Abstract
The fluorescence of silver clusters encapsulated by single stranded oligo-DNA (24 cytosine base pairs, C24:Agn) was used to monitor the transfection of this new silver/DNA fluorophore inside living HeLa cells. For this, the C24:Agn molecules were complexed with a commercially available transfection reagent Lipofectamine and the internalization of C24:Agn was followed with confocal fluorescence microscopy. Bright near-infrared fluorescence was observed from inside the transfected HeLa cells, when exciting with 633 nm excitation, opening up the possibility for the use of these C24:Agn clusters for biological labelling and imaging of living cells and for monitoring the transfection process with limited harm to the living cells.
Introduction
Since advances in fluorescence microscopy greatly expand our understanding of biological systems, numerous organic dyes exhibiting different photophysical properties1 have been employed to investigate processes in living cells.2–6 Imaging intracellular dynamics with organic dyes is limited by the probe brightness7 and photostability8 and strategies for specific labelling. These problems become especially severe in single molecule studies. Advances in nanotechnology have led researchers to use quantum dots as alternative imaging agents.9 Compared with organic dyes, quantum dots are brighter, remarkably resistant to photobleaching and readily excitable with low intensity sources.10–13 However, their toxicity14 and size15 can cause problems. The probe size for example can affect cellular uptake and influence mobility when used for single particle tracking studies.16 Reducing the size of quantum dots without losing their advantageous photophysical properties is ongoing research. Howarth et al. synthesized small quantum dots, having a hydrodynamic diameter of 11.1 ± 0.1 nm and a fluorescence quantum yield of ~0.4.17 Another class of fluorophores that are frequently used are fluorescent proteins, which have gained popularity as fluorescent labels in the last 10 years.18 Key to the success of fluorescent proteins (FP) is the easy introduction inside cells by genetic encoding of the FPs in vectors and plasmids, the non-toxicity and the specificity by fusing the FPs to proteins of interest. A lot of research was performed on improving the fluorescent properties of green fluorescent proteins (GFP)19–21 from Aequorea victoria, GFP mutants and related proteins from other marine species (like, for example, DsRed).22–24 Despite these efforts, FPs suffer from moderate brightness and fast blinking/photobleaching (note that the unwanted blinking properties of GFPs have recently been found to be beneficial for super resolution microscopy schemes).23,25–28
Recently, small silver clusters have emerged as a new class of bright and photostable fluorophores.29–51 In this article we focus on aDNA-stabilized silver cluster system. It has been shown in earlier reports that single-stranded DNA, preferably with cytosine bases, serves as a template and stabilizes and protects Ag nanoclusters from aggregation to large nanoparticles after reduction of the cations.29–31,33,34,36,40,41,47,52 Different sized silver nanoclusters exhibit different emission maxima at blue (480 nm), green (525 nm), yellow (572 nm), red (650 nm), and NIR (720 nm)wavelengths.31,34,41,52 For in vivo imaging, there is a major drawback in using UV and blue light. Several abundant biomolecules in cells show high absorption in these wavelength ranges, thereby reducing light penetration through tissue. Additionally, many intracellular species exhibit weak fluorescence when illuminated at these wavelengths, thereby increasing background. The use of red and NIR emitters is hence preferable. NIR DNA/Ag emitters immobilized in poly(vinyl alcohol) (PVA) films have been shown to be bright and photostable, even at the single molecule (SM) level.31 These SM studies also revealed excursions, on the microsecond time scale, into a dark state for these NIR DNA/Ag emitters. Recently, it was shown that photoinduced electron transfer is the cause of the formation of this blinking on the microsecond time scale.53 Taking advantage of the depopulation of a dark state of these NIR DNA/Ag emitters when exciting with an additional 805 nm excitation source, optical demodulation techniques have been demonstrated to be able to extract the signal of these emitters even in high background conditions.36
Some recent reports have shown that the use of biological scaffolds such as peptides and DNA for encapsulating and stabilizing silver nanoclusters has enabled new biological imaging applications.32,35,37,54 The peptide-encapsulated silver nanoclusters that were used in fixed cell and live cell imaging displayed a relatively low quantum yield of fluorescence (~0.03).35 In contrast to peptide-encapsulated silver nanoclusters, DNA-encapsulated silver nanoclusters have higher fluorescence quantum yields and the red emitting Ag nanoclusters encapsulated with DNA were used for fixed and live cell imaging.33–35,37 Yu et al. demonstrated the imaging of internalized (by endocytosis) antibody-linked red emitting C24:Agn (anti-Heparin sulfate-C24-Ag) inside living NIH 3T3 cells.35 In this report, we focus on the performance of a NIR emitter C24:Agn as a fluorescent marker for in vivo labelling by using Lipofectamine as a transfection agent to incorporate the C24:Agn fluorophores inside living HeLa cells. The latter strategy was chosen because this is an approach that is also used in antisense therapy, where single-stranded oligodeoxyribonucleotides are delivered inside cells to inhibit the expression of a specific gene. In this way, fluorescent single-stranded oligonucleotides could act as a model system to study the performance of different cationic liposomes and cationic polymers as transfection agents. Also the uptake process via endocytosis and dissociation of the liposome/oligonucleotide complexes could be potentially monitored in this way.55 In this contribution, the incorporation is monitored and confirmed by scanning confocal fluorescence microscopy which allows visualizing sections through the cell.
Experimental
Material
Silver nitrate (Sigma-Aldrich, 99.9999%), the reductant sodium borohydride (Aldrich, 98%) and sodium chloride (Sigma-Aldrich, 99.999%) were used without further purification. Oligonucleotides with 24 bases of cytosine, 5′-(C)24-3′ (Eurogentec) were received as solids and dissolved in 0.05 M potassium phosphate buffer (pH = 7.2).
Synthesis of C24:Agn
The C24 and AgNO3 solutions (1 Ag+/2 bases)56,57 were mixed and cooled to 0 °C with ice. After 10 min of cooling, the solutions were reduced with NaBH4 solution and shaken intensively to form silver nanoclusters. For this, NaBH4 was dissolved in 18 MΩ deionized water and added within 30 s of preparation to theC24 and AgNO3 containing solution. Immediately after adding NaBH4, the C24:Agn solution was stored in the dark until it was used in the experiments.52
Steady-state measurements
Fluorescence excitation and emission spectra were obtained using a Photon Technology International spectrofluorimeter (QM-4SE). For these experiments NaCl (the resulting concentration was 130 mM) was added to the potassium phosphate buffered C24:Agn solution. This was done in order to have the same NaCl concentration as in the PBS buffer used for the cell transfection experiments. Chloride is known to precipitate out silver cations in aqueous solution, however the present amount of NaCl does not prevent the emission of the C24:Agn emitter. For the determination of the quantum yield of fluorescence, a Photon Technology International spectrofluorimeter (QM-4SE) and a spectrophotometer (Lambda 40, Perkin-Elmer) were used. Aluminium 2,9,16,23-tetrakis(phenylthio)-29H,31H-phthalocyanine chloride (Fabricolor Holding Int. LLC) in ethanol was used as a reference (quantum yield of fluorescence = 0.35).
Time-resolved measurements
Fluorescence decay times have been determined by time-correlated single photon counting experiments. The setup has been described in detail previously.58 Briefly, a 635 nm pulsed diode laser (pico-quant) was used as an excitation source. Via a double monochromator (Sciencetech 9030, 6 nm bandwidth), the fluorescence was detected with a microchannel plate photomultiplier (MCP-PMT, R3809U,Hamamatsu) connected to a time-correlated single photon counting card (Becker & Hickl, SPC 830).
Transfection and cell cultures
A day after the synthesis of the C24:Agn emitter, the solution was poured into a 3 kDa cut-off filter attached to a centrifuge tube (Millipore) to remove unbound Ag+ from the C24:Agn solution. The C24:Agn solution was centrifuged for 100 min at 14 000 g and about 50 µL of C24:Agn solution remained in the filter. The remaining C24:Agn solution was redissolved in a PBS buffer and adjusted to a concentration of 60 µM (based on the amount of C24 present). The PBS buffered C24:Agn solution was finally passed through a 0.22 µm filter (Millipore) right before transfection as a precaution to remove any microorganisms from the C24:Agn solution.
Lipofectamine TM 2000 (Invitrogen) was used to aid the transfection of HeLa cells with the C24:Agn emitter. 1, 5, 10 or 20 µL of C24:Agn and 1, 5, 10 or 20 µL of Lipofectamine were dissolved, each in 50 µL of OPTI-MEM I Reduced Serum medium without Phenol Red (Invitrogen). The C24:Agn and Lipofectamine solutions were allowed to mix for 20 min to form C24:Agn/Lipofectamine complexes. TheC24:Agn/Lipofectamine complexes were incubated with HeLa cells (90% confluency) in OPTI-MEM I medium in an 8 well plate (0.8 cm2) for 6 h at 37 °C under 5% CO2. The OPTI-MEM I medium was removed from the cells and Dulbecco’s Modified Eagle Medium (D-MEM) without Phenol Red (Invitrogen) supplemented with 10% Donor Bovine Serum (DBS) (Invitrogen), l-glutamine (Invitrogen), Gentamicin (50 mg ml−1, Invitrogen), and sodium bicarbonate 7.5% (Invitrogen) was added to the cells. The HeLa cells were further incubated for 18 h. The plate was removed from the incubator and placed on the confocal microscope for measurements at room temperature conditions.
Stage scanning confocal microscopy
A 633 nm continuous wave (CW) (JDS uniphase, HeNe laser) was used as excitation source. The excitation light, after passing through an excitation filter, was reflected by a dichroic mirror (Z633DCRB dichroic, Chroma) and was focused by a 100×, 1.3 N.A. oil immersion objective (Olympus) onto the sample mounted on an IX70 inverted microscope (Olympus). The fluorescence from the C24:Agn emitters, after passing through an emission filter (665LP, Chroma),was focused on an avalanche photodiode (APD) (SPCM-AQR-14, PerkinElmer). The output signals from the APD were recorded with a SPC-830 card (Becker & Hickl). Confocal fluorescence images were constructed by using a scanning stage (Physik Instrumente,Waldbronn, Germany) and an APD as point detector. Generally, 80 by 80 µm images were created using 256 by 256 pixels and an exposure time of 3 ms for each pixel.
Toxicity test
The HeLa cells in the 8 well plate were detached from the surface by adding 0.5% trypsin (Invitrogen). The detached HeLa cells in the D-MEM medium, used during the transfection, were placed in an Eppendorf tube. 10 µL of the suspended HeLa cells and 10 µL of Trypan Blue (Invitrogen) were placed in an Eppendorf tube and mixed well with pipettes. The 10 µL of mixture was placed in a counting grid chamber (KOVA Glasstic Slide 10 With Grids (HYCOR)) and live cells were counted with a microscope, following the instructions from the manufacturer.
Results and discussion
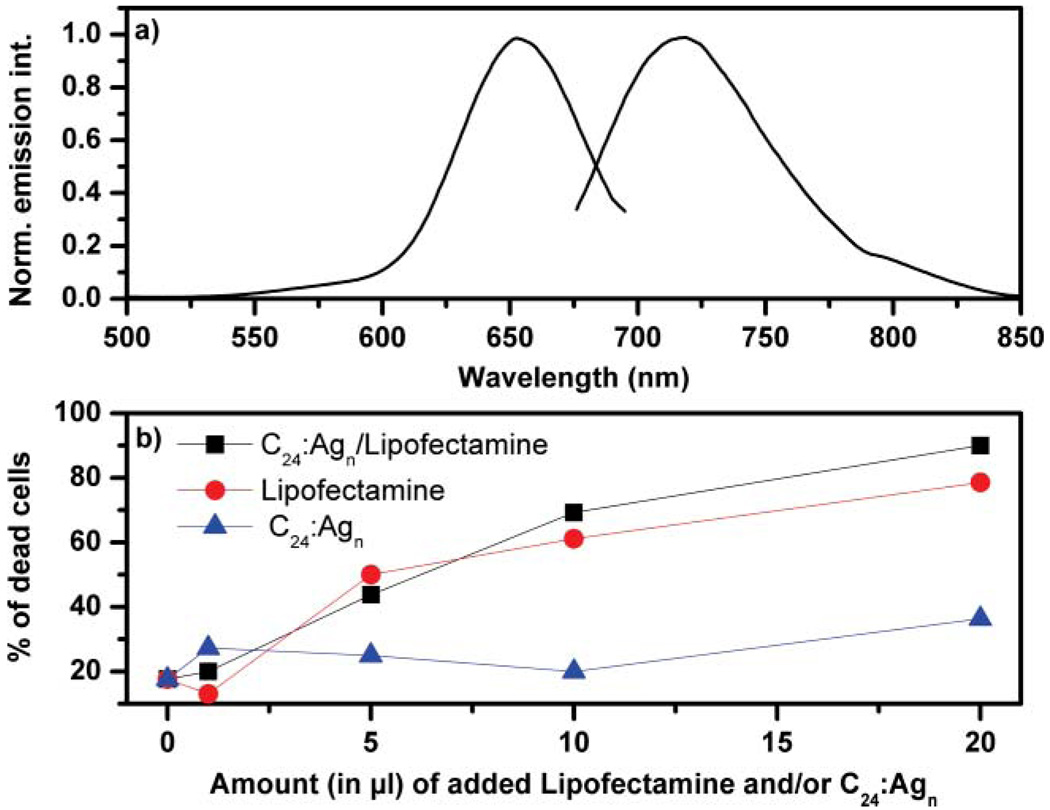
Fig. 1 shows the normalized excitation and emission spectra of the C24:Agn NIR emitter in a NaCl containing potassium phosphate buffered aqueous solution. The NaCl concentration was chosen to be similar as in the PBS buffer used for the cell transfection experiments. The C24:Agn NIR emitter has an excitation maximum of 650 nm and emission maximum of 715 nm. This emitter is spectrally similar to the C12:Agn NIR emitter and has a fluorescence quantum yield of 0.14 and the fluorescence decay consists largely out of a 2.67 ns fluorescence decay time component.31,59
Fig. 1.
(a) Normalized excitation spectrum (detected at 715 nm) and emission spectrum (excited at 650 nm) of C24:Agn in a NaCl containing potassium phosphate buffer. [C24] = 60 µM, [Ag+] = 720 µM, and [BH4−] = 720 µM. (b) Percentage of dead cells after 24 h of incubation with 0, 1, 5, 10 or 20 µL of only Lipofectamine (●), only C24:Agn (▲) or C24:Agn/Lipofectamine (■).
A major challenge when using Cm:Agn as a fluorescent label inside living cells is the poor permeability of Cm:Agn through the cell membrane. Since the cell membrane consists of polar head (hydrophilic) and nonpolar tail (hydrophobic) groups, it is very difficult for the negatively charged DNA to cross the cell membrane. In a way, we face the same problem as when one wants to introduce a piece of single stranded oligonucleotide into the cell, like in antisense therapy for example.55 For this, several strategies have been developed, for example electroporation,60 use of a virus vector,61,62 lipofection,63,64 gene guns,65 and microinjection.66 Here we chose transfection with the commercially available cationic liposome Lipofectamine. The positively charged Lipofectamine forms a complex with the negatively charged DNA by electrostatic interaction.67 The resulting complex, having a positively charged surface, can cross the cell membrane.67
Before looking at the transfection, using confocal fluorescence microscopy, the toxicity of adding Lipofectamine and C24:Agn to the HeLa cells was investigated. To get an idea about the toxicity, living and dead HeLa cells were identified by staining with Trypan Blue and counting them using a hemocytometer. Starting with the same cell density of about 90 percent confluency, Fig. 1b shows the percentage of dead HeLa cells after 24 h of incubation with 0, 1, 5, 10 or 20 µL of C24:Agn and/or 1, 5, 10 or 20 µL of Lipofectamine. When adding 1 µL of only Lipofectamine, only C24:Agn or C24:Agn/Lipofectamine to the HeLa cells, no significant toxicity or increase in the number of dead HeLa cells was observed. However, increasing the amount of Lipofectamine or C24:Agn/Lipofectamine to 5, 10 and 20 µL increases substantially the amount of dead HeLa cells. Increasing the amount of C24:Agn to 5, 10 and 20 µL does not lead to the same increase in dead HeLa cells. Only when adding 20 µL of C24:Agn there might be a small increase in the number of dead HeLa cells with respect to the reference point (0 µL). Based on this data we can conclude that the high toxicity and large number of dead HeLa cells when adding 20 µL of C24:Agn/Lipofectamine is largely due to the high amount of Lipofectamine and not due to the C24:Agn molecules. 20 µL of Lipofectamine is indeed a large amount, since it is about 15 times more than the recommended amount in the protocol provided by Invitrogen. Nevertheless we used this amount for following the transfection of the Hela cells in Fig. 3 because the creation yield of our specific NIR emitting DNA/Ag cluster probe is quite low, meaning that a large fraction of incorporated DNA/silver is not the NIR emitting probe. At this high dose of Lipofectamine we obtained good labelling of the cells, and despite the high toxicity the transfection could be followed clearly in some living HeLa cells. At the recommended concentration (about 1 µL) of Lipofectamine, the toxicity was substantially less, but so was the labelling (see Fig. 2).When the C24:Agn emitter can be created with a higher yield, less Lipofectamine will be needed, so that the transfection can be monitored at lower toxicity levels.
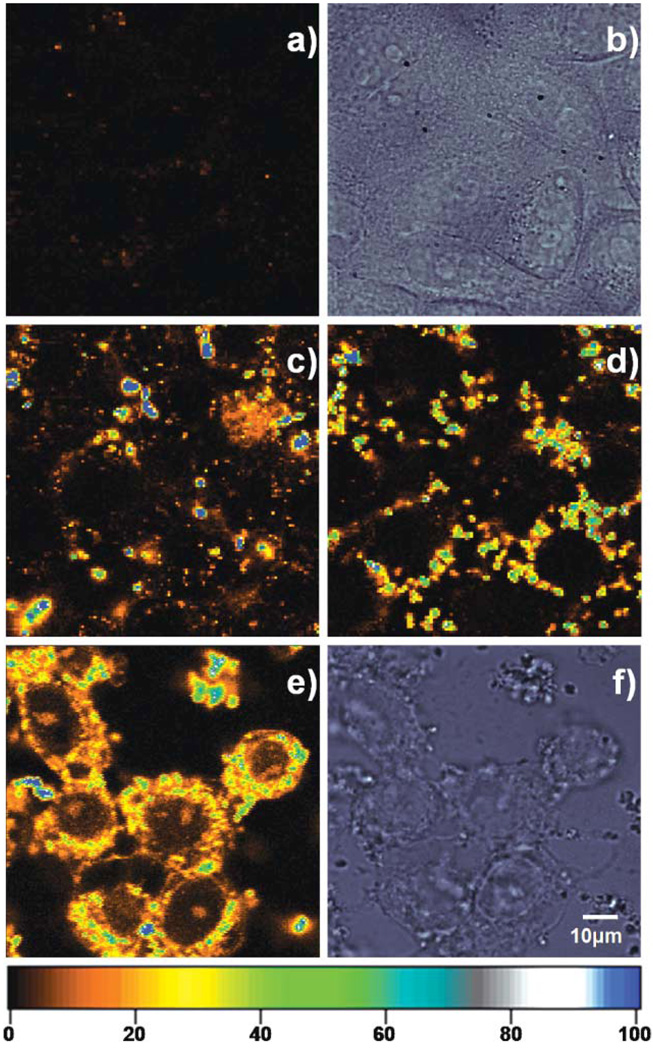
Fig. 3.
(a) Confocal fluorescence and (b) corresponding DIC images of HeLa cells without the addition of Lipofectamine/C24:Agn complexes. Confocal fluorescence images of living HeLa cells incubated for (c) 3 h, (d) 6 h, (e) 24 h with 20 µL of the Lipofectamine/C24:Agn complexes at 37 °C under 5% CO2. (f) Corresponding DIC image of (e). [C24] = 3 µM, [Ag+] = 36 µM, and [BH4−] = 36 µM. The living cells were taken out of the incubator at the specified times and confocal fluorescence images were obtained using a scanning stage (3 ms exposure per pixel, excitation intensity 130 W cm−2). The colour scale bar indicates the emission rate of the confocal fluorescence microscopy images in counts per ms.
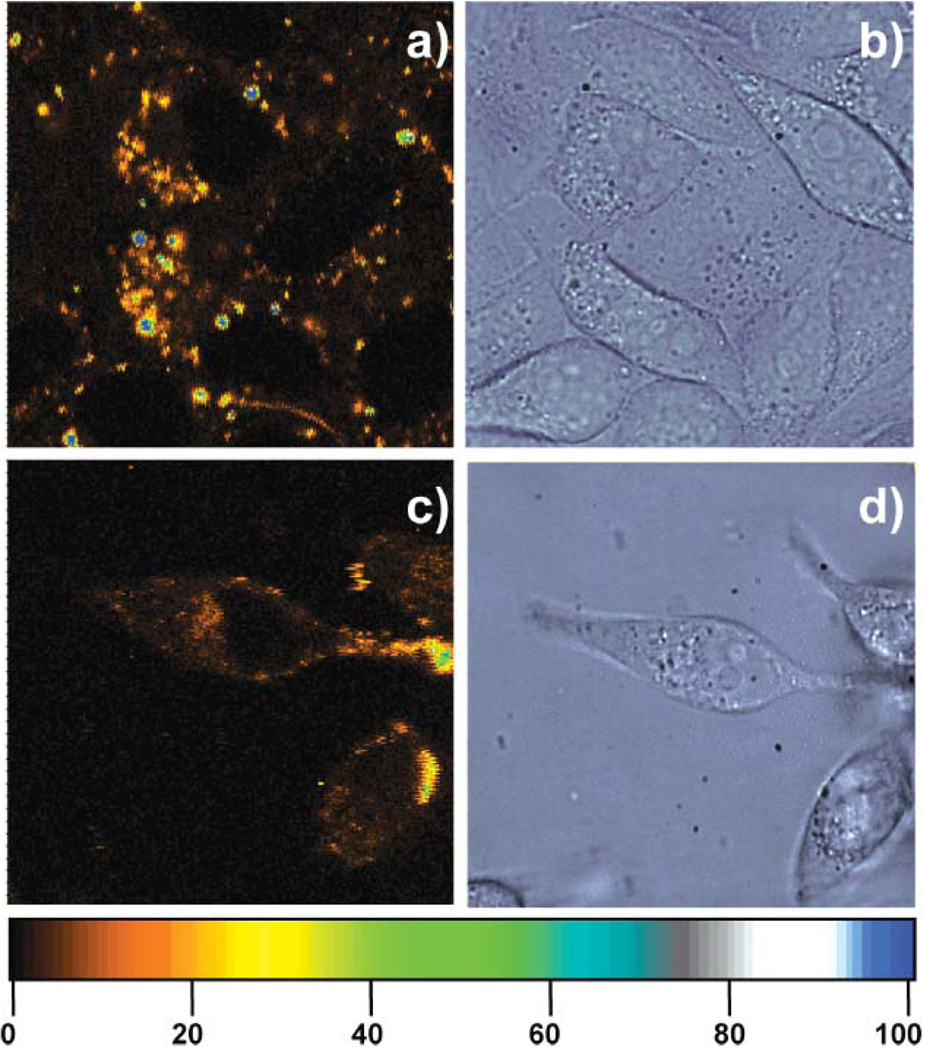
Fig. 2.
(a) Confocal fluorescence and (b) corresponding DIC images of HeLa cells incubated for 24 h with 1 µL of the Lipofectamine/C24:Agn complexes at 37 °C under 5% CO2. [C24] = 0.15 µM, [Ag+] = 1.8 µM, and [BH4−] = 1.8 µM. (c) Confocal fluorescence and (d) corresponding DIC images of HeLa cells incubated for 24 h with 20 µL of the C24:Agn at 37 °C under 5% CO2. [C24] = 3 µM, [Ag+] = 36 µM, and [BH4−] = 36 µM. The living cells were taken out of the incubator after 24 h and confocal fluorescence images were obtained using a scanning stage (3 ms exposure per pixel, excitation intensity 130 W cm−2). The colour scale bar indicates the emission rate of the confocal fluorescence microscopy images in counts per ms.
Fig. 2a and 2b show a confocal fluorescence image and a differential interference contrast (DIC) image of HeLa cells incubated with 1 µL of the C24:Agn/Lipofectamine complexes for 24 h. Some bright C24:Agn/Lipofectamine spots can be observed, but, as mentioned before, no clear labelling of the HeLa cells is observed. Fig. 2c and 2d show a confocal fluorescence image and a DIC image of HeLa cells incubated with 20 µL of the C24:Agn molecules for 24 h. The latter experiment shows the amount of labelling of the HeLa by C24:Agn without using Lipofectamine.
The labelling of the HeLa cells is rather low as can be seen in Fig. 2c. Similarly, it was shown before by Yu et al. that the affinity to and permeability of NIH 3T3 cells to DNA is low, resulting in poor staining of cells by C24:Agn alone.35
Fig. 3 shows confocal fluorescence images of HeLa cells incubated with 20 µL of the C24:Agn/Lipofectamine complexes. Without C24:Agn/Lipofectamine complexes, no emission was observed from the HeLa cells as is shown in Fig. 3a.A corresponding DIC image for Fig. 3a can be seen in Fig. 3b, showing the presence of the HeLa cells. The fluorescence images in Fig. 3c–e represent different HeLa cells that were taken out of the incubator after 3, 6 and 24 h. After 3 h incubation of HeLa cells, the majority of C24:Agn/Lipofectamine complexes had not interacted with cell membranes as can be seen by the very bright (blue colored) micrometre sized spheres. The outline of the HeLa cells can not be clearly determined (see Fig. 3c). After 6 h of incubation with the C24:Agn/Lipofectamine complexes, the complexes can be seen mainly at the cell surface, starting their incorporation into the HeLa cell, as is demonstrated in Fig. 3d. There is at this point however no significant fluorescence visible from inside the nucleus. After 24 h of incubation, shown in Fig. 3e, the fluorescence can be found back in the intracellular compartments. At this point the living HeLa cells are non specifically labelled with the C24:Agn molecules. The observed time for successful labelling of the HeLa cells is in agreement with the provided Lipofectamine protocol that specifies a minimum incubation time of 24 h for successful transfection. It is interesting to note that it looks like some C24:Agn molecules somehow escaped from vesicle like structures and were able to enter the nucleus.35 Amore detailed time series could may be provide an answer on how the C24:Agn molecules end up in the nucleus. The latter could provide also insight for antisense therapy studies.55
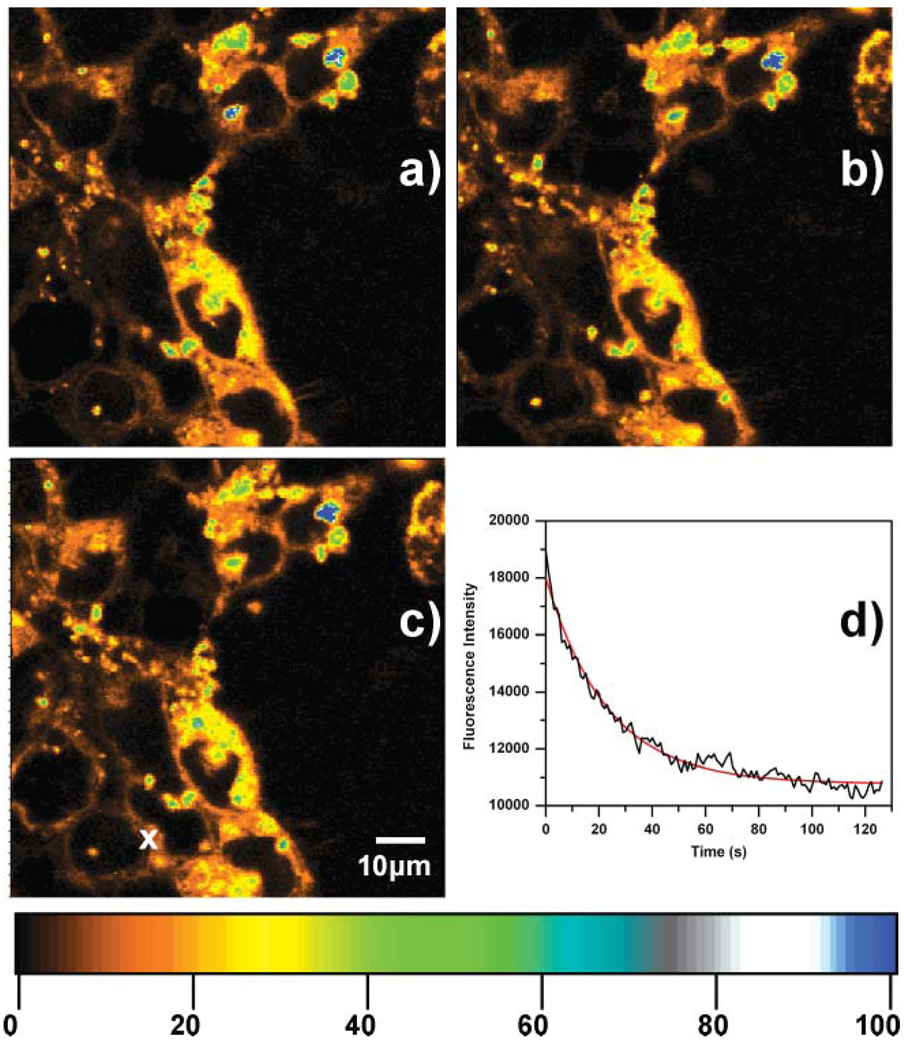
The fluorescence stability of the emissive C24:Agn labels in the transfected HeLa cells was tested in two ways and the results are depicted in Fig. 4. Fig. 4a–c show 3 fluorescence images (first, middle and last of a sequence of 18 images) of HeLa cells transfected with C24:Agn/Lipofectamine complexes for 24 h at 37 °C under 5% CO2. This 80 by 80 µm region was scanned 18 times over a period of 80 min and the average fluorescence intensity remained unchanged (excitation power: 46 W cm−2). In this way, each pixel is of course only exposed for 3 ms per frame. Therefore we moved the stage scanning to a certain spot (see white x in Fig. 4c) to investigate the photostability of the C24:Agn inside the HeLa cells. When exposed to constant laser irradiation (72 W cm−2), a decrease of the emission intensity was observed at the marked spot in Fig. 4c as can be seen in the graph, shown in Fig. 4d. The fluorescence intensity decreased from19 000 to 11 000 counts per second (cps) in 130 s. This 130 s fluorescence intensity trajectory can be fitted with a mono-exponential decaying function with a decay time of 23 s (red curve in Fig. 4d). The background fluorescence at the dark areas between the HeLa cells in Fig. 4c or when no C24:Agn/Lipofectamine complexes are present (like in Fig. 3a) is approximately 100 cps. Therefore, the 11 000 cps, observed after 130 s, is clearly is not the background intensity level but emission from the C24:Agn that decays more slowly. The latter can be due to the fact that a fraction of the C24:Agn might be in a different environment where they are more photostable or protected. A red emitting DNA/silver was found to display an exponential like bleaching when immobilized in PVA.34 Excellent photostabilities were observed previously for red and NIR emitting DNA/silver cluster complexes, outperforming organic dyes like Cy3 and Cy5, when immobilized in PVA.31,34
Fig. 4.
(a)–(c) First, middle and last image of a sequence of 18 confocal fluorescence images of HeLa cells incubated for 24 h with Lipofectamine/C24:Agn complexes at 37 °C under 5% CO2. Excitation intensity was 46 W cm−2 and 3 ms exposure per pixel. (d) Fluorescence intensity trajectory of Lipofectamine/C24:Agn complexes excited at 633 nm CW excitation and an excitation intensity of 72.5 W cm−2. [C24] = 3 µM, [Ag+] = 36 µM, and [BH4−] = 36 µM. The colour scale bar indicates the emission rate of the confocal fluorescence microscopy images in counts per ms.
Conclusions
In this paper we demonstrated that the near infrared emitting C24:Agn can be introduced into living HeLa by using Lipofectamine as a transfection agent. Bright luminescence from the C24:Agn was observed in the endocytosomes and nucleus of the living HeLa cells after an incubation period of 24 h. The use of these fluorescent silver cluster containing oligonucleotides could provide an interesting new way to study the transfection mechanism and dynamics (movement to the nucleus). Photostability of the C24:Agn label inside the living HeLa cells was investigated, resulting in exponential bleaching behaviour with a decay time of 23 s at 72.5 W cm−2 irradiation intensity and a much slower component. Also, evidence was brought forward indicating that the C24:Agn labels do not seem to be very toxic for the HeLa cells. This opens the possibility of conjugating this fluorescent C24:Agn probe with site-specific proteins to create site specific labelling for in vivo labelling and single particle tracking applications.
Acknowledgements
The authors acknowledge the “Fonds voor Wetenschappelijk Onderzoek (FWO)” (GrantG.0366.06), the K.U.Leuven Research Fund (GOA 2006/2, Center of Excellence CECAT, CREA2007) and the Federal Science Policy of Belgium (IAP-VI/27). This work, as part of the European Science Foundation EUROCORES Program SONS, was supported from funds by the FWO and the EC 6th Framework Program (ERAS-CT-2003-980409). Y.A. thanks Jelle Hendrix for fruitful discussion, Prof. Yves Engel-borghs for use of his cell facility and Lotte Bral and Dr Dirk Daelemans for providing HeLa cells and cell training. R.M.D. acknowledges NIH R01-GM086195. T.V. thanks the F.W.O. for a postdoctoral fellowship.
Contributor Information
Johan Hofkens, Email: Johan.Hofkens@chem.kuleuven.be.
Tom Vosch, Email: Tom.Vosch@chem.kuleuven.be.
Notes and references
- 1.Schmidt T, Kubitscheck U, Rohler D, Nienhaus U. Single Mol. 2002;3:327. [Google Scholar]
- 2.Haugland RP. The Handbook—A Guide to Fluorescent Probes and Labeling Technologies. Eugene OR: Molecular Probes; 2005. [Google Scholar]
- 3.Margineanu A, Hotta JI, Van Der Auweraer M, Ameloot M, Stefan A, Beljonne D, Engelborghs Y, Herrmann A, Muellen K, De Schryver FC, Hofkens J. Biophys. J. 2007;93:2877–2891. doi: 10.1529/biophysj.106.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin M, Kuhlmann C, Sorokina K, Li C, Mihov G, Pietrowski E, Koynov K, Klapper M, Luhmann H, Müllen K, Weil T. Biomacromolecules. 2008;9:1381–1389. doi: 10.1021/bm701138g. [DOI] [PubMed] [Google Scholar]
- 5.Margineanu A, Hofkens J, Cotlet M, Habuchi S, Stefan A, Qu JQ, Kohl C, Mullen K, Vercammen J, Engelborghs Y, Gensch T, De Schryver FC. J. Phys. Chem. B. 2004;108:12242–12251. [Google Scholar]
- 6.Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 7.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 8.Eggeling C, Widengren J, Rigler R, Seidel CAM. Anal. Chem. 1998;70:2651–2659. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat. Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 11.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AM, Ruan G, Rhyner MN, Nie SM. Ann. Biomed. Eng. 2006;34:3–14. doi: 10.1007/s10439-005-9000-9. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Gao XH, Yang LL, Petros JA, Marshal FF, Simons JW, Nie SM. Curr. Opin. Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 16.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howarth M, Liu WH, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY. Nat. Methods. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum C, Subramaniam V. Anal. Bioanal.Chem. 2009;393:527–541. doi: 10.1007/s00216-008-2425-x. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer M. Chem. Soc. Rev. 2009;38:2823–2832. doi: 10.1039/b904023d. [DOI] [PubMed] [Google Scholar]
- 20.Seward HE, Bagshaw CR. Chem. Soc. Rev. 2009;38:2842–2851. doi: 10.1039/b901355p. [DOI] [PubMed] [Google Scholar]
- 21.Day RN, Davidson MW. Chem. Soc. Rev. 2009;38:2887–2921. doi: 10.1039/b901966a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotlet M, Hofkens J, Habuchi S, Dirix G, Van Guyse M, Michiels J, Vanderleyden J, De Schryver FC. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14398–14403. doi: 10.1073/pnas.251532698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 24.Mocz G. Mar. Biotechnol. 2007;9:305–328. doi: 10.1007/s10126-006-7145-7. [DOI] [PubMed] [Google Scholar]
- 25.Flors C, Hotta J, Uji-I H, Dedecker P, Ando R, Mizuno H, Miyawaki A, Hofkens J. J. Am. Chem. Soc. 2007;129:13970–13977. doi: 10.1021/ja074704l. [DOI] [PubMed] [Google Scholar]
- 26.Folling J, Bossi M, Bock H, Medda R, Wurm CA, Hein B, Jakobs S, Eggeling C, Hell SW. Nat. Methods. 2008;5:943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- 27.Hell SW. Science. 2007;316:1153–1158. doi: 10.1126/science.1137395. [DOI] [PubMed] [Google Scholar]
- 28.Hess ST, Girirajan TPK, Mason MD. Biophys. J. 2006;91:4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty JT, Zheng J, Hud NV, Dickson RM. J. Am. Chem. Soc. 2004;126:5207–5212. doi: 10.1021/ja031931o. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie CM, Johnsen KR, Kiser JR, Antoku Y, Dickson RM, Petty JT. J. Phys. Chem. C. 2007;111:175–181. doi: 10.1021/jp0648487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vosch T, Antoku Y, Hsiang JC, Richards CI, Gonzalez JI, Dickson RM. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12616–12621. doi: 10.1073/pnas.0610677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Patel SA, Dickson RM. Angew. Chem., Int. Ed. 2007;46:2028–2030. doi: 10.1002/anie.200123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SA, Richards CI, Hsiang JC, Dickson RM. J. Am. Chem. Soc. 2008;130:11602–11603. doi: 10.1021/ja804710r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards CI, Choi S, Hsiang JC, Antoku Y, Vosch T, Bongiorno A, Tzeng YL, Dickson RM. J. Am. Chem. Soc. 2008;130:5038–5039. doi: 10.1021/ja8005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Choi S, Richards CI, Antoku Y, Dickson RM. Photochem. Photobiol. 2008;84:1435–1439. doi: 10.1111/j.1751-1097.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards CI, Hsiang JC, Senapati D, Patel S, Yu J, Vosch T, Dickson RM. J. Am. Chem. Soc. 2009;131:4619–4621. doi: 10.1021/ja809785s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Choi S, Dickson RM. Angew. Chem., Int. Ed. 2009;48:318–320. doi: 10.1002/anie.200804137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyser LA, Vinson AE, Bartko AP, Dickson RM. Science. 2001;291:103–106. doi: 10.1126/science.291.5501.103. [DOI] [PubMed] [Google Scholar]
- 39.Guo WW, Yuan JP, Wang EK. Chem. Commun. 2009:3395–3397. doi: 10.1039/b821518a. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta B, Ritchie CM, Buckman JG, Johnsen KR, Goodwin PM, Petty JT. J. Phys. Chem. C. 2008;112:18776–18782. doi: 10.1021/jp804031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill PR, Velazquez LR, Dunn DG, Gwinn EG, Fygenson DK. J. Phys. Chem. C. 2009;113:4229–4233. [Google Scholar]
- 42.Shang L, Dong SJ. Biosens. Bioelectron. 2009;24:1569–1573. doi: 10.1016/j.bios.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JG, Xu SQ, Kumacheva E. Adv. Mater. 2005;17:2336–2340. [Google Scholar]
- 44.Mrudula KV, Rao TUB, Pradeep T. J. Mater. Chem. 2009;19:4335–4342. [Google Scholar]
- 45.Takimoto B, Nabika H, Murakoshi K. J. Phys. Chem. C. 2009;113:11751–11755. [Google Scholar]
- 46.Narayanan SS, Pal SK. J. Phys. Chem. C. 2008;112:4874–4879. doi: 10.1021/jp710127s. [DOI] [PubMed] [Google Scholar]
- 47.Gwinn EG, O’Neill P, Guerrero AJ, Bouwmeester D, Fygenson DK. Adv. Mater. 2008;20:279–283. [Google Scholar]
- 48.Diez I, Pusa M, Kulmala S, Jiang H, Walther A, Goldmann AS, Muller AHE, Ikkala O, Ras RHA. Angew. Chem., Int. Ed. 2009;48:2122–2125. doi: 10.1002/anie.200806210. [DOI] [PubMed] [Google Scholar]
- 49.Treguer M, Rocco F, Lelong G, Le Nestour A, Cardinal T, Maali A, Lounis B. Solid State Sci. 2005;7:812–818. [Google Scholar]
- 50.De Cremer G, Coutiño-Gonzalez E, Roeffaers MBJ, Moens B, Ollevier J, Van Der Auweraer M, Schoonheydt R, Jacobs PA, De Schryver FC, Hofkens J, De Vos DE, Sels BF, Vosch T. J. Am. Chem. Soc. 2009;131:3049–3056. doi: 10.1021/ja810071s. [DOI] [PubMed] [Google Scholar]
- 51.De Cremer G, Antoku Y, Roeffaers MBJ, Sliwa M, Van Noyen J, Smout S, Hofkens J, De Vos DE, Sels BF, Vosch T. Angew. Chem., Int. Ed. 2008;47:2813–2816. doi: 10.1002/anie.200704861. [DOI] [PubMed] [Google Scholar]
- 52.Antoku Y. PhD thesis. Atlanta: Georgia Tech; 2007. [Google Scholar]
- 53.Patel SA, Cozzuol M, Hales JM, Richards CI, Sartin M, Hsiang JC, Vosch T, Perry JW, Dickson RM. J. Phys. Chem. C. 2009;113:20264–20270. doi: 10.1021/jp9079537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makarava N, Parfenov A, Baskakov IV. Biophys. J. 2005;89:572–580. doi: 10.1529/biophysj.104.049627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas B, Remaut K, Sanders NN, Braeckmans K, De Smedt SC, Demeester J. J. Controlled Release. 2005;103:435–450. doi: 10.1016/j.jconrel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Dattagupta N, Crothers DM. Nucleic Acids Res. 1981;9:2971–2985. doi: 10.1093/nar/9.12.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arya SK, Yang JT. Biopolymers. 1975;14:1847–1861. [Google Scholar]
- 58.Maus M, Rousseau E, Cotlet M, Schweitzer G, Hofkens J, Van Der Auweraer M, De Schryver FC, Krueger A. Rev. Sci. Instrum. 2001;72:36–40. [Google Scholar]
- 59.A tri-exponential function best fitted the fluorescence decay trace. The three components were 0.27 ns (6.27%), 1.77 ns (27.75%) and 2.67 ns (65.98%) with a χ2 value of 1.009.
- 60.Neumann E, Schaeferridder M, Wang Y, Hofschneider PH. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamer DH, Leder P. Cell. 1979;18:1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- 62.Mulligan RC, Howard BH, Berg P. Nature. 1979;277:108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- 63.Fraley R, Subramani S, Berg P, Papahadjopoulos D. J. Biol. Chem. 1980;255:431–435. [PubMed] [Google Scholar]
- 64.Wong TK, Nicolau C, Hofschneider PH. Gene. 1980;10:87–94. doi: 10.1016/0378-1119(80)90126-2. [DOI] [PubMed] [Google Scholar]
- 65.Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Hum. Gene Ther. 1997;8:1303–1311. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 66.Capecchi MR. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- 67.Hirko A, Tang F, Hughes JA. Curr. Med. Chem. 2003;10:1185–1193. doi: 10.2174/0929867033457412. [DOI] [PubMed] [Google Scholar]