1, 23) | 14 (3, 35) | 18 (5, 40) | 11 (1, 33) | 37 (16, 62) |
| 15 mcg IM | 4 (<1, 20) | 12 (3, 31) | 20 (7, 41) | 8 (1, 27) | 6 (<1, 30) | 38 (15, 65) |
| 45 mcg IM | 0 (0, 14) | 12 (3, 31) | 32 (15, 54) | 16 (5, 36) | 14 (3, 36) | 76 (53, 92) |
| % ≥4 fold rise | ||||||
| (95%CI) | ||||||
| 3 mcg ID | N/A | 4 (<1, 20) | 12 (3, 31) | 0 (0, 14) | 0 (0, 17) | 10 (1, 32) |
| 9 mcg ID | N/A | 0 (0, 15) | 9 (1, 29) | 14 (3, 35) | 5 (<1, 26) | 37 (16, 62) |
| 15 mcg IM | N/A | 8 (1, 26) | 20 (7, 41) | 4 (<1, 21) | 0 (0, 21) | 31 (11, 59) |
| 45 mcg IM | N/A | 12 (3, 31) | 32 (15, 54) | 12 (3, 31) | 10 (1, 30) | 71 (48, 89) |
Antibody responses were infrequent after 1 (<12%) or 2 doses (<20%) of both dosages of ID (3 or 9µg) and after the 15µg IM . In contrast, HAI and Neut antibody response frequencies were higher in the group receiving 45µg IM (Table 2): two IM doses of 45µg elicited ≥4-fold increases in HAI and Neut antibody titers in 56% and 32% of subjects, respectively.
Antibody responses among the 77 subjects who received three doses of vaccine are shown in Table 2. As expected, the antibody levels declined before the receipt of the third dose (Table 2: ~ 6 months post dose 2). After the third dose antibody responses were higher among all the vaccine groups; Neut responses were generally higher than HAI responses. HAI responses after three doses were not significantly different than responses observed after two doses. Immune responses 28 days after the third dose (Day 243) were highest among the 45µg IM group. When assessing by Neut immune responses, three doses of vaccine appeared to be more immunogenic than two doses of vaccine. Neut GMTs after three doses were higher for each group with significantly higher GMTs achieved in the 45µg IM group [65.6 (95% CI 40.4, 106.6) after three doses compared to 21.9 (95% CI 13.0, 37.0) after two doses]. Substantial increases in Neut antibody titers were also observed among the 3, 9, and 15µg groups and titers after three doses of 9µg ID were similar to responses after two doses of 45µg IM.
4 Discussion
We evaluated the safety and immunogenicity of intradermal immunization with a subvirion influenza A/H5N1 vaccine in young healthy adults. Intradermal administration of vaccine was associated with higher frequencies of transient injection site redness and hyperpigmentation when compared to intramuscular administration; however, all vaccine dosages and routes were well tolerated. Seroconversion frequencies after a single dose of vaccine by either the IM or ID route were low. After two doses, the immune responses were similar and low in the 3µg and 9µg ID groups and the15µg IM group, with the greatest immune response elicited after two doses of 45µg IM [56% (95% CI 35, 76)]. Treanor et al. reported similar antibody responses of 41% (95% CI 31, 52) after two doses of 45µg IM of the same vaccine[4]. A third dose increased antibody responses from baseline prior to the Dose 3. Among the lower doses, the third dose did not increase the overall immune responses among the vaccine groups above those observed after two doses of vaccine.
Intradermal immunization has been investigated as a vaccination strategy since the 1930s. In 1931, Tuft proposed the ID route as a method of immunization for typhoid vaccine.[11] In 1937, Francis and Magill inoculated seven subjects with three ID doses at weekly intervals of 0.5 cc of an influenza A/PR8 virus cultivated in tissue culture medium. Significant antibody responses were observed. [12] Van Gelder et al and Bruyn et al evaluated a bivalent influenza vaccines (type A and B) of unspecified chicken cell agglutinating (C CA) units given by the ID or subcutaneous (SC) route.[13–15] Although the ID dosage administered was one-tenth (0.1 mL) of the SC dosage (1.0 mL), the ID route elicited fewer systemic reactions and induced greater mean antibody responses. Weller et al. administered an even lower dose (1/50th of the usual SC dose) of influenza vaccine by the ID route and noted responses similar to 1.0 mL SC.[16] In all of these early influenza vaccine studies, a higher frequency of mild, transient injection site reactions (erythema and swelling) and fewer systemic symptoms were reported with ID immunization, and immune responses were comparable to those observed following immunization with standard SC dosages of vaccine.
The studies described above were performed among primed individuals; i.e., subjects who had preexisting immunity to the influenza vaccine viruses. A series of investigations of ID immunization were conducted among unprimed individuals (no preexisting immunity) in the 1950s in response to the H2N2 pandemic of 1957, “Asian Flu”. Boger et al. compared 0.1 ml ID to 1.0 mL IM of an H2N2 vaccine[17], and McCarroll and Kilbourne evaluated low dosages of H2N2 vaccine by the ID route in unprimed individuals.[18] Results of both trials indicated that immunization with lower dosages of a novel influenza vaccine strain administered by the ID route elicited antibody responses that were inferior when compared with those following immunization with standard SC dosages of vaccine in an unprimed population.
More recently, Belshe et al. and Kenney et al. investigated the ID route for immunization with interpandemic influenza vaccines in response to the 2003 vaccine supply shortage in the United States.[6,8] Both reported similar immune responses induced when comparing ID vaccination of lower dosages of seasonal influenza vaccine in healthy adults to standard IM dosages. Since then, several groups have reported similar findings, with comparable serum antibody responses achieved with lower ID dosages and standard IM dosages for interpandemic influenza vaccines in special populations (i.e. pediatrics, HIV, lung transplant).[7,9,19] However, Auewarakul et al. [5] in a study of 500 healthy volunteers observed significantly lower immune responses after a single 0.1 mL ID dose (3µg HA of each viral antigen) of inactivated split-virion influenza vaccine compared to 0.5 mL IM (15µg HA of each viral antigen).
The results of previous studies for influenza have varied, while ID vaccination has been successfully used for rabies and Hepatitis B. It is difficult to assess the potential advantage of the ID route from earlier studies for influenza vaccines due to differences in study designs, sample sizes, preexisting immunity, age of individuals, underlying illnesses, antigen content of vaccines, vaccine preparation, and variability in serological assays. ID vaccination for influenza has not been extensively studied with conventional vaccines and further evaluations should be pursued.
Ours is the first study conducted to explore ID vaccination of an influenza A/H5N1 vaccine. HAI antibody responses following immunization with two doses of ≤15µg were infrequent (<30%) by either route of administration. The study did have some limitations. First, this was a pilot study designed to assess safety and reactogenicity. The study was not powered to evaluate differences in immunogenicity. Second, available formulations only permitted administration of 3µg and 9µg of H5N1 hemagglutinin (HA) by the ID route in 0.1 mL. The majority of the previous ID studies administered one-fifth the optimal IM dosage by the ID route; in which case we would want to give at least 18µg ID per dose (one-fifth of 90µg). Third, results may vary because of the Mantoux technique used for ID injections. This technique requires experience and consistency. To control for this limitation in our study, a single experienced vaccinator administered all ID and IM injections and the presence of wheal formation was noted for all individuals in the ID vaccine groups. Investigations of intradermal delivery devices are underway and are a potential solution, but cost of devices and the financial constraints of developing countries are important considerations. Fourth, cellular immune responses were not assessed in this study and could be a potential area of investigation in future studies. Finally, we did not compare the same dosages administered by the IM and the ID routes. Therefore, the study was not designed to answer the question whether the ID route is superior to the IM route.
In conclusion, IM and ID immunization with a subvirion inactivated influenza A/H5N1 was safe and well tolerated. Low dosages (3- or 9µg) of vaccine administered ID were inferior to the immune responses elicited by 45µg IM. Alternative strategies to improve immune response will need to be assessed and may include higher concentrations of HA and/or inclusion of an adjuvant administered by the ID route. A phase II study designed to compare similar dosages (30µg) of a subvirion, influenza A/H5N1 vaccine administered by the ID and IM route in young healthy adults is underway.
Acknowledgments
The authors gratefully acknowledge the participants and the following persons for their contributions to the study: Coni Cheesman, P.A., Diane Nino, and the staff of the BCM Vaccine Research Center (BCM), Robin Cessna, Heather Hill and colleagues at EMMES, PPD staff, and the Southern Research Institute. The authors would also like to thank the members of the Safety Monitoring Committee for their valuable input (Karen Kotloff, MD, Chair; Carol Baker, MD and Kenneth Zangwill, MD) and their colleagues at the NIH/NIAID/DMID (Linda Lambert, PhD, Katherine Muth, Shy Shorer, MD, and Jean Hu-Primmer).
Financial Support: National Institute of Allergy and Infectious Diseases; Division of Microbiology and Infectious Diseases (contract NO1-AI-25465) from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Identifier: NCT00310206
Potential Conflicts of Interest:
WAK, SMP, and HES: Research support from Protein Sciences, GlaxoSmithKline, Novartis
RLA: Consultant to GlaxoSmithKline
TRC: None
Presented in part the VIII International Symposium for Respiratory Viral Infections, Big Island, Hawaii, May 16–19, 2006 (oral presentation) and the 44th Annual IDSA Meeting, Toronto, Ontario, Canada, October 12–15, 2006, late breaker presentation LB-5.
References
- 1.World Health Organization. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. 2008 http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_01_15/en/index.html.
- 2.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 3.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 4.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 5.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–663. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 7.Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 2007;119:1076–1082. doi: 10.1542/peds.2006-3176. [DOI] [PubMed] [Google Scholar]
- 8.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 9.Khanlou H, Sanchez S, Babaie M, et al. The safety and efficacy of dose-sparing intradermal administration of influenza vaccine in human immunodeficiency virus-positive patients. Arch Intern Med. 2006;166:1417. doi: 10.1001/archinte.166.13.1417. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt KN, Ryan GJ, Sheerin KA. Reduced-dose influenza vaccine. Ann Pharmacother. 2006;40:1635–1639. doi: 10.1345/aph.1G645. [DOI] [PubMed] [Google Scholar]
- 11.Tuft L. Active immunization against typhoid fever, with particular reference to an intradermal method. J Lab & Clin Med. 1931;16:552–556. [Google Scholar]
- 12.Francis T, Jr, Magill TP. The antibody response of human subjects vaccinated with the virus of human influenza. J Exper Med. 1937;65:251–259. doi: 10.1084/jem.65.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Gelder DW, Greenspan FS, Dufresne NE. Influenza vaccination: comparison of intracutaneous and subcutaneous methods. U S Nav Med Bull. 1947;47:197–206. [PubMed] [Google Scholar]
- 14.Bruyn H, Meiklejohn G, Brainerd H. Influenza vaccination; a comparison of antibody response obtained by various methods of administration. J Immunol. 1949;62:1–11. [PubMed] [Google Scholar]
- 15.Bruyn H, Meiklejohn G, Brainerd HD. Influenza vaccine; a study of serologic responses and incidence of reactions following subcutaneous and intradermal inoculation. Am J Dis Child. 1949;77:149–163. [PubMed] [Google Scholar]
- 16.Weller TH, Cheever FS, Enders JF. Immunologic reactions following the intradermal inoculation of influenza A and B vaccine. Proc Soc Exp Biol and Med. 1948;67:96–101. doi: 10.3181/00379727-67-16216. [DOI] [PubMed] [Google Scholar]
- 17.Boger W, Liu O. Subcutaneous and intradermal vaccination with Asian influenza vaccine. J Am Med Assoc. 1957;165:1687–1689. doi: 10.1001/jama.1957.72980310001010. [DOI] [PubMed] [Google Scholar]
- 18.McCarroll J, Kilbourne E. Immunization with Asian-strain influenza vaccine: equivalence of the subcutaneous and intradermal routes. N Engl J Med. 1958;259:618–621. doi: 10.1056/NEJM195809252591304. [DOI] [PubMed] [Google Scholar]
- 19.Manuel O, Humar A, Chen MH, et al. Immunogenicity and safety of an intradermal boosting strategy for vaccination against influenza in lung transplant recipients. Am J Transplant. 2007;7:2567–2572. doi: 10.1111/j.1600-6143.2007.01982.x. [DOI] [PubMed] [Google Scholar]