Abstract
Rationale
Vascular fibrosis and calcification contribute to diabetic arteriosclerosis, impairing Windkessel physiology necessary for distal tissue perfusion. Wnt family members up-regulated in arteries by the low-grade inflammation of “diabesity” -- stimulate type I collagen expression and osteogenic mineralization of mesenchymal progenitors via β-catenin. Conversely, parathyroid hormone (PTH) inhibits aortic calcification in LDLR (low density lipoprotein receptor)-deficient mice fed high fat diabetogenic diets (HFD).
Objective
We wished to determine the impact of vascular PTH receptor (PTH1R) activity on arteriosclerotic Wnt/β-catenin signaling in vitro and in vivo. We generated SM-caPTH1R transgenic mice, a model in which the constitutively active PTH1R variant H223R (caPTH1R) is expressed only in the vasculature.
Methods and Results
The caPTH1R inhibited Wnt/β-catenin signaling, collagen production, and vascular smooth muscle cell (VSMC) proliferation and calcification in vitro. Transgenic SM-caPTH1R;LDLR+/− mice fed HFD develop “diabesity,” with no improvements in fasting serum glucose, cholesterol, weight, body composition, or bone mass vs. LDLR+/− siblings. SM-caPTH1R down-regulated aortic Col1A1, Runx2, and Nox1 expression without altering TNF, Msx2, Wnt7a/b, or Nox4. The SM-caPTH1R transgene decreased aortic β-catenin protein accumulation and signaling in diabetic LDLR+/− mice. Levels of aortic superoxide -- a precursor of peroxide that activates pro-MMP9 and osteogenic signaling in VSMCs -- were suppressed by the SM-caPTH1R transgene. Aortic calcification, collagen accumulation, and wall thickness were concomitantly reduced, enhancing vessel distensibility.
Conclusions
Cell-autonomous VSMC PTH1R activity inhibits arteriosclerotic Wnt/β-catenin signaling and reduces vascular oxidative stress, thus limiting aortic type I collagen and calcium accrual in diabetic LDLR-deficient mice.
Keywords: arteriosclerosis, β-catenin, diabetes, parathyroid hormone, Wnt
INTRODUCTION
Atherosclerosis and medial artery calcification are two contributors to arteriosclerosis ---the vascular stiffening that impairs Windkessel physiology necessary for smooth distal tissue perfusion and efficient cardiac performance1. Arteriosclerotic changes in mural biochemical and geometric properties are responsible for functional ageing of conduit arteries1. In the musculoskeletal system, the lower extremities bear the brunt of disease burden arising from arteriosclerosis2,3. Claudication, critical limb ischemia, and amputation are salient manifestations that reduce mobility and increase morbidity in our patients2,3. In either the presence or absence of type II diabetes (T2DM), the presence and extent of vascular calcification portends lower extremity amputation risk3. Bostrom, Demer, Shanahan and colleagues demonstrated the elaboration of osteochondrogenic regulatory programs in calcifying segments of patients with vascular disease, indicating that active biomineralization programs contribute to arterial calcium accrual4,5. Rajamannan, Miller, Heistad et al 6,7,8 have made similar observations in calcific aortic stenosis, a lethal sclerotic vasculopathy also increased by T2DM and advancing age9. A fundamental understanding of the pro-calcific and pro-fibrotic mechanisms of diabetic arteriosclerosis is necessary to develop new strategies to address the burgeoning vascular disease burden afflicting our population4.
In our pre-clinical studies of arterial calcification, we have implemented the LDLR (low density lipoprotein receptor)-deficient mouse model10. When LDLR−/− mice are fed high fat Western diets (HFD), male animals develop obesity, diabetes, hyperinsulinemia, hypertriglyceridemia and hypercholesterolemia, with progressively severe arterial calcification11. While medial calcification predominates at early disease stages, at later stages atherosclerotic intimal calcification increasingly accounts for vascular calcium load11,12. Without HFD-induced “diabesity,” vascular disease phenotypes are markedly attenuated. However, in the presence of selective apolipoprotein (Apo) B100 expression, LDLR-deficient mice develop hemodynamically significant calcific aortic stenosis -- even when aged on standard rodent chow diets13. In LDLR−/−;ApoB(100/100) mice, cholesterol – dependent initiation and reversal of age-associated aortic valve calcification is heralded by vascular changes in osteogenic transcription factors, including Msx2 (muscle segment homeobox protein homolog 2)14. Similarly, at the very earliest stages of HFD-induced “diabesity” and dyslipidemia, osteogenic gene regulatory programs are upregulated in the aortas of LDLR-deficient mice15,16. Within two weeks of HFD challenge, the osteogenic transcription factor Msx2 is ectopically induced in aortic valve fibrosa and aortic adventitia15,16. Msx2 enhances cardiovascular calcification by activating paracrine Wnt (wingless-type integration site family member) signals that drive osteogenic17 and myofibroblast17,18 differentiation programs in adjacent mesenchymal progenitors16–18. Inflammatory cytokines, matrix metalloproteinases (MMPs), and reactive oxygen species (ROS) initiate and propagate these processes, activating mural β-catenin signals11,19.
β-catenin is a transcriptional co-adaptor indispensable for osteogenic tissue mineralization20 and participates in osteochondrogenic differentiation of mural mesenchymal progenitors21. In the vasculature, β-catenin activity is not only entrained to TNF (tumor necrosis factor) and Msx2-Wnt activity 11; N-cadherin- and MMP-regulated cell-cell interactions reciprocally control β-catenin signals that drive VSMC (vascular smooth muscle cell) proliferation and neointima formation19,22. Moreover, ROS activate pro-MMP923 which in turn cleaves cell-surface N-cadherin and liberates the cadherin-sequestered pool of β-catenin to promote signaling19. Thus, data from multiple laboratories have converged upon β-catenin activation as a key component of arteriosclerotic physiology8,16,19,21,24.
Previously, we demonstrated that dosing with the bioactive human parathyroid hormone (PTH) fragment PTH(1–34) reduced vascular mineralization, down-regulated aortic Msx2-Wnt expression, and inhibited Wnt-activated β-catenin signaling16,25. However, in vivo PTH(1–34) administration may exert both direct and indirect actions on vascular physiology. While the PTH/PTHrP receptor (PTH1R) is expressed and biologically active in VSMC26,27, PTH1R activation by PTH(1–34) in kidney, bone, and hematopoietic niche may also influence arteriosclerotic processes28. Thus, to better understand the role of VSMC PTH1R signaling in arteriosclerosis, we generated SM-caPTH1R transgenic mice. In this model, the constitutively active Jansen PTH1R (caPTH1R) variant PTH1R(H223R)29 is expressed in VSMC using the 0.5 kb SM22 (transgelin) promoter11,30. We find that VSMC-autonomous PTH1R signaling decreases aortic activation of pro-calcific and pro-fibrotic β-catenin signals, diminishes vascular oxidative stress, and reduces aortic collagen and calcium accrual the LDLR-deficient mouse model of diabetic vascular disease.
METHODS
Materials, methods, and statistics implemented are presented in detail in the Online Supplement. Procedures for generating and evaluating mice transgenic for SM-caPTH1R were approved by the Washington University Animal Studies Committee. LDLR −/− mice (Stock #002207) and TOPGAL mice (Stock #004623) were obtained from the Jackson Laboratories. Diet-induced diabetes was initiated in male sibling cohorts at 5 – 8 weeks of age by ad libitum feeding with the “Western” HFD (Harlan TD.88137; 42% of calories from fat). Following 3 – 4 months of dietary challenge, body composition, serum biochemistries, aortic pathology, and aortic gene expression were analyzed11. Data are presented as the mean +/− S.E. of independent replicates (n = 3 to 22, dependent upon the assay).
RESULTS
Wnt/β-catenin signaling elicits pro-fibrotic and pro-calcific responses in C3H10T1/2 multipotent mural mesenchymal progenitors
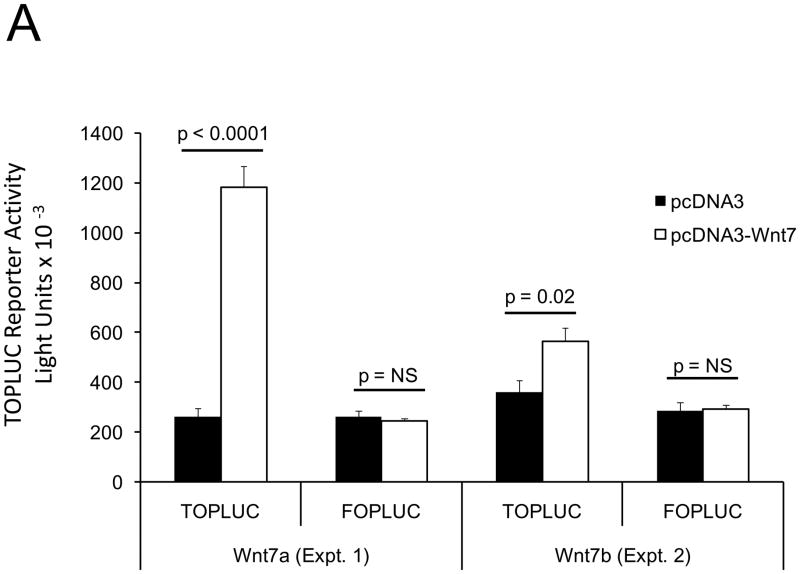
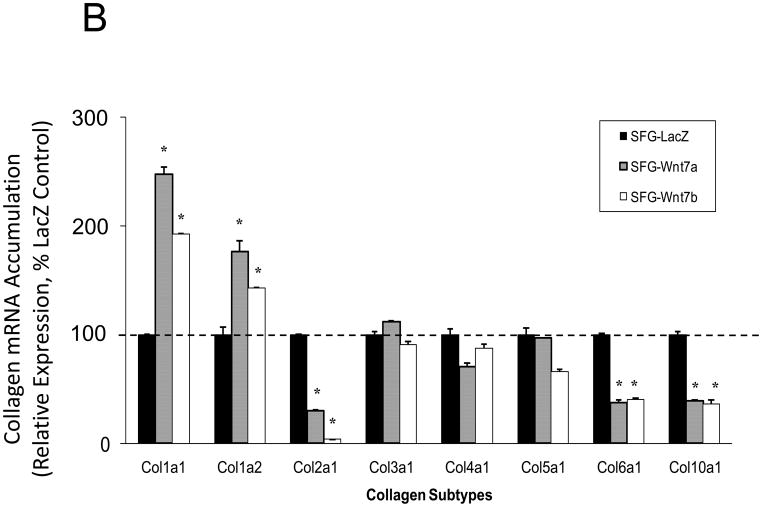
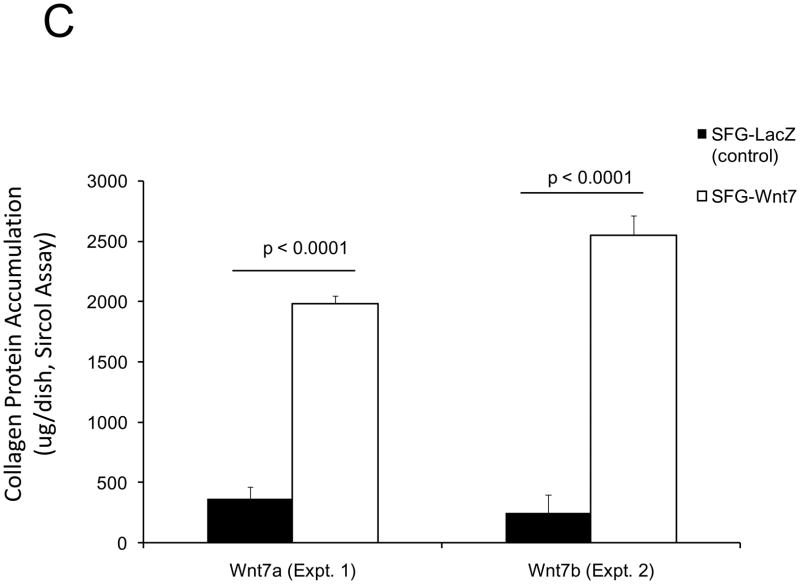
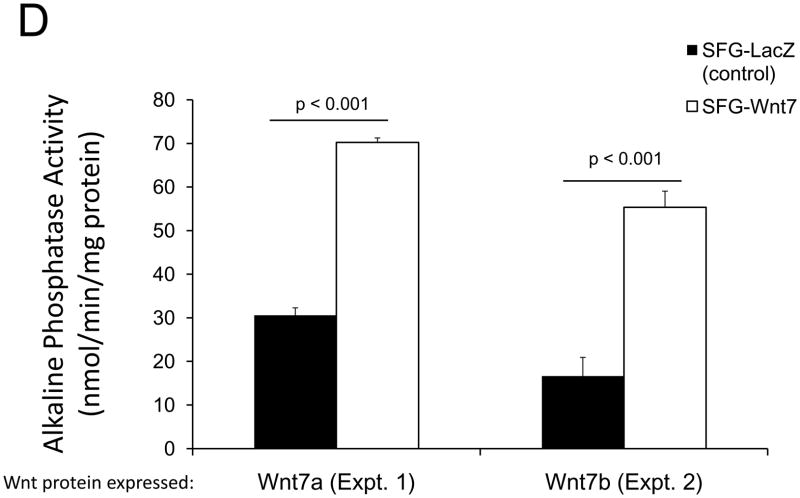
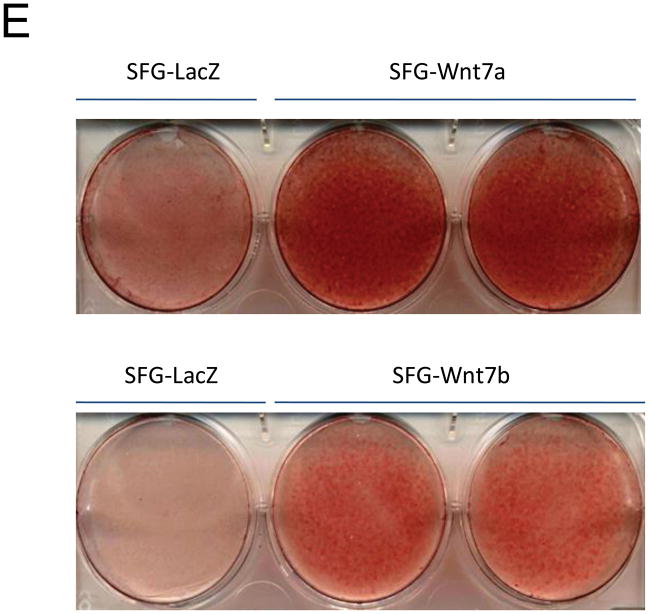
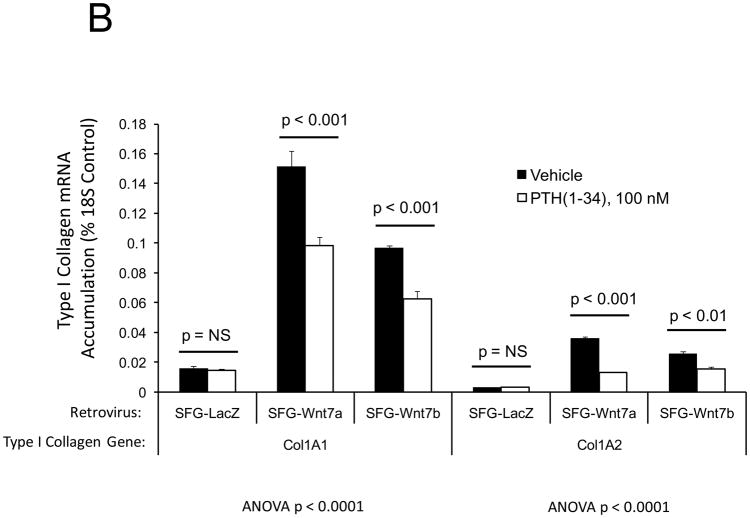
In male LDLR −/− mice, diabetogenic HFD upregulates TNF-dependent aortic Msx2-Wnt signaling cascades that promote vascular calcium deposition11,25. Wnt7a and Wnt7b are vascular Wnts11,16,31,32 that participate in Msx2 pro-osteogenic actions16,33. Like Wnt3a, Wnt7a and Wnt7b also stimulate canonical β-catenin signaling in C3H10T1/2 cells, as evident in TCF/LEF optimal promoter (TOP) – luciferase reporter (LUC) activation (Figure 1A). To better understand the contributions of the Wnt7 family to vascular fibrosis and calcification, we examined Wnt7 effects on C3H10T1/2, a multipotent mural mesenchymal progenitor34 that elaborates both osteogenic17 and vascular smooth muscle18,34 phenotypes. We generated SFG-Wnt7a and SFG-Wnt7b -- vesicular stomatitis virus G protein pseudotyped retroviral vectors (SFG35) for Wnt7 expression 17 -- and assessed the impact of SFG-Wnt7 transduction on collagen gene expression, collagen protein production, and mineral deposition. As compared to cultures transduced with SFG- LacZ (β-galactosidase; control), cultures transduced with SFG-Wnt7a or SFG–Wnt7b exhibited increased expression of type I collagen (Col1A1 and Col1A2; Figure 1B) and increased collagen protein content (Figure 1C). Col2A1, Col6A1, and Col10A1 were concomitantly suppressed, indicating selective activation of a pro-osteogenic36 collagen gene regulatory program (Figure 1B). Bone alkaline phosphatase enzyme activity (Figure 1D) and mRNA (not shown) were also induced by SFG-Wnt7. Moreover, when cultured under conditions permissive for matrix mineralization, SFG-Wnt7 significantly increased calcium deposition in cultured cells as compared to SFG-LacZ control (Figure 1E). Thus, signals provided by Wnt7a and Wnt7b elicit pro-fibrotic and pro-calcific responses characteristic of osteogenic differentiation in C3H10T1/2 mural mesenchymal progenitor cells34.
Figure 1. Wnt7 family members activate canonical β-catenin signaling, upregulate type I collagen expression, and promote matrix mineralization in C3H10T1/2 cells.
Panel A, C3H10T1/2 cells were co-transfected with pcDNA3-Wnt7 expression vectors and LUC reporters as indicated. Both pcDNA-Wnt7a and pcDNA3-Wnt7b upregulated TOPLUC activity vs. vector control. FOPLUC (lacks intact TCF/LEF cognate) was not regulated (n = 6 per condition, replicated > 3 times). Panel B, C3H10T1/2 cells were transduced with SFG vectors expressing either Wnt7a or Wnt7b, and gene expression assessed by RT-qPCR. SFG-Wnt7a and SFG-Wnt7b upregulated Col1A1 and Col1A2 vs. the control virus, SFG-LacZ. *, p < 0.05 vs. SGF-LacZ (n = 3 per condition, replicated twice). Panel C, collagen protein was also increased in monolayers transduced with SFG-Wnt7 vectors (n= 3 per condition, replicated ≥ twice). Panel D, alkaline phosphatase enzyme activity was upregulated by Wnt7a and Wnt7b (n = 4 per condition). Panel E, SFG-Wnt7 transduction also increased calcium deposition, revealed by Alizarin red staining (n = 2 per condition, replicated > 5 times).
Canonical β-catenin transcriptional responses elicited by Wnt signaling are inhibited by PTH1R activation
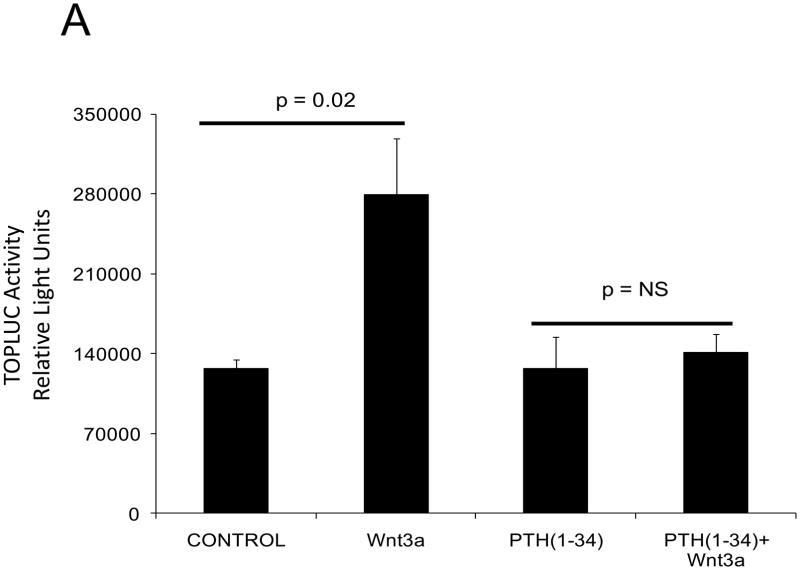
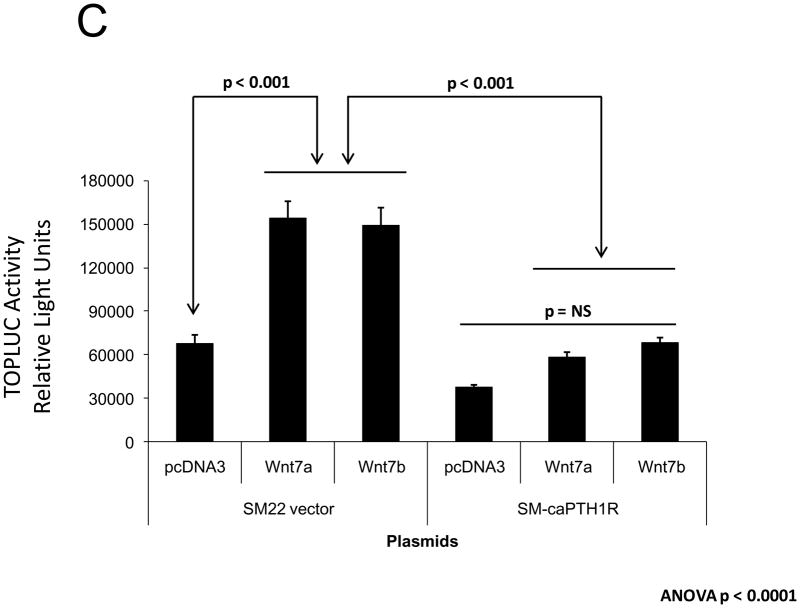
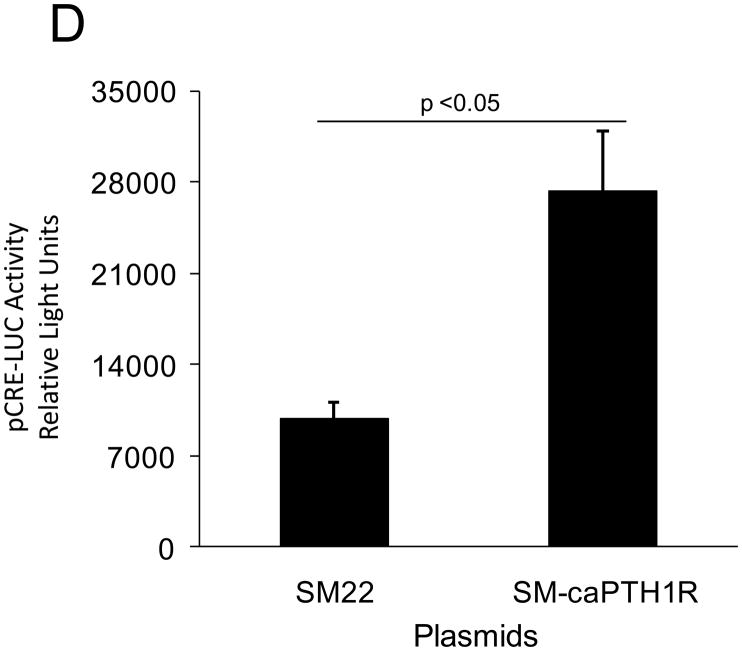
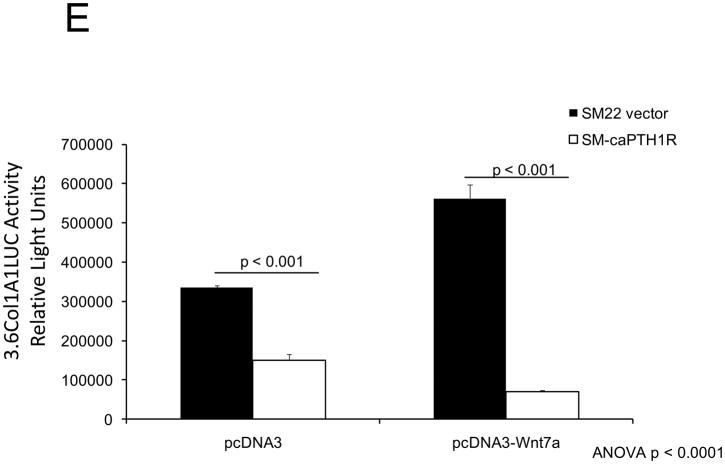
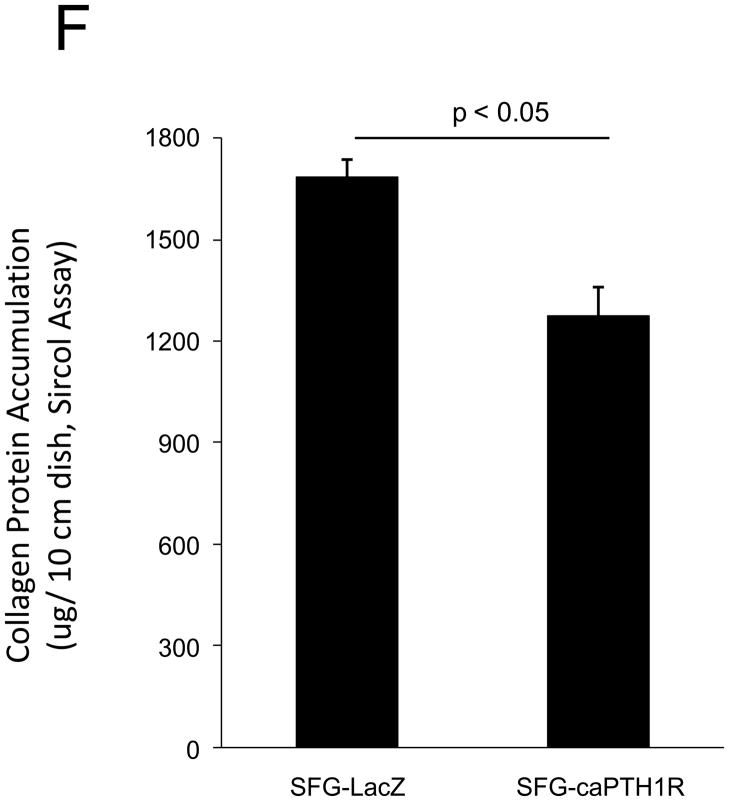
PTH(1–34) – a.k.a. teriparatide – is a clinically useful agonist of the PTH1R that simultaneously stimulates bone formation and inhibits vascular calcification in diabetic25 and uremic37 rodent models. Since Wnt/β-catenin cascades24 participate in diabetic arteriosclerosis11,16, we examined the impact of PTH/PTH1R activation on Wnt/β-catenin-regulated transcription, including type I collagen expression. For these experiments, we implemented primary mouse aorta VSMCs and A7r5 cells, a SM22α-expressing rat aorta VSMC line that faithfully recapitulates key features of VSMC-specific gene transcription38. PTH(1–34) inhibits TOPLUC activation by either Wnt3a (Figure 2A) or by Wnt7a/b (data not shown), indicating that Wnt7a, Wnt7b, and Wnt3a elaborate similar PTH-sensitive canonical signals in VSMCs. Likewise, PTH(1–34) significantly reduces Wnt7a- and Wnt7b-stimulated Col1A1 and Col1A2 expression in primary aortic VSMCs (Figure 2B). Moreover, using the 0.5 kb SM22-promoter30 to drive expression, co-expression of PTH1R(H223R) -- a constitutively active form of the PTH1R (caPTH1R) that does not require ligand for activation29 -- inhibited Wnt7-activated TOPLUC transcription in A7r5 VSMCs (Figure 2C) and primary aortic VSMC cultures (data not shown). Suppression of transcription by caPTH1R was promoter-specific, since SM-caPTH1R stimulated cyclic AMP responsive pCRE-LUC as expected in transient co-transfection assays (Figure 2D). The caPTH1R also inhibited basal (Figure 2E), Wnt3a (not shown), and Wnt7a-stimulated (Figure 2E) Col1A1 promoter activity as reflected by 3.6ColA1-LUC activity in aortic VSMCs. Similar responses were observed with the 2.3 kb Col1A1 promoter fragment (not shown)1. Finally, retroviral transduction of primary aortic VSMC with SFG-caPTH1R reduced basal collagen protein accumulation vs. cultures transduced with SFG-LacZ (Figure 2F). Thus, PTH/PTH1R activation inhibits Wnt/β-catenin signaling, type I collagen expression and collagen protein accumulation in aortic VSMC cultures.
Figure 2. PTH and the constitutively active receptor caPTH1R inhibit canonical Wnt/β-catenin signaling, Col1A1 transcription, and collagen accumulation.
Panel A, A7r5 aortic VSMCs were transfected with the TOPLUC reporter and treated with 10 ng/ml Wnt3a and 100 nM PTH either alone or in combination. PTH(1–34) abrogated Wnt3a-mediated activation (n = 6 per condition, replicated twice). Panel B, PTH(1–34) inhibits SFG-Wnt7a and SFG-Wnt7b induction of Col1A1 and Col1A2 expression in transduced primary aortic VSMCs (n = 3 per condition, replicated twice). Panel C, co-transfection of SM-caPTH1R, a VSMC expression plasmid for constitutively active caPTH1R, also inhibits TOPLUC activation by pcDNA3-Wnt7a or pcDNA-Wnt7b (n = 6 per condition, replicated twice). Panel D, TOPLUC inhibition by SM-caPTH1R was promoter-specific, since SM-caPTH1R stimulates pCRE-LUC (n = 6 per condition, replicated twice). Panel E, SM-caPTH1R inhibits basal and pcDNA3-Wnt7a induced Col1A1 promoter activity (n = 6 per condition, replicated 4 times). Panel F, primary aortic VSMC transduced with SFG-caPTH1R exhibit decreased collagen protein accumulation vs. SFG-LacZ controls (n = 3 per condition).
SM-caPTH1R transgene expression down-regulates aortic β-catenin signaling and aortic β-catenin protein accumulation in vivo
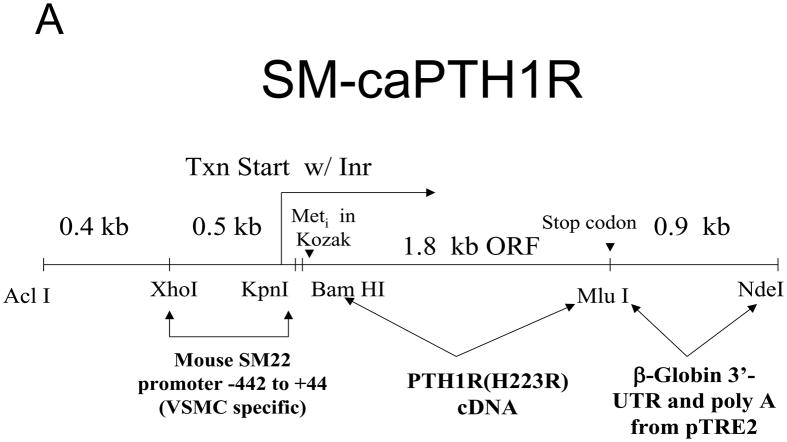
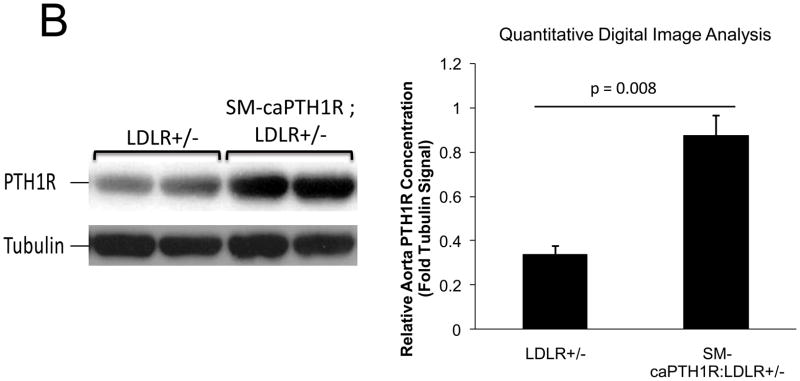
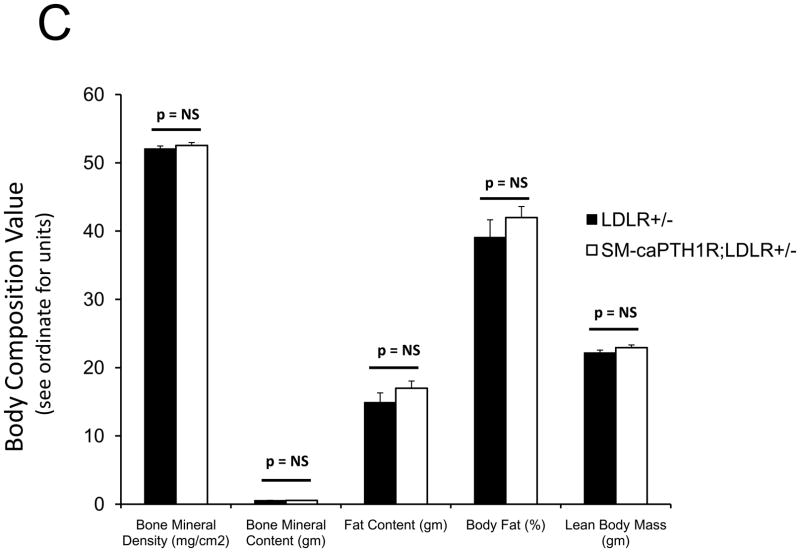
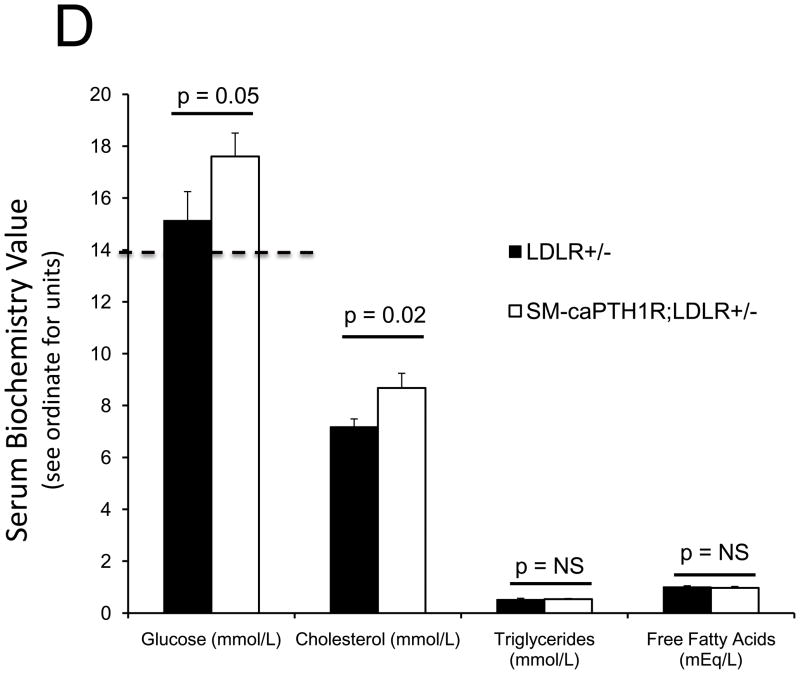
PTH(1–34) administration reciprocally regulates aortic vs. skeletal osteogenic programs16,25. Specifically, PTH(1–34) suppresses aortic OPN (osteopontin), Msx2, and Wnt7a, but stimulates bone formation, skeletal OPN expression, and circulating OPN levels in LDLR-deficient mice on HFD25. PTH(1–34) down-regulates aortic calcium accumulation in diabetic LDLR-deficient mice (Online Figure I and ref. 25). However, PTH(1–34) dosing also exerts global effects – e.g., changes in skeletal hematopoietic niche size28, circulating OPN25,39, and ROS-generated oxylipid signals23 (Online Figure II) – that control vascular disease processes4,39. We wished to assess whether VSMC-autonomous PTH1R signaling could convey a subset of PTH(1–34) responses; therefore, we generated mice transgenic for a constitutively active PTH1R (caPTH1R)29, using the 0.5 kb SM22 promoter30 as a delivery module (SM-caPTH1R; Figure 3A). The SM-caPTH1R expression vector we validated by A7r5 transient transfection (Figure 2D) was digested with Acl I and Nde I to liberate the SM-caPTH1R-βglobin UTR fragment (Figure 3A), and transgenic mice were generated (see “Methods”). Subsequently, we bred the SM-caPTH1R transgene onto the heterozygous LDLR+/− background, a LDLR-deficient background of intermediate vascular disease severity40. As in LDLR−/− mice, HFD upregulates aortic expression of Msx2 in LDLR+/− mice (supplement Online Figure III); however, 3–4 months of HFD feeding in LDLR+/− mice was required to achieve significant diabetes, and cholesterol levels were much lower in LDLR+/− vs. LDLR−/− mice (Online Figure IV). Analysis of aortic extracts from SM-caPTH1R;LDLR+/− transgenic mice demonstrated a 2.5-fold increase in PTH1R protein levels vs. non-transgenic siblings (Figure 3B; p < 0.01). We then challenged male SM-caPTH1R;LDLR+/− and LDLR+/− sibling cohorts with 4 months of HFD, assessing the impact on body composition and metabolic profiles. As shown in Figure 3C, SM-caPTH1R;LDLR+/− mice exhibited no significant differences in bone mineral content, lean body mass, or fat mass as assessed by dual electron X-ray absorptiometry (DXA)25. Serum glucose and cholesterol levels were not reduced by the transgene, and were in fact slightly higher in SM-caPTH1R animals (Figure 3D). No significant differences were observed in serum triglycerides and free fatty acids. Similarly, no significant differences were observed in fasting serum calcium, PTH, 8-F-isoprostane, or markers of bone turnover (Online Table I).
Figure 3. LDLR+/− mice transgenic for SM-caPTH1R exhibit increased levels of aortic PTH1R protein without improvements in “gdiabesity”h phenotype or alterations in bone turnover.
Panel A, schematic of the SM-caPTH1R expression construct and Acl1-NdeI fragment used to generate transgenic mice. Panel B, immunoreactive PTH1R protein was significantly increased in aortic extracts from SM-caPTH1R;LDLR+/− mice vs. non-transgenic LDLR+/− littermates (n = 4). Panel C, body composition assessed by DXA was unaffected by the SM-caPTH1R transgene (n = 14–16/group; 4 months HFD). Panel D, likewise, serum fasting glucose, cholesterol, triglycerides, and free fatty acids were not improved by the SM-caPTH1R transgene.
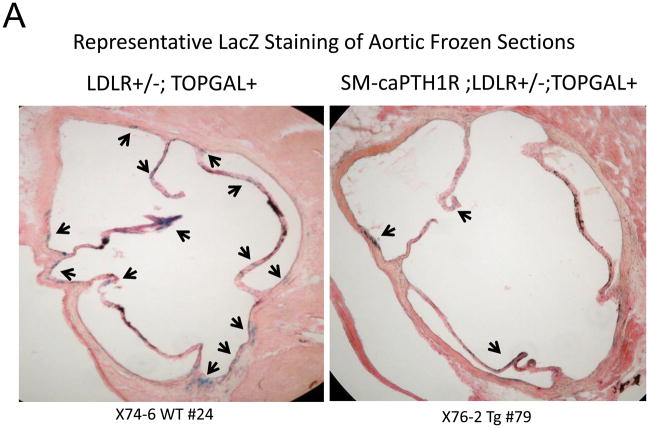
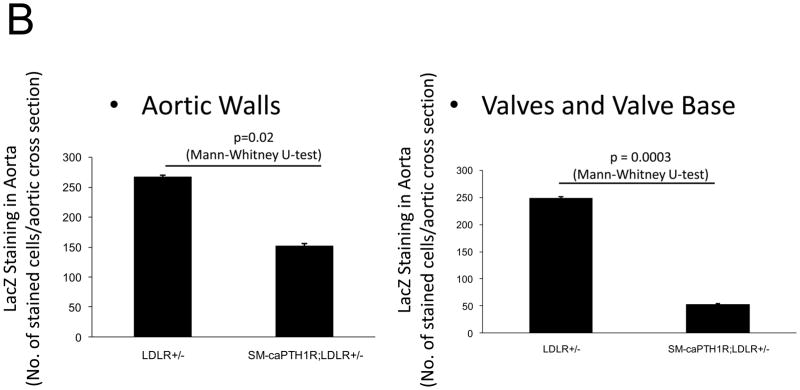
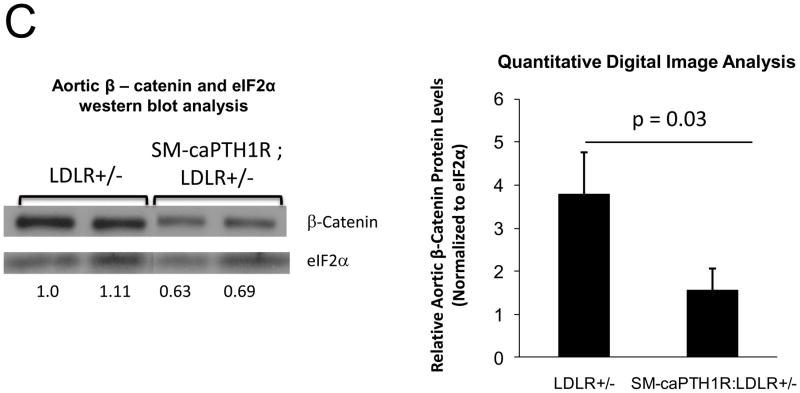
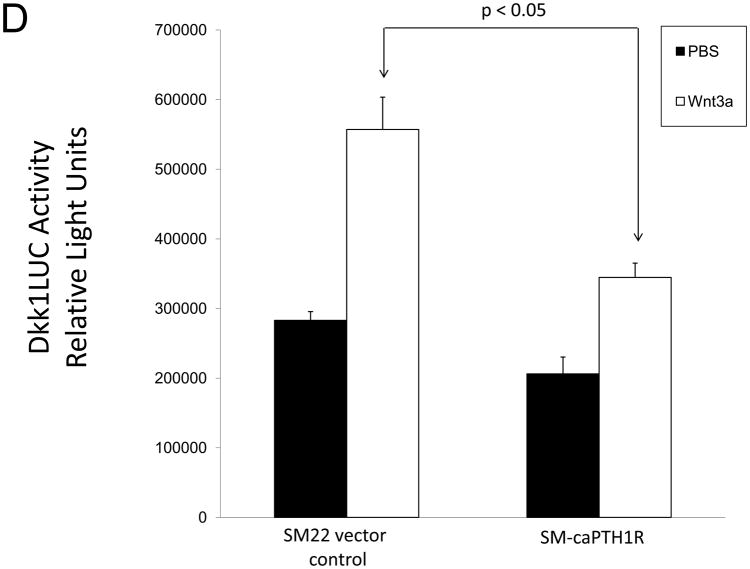
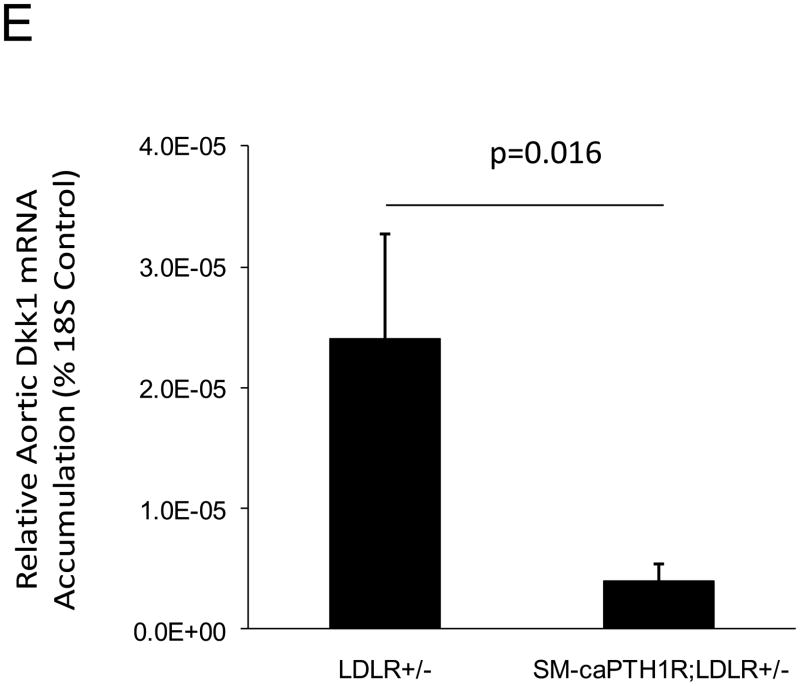
Because SM-caPTH1R inhibited Wnt activation of TCF/LEF-LUC in A7r5 VSMCs, we hypothesized that SM-caPTH1R may inhibit β-catenin activation signaling in VSMCs of the aortic tunica media11,16. To test this notion, we generated SM-caPTH1R; TOPGAL+;LDLR+/− mice, and scored the aortic extent of LacZ (β-galactosidase) staining as an index of mural β-catenin activation registered by the TOPGAL reporter41. As shown in Figures 4A and 4B, SM-caPTH1R; TOPGAL+;LDLR+/− mice exhibited significantly fewer aortic LacZ+ cells vs. non-transgenic TOPGAL+;LDLR+/− sibs fed HFD. Furthermore, by western blot analysis16 aortic β-catenin protein levels were significantly reduced in SM-caPTH1R transgenics (Figure 4C), confirming the inhibition of β-catenin signaling. We next examined Dkk1 regulation. Dkk1 is highly expressed in aorta42, and is a direct endogenous target of β-catenin signaling43 that participates in a negative feedback loop to curtail excessive Wnt receptor activation24. In vitro, Wnt3a upregulates Dkk1 promoter (LUC reporter) activity in aortic VSMCs, and caPTH1R inhibits Wnt3a-dependent induction (Figure 4D). In vivo, the SM-caPTH1R transgene also significantly reduced aortic Dkk1 mRNA accumulation (Figure 4E), and circulating levels of Dkk1 protein (data not shown). Thus, caPTH1R signaling down-regulates aortic VSMC β-catenin signaling in vivo.
Figure 4. The SM-caPTH1R transgene reduces aortic β-catenin signaling and β-catenin protein accumulation in diabetic LDLR+/− mice.
SM-caPTH1R;LDLR−/− transgenic mice were bred with TOPGAL+;LDLR+/+ reporter mice, and the extent of aortic LacZ staining assessed in SM-caPTH1R;LDLR+/−;TOPGAL+ mice vs. LDLR+/−;TOPGAL+ siblings following 1.5 months of HFD challenge. Panel A, representative aortic LacZ reporter visualization by staining frozen sections from these cohorts. Panel B, the extent of LacZ staining was reduced in aortas of LDLR+/−;TOPGAL+ reporter mice possessing the SM-caPTH1R transgene (n = 12). Panel C, the accumulation of aortic β-catenin was reduced in LDLR+/− mice possessing the SM-caPTH1R transgene (n = 4). Panel D, Wnt-induced Dkk1 promoter activity is inhibited by SM-caPTH1R in aortic VSMCs in vitro. Panel E, the expression of aortic Dkk1, an endogenous target of Wnt/β-catenin signaling, was reduced in vivo in mice possessing the SM-caPTH1R transgene (n = 12).
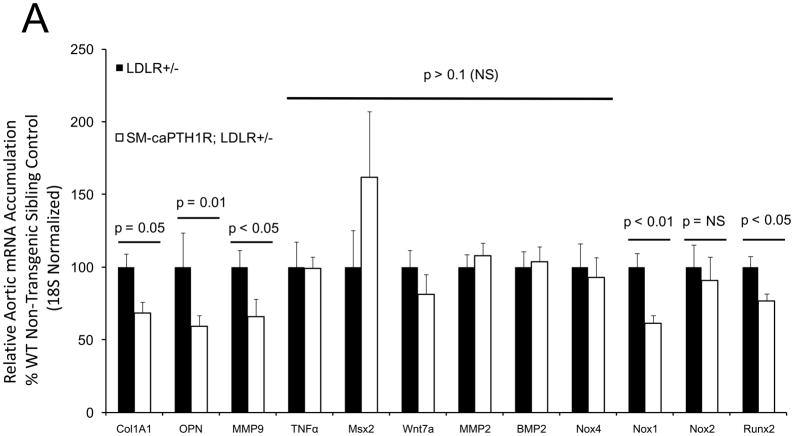
The SM-caPTH1R transgene reduces diabetic arteriosclerotic gene expression and fibrosis without altering expression of vascular TNF, Msx2, BMP2, or Wnt7
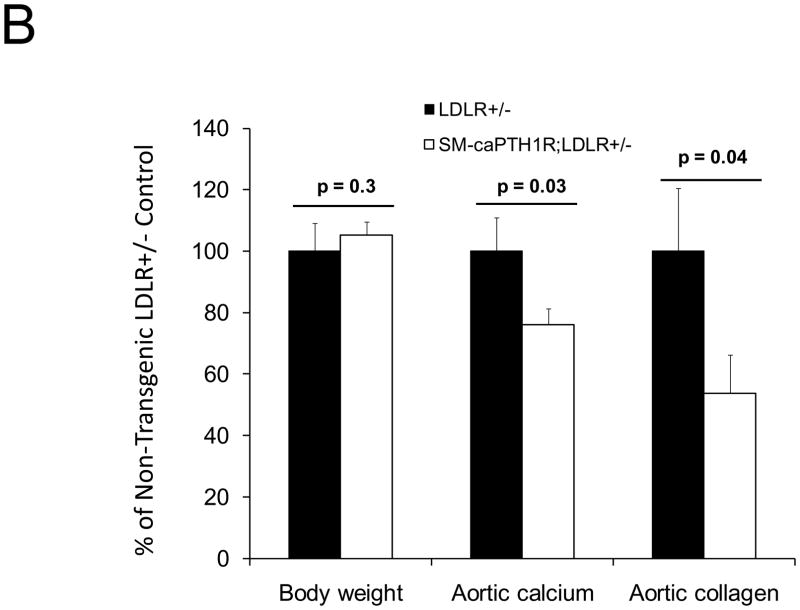
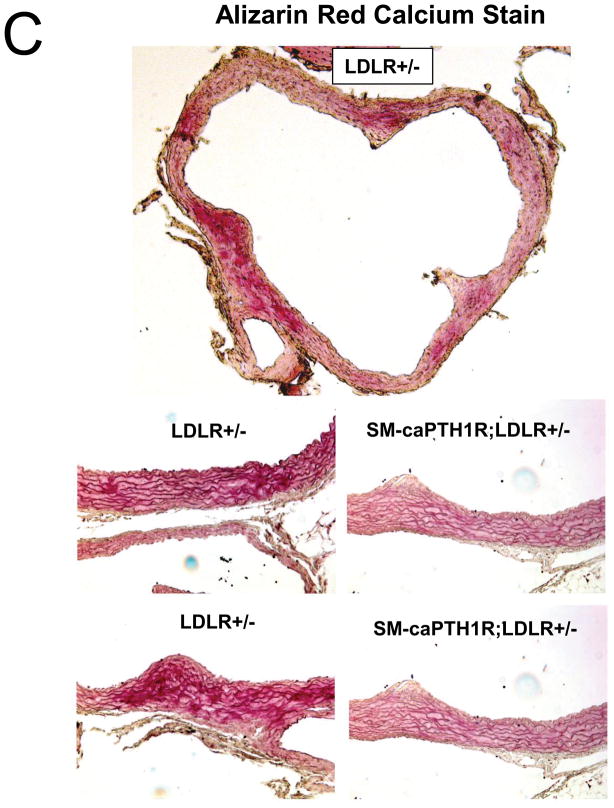
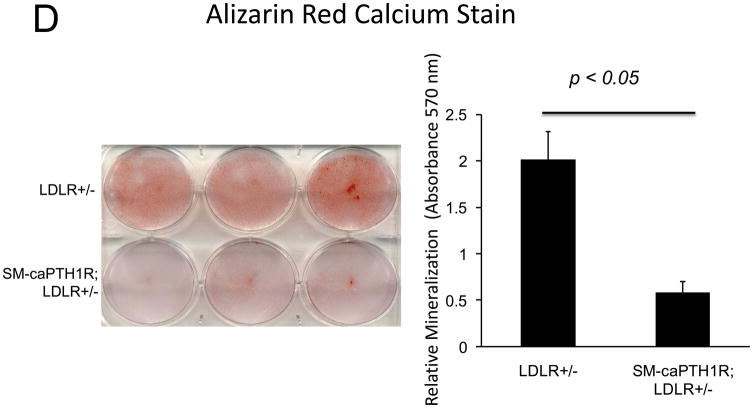
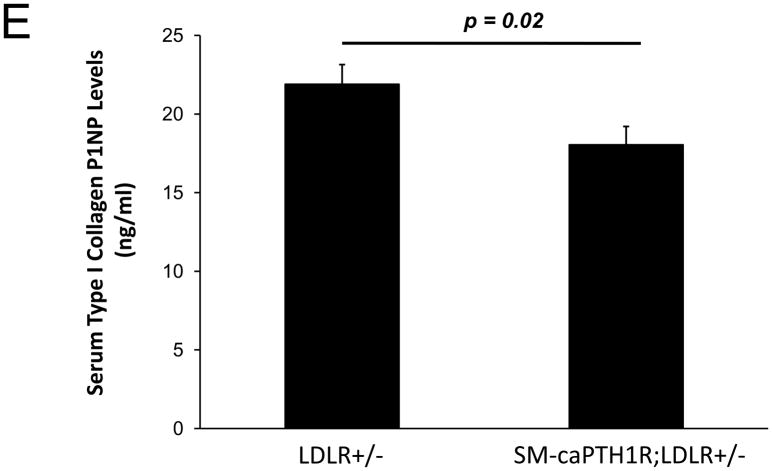
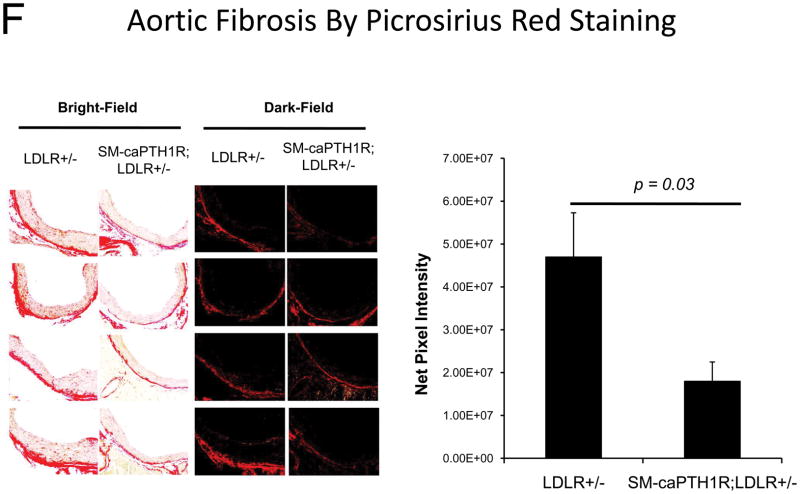
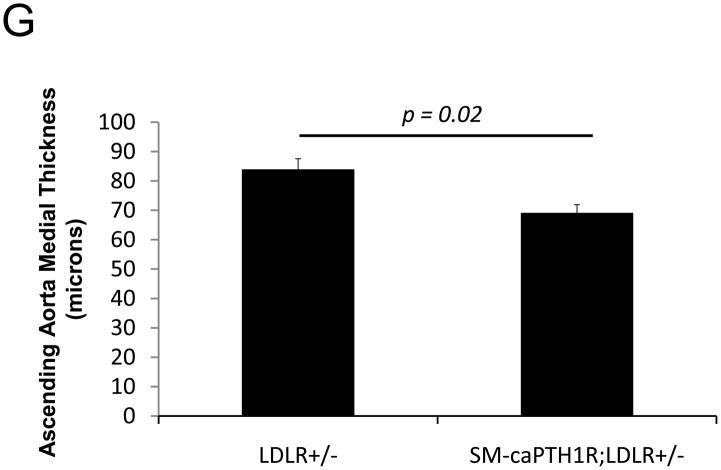
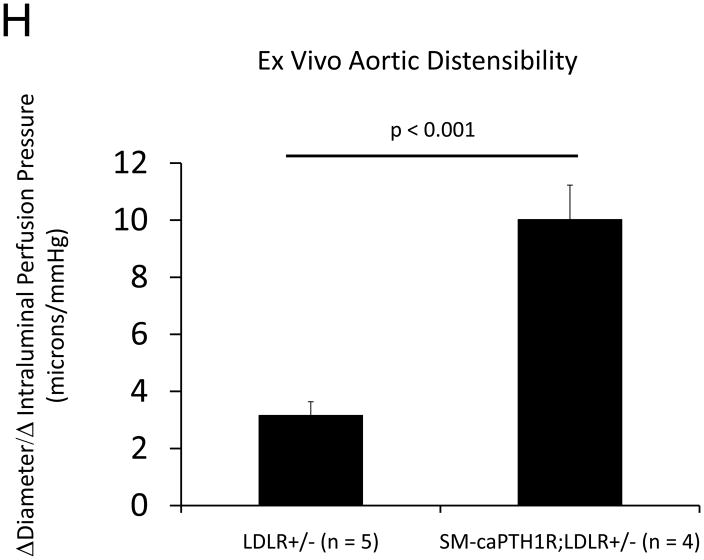
Diabesity-induced TNF upregulates vascular BMP2 and Msx2-Wnt expression and activates Wnt/β-catenin signals in vivo11,16. Thus, reductions in vascular β-catenin signaling in response to the SM-caPTH1R transgene could be mediated via reductions in aortic TNF, Msx2, BMP2, or Wnt7. To test this, we extracted whole aorta RNA from SM-caPTH1R;LDLR+/− transgenic and non-transgenic LDLR+/− siblings fed HFD, and evaluated gene expression by RT-qPCR. Unlike results obtained with PTH(1–34)16,25 or infliximab11 administration, aortic expression of TNF, Msx2, BMP2, and Wnt7a were not significantly altered by the SM-caPTH1R transgene (Figure 5A). The expression of other vascular Wnt ligands such as Wnt3a and Wnt7b were also unaltered (data not shown). By contrast, as compared with non-transgenic sibling cohorts, SM-caPTH1R mice on HFD exhibited reduced aortic OPN and COL1A1 gene expression (Figure 5A). MMP9 – a target of OPN-dependent ROS signaling in diabetic arteriosclerosis 23,44-- was also diminished by caPTH1R (Figure 5A), while MMP2 and Nox4 – the most abundant NADPH oxidase of VSMCs -- was unaffected. However, Nox1, a NADPH oxidase that selectively conveys VSMC activation by advanced glycosylation products in diabetes45, was decreased. Moreover, expression of Runx2, an osteochondrogenic transcription factor targeted ROS signaling in VSMCs46, was significantly diminished (confirmed by Western blot; data not shown). The reductions in aortic β-catenin signaling and Runx2 expression without changes in upstream TNF, Msx2, BMP2, or Wnt7 arteriosclerotic stimuli suggested that mural PTH1R activation might reduce aortic collagen accumulation and calcium accrual in vivo as in vitro. To test this notion, SM-caPTH1R;LDLR+/− mice and non-transgenic LDLR+/− siblings were placed on diabetogenic HFD for 12 weeks, and aortas extracted for calcium and collagen content. As shown in Figure 5B, no difference in body weight was observed between cohorts. However, aortic calcium content (p = 0.03) and collagen content (p = 0.04) were significantly reduced in SM-caPTH1R;LDLR+/− transgenic mice vs. non-transgenic siblings. Histological staining for calcium deposition with Alizarin red demonstrated that transgene-regulated calcium deposition occurred primarily within the aortic tunica media (Figure 5C). Furthermore cultures of transgenic aortic VSMCs exhibited reduced mineralization in vitro as compared with non-transgenic LDLR+/− “WT” controls (Figure 5D). Twenty-four hour fasting urinary calcium/creatinine ratios were not altered, indicating that the transgene did not enhance urinary calcium clearance (Online Figure V). Serum P1NP, a marker of type I collagen biosynthesis36, was reduced in SM-caPTH1R;LDLR+/− mice on HFD (Figure 5E). Histological staining of collagen with Picrosirius red confirmed reductions in aortic fibrosis by the SM-caPTH1R transgene (Figure 5F). Diabetic SM-caPTH1R;LDLR−/− mice also exhibited reduced aortic collagen content as compared to non-trangenic LDLR−/− siblings (Online Figure VI). Histomorphometric analysis revealed reduced aortic wall thickness in SM-caPTH1R;LDLR+/− mice (Figure 5G), reflecting transgene-induced reductions in VSMC proliferation (Online Figure VII). Consistent with reductions in collagen, calcification, and wall thickness, aortas from SM-caPTH1R transgenic mice exhibited increased distensibility by ex vivio plethysmography (Figure 5H). Thus, VSMC PTH1R activity directly inhibits pro-calcific and pro-fibrotic β-catenin signaling in aortas of diabetic LDLR-deficient mice, down-regulating Nox1 and Runx2 without altering TNF, Msx2, BMP2, or Wnt7 expression. VSMC-autonomous PTH1R signaling recapitulates many, but not all, effects of systemic PTH(1–34) on diabetic arteriosclerosis25.
Figure 5. The SM-caPTH1R transgene down-regulates aortic fibrosis and calcification without inhibiting TNF, BMP2, Wnt, and Msx2 gene expression in diabetic LDLR+/− mice.
Panel A, aortic Col1A1, OPN, MMP9, Nox1, and Runx2 were reduced in LDLR+/− mice possessing the SM-caPTH1R transgene. Nox4 and upstream osteogenic signals were not altered (n = 10; 4 months HFD). Panel B, aortic calcium and collagen content were decreased in aortic extracts from diabetic LDLR+/− mice possessing the SM-caPTH1R transgene (n = 6–12 per group; 3 months HFD). Panel C, aortic calcium deposition occurred primarily within the tunica media. Panel D, the SM-caPTH1R transgene suppressed VSMC calcification in vitro. Panel E, serum P1NP, a marker of collagen biosynthesis, was also decreased (n = 19–22 per group). Panel F, aortic collagen content assessed by Picrosirius histochemistry and digital image analysis confirmed that SM-caPTH1R transgene decreased fibrosis (n = 4). Panel G, thickness of the aortic tunica media was reduced by the SM-caPTH1R transgene. Panel H, aortic distensibility was increased by the SM-caPTH1R transgene.
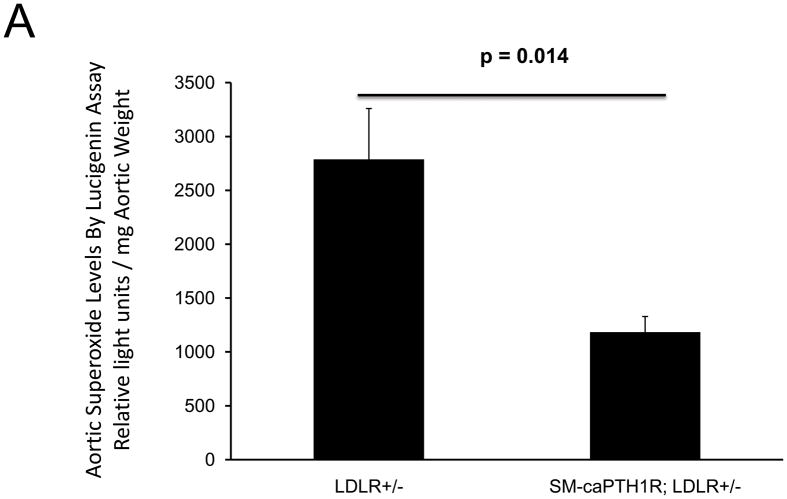
The SM-caPTH1R transgene reduces aortic superoxide accumulation
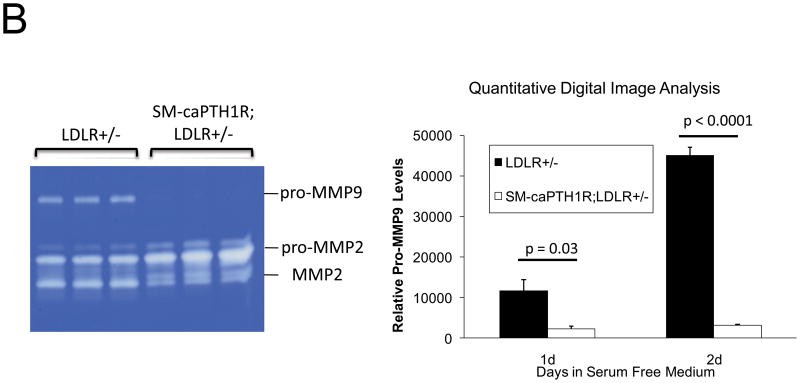
Oxidative stress plays a key role in diabetic arteriosclerosis6,44,45, and systemic PTH(1–34) administration globally reduced oxidative stress reflected in serum 8-F-isoprostane in LDLR−/− mice (supplement Figure S2)23. SM-caPTH1R transgene did not globally reduce oxidative stress (Table I); however, the SM-caPTH1R transgene did reduce aortic Nox1 -- a key VSMC source of superoxide 45 -- and aortic OPN -- a stimulus for Nox activation23,44. Therefore, we assessed the impact of SM-caPTH1R transgene on aortic superoxide, a direct measure of local tissue oxidative stress. As shown in Figure 6A, the SM-PTH1R transgene significantly reduced aortic superoxide levels in LDLR+/− mice as measured by lucigenin assay45; similar inhibition was observed in LDLR−/− mice (Online Figure VIII). Moreover, VSMC pro-MMP9 activation -- a target and index of Nox/ROS signaling19 -- was also down-regulated by the caPTH1R transgene (Figure 6B). Dihydroethidium staining23 confirmed reductions in superoxide in VSMC expressing caPTH1R (data not shown). Thus, VSMC PTH1R signaling reduces aortic VSMC superoxide levels in LDLR-deficient mice fed diabetogenic diets.
Figure 6. The SM-caPTH1R transgene reduces aortic oxidative stress.
Panel A, aortic superoxide levels were reduced in LDLR+/− mice possessing the SM-caPTH1R transgene (n = 4/group). Panel B, pro-MMP9 activity, an index of ROS signaling down-stream of TNF and OPN23, was reduced in VSMCs from LDLR+/− mice possessing the SM-caPTH1R transgene. Representative zymogram (left) and quantitative digital image analysis (right).
DISCUSSION
Once considered a passive process of dead and dying cells, research from laboratories worldwide has identified that arteriosclerotic matrix calcification is an actively regulated form of tissue biomineralization4. As in the developing skeleton36, molecular and histoanatomic heterogeneity exists in vascular calcium deposition15. In advanced atherosclerosis, such as that recapitulated by the ApoE-null mouse47 and LDLR-null mice on fatty diets for 4 months or more12, osteochondrogenic regulatory programs drive endochondral-type vascular mineralization, with contributions of dystrophic calcification also observed within cholesterol-laden lipid deposits11. With time, advanced lesions can remodel to form woven bone with active hematopoiesis – i.e. vascular osteogenesis4,48. Elegant data from Giachelli demonstrate that osteochondrogenic trans-differentiation of VSMCs drives atherosclerotic calcification process in ApoE-deficient mice47. In the setting of chronic renal insufficiency, the hyperphosphatemic milieu of uremia further enhances osteochondrogenic trans-differentiation49, with VSMC apoptosis contributing to medial and intimal calcium load50. In T2DM, medial calcification predominates, driven by low-grade systemic inflammation of obesity and diabetes4,15. These clinically relevant stimuli are recapitulated in male LDLR-deficient mice fed HFD15,25,51 but not in ApoE-deficient mice15,51. When fed the HFD, male LDLR−/− mice become increasingly obese, hyperglycemic, hyperinsulinemic, and dyslipidemic with concomitant aortic calcification11. During early disease stages, medial artery calcification predominates as revealed by Alizarin red staining, without significant endochondral contributions11. Aortic Msx2-Wnt signaling, reminiscent of membranous ossification36, is upregulated early on in the LDLR-deficient model11,15,16. At later stages, aortic medial calcification is progressively accompanied by atherosclerotic calcification, including mineralization of cholesterol-laden lipid deposits11. Thus, in response to clinically relevant stimuli – diet-induced diabetes, obesity, and dyslipidemia --initiation and progression phases of arterial calcification in T2DM can be studied in detail15. In both diabetic LDLR-deficient and atherosclerotic ApoE-deficient models, inflammation, oxidative stress and MMP proteolytic remodeling have emerged as contributors to vascular calcium deposition4,15.
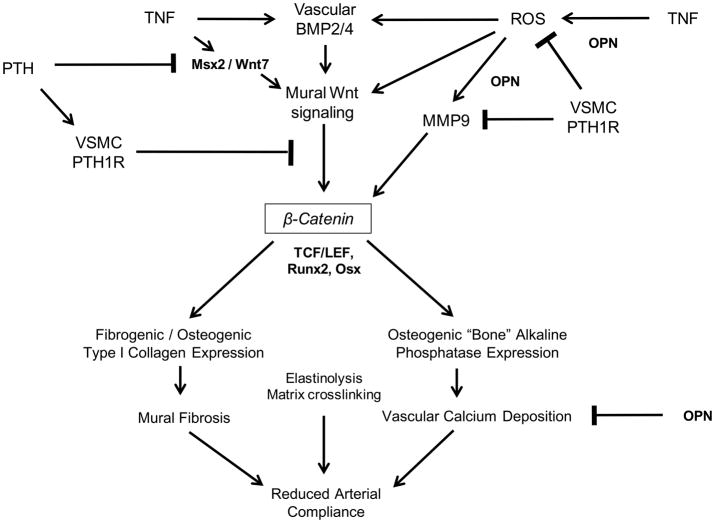
The endocrine mechanisms controlling arteriosclerotic mineralization are only beginning to be understood4. In this study, we focused upon VSMC-autonomous PTH1R signaling and its impact upon diabetic vascular disease. Daily PTH(1–34) administration -- a potent stimulus for PTH1R activation and bone formation -- reduces aortic OPN expression, down-regulates aortic osteogenic Msx2-Wnt signaling, and decreases aortic calcification in LDLR−/− mice16,25. Since the PTH1R is expressed in VSMC as well as in osteoblasts, reductions in vascular mineralization could arise from direct vascular actions of PTH(1–34) as well as indirect actions via the hematopoietic bone marrow-vascular axis 4,12,25. Implementing the VSMC-specific 0.5 kb SM22 promoter30 to express constitutively active Jansen receptor PTH1R(H223R)29, we now demonstrate that the PTH1R exerts VSMC-autonomous actions that inhibit pro-osteogenic and pro-fibroblast Wnt/β-catenin signaling. Mural oxidative stress was concomitantly reduced by the SM-caPTH1R transgene. Because ROS inactivate the auto-inhibitory MMP9 pro-peptide, oxidative stress enhances matrix turnover23. Guzman demonstrated the critical role for MMPs in vascular calcification, using a vitamin D toxicity model52. Elastinolysis is certainly one contributor4; however, seminal studies by George and colleagues have demonstrated that MMP9 and MMP12 stimulate β-catenin signaling by proteolytically liberating the N-cadherin-associated VSMC β-catenin pool19. Thus, in addition to direct inhibition of β-catenin signaling, PTH1R-mediated down-regulation of vascular ROS and MMP9 may also help preserve N-cadherin-dependent restraint of β-catenin in diabetic arteriosclerosis (Figure 7).
Figure 7. The VSMC PTH1R and β-Catenin Signaling in Diabetic Arteriosclerosis: A Working Model.
Low-grade vascular inflammation and ROS that accompany T2DM promote mural elaboration of TNF, BMP2/4, and Msx2-Wnt signaling cascades that direct osteogenic differentiation of arterial mesenchymal progenitors15. VSMC-autonomous PTH1R activation down-regulates ROS accumulation, MMP9 activation (degrades N-cadherin19), β-catenin protein accumulation and signaling, and Runx2 expression. By mechanisms yet to be identified, systemic PTH(1–34) administration additionally reduces adventitial Msx2 and Wnt mRNA accumulation16,25. The impact of PTH/PTH1R signaling on elastinolysis and matrix crosslinking has yet to be evaluated. Not shown is the important role of Sox9 in specifying the common osteo-/chondro- progenitor, or the Smad signals that convey vascular BMP actions4,58.
The precise intracellular signaling cascade whereby the PTH1R inhibits VSMC β-catenin actions has yet to be delineated. Since β-catenin protein levels are reduced by PTH1R activity in VSMCs, ubiquitin-dependent β-catenin proteolysis is likely involved53. However, if so this mechanism must be cell-type specific since protein kinase A (PKA) -- a key PTH1R mediator that inhibits Wnt/β-catenin signaling in VSMCs -- augments β-catenin accumulation in other cell types53,54. Cell type-specific protein-protein interactions between PTH1R and LRP654, the Wnt co-receptor important in paracrine Msx2-Wnt signaling16,33, may also participate. Recently, however, peroxide has been shown to upregulate β-catenin levels and signaling via a ROS-sensitive nucleoredoxin-disheveled relay55. Of note, PKA phosphorylates NoxA1 and thereby reduces Nox1 activity56 – a key source of ROS in diabetic VSMCs45. Thus, it is tempting to speculate that inhibition of Nox1-dependent ROS signaling55 mediates PTH1R down-regulation of aortic β-catenin and pro-MMP9. Alternatively, PTH1R activity may upregulate antioxidant defenses in VSMCs6. These potential mechanisms remain to be evaluated.
There are limitations to our study. We have emphasized the impact of VSMC PTH1R signaling on diabetic arterial calcification in the absence of significant renal insufficiency. Chronic renal insufficiency is a common consequence of long-standing T2DM, and profoundly accelerates vascular calcium accrual57. The phosphate retention that characterizes uremia is a powerful stimulus not only for osteochondrogenic signaling, but also VSMC apoptosis50. Thus, it remains to be determined whether VSMC-autonomous PTH1R signaling has any impact on vascular calcification with uremia. Anatomically distinct vascular beds may be differentially impacted by VSMC PTH1R activation15. Although MMP9, BMPs, and Wnt7 family members are prominent in diseased vessels8,11,16,23,32,58, the relative contributions of individual components to the biology of diabetic arteriosclerosis have yet to be established. Moreover, although increased aortic distensibility was observed ex vivo, improvements in vascular compliance in response to VSMC PTH1R actions have yet to be demonstrated in vivo – and reductions in vascular ROS may potentially improve endothelium-dependent vasodilatation. Because systemic PTH(1–34) administration reduces adventitial Msx2 and Wnt7 expression16 -- and regulates cell products released from the skeletal hematopoietic niche 28 -- PTH1R signaling beyond medial VSMCs may play even more important roles in the regulation of vascular calcification. Nevertheless, our study newly identifies the important contributions of VSMC-autonomous PTH1R signaling in limiting diabetic arteriosclerosis. This adds to accumulating data indicating that modulation of vascular PTH1R signaling may be useful as pharmacotherapy for treating cardiovascular disease16,25,27,28.
Novelty and Significance.
What is known?
Arteriosclerotic fibrosis and calcification of conduit vessels compromises smooth distal tissue perfusion, increasing the risk of lower extremity amputation.
Arteriosclerosis is an actively regulated process potentiated in part via oxidative stress and osteogenic Wnt/β-catenin signaling.
The PTH receptor (PTH1R) is expressed in bone and vascular smooth muscle, and intermittent PTH administration stimulates bone formation but suppresses arterial calcification.
What new information does this article contribute?
PTH inhibits transduction of pro-fibrotic canonical Wnt/β-catenin signals.
Vascular smooth muscle cell-autonomous PTH1R signaling inhibits pro-calcific and pro-fibrotic Wnt/β-catenin signaling.
Vascular smooth muscle cell-autonomous PTH1R signaling reduces arterial oxidative stress.
Inhibition of vascular Wnt/β-catenin and ROS signaling with PTH1R agonists may exert beneficial actions in diabetic arteriosclerosis.
Summary.
Arteriosclerotic calcification and fibrosis reduce conduit vessel compliance and distal tissue perfusion, thereby increasing the risk for amputation. Osteogenic gene regulatory programs control matrix deposition and calcification in both bone and vasculature, and are reciprocally regulated by the osteotropic hormone, PTH. We demonstrate that pro-calcific and pro-osteogenic Wnt/β-catenin signaling cascades are inhibited in vascular smooth muscle cells by cell-autonomous PTH receptor actions that reduce β-catenin signaling and vascular oxidative stress.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by NIH grants HL069229 and HL081138 to D.A.T., and the Barnes-Jewish Hospital Foundation.
Non-standard Abbreviations and Acronyms
- Apo
apolipoprotein
- BMP
bone morphogenetic protein
- caPTH1R
constitutively active Jansen PTH1R variant PTH1R(H223R)
- Col
collagen gene
- Col1A1
type I collagen gene for α-1 chain
- Col1A2
type I collagen gene for α-2 chain
- 3.6 Col1A1-LUC
3.6 kb Col1A1 promoter – LUC reporter construct
- CRE
cyclic AMP response element
- DXA
dual electron X-ray absorptiometry
- Dkk1
dickkopf homolog 1
- FOPLUC
mutated negative control TCF/LEF optimal promoter –luciferase reporter plasmid
- HFD
diabetogenic high fat western diet
- LacZ
β-galactosidase
- LDLR
low density lipoprotein receptor
- LEF
lymphoid enhancer binding factor
- LRP
LDLR related protein
- LUC
luciferase reporter gene
- MESA
Multiethnic Study of Atherosclerosis
- MMP
matrix metalloproteinase
- Msx
muscle segment homeobox protein homolog
- Osx
osterix
- OPN
osteopontin
- pcDNA3
cytomegalovirus promoter/enhancer eukaryotic expression vector
- PKA
protein kinase A
- PTH
parathyroid hormone
- PTHrP
PTH-related protein
- PTH1R
PTH/PTHrP receptor
- ROS
reactive oxygen specifies
- Runx2
Runt-related transcription factor 2
- SFG
vesicular stomatitis virus G-protein pseudotyped Moloney murine leukemia virus retroviral vector
- SM22
transgelin
- T2DM
type II diabetes mellitus
- TCF
T cell transcription factor
- TNF
tumor necrosis factor alpha
- TOPGAL
TCF/LEF optimal promoter-galactosidase reporter mouse
- TOPLUC
TCF/LEF optimal promoter –luciferase reporter plasmid
- UTR
untranslated region
- VSMC
vascular smooth muscle cell
- Wnt
wingless-type MMTV integration site family member
Footnotes
Disclosures
D.A.T. has served as a paid consultant to Merck, Pfizer, and the Institute of Medicine’s Committee to Review Daily Recommended Intake for Calcium and Vitamin D.
References
- 1.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 2.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 3.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–74. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 6.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–50. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–9. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 12.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–20. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–9. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 14.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the Osteogenic Regulation of Vascular Calcification. A Review and Perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–77. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 18.George SJ. Regulation of myofibroblast differentiation by convergence of the Wnt and TGF-beta1/Smad signaling pathways. J Mol Cell Cardiol. 2009;46:610–1. doi: 10.1016/j.yjmcc.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2009;81:178–86. doi: 10.1093/cvr/cvn278. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 21.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–9. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 22.Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res. 2006;99:1329–37. doi: 10.1161/01.RES.0000253533.65446.33. [DOI] [PubMed] [Google Scholar]
- 23.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–89. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–9. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 26.Maeda S, Sutliff RL, Qian J, Lorenz JN, Wang J, Tang H, Nakayama T, Weber C, Witte D, Strauch AR, Paul RJ, Fagin JA, Clemens TL. Targeted overexpression of parathyroid hormone-related protein (PTHrP) to vascular smooth muscle in transgenic mice lowers blood pressure and alters vascular contractility. Endocrinology. 1999;140:1815–25. doi: 10.1210/endo.140.4.6646. [DOI] [PubMed] [Google Scholar]
- 27.Fiaschi-Taesch N, Sicari B, Ubriani K, Cozar-Castellano I, Takane KK, Stewart AF. Mutant parathyroid hormone-related protein, devoid of the nuclear localization signal, markedly inhibits arterial smooth muscle cell cycle and neointima formation by coordinate up-regulation of p15Ink4b and p27kip1. Endocrinology. 2009;150:1429–39. doi: 10.1210/en.2008-0737. [DOI] [PubMed] [Google Scholar]
- 28.Napoli C, William-Ignarro S, Byrns R, Balestrieri ML, Crimi E, Farzati B, Mancini FP, de Nigris F, Matarazzo A, D’Amora M, Abbondanza C, Fiorito C, Giovane A, Florio A, Varricchio E, Palagiano A, Minucci PB, Tecce MF, Giordano A, Pavan A, Ignarro LJ. Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat Clin Pract Cardiovasc Med. 2008;5:571–9. doi: 10.1038/ncpcardio1214. [DOI] [PubMed] [Google Scholar]
- 29.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–59. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–50. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–20. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283:20505–22. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–6. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 37.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1–34) and PTH(7–34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–30. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS. Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J Biol Chem. 1995;270:13460–9. doi: 10.1074/jbc.270.22.13460. [DOI] [PubMed] [Google Scholar]
- 39.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–9. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 40.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–43. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 42.Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 43.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–6. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 44.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–82. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 45.San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–9. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–27. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 49.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–6. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 50.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–57. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 51.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–14. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 52.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–6. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 53.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–72. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–79. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–8. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 56.Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J Biol Chem. 2007;282:34787–800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 57.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–28. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 58.Yao Y, Watson AD, Ji S, Bostrom KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res. 2009;105:575–84. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.