Abstract
Inhibition of anti-tumor T cell responses can be mediated by the productive interaction between the programmed death-1 (PD-1) receptor on T cells and its ligand PD-L1. PD-L1 is highly expressed on both murine bone marrow-derived dendritic cells (DC) and B16 melanoma. In this study, in vitro blockade of PD-L1 interaction on DC led to enhanced IFN-gamma production and cytotoxicity by antigen-specific T cells. In vivo, the systemic administration of anti-PD-L1 antibody plus melanoma peptide-pulsed DC resulted in a higher number of melanoma peptide-specific CD8+ T cells but this combination was insufficient to delay the growth of established B16 melanoma. While the addition of 600 rad of total body irradiation (TBI) delayed tumor growth, further adoptive transfer of antigen-specific CD8+ T cells was needed to achieve tumor regression and long-term survival of the treated mice. Lymphopenic mice treated with anti-PD-L1 antibody demonstrated increased activation and persistence of adoptively transferred T cells, including a higher number of CD8+ T cells infiltrating the tumor mass. Together, these studies support the blocking of PD-L1 signaling as a means to enhance combined immunotherapy approaches against melanoma.
INTRODUCTION
Certain immunotherapeutic strategies have been designed to activate and expand tumor-reactive T cells. For example, tumor antigen(s)-loaded dendritic cells (DC) have been shown to induce specific CD8+ T cell responses against a variety of distinct tumor types (1–3). It is evident that even in the face of strongly induced anti-tumor T cell responses there are distinct mechanisms that allow tumors to escape immune destruction. Among these mechanisms, tumor expression of Programmed Death Ligand 1 (PD-L1, B7-H1) may contribute to the downregulation of immune responses by limiting the expansion or survival of effector T cells (4). PD-L1 is normally expressed on resting B cells, T cells, myeloid cells, and DC and is important for the maintenance of peripheral tolerance (5, 6). Many tumors express PD-L1, including lung, ovarian, melanoma, and pancreatic tumors (4, 7). Several receptors have been identified that bind to PD-L1, including PD-1, expressed on activated T cells, and CD80, expressed on antigen presenting cells, including DC. Ligation of PD-L1 with its binding partners results in T cell apoptosis, downregulation of proliferative responses, and decreased cytokine secretion (4, 8, 9). In melanoma patients, tumor-infiltrating CD8+ T cells express high levels of PD-1 that correlates with impaired effector cell function (10).
Strategies to block PD-L1 signaling and enhance anti-tumor CD8+ T cell responses have yielded promising results. Blocking PD-L1 on myeloid DC isolated from ovarian cancer patients leads to enhanced T cell activation in vitro (11). In murine tumor models, PD-L1 blockade enhances tumor-specific cytotoxic T lymphocyte (CTL) killing (12). In some murine tumor models, sole administration of specific antibody recognizing PD-1 or PD-L1 leads to successful treatment of established tumors (12–14). In other models, combination treatment with anti-PD-L1 antibody improves immunotherapeutic approaches to induce tumor regressions (12, 15).
Studies have shown that active vaccination during homeostatic proliferation skews the T cell repertoire towards self- or tumor associated-antigens (16–18). Transfer of naïve or activated T cells in a lymphopenic setting is an effective means of generating anti-tumor immunity and is successful at inducing tumor regression in both murine cancer models and human clinical trials (19–22). Active vaccination in combination with lymphodepletion and adoptive T cell transfer can further enhance anti-tumor immunity (23, 24). Our studies have shown that DC-based vaccination following total body irradiation and bone marrow transplant is an effective means to induce regression of established tumors and long-term survival of mice (25). In this study, we show that blocking PD-L1 signaling following lymphodepletion in melanoma-bearing mice enhances the efficacy of adoptively transferred T cells in combination with a DC-based vaccine.
MATERIALS AND METHODS
Animals
Six- to eight-week-old C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN). B6Ly5.2Cr mice expressing CD45.1, were obtained from the National Institutes of Health-Frederick Cancer Research and Development Center (Frederick, MD). Pmel, OT-I, and OT-II mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and housed at the Animal Research Facility of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Florida, Tampa, FL.
Culture Medium and Tumor Cell Lines
Complete medium (CM) consisted of RPMI 1640 supplemented with 10% heat inactivated FCS, 0.1 mM nonessential amino acids, 1 µM sodium pyruvate, 2 mM fresh L-glutamine, 100 µg/ml streptomycin, 100 units/ml penicillin, 50 µg/ml gentamycin, 0.5 µg/ml fungizone (all from Life Technologies, Inc. Rockville, MD) and 0.05 mM 2-mercaptoethanol (Sigma Chemical Co., St. Louis, MO.). The B16 melanoma is a tumor of spontaneous origin. A poorly immunogenic, highly metastatic subclone of B16-D5, herein denoted B16, has been previously characterized. B16-D5 expresses a low level of MHC class I molecules and no detectable MHC class II molecules (26). EL4 is a T cell thymoma of C57BL/6 origin. B16 and EL4 cells were maintained by serial in vitro passages in CM. M05 tumor was generated by transfection of B16 melanoma with pAc-neo-OVA plasmid and was provided by Dr. Kenneth Rock (Dana-Farber Cancer Institute). M05 cells were maintained by serial in vitro passages in CM supplemented with 0.8 mg/ml G418.
Generation of Bone Marrow-Derived DC and Antigen Pulsing
Erythrocyte-depleted mouse bone marrow cells were cultured in complete medium supplemented with 20 ng/ml GM-CSF and 10 ng/ml IL-4 (R&D Systems, Minneapolis, MN) as described previously (1). On day 5, cells were harvested by gentle pipeting and layered onto an Optiprep gradient (Axis-Shield, Oslo, Norway). The low-density cell interface was collected and washed twice. For PD-L1 blockade experiments, DC were resuspended at 1×107 cells/ml in PBS and incubated with 10 ug/ml normal rat IgG (NrIgG) or rat anti-mouse PD-L1 antibody (anti-PD-L1, clone 10F.9G2, BioXcell, West Lebanon, NH). DC were washed one time and resuspended at 1×106cells/ml CM. For antigen-pulsing, DC were pulsed overnight with 10 µg/ml of either hgp10025–33, OVA257–264 (OVASIINFEKL), or OVA323–339 peptide (Invitrogen, Carlsbad, CA).
In Vitro T cell Stimulation and 51Cr Assay
Splenocytes from OT-I or OT-II mice were incubated with DC pulsed with OVA peptide at a 10:1 ratio. Supernatants were collected after 48 hours and IFN-gamma was measured in an ELISA assay. To measure cytotoxicity, splenocytes from pmel mice were co-cultured for 5 days with NrIgG or anti-PD-L1 pre-treated DC pulsed with gp10025–33 peptide at a 10 to 1 ratio. On day 5, pmel cells were collected and cell cytotoxicity was measured in a 51Cr assay. EL4 cells pulsed with hgp10025–33 or OVA257–264 peptide were labeled with 51Cr for 90 min at 37 °C (100 µCi / 2×106 cells). Pmel effector cells were co-cultured with labeled EL4 target cells at various effector to target ratios in a 96 well plate for 5 hours. Percent specific lysis was calculated as 100 [(experimental CPM –spontaneous CPM)/(maximal CPM - spontaneous CPM)]. Labeled target cells cultured in CM alone or in 10% SDS were used to determine the spontaneous and maximum release, respectively.
In Vivo Immunizations
C57BL/6 mice were immunized three times at one week intervals with DC pulsed with OVA257–264. Mice also received 20 mg/kg NrIgG or anti-PD-L1 antibody i.p. beginning on the first day of immunization and continuing every 3–4 days thereafter. Splenocytes were then prepared from these mice one week after the final immunization. The percentage of CD8+OVA tetramer+ cells was measured by flow cytometry. In addition, T cells were plated either alone or co-cultured with DC pulsed with OVA257–264 peptide at a 10:1 ratio for 48 hours. Supernatants were then collected and IFN-gamma secretion was measured by ELISA.
In Vivo Treatment Model
A total of 1×105 B16 tumor cells were injected s.c. in the left flank of either C57BL/6 or Ly5.2 mice. Mice received i.p. injections of 20 mg/kg NrIgG or anti-PD-L1 antibodies on day 3. Additional treatments were given every 3–4 days until the end of the experiment. In some experiments, mice also received injections of 1×106 gp100 peptide-pulsed DC on days 3 and 7 after injection of tumor cells. Mice were humanely euthanized when tumors exceeded 1.5 cm in diameter, appeared necrotic, or interfered with locomotion.
Lymphopenia Model
A total of 1×105 B16 or 3×105 M05 tumor cells were injected s.c. in the left flank of C57BL/6 or Ly5.2 mice. Three days later, mice received a sublethal dose (600cGy) of total body irradiation (TBI) administered by a 137Cs γ radiation source. For adoptive transfer experiments, T cells from the spleens of either naïve pmel mice or C57BL/6 mice vaccinated 3–4 times with DC-OVA257–264 were enriched on a T cell column (R&D Systems). On day 4 following tumor injection, T cells (1×107) were transferred i.v. and mice were also immunized s.c. with 1×106 gp100 or OVA257–264 pulsed DC. A second DC treatment was given on day 11. In addition, mice were treated i.p. with 20 mg/kg NrIgG or anti-PD-L1 antibody starting on day 4 and continuing every 3–4 days for 60 days or until the end of the experiment.
Isolation of Tumor Infiltrating Lymphocytes (TIL)
Tumor cell suspensions were prepared from solid tumors by enzymatic digestion in HBSS (Life Technologies, Inc.) containing 1 mg/ml collagenase, 0.1 mg/ml DNAse I, and 2.5 units/ml of hyaluronidase (all from Sigma) with constant stirring for 2 hours at room temperature. The resulting suspension was passed through a 70 µm cell strainer, washed once with HBSS, and resuspended in PBS + 3% BSA to a concentration of 1×106 cells/ml for flow cytometric analysis.
Proliferation Assay
Splenocytes were collected at various timepoints after adoptive T cell transfer and co-cultured with 10 µg/ml gp100 peptide in 96 well plates for 48 hours. 3H-thymidine (1µCi per well) was added for the final 18 hours of incubation. Cells were harvested and the amount of thymidine incorporated was measured on a Trilux, Beta Scintillation Counter (Wallac, Finland).
Antibodies and Flow Cytometric Analysis
For FACS analyses, DC were stained with FITC conjugated anti-IAb and PE-conjugated anti-PD-L1 monoclonal antibodies (mAbs) after incubation with purified anti-CD16/32 Fc blocking antibody. Splenocytes and TIL were stained with FITC conjugated anti-CD8 and APC conjugated anti-CD45.2 mAbs after incubation with purified anti-CD16/32 Fc blocking antibody. All antibodies were purchased from BD Pharmingen (San Diego, CA). For tetramer analysis, splenocytes were stained with PE conjugated anti-CD8 mAb and anti-Kb OVA tetramer (Beckman Coulter Inc., Fullerton, CA). Data were acquired on a FACSCalibur and analyzed using CellQuest Pro software (BD Bioscience, San Jose, CA).
Statistical Analysis
A Mann-Whitney test (unpaired) or a Student’s paired t-test was used to compare between two treatment groups. All statistical evaluations of data were performed using GraphPad Prism software. Statistical significance was achieved at p<0.05.
RESULTS
Expression of PD-L1 on DC and T cell stimulation in vitro
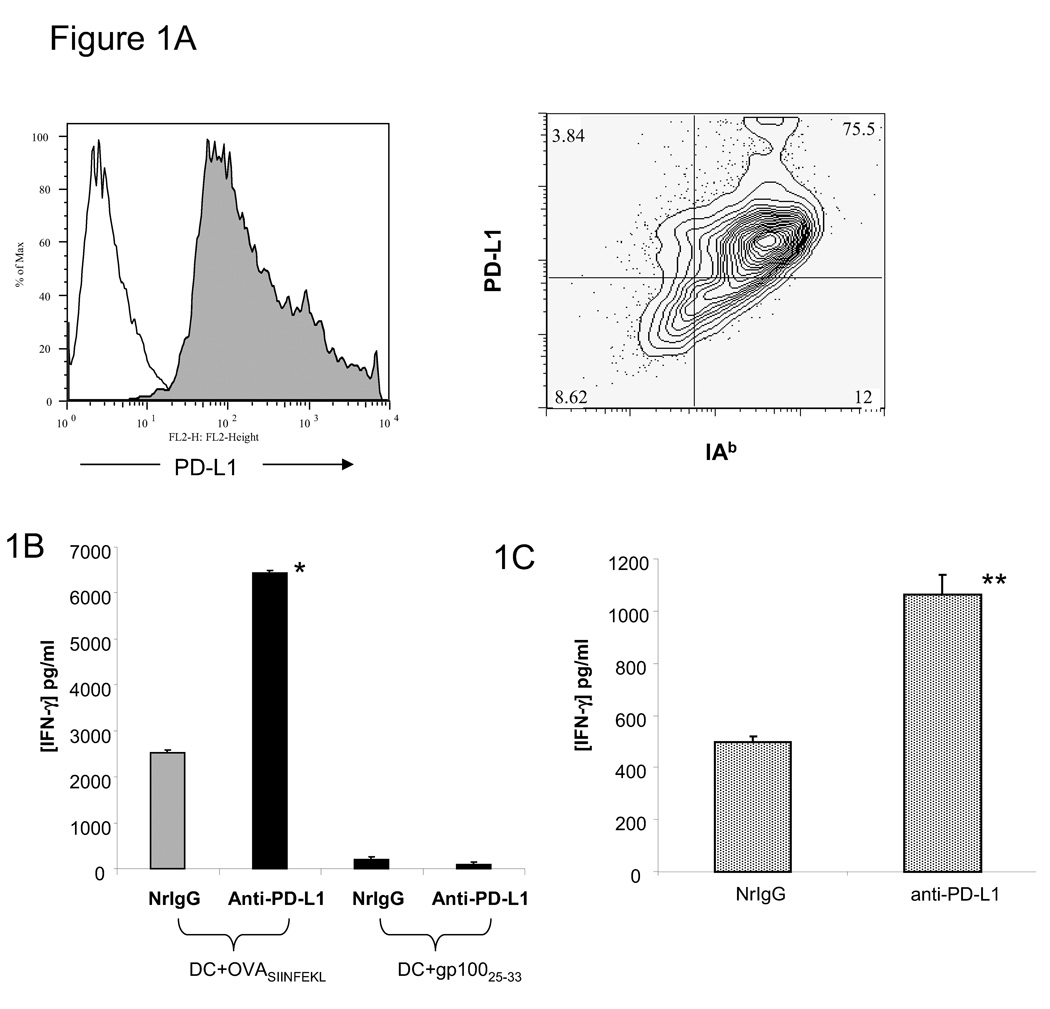
After five days of culture, approximately 75% of bone marrow-derived DC expressed high levels of PD-L1 (Figure 1A). We first examined IFN-γ production by T cells after co-culturing with DC treated with anti-PD-L1 antibody. Co-culture of OVA257–264 peptide-pulsed DC pre-treated with NrIgG resulted in the production of IFN-γ by CD8+ OT-I T cells (2,535 ± 55 pg/ml, Figure 1B). Co-culture after pre-treatment of DC with anti-PD-L1 antibody resulted in increased production of IFN-γ by CD8+ OT-I cells (6,445 ± 116 pg/ml, p<0.001 compared to DC treated with NrIgG). OT-I T cells did not produce IFN-γ in response to gp100 peptide-pulsed DC pre-treated with NrIgG or anti-PD-L1 antibody. Co-culture of OVA323–339 peptide-pulsed DC pre-treated with anti-PD-L1 antibody led to enhanced stimulation of CD4+ OT-II T cells (1,063 ± 76 pg/ml, Figure 1C) compared to NrIgG pre-treated DC (498 ± 21 pg/ml, p<0.01).
Figure 1.
Peptide-loaded DC pre-treated with anti-PD-L1 antibody enhance stimulation of a specific T cell response. (A) After 5 days of in vitro culture, bone marrow-derived DC were stained with anti-IAb and PD-L1 antibodies and analyzed by flow cytometry. Filled histogram = anti-PD-L1, open histogram = isotype control. (B) DC were pulsed with OVA257–264 or gp10025–33 peptide and treated with either 10 µg/ml NrIgG or anti-PD-L1 antibody for 24 hours. These DC were then co-cultured with OT-I splenocytes at a 1:10 ratio for 48 hours. (C) DC were pulsed with OVA323–337 peptide and treated with either 10 µg/ml NrIgG or anti-PD-L1 antibody for 24 hours. These DC were co-cultured with OT-II splenocytes at a 1:10 ratio for 48 hours. Supernatants were collected and tested in an IFN-gamma ELISA assay. One of 2 representative experiments is shown. *indicates p<0.001, **indicates p<0.01.
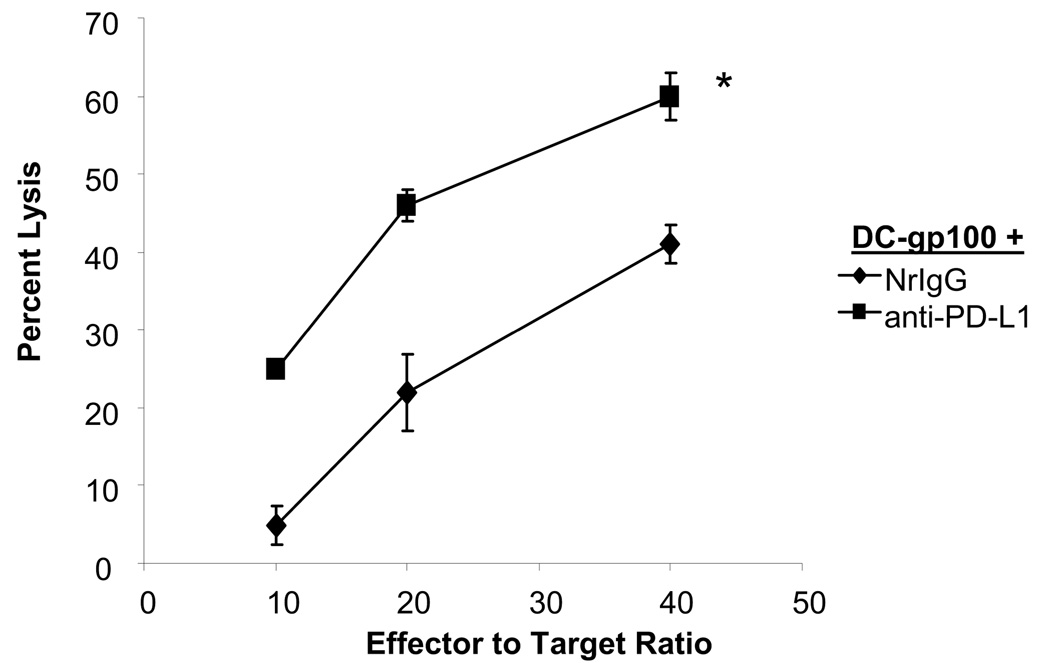
Using a second experimental system, we also examined cytotoxicity of pmel T cells after stimulation with gp100 peptide-pulsed DC pre-treated with NrIgG or anti-PD-L1 antibody. After 5 days of co-culture, we assessed the ability of pmel T cells to kill gp100-loaded target cells. As shown in Figure 2, pmel T cells stimulated in vitro with DC-gp100-anti-PD-L1 antibody demonstrated enhanced killing of EL4-gp100 compared to pmel T cells stimulated with DC-gp100-NrIgG (p<0.01). Pmel T cells did not kill EL4 cells loaded with an irrelevant peptide (not shown). Together, these experiments demonstrated that blocking PD-L1 on DC in vitro could enhance T cell effector functions.
Figure 2.
Stimulation with anti-PD-L1 antibody-treated DC enhances T cell cytotoxicity. Purified pmel T cells were stimulated in vitro for 5 days with gp100-pulsed DC pre-treated with NrIgG or anti-PD-L1 antibody. Cell cytotoxicity against gp100-coated EL4 target cells was measured in a 5 hour 51Cr release assay. *indicates p<0.01.
Immunization with peptide-pulsed DC and administration of anti-PD-L1 antibody
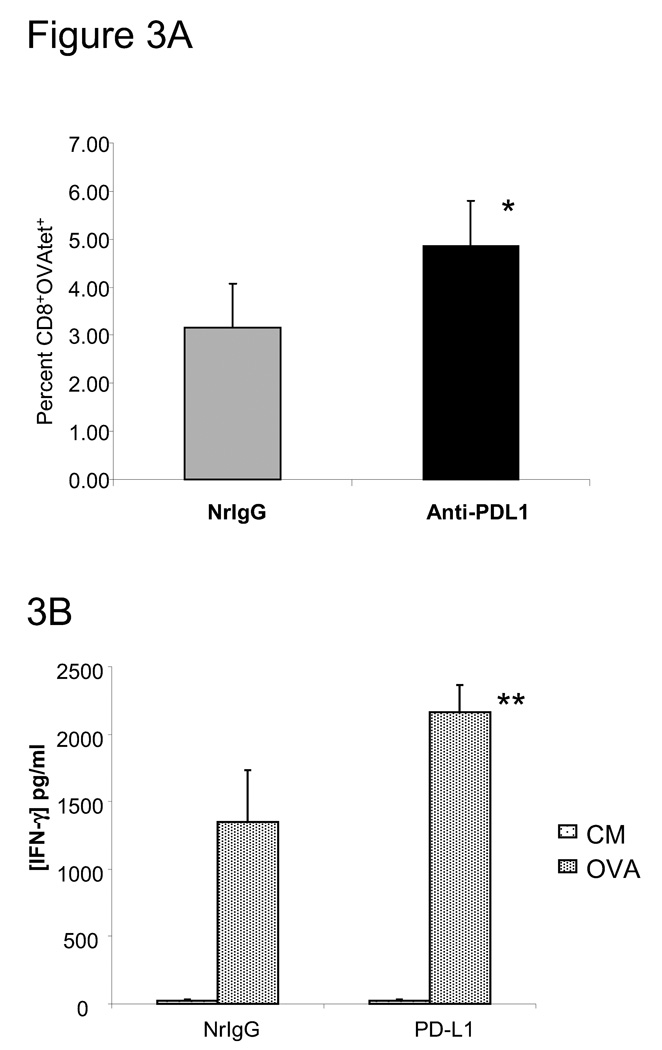
To examine the efficacy of peptide-pulsed DC immunization in combination with anti-PD-L1 antibody to enhance T cell responses in vivo, we immunized mice three times with DC pulsed with OVA peptide. Mice also received NrIgG or anti-PD-L1 antibody beginning on the first day of immunization and continuing every 3–4 days. Splenocytes were collected one week after the final immunization. The percentage of CD8+, OVA tetramer positive T cells was measured by flow cytometry. As shown in Figure 3A, a higher percentage of OVA-specific CD8+ T cells was measured in the splenocytes of mice that received anti-PD-L1 antibody in combination with DC-OVA peptide immunization (p<0.01 compared to NrIgG treated mice). Restimulation with OVA peptide for 48 hours resulted in higher production of IFN-γ by splenocytes of anti-PD-L1 antibody treated, immunized mice (2,164 ± 200 pg/ml, Figure 3B) compared to splenocytes of NrIgG antibody treated, immunized mice (1,348 ± 389 pg/ml, p<0.05). These studies demonstrated that blocking PD-L1 in vivo can enhance T cell activation induced by peptide-pulsed DC immunization.
Figure 3.
Immunization with OVA peptide-pulsed DC in combination with anti-PD-L1 antibody treatment leads to enhanced CD8+ T cell response. C57BL/6 mice (n=4) were immunized s.c. three times with DC pulsed with OVA257–264 peptide at one week intervals. Mice also received NrIgG or anti-PD-L1 antibody every 3–4 days. Splenocytes were collected one week after the final peptide-pulsed DC immunization. (A) The percentage of CD8+ and OVA tetramer+ cells was measured by flow cytometry. * indicates p<0.01. (B) Splenocytes alone or co-cultured with 10 µg/ml OVA257–264 peptide in vitro for 48 hours. Supernatants were collected and IFN-gamma was measured by an ELISA assay. This experiment was repeated twice with similar results. ** indicates p<0.05.
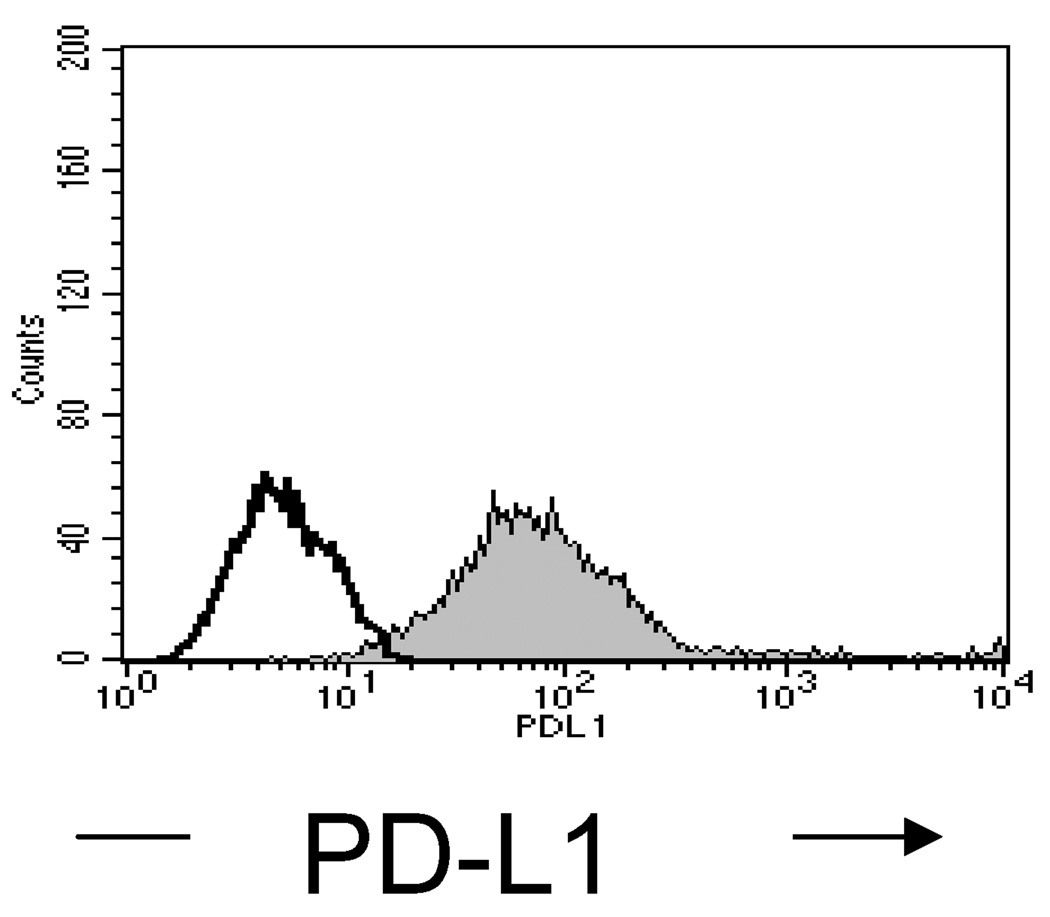
Treatment of B16 melanoma with anti-PD-L1 antibody and peptide-pulsed DC
PD-L1 is expressed by B16 melanoma (MFI 173, Figure 4), which could lead to the inhibition of B16-specific T cells. To test this possibility, we injected B16 melanoma cells s.c. into C57BL/6 mice. On day 3, mice received NrIgG or anti-PD-L1 antibody alone or in combination with DC pulsed with gp100 peptide (DC-gp100). Mice received NrIgG or anti-PD-L1 antibody every 3–4 days until the end of the experiment. In addition, mice received a second DC-gp100 immunization on day 10. As shown in Table I, treatment with anti-PD-L1 antibody alone or in combination with DC-gp100 immunization was unable to induce a significant delay in the growth of B16 tumor or to improve mice survival.
Figure 4.
Expression of PD-L1 on B16 melanoma cells. B16 cells were stained with anti-PD-L1 antibody. Expression was measured by flow cytometry. Open histogram = Isotype control; filled histogram: PD-L1 expression.
Table I.
Treatment of B16 melanoma with peptide-pulsed DC and anti-PD-L1 antibody
| Treatment1 (n=6) | Mean Tumor Area (mm2)2 | Mean Survival (days) |
|---|---|---|
| NrIgG | 392 ± 8 | 27 ± 1 |
| anti-PD-L1 | 347 ± 53 | 24 ± 6 |
| DC-gp100 + NrIgG | 333 ± 67 | 26 ± 4 |
| DC-gp100 + anti-PD-L1 | 268 ± 72 | 31 ± 15 |
Mice were treated beginning on day 3 after s.c. injection of B16 cells
Measurements recorded on day 28
Treatment of lymphopenic mice with anti-PD-L1 antibody
It has been shown that PD-1 expression is downregulated on T cells undergoing acute homeostatic proliferation; however, by day 21, PD-1 is upregulated on T cells that are highly reactive to self antigens (27). We examined whether treatment with anti-PD-L1 antibody during homeostatic proliferation could lead to a reduction in the growth of B16 melanoma. Mice were treated with 600 rad TBI three days after injection of B16 tumor cells. On the following day, mice were immunized with DC-gp100 alone or in combination with anti-PD-L1 antibody treatment. An additional DC-gp100 immunization was given 7 days later. Anti-PD-L1 antibody (or NrIgG) treatments were given i.p. every 3–4 days until the end of the experiment. As shown in Table II, combination therapy with DC-gp100 and anti-PD-L1 antibody was able to delay the growth of B16 in lymphopenic mice and enhance survival (p<0.05 compared to all other groups); however, all mice eventually succumbed to tumor growth.
Table II.
Treatment of B16 melanoma with TBI, peptide-pulsed DC and anti-PD-L1 antibody
| Treatment1 | Mean tumor size (mm2)2 | Survival (days) |
|---|---|---|
| NrIgG | 325 ± 4 | 33 ± 4 |
| anti-PD-L1 | 371 ± 46 | 37 ± 3 |
| DC-gp100 + NrIgG | 315 ± 52 | 34 ± 4 |
| DC-gp100 + anti-PD-L1 | 175 ± 41* | 46 ± 9* |
Mice (n=6) were treated with TBI on day 3 after B16 injection. Mice received DC injections on days 4 and 11. Mice received NrIgG or anti-PD-L1 antibody every 3–4 days beginning on day 4
Tumor measurements were recorded on day 32
indicates p<0.05 compared to all other groups
Adoptive transfer of melanoma-specific T cells in combination with anti-PD-L1 antibody and DC immunization in lymphopenic mice
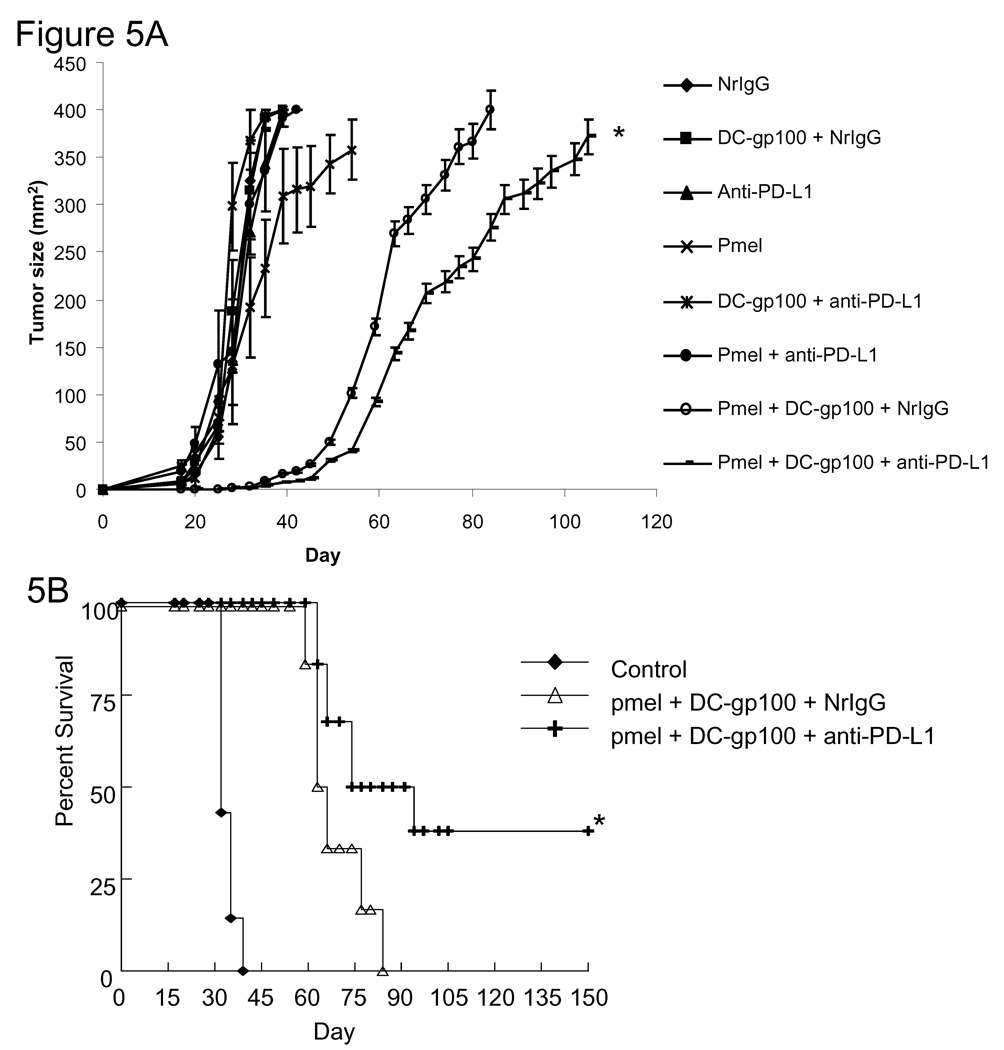
As a means to enhance the effect of peptide-pulsed DC immunization in the setting of lymphopenia, we adoptively transferred melanoma-specific T cells isolated from the spleens of pmel mice. Three days after injection of B16 tumor cells, mice were treated with 600 rad of TBI. On day 4, mice received an i.v. injection of 1×107 pmel T cells in combination with s.c. injection of DC-gp100. In addition, mice were treated i.p. with NrIgG or anti-PD-L1 antibody starting on day 4 and continuing every 3–4 days for 60 days. An additional DC-gp100 treatment was given on day 11. As shown in Figure 5A, tumors in mice treated with pmel T cells, DC-gp100, and anti-PD-L1 antibody demonstrated a significantly slower growth rate. Moreover, 40% of mice treated with pmel T cells, DC-gp100, and anti-PD-L1 antibody went on to show prolonged survival past 150 days (Figure 5B).
Figure 5.
Adoptive transfer of pmel T cells enhances the anti-B16 tumor response in tumor-bearing mice treated with anti-PD-L1 antibody and peptide-pulsed DC. C57BL/6 (n=6 per group) mice were injected s.c. with B16 cells. On day 3, mice received 600 rad of TBI. On day 4, mice received 1×107 pmel T cells i.v. Beginning on day 4 and continuing every 3–4 days until day 60, mice received i.p. injections of NrIgG or anti-PD-L1 antibody. Mice also received s.c. injections of DC pulsed with gp100 peptide on days 4 and 11 after B16 injection. Tumor sizes were measured twice each week. (A) Tumor growth. (B) Survival. The results are representative of two similar experiments. * indicates p<0.05 compared to pmel + DC-gp100 + NrIgG treated mice.
Persistence of melanoma-specific T cells in mice treated with anti-PD-L1 antibody
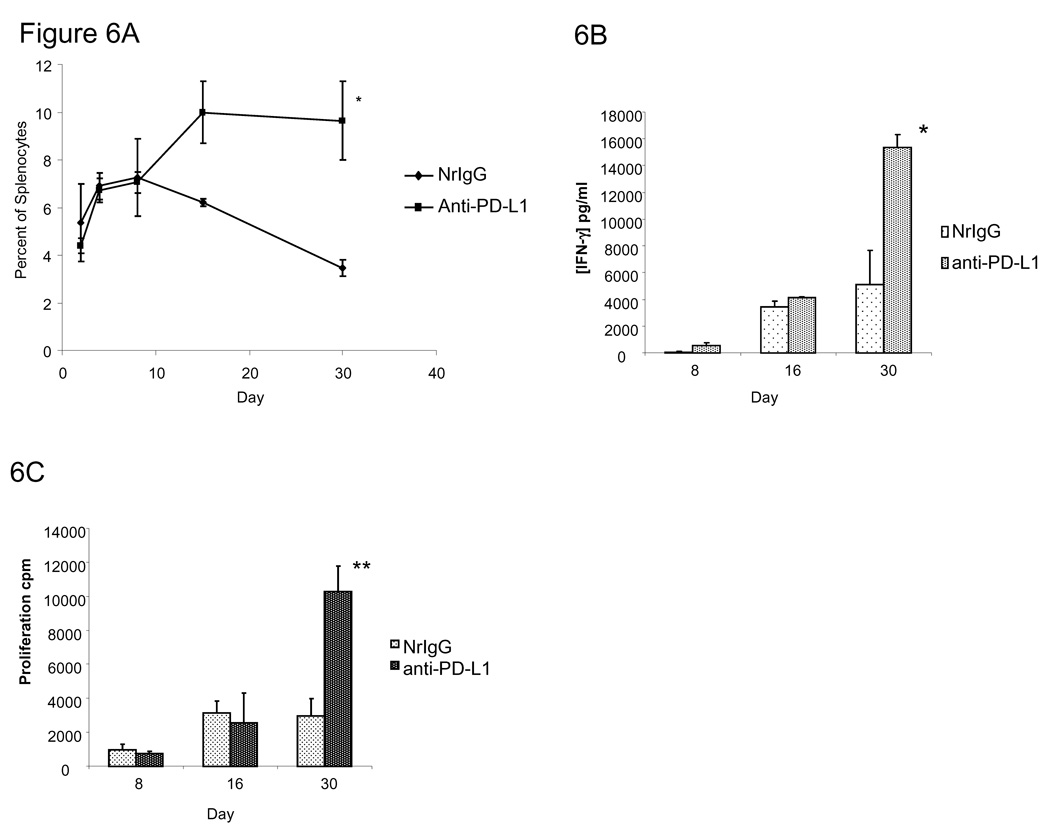
We next examined whether pmel T cells persisted for a longer duration in mice treated with anti-PD-L1 antibody. Ly5.2 (CD45.1+) mice were treated with TBI. Mice received 1×107 pmel T cells (CD45.2+) one day later in combination with either NrIgG or anti-PD-L1 antibody. Antibodies were given every 3–4 days for 4 weeks. Splenocytes were collected at various timepoints and the percentage of CD45.2+ T cells was measured by flow cytometry. As shown in Figure 6A, mice treated with anti-PD-L1 antibody had a higher percentage of CD45.2+ T cells remaining in the spleen at days 16 and 30 (p<0.05). This increased persistence correlated with an enhanced secretion of IFN-gamma by splenocytes from mice treated with pmel T cells and anti-PD-L1 antibody at day 30 (Figure 6B, p<0.05 compared to NrIgG treated mice). In addition, splenocytes from mice treated with anti-PD-L1 antibody demonstrated enhanced proliferation against gp100 peptide at day 30 (Figure 6c, 10,264 ± 1,538) compared to NrIgG treated mice (2,970 ± 1,017 cpm, p<0.05). Together, these data indicated that enhanced persistence and activation of pmel T cells in mice could be achieved by administration of anti-PD-L1 antibody.
Figure 6.
Pmel T cells persist in mice treated with anti-PD-L1 antibody. Ly5.2 (CD45.1+) mice (n=20) were treated with 600 rad of TBI. On day 3, mice received i.v. 1×107 pmel T cells. Beginning on day 3 and continuing every 3–4 days, mice received i.p. injections of either NrIgG or anti-PD-L1 antibody. Spleens (n=4) were collected at each time point and (A) the percentage of CD45.2+ pmel T cells was measured by flow cytometry. Splenocytes were restimulated in vitro with gp100-peptide pulsed DC for 48 hours. (B) Supernatants were collected and IFN-gamma was measured by an ELISA assay. (C) 3H-thymidine was added for an additional 18 hours. Cells were harvested and the amount of thymidine incorporation was measured. These experiments were repeated two times. * indicates p<0.01, **indicates p<0.05.
Trafficking of melanoma-specific T cells to tumor after treatment with anti-PD-L1 antibody
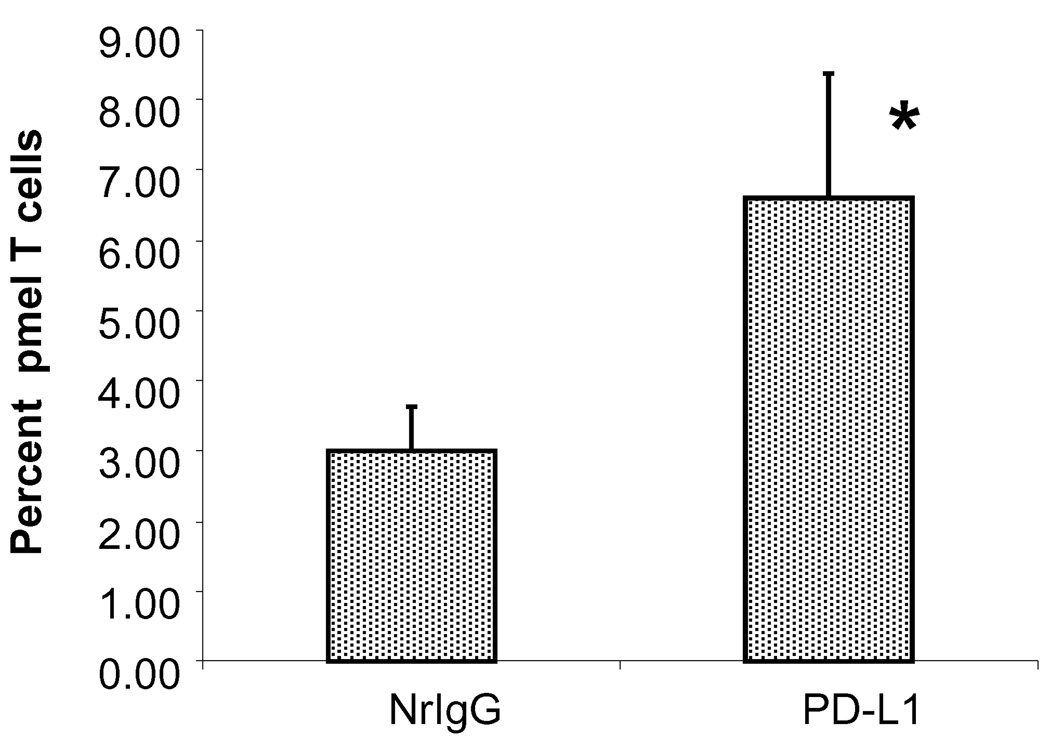
We next examined the percentage of pmel T cells present in the tumor infiltration lymphocytes (TIL) of mice treated with anti-PD-L1 antibody. Mice were injected s.c. with B16 cells and received 600 rad of TBI on day 11. On day 12, mice received 1×107 pmel T cells i.v. in combination with NrIgG or anti-PD-L1 antibody. Antibody treatment was continued every 3–4 days until spleens and tumors were collected on day 19. CD45.2+ T cells present in the spleen and TIL were analyzed by flow cytometry. As shown in Figure 7, mice treated with anti-PD-L1 antibody had a higher percentage of CD45.2+ T cells in TIL (6.6% compared to 3% in mice treated with NrIgG, p<0.05).
Figure 7.
Higher numbers of pmel T cells infiltrate B16 tumor in mice treated with anti-PD-L1 antibody. Ly5.2 mice (n=10) were injected s.c. with B16 tumor cells. On day 11, mice received 1×107 pmel T cells i.v. Mice also received i.p. injections of either NrIgG or anti-PD-L1 antibody on days 11, 14, and 17. Spleens and tumors were collected on day 19 and the percentage of CD45.2+ pmel T cells was measured. * indicates p<0.05 compared to NrIgG treated mice. This experiment was repeated two times with similar results.
Adoptive transfer of effector T cells in combination with anti-PD-L1 antibody treatment
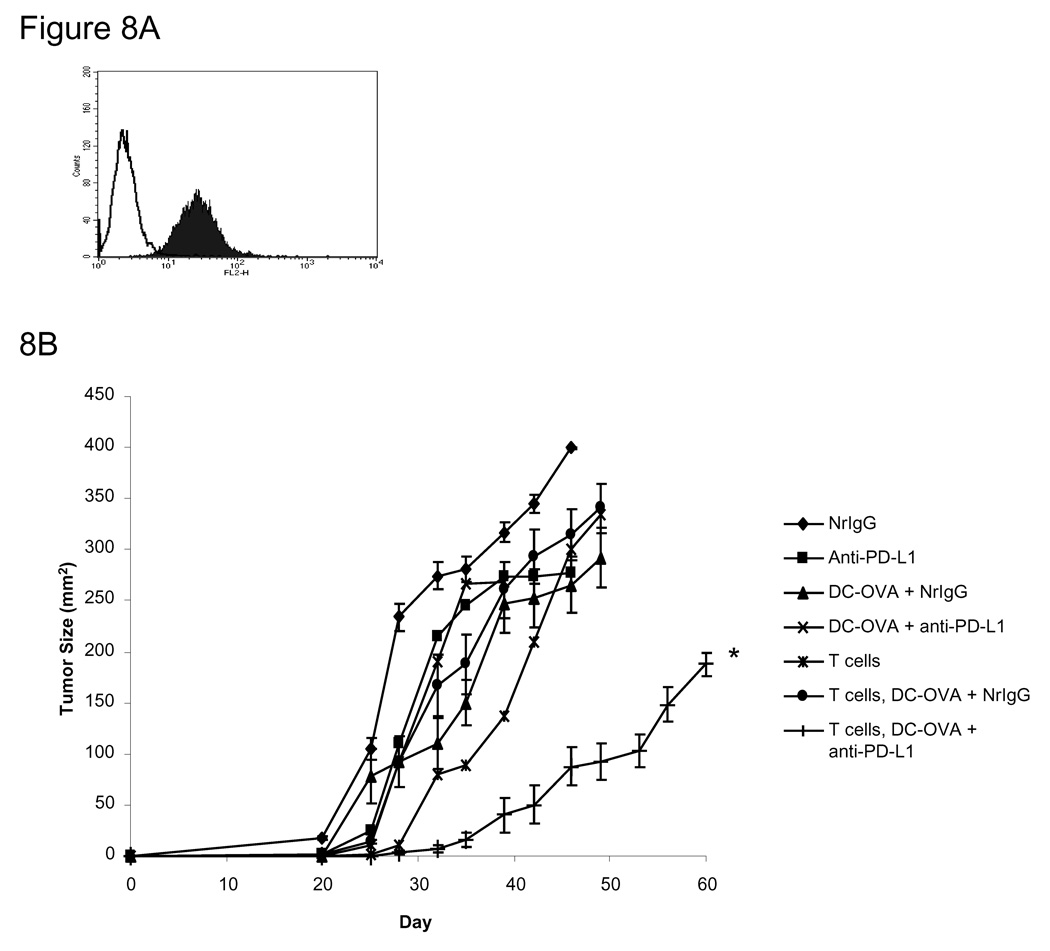
We next examined whether treatment with anti-PD-L1 antibody could enhance the therapeutic efficacy of adoptively transferred T cells isolated from mice previously immunized with DC pulsed with OVASIINFEKL (DC-OVA). Figure 8A shows that PD-L1 is indeed expressed by M05 melanoma cells (MFI 31.5). Mice were treated with TBI on day 3 after injection of M05 cells. On day 4, mice received 1×107 T cells isolated from the spleens of DC-OVA immunized donor mice. In addition, DC-OVA immunizations were given s.c. to the recipients on days 4 and 11 along with NrIgG or anti-PD-L1 antibody beginning on day 4 and continuing every 3–4 days until the end of the experiment. Mice treated with the adoptive transfer of OVA-immune T cells in combination with anti-PD-L1 antibody displayed a significant delay in the growth of M05 tumor (Figure 8B, p<0.05 compared to all other groups).
Figure 8.
Adoptive transfer of OVA-immune T cells enhances the anti-tumor response in M05-bearing mice treated with anti-PD-L1 antibody and peptide-pulsed DC. (A) Expression of PD-L1 on the surface of M05 cells. Filled histogram: anti-PD-L1, open histogram: NrIgG. (B) C57BL/6 (n=6 per group) mice were injected s.c. with M05 cells. On day 3, mice received 600 rad of TBI. On day 4, mice received 1×107 T cells i.v. that were isolated from mice immunized four times with DC-OVASIINFEKL. Beginning on day 4 and continuing every 3–4 days until day 60, mice received i.p. injections of either NrIgG or anti-PD-L1 antibody. Mice also received DC pulsed with OVASIINFEKL peptide s.c. on days 4 and 11 after B16 injection. Tumors were measured twice each week. The results are representative of two experiments. * indicates p<0.05 compared to T cells + DC-OVA + NrIgG treated mice.
DISCUSSION
DC-based vaccines are an effective means to induce anti-tumor T cell responses. However, their use rarely translates into clinically meaningful responses in patients with cancer. The expression of PD-L1 on DC and tumor cells may limit the induction of robust anti-tumor T cell responses. PD-L1 binds to PD-1 on activated T cells to induce and maintain peripheral tolerance (5). Pulko et al. have shown that DC generated from the bone marrow of PD-L1 knock-out mice are superior to wild-type DC at inducing both antigen-specific CD8+ T cell responses and tumor suppression. (28). In our current study, blocking PD-L1 expression on DC led to enhanced secretion of IFN-gamma and cytotoxicity by antigen-specific T cells. In addition, vaccination with OVA peptide-pulsed DC in combination with anti-PD-L1 antibody treatment induced an increased number of OVA-specific CD8+ T cells and enhanced IFN-gamma secretion. Despite these positive effects, treatment of mice bearing B16 tumors with gp100 peptide-pulsed DC in combination with anti-PD-L1 antibody did not result in tumor regression. This result suggests that in addition to expressing high levels of PD-L1, B16 melanoma employs additional operative mechanisms of immune evasion.
In an effort to improve immunity, we treated B16 melanoma bearing mice with anti-PD-L1 antibody and administered peptide-pulsed DC in a lymphopenic environment. In this setting, the opportunity exists to educate reconstituting T cells during homeostatis-driven proliferation. Vaccination after the induction of lymphopenia has been shown to enhance the activation and expansion of anti-tumor T cells (20), which may be due to an increased availability of immune stimulating cytokines, such as IL-15 and IL-7 (29), and the downregulation of inhibitory molecules on T cells during acute homeostatic proliferation (30). It has been shown that PD-1 expression is deficient on T cells undergoing acute homeostatic proliferation (27). Normal expression of PD-1 is restored by day 21 and is found to be highly expressed on autoreactive T cells, leading to the elimination of these cells (30). In our tumor model, treatment with anti-PD-L1 antibody alone after the induction of lymphopenia had no effect on tumor growth. Combination therapy with anti-PD-L1 antibody and peptide-pulsed DC immunizations resulted in a significant delay in B16 tumor growth. The addition of adoptively transferred, tumor-specific T cells enhanced this effect and led to an improved survival in mice bearing either B16 or the M05 melanoma.
The efficacy of T cell adoptive transfer in the setting of lymphopenia depends on the persistence of the transferred T cells (31). In viral models, blocking PD-L1 led to enhanced activation and persistence of viral-specific T cells (32). In the current study, there was no difference in the initial proliferative rate of adoptively transferred pmel T cells in mice treated with NrIgG or PD-L1 antibody (data not shown). However, pmel T cells persisted longer in mice treated with anti-PD-L1 antibody. By day 30 after adoptive transfer, the number of pmel T cells was significantly lower in the spleens of mice treated with NrIgG, which correlated inversely with the growth of B16 tumors.
The expression of PD-L1 late in anti-tumor immune responses may regulate and suppress long-term immunity. Upregulation of PD-L1 expression in the spleen following vaccination has been linked to lower numbers and impaired function of both memory and effector T cells (33). In mice bearing renal cell or lung carcinomas, upregulation of PD-L1 on splenocytes correlated with an IFN-γ dependent loss of CD4+ T cells and impaired anti-tumor memory T cell responses (34). In our current study, adoptive transfer of pmel T cells in combination with DC vaccination led to a delay in tumor growth in mice receiving either NrIgG or anti-PD-L1 antibody, but eventually all of the NrIgG treated mice grew tumors and died. In contrast, some mice treated with anti-PD-L1 antibody went on to be tumor-free. This correlated with an enhanced number of T cells infiltrating the tumor and increased proliferation and IFN-γ production by tumor-specific T cells.
Collectively, our studies support the addition of anti-PD-L1 antibody to immunotherapeutic approaches that employ the adoptive transfer of T cells and active vaccination, particularly in the setting of lymphopenia. Blocking PD-L1 signaling allows longer persistence and enhanced infiltration of T cells into PD-L1-expressing tumor. This approach may be valuable as a means to enhance clinical responses in patients with melanoma.
ACKNOWLEDGEMENTS
We thank the Ocala Royal Dames for Cancer Research for their generous support. We also thank the Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center and Research Institute for its contribution to this work.
Footnotes
This work was supported by the National Cancer Institute Grant K99CA128825-01 (SPT).
REFERENCES
- 1.Pilon-Thomas S, Verhaegen M, Kuhn L, Riker A, Mulé JJ. Induction of anti-tumor immunity by vaccination with dendritic cells pulsed with anti-CD44 IgG opsonized tumor cells. Cancer Immunol Immunother. 2006;55:1238–1246. doi: 10.1007/s00262-005-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature medicine. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 3.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mulé JJ. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 9.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature medicine. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 12.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer research. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 13.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer research. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 16.La Gruta NL, Driel IR, Gleeson PA. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. European journal of immunology. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/ MHC epitopes and strength of their interaction with T cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 19.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. The Journal of experimental medicine. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koike N, Pilon-Thomas S, Mulé JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother. 2008;31:402–412. doi: 10.1097/CJI.0b013e31816cabbb. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (New York, N.Y. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Wang YL, Hu HM, Fox BA, Si LS. Mechanism of augmented anti-tumor immunity in reconstituted lymphopenic mice immunized with melanoma vaccine. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2005;27:708–712. [PubMed] [Google Scholar]
- 24.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, Veloso E, Zheng Z, Westphal S, Mair R, Mair R, Chi N, Ratterree B, Pochran MF, Natt S, Hinkle J, Sickles C, Sohal A, Ruehle K, Lynch C, Zhang L, Porter DL, Luger S, Guo C, Fang HB, Blackwelder W, Hankey K, Mann D, Edelman R, Frasch C, Levine BL, Cross A, June CH. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nature medicine. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 25.Asavaroengchai W, Kotera Y, Koike N, Pilon-Thomas S, Mulé JJ. Augmentation of antitumor immune responses after adoptive transfer of bone marrow derived from donors immunized with tumor lysate-pulsed dendritic cells. Biol Blood Marrow Transplant. 2004;10:524–533. doi: 10.1016/j.bbmt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer gene therapy. 1996;3:39–47. [PubMed] [Google Scholar]
- 27.Shvets A, Chakrabarti R, Gonzalez-Quintial R, Baccala R, Theofilopoulos AN, Prud'homme GJ. Impaired negative regulation of homeostatically proliferating T cells. Blood. 2009;113:622–625. doi: 10.1182/blood-2008-03-139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, Dong H. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. 2009;183:3634–3641. doi: 10.4049/jimmunol.0900974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of experimental medicine. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. The Journal of experimental medicine. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. The Journal of experimental medicine. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster WS, Thompson RH, Harris KJ, Frigola X, Kuntz S, Inman BA, Dong H. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. J Immunol. 2007;179:2860–2869. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]
- 34.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nature medicine. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]