Abstract
We conducted a phase II trial of doxercalciferol, a vitamin D2 analogue, in 15 patients with MDS. Each received doxercalciferol 12.5 μg orally daily for 12 weeks. Nine of 15 patients completed the prescribed course and of these, six had stable disease. No patient had a response (IWG criteria) and overall eight patients experienced progressive disease while on therapy. Two patients with chronic myelomonocytic leukemia (CMML) had a marked rise in monocytes on study. Overall the treatment was well tolerated. One patient was removed from study due to hypercalcemia. We conclude that short-term treatment with doxercalciferol has limited activity in patients with MDS.
Keywords: Doxercalciferol, myelodysplastic syndrome, phase II study
Introduction
The myelodysplastic syndromes (MDS) are a group of disorders characterized by one or more peripheral blood cytopenias secondary to bone marrow dysfunction [1]. Prognosis is directly related to the number of bone marrow blasts, the degree of peripheral blood cytopenias, and findings on cytogenetic analysis [2].
Small phase II studies have suggested that calcitriol may improve the blood counts in patients with MDS, but may cause hypercalcemia or hypercalciuria. Doxercalciferol, a synthetic analog of vitamin D2, has equivalent affinity for the vitamin D receptor (VDR), can safely be given to human subjects [3,4], and has demonstrated a lower propensity to cause hypercalcemia and hypercalcuria [3]. Given the lower likelihood of toxicity compared to calcitriol, we reasoned that the higher tolerable doses of doxercalciferol would result in a greater likelihood of benefit to those with MDS. To address this question, we performed a small pilot study to test the short-term efficacy and toxicity of doxercalciferol in patients with MDS.
Methods
Patients
The research protocol was approved by the Institutional Review Board of the University of Wisconsin Hospitals and Clinics. Adult patients with MDS as defined by the World Health Organization or French-American-British Classification were considered eligible. Eligibility required: morphologic evidence of disease on bone marrow examination; one or more cytopenia; creatinine clearance >50 mL/min/1.73 M2; ECOG performance status 0 – 2; and no cytokine treatment within 4 weeks. Patients with CMML or secondary MDS were permitted to participate.
Patients were excluded for a history hypercalcemia or any condition that may predispose to hypercalcemia or hypercalciuira (renal stone disease within 5 years of study entry, thiazide diuretic use, calcium or vitamin D supplements, glucocorticoids, fluoride, or lithium). Patients with uncontrolled malignancy or prior exposure to doxercalciferol were also excluded.
Treatment
At enrollment, patients received 12.5 μg of doxercalciferol daily for 12 weeks, and were advised to take the medication orally prior to the morning meal. Patients could not receive erythropoietin. Therapy was terminated for the following reasons: development of grade 4 nonhematologic toxicity; recurrence of grade 3 nonhematological toxicity after dose reduction; three episodes of grade 1 or 2 hypercalcemia or creatinine elevation; or treatment failure (defined as worsening cytopenias, increase in bone marrow blasts, or rising monocytosis in those with CMML). Patients who experienced a ≥ 50% drop in their baseline platelet or neutrophil count and those who required a ≥ 50% increase in RBC or platelet transfusion requirements from baseline were removed from study and considered to have progression. Patients who did not demonstrate improvement of peripheral blood counts by the completion of 12 weeks of therapy were not required to undergo repeat bone marrow biopsy evaluation.
Assessment of efficacy and safety
Response criteria used were those set forth by an international working group for MDS (IWG criteria) [5]. Patients were followed with baseline and monthly examinations, toxicity assessments (NCI CTC, version 2.0), blood counts (including reticulocytes), and serum chemistries.
Statistical analysis
The primary objective of this phase II study was to evaluate the response rate and to further evaluate the safety of doxercalciferol in patients with MDS. A two-stage Simon’s minimax design [6] was implemented in which if there were fewer than 2 responses among the initial 16 patients, the study was to be terminated due to lack of efficacy. Otherwise the study was to continue with an additional nine patients. This design tests the null hypothesis that the true probability of response is no more than 0.1 at a one-sided significance level of 0.1 against the alternative hypothesis that it is at least 0.3 with a power of 0.9.
Results
Study population
The characteristics of the study population are presented in Table I. Because of the lack of responses, the study was terminated early after enrollment of 15 patients between January 2003 and October 2004. The patients were between 51 and 92 years of age (median 77 years) and each FAB MDS subtype was represented including one patient with refractory anemia with excess blasts in transformation (RAEB-t). Seven patients had normal cytogenetics, two had monosomy 7, and three had 5q deletions (two of whom had 5q minus syndrome). Twelve of 15 patients had international prognostic scoring system (IPSS) scores of low or intermediate-1. All but one patient was red-cell transfusion dependent at the time of study entry.
Table I.
Patient characteristics.
| Gender | 10 male/5 female |
| FAB diagnosis | |
| RA | 7 |
| RARS | 3 |
| RAEB | 2 |
| RAEB-t | 1 |
| CMML | 2 |
| Cytogenetics | |
| Monosomy 7 | 2 |
| 5q minus | 3 |
| Trisomy 8 | 2 |
| Normal | 7 |
| Unavailable | 1 |
| IPSS | |
| Low | 5 |
| Intermediate-1 | 7 |
| Intermediate-2 | 1 |
| High | 1 |
Toxicity
Overall, doxercalciferol was well tolerated. One patient had significant nonhematologic toxicity (grade 3 rash, grade 3 hypercalcemia), increasing red cell transfusion requirements, and was removed from study after 4 weeks of therapy. All toxicity resolved within 2 weeks of discontinuing doxercalciferol. No other patient underwent dose-reduction or discontinuation for nonhematologic side effects.
Eight patients experienced disease progression while on doxercalciferol. Two patients with CMML developed marked monocytosis while on study, which improved after discontinuing doxercalciferol. The patient with RAEB-t experienced worsening blood counts and a bone marrow performed after 6 weeks showed increasing blasts. The other five patients experienced a fall in the peripheral blood counts while on doxercalciferol. Two patients had worsening hemoglobin, three patients developed worsening neutropenia, and two patients developed worsening thrombocytopenia. The fall in blood counts lead to the early removal of the patients from the study in four of the five cases. In all cases, the blood counts did not improve of off therapy and the deterioration in blood counts was attributed to disease progression.
Efficacy
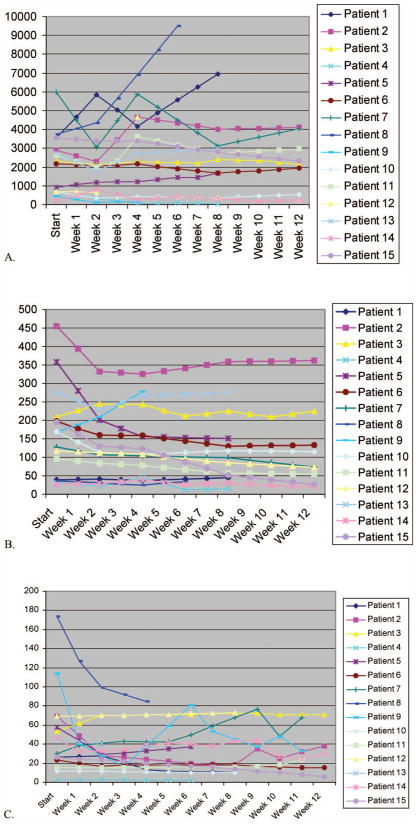
Nine of 15 patients completed a full 12-week course of doxercalciferol. Of these, six had stable disease, with the remainder showing progression based upon changes in the peripheral blood counts. There were no hematologic responses. As noted above, a total of six patients were taken off study before completion, five of whom were taken off for MDS progression or transformation to AML and one due to toxicity. In all, eight of the 15 patients on study showed evidence of disease progression due to worsening peripheral blood counts (six patients), symptomatic monocytosis (two patients with CMML), or increasing blast percentage in the bone marrow (one patient). There were a few instances of apparent response in reticulocyte counts (see Figure 1), although none of these translated into hemoglobin responses.
Figure 1.
Depicts the changes in peripheral blood counts over the 12 week course of doxercalciferal treatment: (A) Leucocytes per microlitre, (B) Platelets×1000 per microlitre, (C) reticulocytes×1000 per microlitre.
Discussion
Naturally occurring vitamin D and analogues have been found to induce the maturation of various leukemia cell lines through the action on the VDR [7], and vitamin D metabolites appear to play an important role in the regulation of growth and promotion of differentiation of these tissue types and the malignant cells derived from them [8 – 10]. Unfortunately, vitamin D3 (calcitriol or 1α,25 dihydroxyvitamin D3) at doses that may influence hematopoiesis also may cause hypercalcemia and hypercalcuria. Doxercalciferol, a synthetic analog of vitamin D2, has equivalent affinity for the VDR [7], and clinical trials have shown that it has a lower propensity to cause hypercalcemia and hypercalcuria [3]. Furthermore, a phase II trial of doxercalciferol has shown safety with chronic daily dosing at ≥ 10 mcg daily [4].
Several studies have examined the use of calcitriol to treat MDS. Motomura et al. [11] performed a nonrandomized clinical trial of calcitriol in MDS at a dosage of 4 – 6 μg per day for a median of 17 months. Although overall survival was not affected, the patients who received calcitriol had a significantly lower chance of developing AML in a Kaplan-Meyer survival analysis. Slapak et al. [12] treated 29 elderly patients with AML using a protocol that combined low-dose cytarabine and hydroxyurea for 21 days with calcitriol (0.25 μg twice per day) until relapse. The CR rate was 45% with median remission duration of 9.8 months. Mellibovsky et al. [13] treated 14 patients with low risk MDS with calcitriol. Ten of the 14 patients responded to the treatment with either improved blood counts or transfusion independence. They reported no episodes of hyper-calcemia and no patient stopped treatment because of toxicity. In addition, at least one other vitamin D2 analog has been studied for use in MDS. Koeffler et al. [14] found no clinical responses among 12 patients treated with paricalcitol (which is widely used to treat secondary hyperparathyroidism) in spite of promising in vitro results [15].
Our study has shown that doxercalciferol, when given for 12 weeks to patients with MDS, did not lead to hematologic improvement by IWG criteria. In fact, we found that two patients with CMML experienced a rise in the monocyte count while on therapy, suggesting the doxercalciferol may encourage monocytopoiesis. Our study design was intended to explore whether doxercalciferol may improve blood counts over a 12-week period. The short-term endpoint could miss the possibility that a longer duration of therapy may be effective or that a short-term fall in counts may reflect a cytotoxic effect that will later result in the establishment of improved hematopoiesis, such as seen following methyl-transferase inhibitor therapy, or lenalidomide. Also, we did not follow blood levels of the active vitamin D metabolites and cannot exclude the possibility that unfavorable pharmacokinetics may have dampened any potential effect. We can conclude that doxercalciferol in the dose and schedule studied is unlikely to improve blood counts for patients with MDS.
References
- 1.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649 – 1660. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079 – 2088. [PubMed] [Google Scholar]
- 3.Gallagher JC, Bishop CW, Knutson JC, Mazess RB, DeLuca HF. Effects of increasing doses of 1 α-hydroxyvitamin D2 on calcium homeostasis in postmenopausal osteopenic women. J Bone Miner Res. 1994;9:607 – 614. doi: 10.1002/jbmr.5650090504. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Wilding G, Staab MJ, Horvath D, Miller K, Dresen A, et al. Phase II study of 1-α-hydroxyvitamin D(2) in the treatment of advanced androgen-indiependent prostate cancer. Clin Cancer Res. 2003;9:4077 – 4083. [PubMed] [Google Scholar]
- 5.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671 – 3674. [PubMed] [Google Scholar]
- 6.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1 – 10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa K, Kurobe M, Ozono K, Konno K, Fujishima T, Takayama H, et al. Novel ring A stereoisomers of 2-methyl-1α,25-dihydroxyvitamin D(3) and 2-methyl-20-epi-1α,25-di-hydroxyvitamin D(3): transactivation of target genes and modulation of differentiation in human promyelocytic leukemia (HL-60) cells. Biochem Pharmacol. 2000;59:691 – 702. doi: 10.1016/s0006-2952(99)00357-3. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD. 1,25(OH)2D3-modulated calcium induced keratinocyte differentiation. J Investig Dermatol Symp Proc. 1996;1:22 – 27. [PubMed] [Google Scholar]
- 9.Ripple GH, Wilding G. Drug development in prostate cancer. Semin Oncol. 1999;26:217 – 226. [PubMed] [Google Scholar]
- 10.Mehta RG, Moriarty RM, Mehta RR, Penmasta R, Lazzaro G, Constantinou A, et al. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1α-hydroxyvitamin D5. J Natl Cancer Inst. 1997;89:212 – 218. doi: 10.1093/jnci/89.3.212. [DOI] [PubMed] [Google Scholar]
- 11.Motomura S, Kanamori H, Maruta A, Kodama F, Ohkubo T. The effect of 1-hydroxyvitamin D3 for prolongation of leukemic transformation-free survival in myelodysplastic syndromes. Am J Hematol. 1991;38:67 – 68. doi: 10.1002/ajh.2830380112. [DOI] [PubMed] [Google Scholar]
- 12.Slapak CA, Desforges JF, Fogaren T, Miller KB. Treatment of acute myeloid leukemia in the elderly with low-dose cytarabine, hydroxyurea, and calcitriol. Am J Hematol. 1992;41:178 – 183. doi: 10.1002/ajh.2830410307. [DOI] [PubMed] [Google Scholar]
- 13.Mellibovsky L, Diez A, Perez-Vila E, Serrano S, Nacher M, Aubia J, et al. Vitamin D treatment in myelodysplastic syndromes. Br J Haematol. 1998;100:516 – 520. doi: 10.1046/j.1365-2141.1998.00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Koeffler HP, Aslanian N, O’Kelly J. Vitamin D(2) analog (Paricalcitol; Zemplar) for treatment of myelodysplastic syndrome. Leuk Res. 2005;29:1259 – 1262. doi: 10.1016/j.leukres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai T, Shih LY, Hughes SV, Desmond JC, O’Kelly J, Hewison M, et al. 19-Nor-1,25(OH)2D2 (a novel, noncalcemic vitamin D analogue), combined with arsenic trioxide, has potent antitumor activity against myeloid leukemia. Cancer Res. 2005;15(65):2488–2497. doi: 10.1158/0008-5472.CAN-04-2800. [DOI] [PubMed] [Google Scholar]