Abstract
Because both iron deficiency and iron excess are deleterious to normal cell function, the intracellular level of iron must be tightly controlled. Ferritin, an iron binding protein, regulates iron balance by storing iron in a bioavailable but non-toxic form. Ferritin protein comprises two subunits: ferritin H, which contains ferroxidase activity, and ferritin L. Here we demonstrate that ferritin H mRNA and protein are induced by histone deacetylase inhibitors (HDAC inhibitors), a promising class of anti-cancer drugs, in cultured human cancer cells. Deletion analysis and EMSA assays reveal that the induction of ferritin H occurs at a transcriptional level via Sp1 and NF-Y binding sites near the transcriptional start site of the human ferritin H promoter. Classically, HDAC inhibitors modulate gene expression by increasing histone acetylation. However, ChIP assays demonstrate that HDAC inhibitors induce ferritin H transcription by increasing NF-Y binding to the ferritin H promoter without changes in histone acetylation. These results identify ferritin H as a new target of HDAC inhibitors, and recruitment of NF-Y as a novel mechanism of action of HDAC inhibitors.
Keywords: Ferritin H, histone acetylation, chromatin immunoprecipitation, cancer, HDAC inhibitors, transcription
1. Introduction
Ferritin H plays an important role in iron metabolism. Iron is essential for normal cell growth, proliferation, energy metabolism and other critical functions of cells and tissues. However, excess iron is harmful, and can catalyze the formation of toxic reactive oxygen species (ROS) via Fenton chemistry and other mechanisms. Therefore, iron must be tightly controlled and compartmentalized [1]. Ferritin is the major iron storage protein of the cell. By sequestering excess iron in a nontoxic form, ferritin plays a critical role in the maintenance of intracellular iron balance. Ferritin consists of two subunit types, termed H and L, which are encoded by separate genes [2]. Twenty four of these subunits assemble to form the apoferritin shell. Each apoferritin molecule can sequester up to 4500 iron atoms. The H subunit of ferritin, ferritin H, has inherent ferroxidase activity, and converts Fe(II) to Fe(III) as iron is internalized and sequestered in the ferritin mineral core [2]. Not surprisingly, homozygous murine knockouts of ferritin H are lethal [3]. Overexpression of ferritin H has been shown to cause an iron deficient phenotype and reduce cell growth [4, 5].
Histone acetylation plays an important role in regulation of transcription [6]. Histone acetylation reduces the binding between histones and DNA, thus loosening chromatin structure and facilitating the access of RNA polymerase and other transcription factors to promoter regions. In contrast, histone deacetylation represses transcription by condensing chromatin structure. Histone acetylation and histone deacetylation are catalyzed by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. HATs and HDACs do not directly bind to DNA, but are recruited to gene promoters by transcription factors, such as Sp1, Sp3, and NF-Y [7].
Histone deacetylase inhibitors (HDAC inhibitors) are a promising new class of anti-cancer drug. HDAC inhibitors inhibit cancer cell proliferation and lead to differentiation or apoptosis of cancer cells in vitro and in vivo [8]. Several HDAC inhibitors are currently in clinical trials and show significant anticancer activity [8, 9]. HDAC inhibitors not only induce cancer cells to undergo growth arrest and/or apoptosis, but also exhibit low toxicity against normal cells [10, 11]. HDAC inhibitors are of several chemical types, and range from simple chemicals (such as butyrate) to more complex agents such as hydroximates (such as trichostatin A [TSA], suberoylanilide hydroxamic acid [SAHA]), cyclic peptides (such as depsipeptide, apicidin), and benzamides (such as MS-275)[12, 13].
Regulation of gene expression is essential for the anti-tumor function of HDAC inhibitors, because inhibition of de novo protein synthesis suppresses HDAC inhibitor-induced apoptosis [14]. However, the detailed mechanism of HDAC inhibitor-induced cell death is not fully defined, and may also involve histone acetylation-independent mechanisms [15, 16]. Identification of target genes critical to the function of HDAC inhibitors will not only improve understanding of their fundamental mechanism of action, but may ultimately assist in their clinical application.
Here we reported that ferritin H is transcriptionally induced by HDAC inhibitors in human cancer cells. Unexpectedly, chromatin immunoprecipitation assays demonstrate that HDAC inhibitors do not act by increasing histone acetylation of the ferritin H promoter, but rather by recruiting NF-Y to the promoter. These results identify a novel mechanism of action of this important class of anti-cancer agent.
2. Materials and Methods
2.1 Chemicals and Cell Culture
Sodium butyrate and tricostatin A (TSA) were purchased from Sigma (St. Louis, MO). Human cervical carcinoma cells (HeLa) were obtained from American Type Culture Collection (ATCC) (Manassas, VA) and were maintained in DMEM (Invitrogen, Carlsbad, California) supplemented with 10% FBS (HyClone, Logan, UT), 100 units/ml penicillin, and 100ug/ml streptomycin. PC3 cells were obtained from the ATCC and maintained in RPMI 1640 medium (Invitrogen) containing 10% FBS, 100 units/ml penicillin, and 100ug/ml streptomycin. HME cells were maintained in MEBM (Lonza, MD) supplemented with MEGM SingleQuots (Lonza, MD). Cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C.
2.2 Plasmid construction
To generate human ferritin H promoter-luciferase reporter constructs, a 1.38-kb (−1175- + 209) human ferritin H promoter fragment was cloned from human genomic DNA by PCR amplification. The following primers were used: forward primer, from −1175, 5′-GCGCGGTACCCAGGTTTGTGAGCATCCTGAA; reverse primer, from +209, 5′-GCGCAGATCTTGGCGGCGACTAAGGAGAGG. The forward primer contained Acc65I recognition site, while the reverse primer contained BglII recognition site. The PCR product was purified from an agarose gel, digested and cloned into the pGL3-basic vector (Promega, Madison, WI) at Acc65I and BglII site to generate pGL3-1384. The serial deletion constructs pGL3-275, pGL3-83, pGL3-60, pGL3-48 were generated from pGL3-1384 construct by PCR using primers with an Acc65I recognition site and BglII site for the forward and reverse primers, respectively. The same reverse primer was used in all cases; the sequence is: from +4, 5′-GCGCAGATCTCTGGCCCTGCGGGTCGCTT G-3′. The forward primers are: for pGL3-275, 5′-GCGCGGTACCAGGTGGACTTCCTGCGCCTC-3′; for pGL3-83, 5′-GCGCGGTACCCTCGGGGCGGGCGGCGCTGA-3′; for pGL3-60, 5′-GCGCGGTACCGCCGGGGCGGGCCTGACG-3′; for pGL3-48,5′-GCGCGGTACCCTGACGCCGACGCGGCTATA-3′. The amplified promoter fragments were then inserted into pGL3-basic vector as described above. Mutations of the ferritin H promoter were generated using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer’s instructions. pGL3-83-Sp1-Mutant (pGL3-83-Sp1M) and pGL3-83-NF-Y-Mutant (pGL3-83-NF-YM) were generated from the pGL3-83 construct. The following primers were used: pGL3-83-SpM sense, 5′-CTCGGGGCAAACGGCGCTGATTGGCCG-3′, antisense, 5′-CGGCCAATCAGCGCCGTTTGCCCCGAG-3′; pGL3-83-NFYM sense, 5′-CGGCGCTGAT CGGCCGGGGCGGGCCTG-3′, antisense, 5′-CAGGCCCGCCCCGGCCGATCAGCGCCG-3′. The double mutant construct pGL3-83DM (Sp1M and NFYM) was based on pGL3-Sp1M. The following primers were used: pGL3-83DM sense, 5′-CTCGGGGCAAACGGCGCTGATCGGCCG-3′, antisense, 5′-CGGCCGATCAGCGCCGTTTGCCCCGAG-3′. All ferritin H promoter-luciferase constructs were confirmed by DNA sequencing. EndoFree plasmid maxi kit (Qiagen, Valencia, CA) was used to prepare plasmids for transfection.
2.3 Transfection and luciferase assay
All transfections were performed using Fugene6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. 2×105 cells/well were plated in 6-well plates and incubated in DMEM medium containing 10% FBS overnight. 0.5ug ferritin H promoter-luciferase constructs were cotransfected with 0.5ng pRL-β-actin (human β-actin promoter driven renilla luciferase, a kind gift of Kazuo Yamamoto)[17] as an internal control for transfection efficiency. Neither levels of endogenous beta actin mRNA nor expression driven by the pRL-β-actin control promoter were appreciably induced in response to HDAC inhibitors (≤ 5%) as measured by RT-PCR. Eight hours after transfection, the cells were treated with 100ng/ml TSA or vehicle alone for 16 hours. Ferritin H promoter activities were measured using the Dual Luciferase Assay Kit (Promega, Madison, WI).
2.4 Northern Blot analysis
Northern-blot analysis was performed as described previously[18].
2.5 Real-time RT-PCR
Real-time PCR was carried out on the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, California) as described previously [19]. Primers for PCR were designed with IDT PrimerQuest software (Integrated DNA Technologies, Inc., Coralville, IA). For ferritin H, forward, 5′-CTTTGACCGCGATGATGTGGCTTT-3′ and reverse, 5′-TTTGTCAGTGGCCAGTTTGTGCAG-3′; For ferritin L, forward, 5′-TTGGATCTTCATGCCCTGGGTTCT-3′ and reverse, 5′-AGTCGTGCTTGAGAGTGAGCCTTT-3′; For s-actin, forward, 5′-TTGCCGACAGGATGCAGAAGGA-3′; reverse, 5′-AGGTGGACAGCGAGGCCAGGAT-3′. Intron 2 was amplified to detect ferritin H primary transcript (Fig 3). The sequences are: forward,5′-TTCATCATCTGGCAGTGTTCGGGT-3′; reverse, 5′-ACCTAGAAGTCAGCAAGCCCATCA-3′.
Figure 3. HDAC inhibitors transcriptionally induce ferritin H.
HeLa cells were treated with either vehicle or 200 ng/ml TSA for 6 hours. Production of primary ferritin H transcript was monitored by real-time RT-PCR using primers located in the ferritin H intron 2 region as described in Materials and Methods. Means and standard deviations of 3 independent experiments are shown.
2.6 Western blot analysis
Cells were harvested by scraping into medium, washed in ice-cold PBS, and pellets were frozen until analysis. Cytosolic and nuclear extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagent (Pierce, Rockford, IL). 10–20ug protein was separated on 12% SDS-polyacrylamide gels and transferred to PVDF membranes. Antibodies used in western blots were anti-acetyl histone H3 and H4 (Upstate, Charlottesville, VA), NF-YA (Santa Cruz Biotechnology), polyclonal rabbit antibody to ferritin H [20], and antibody to ferritin L[21]. Blots were incubated with HRP-conjugated anti-secondary antibodies (Bio-Rad, CA) followed by ECL (Thermo Scientific, IL).
2.7 Electrophoretic Mobility Shift Assay (EMSA)
HeLa cell nuclear extract was purchased from Promega. The sequences of the oligonucleotides used in EMSA corresponding to the TSA responsive element are shown in Fig 5A. The two oligonucleotides were annealed and end-labeled with [γ-32P] ATP (PerkinElmer, Waltham Massachusetts) using T4 polynucleotide kinase (Promega, Madison, WI). The binding reactions were performed at room temperature for 15 min by incubating 10–20 ug of nuclear extracts with 10,000~50,000 cpm of end-labeled probes in 20ul of a solution (20 mM HEPES, 1.5 mM MgCl2, 100 mM KCl, 20% glycerol, 0.2 mM EDTA) containing 1ug of poly(dI-dC) and 10 ug of bovine serum albumin. Competition experiments were performed by mixing 25–100 fold molar excess of unlabeled oligonuclotide with nuclear extract prior to the addition of the probe. The sequences of Sp1 oligo used in competition assay are: sense, 5′-ATTCGATCGGGGCGGGGCGAGC-3′, antisense, 5′-GCTCGCCCCGCCCCGATCGAAT -3′. The sequences of NF-Y oligo used in competition assay are: sense, 5′-AGACCGTACGTGATTGGTTAATCTCTT-3′, antisense, 5′-AAGAGATTAACCAATCACGTACGGTCT -3′. In supershift experiments, antibodies against Sp1, Sp3 and NFY-A (Santa Cruz Biotechnology, Santa Cruz, CA) were preincubated with nuclear extracts for 30 minutes before the addition of the probe. DNA-protein complexes were isolated on a native 5% polyacrylamide gel (29:1, acrylamide:bisacrylamide). Gels were dried, and DNA-protein complexes were visualized by autoradiography.
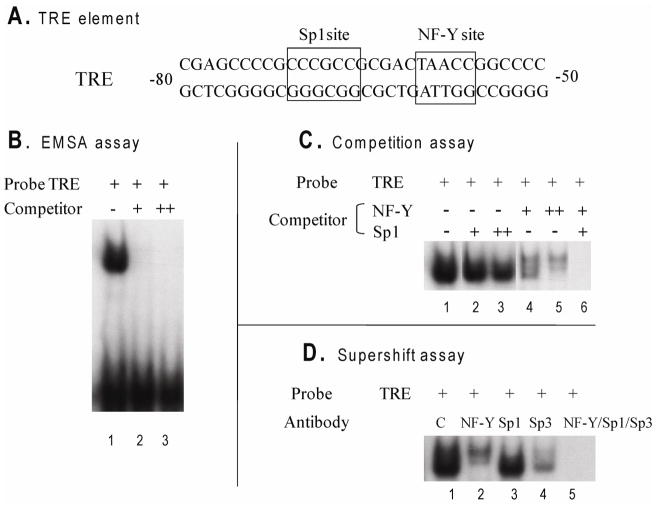
Figure 5. NF-Y, Sp1, and Sp3 bind to the TSA responsive element of ferritin H promoter.
A, Sequences of TSA responsive element (TRE) used in EMSAs. B, EMSAs were performed with nuclear extracts from HeLa cells. 32P-labeled oligonucleotide (TRE) was used as a probe. Nuclear extracts were incubated with labeled probe in the absence (lane 1) or in the presence of unlabelled competitor (lane 2, 3). C, EMSAs were performed as B. Unlabelled oligonucletides (Sp1 oligo in lane 2, 3 and 6, NF-Y oligo in lane 4, 5 and 6) were added as the competitors. “+” indicates 25- and “++” indicates 50-fold molar excess of unlabelled competitor. D, Supershift assay was performed in the presence of NF-Y antibody (lane 2), Sp1 antibody (lane 3), Sp3 antibody (lane 4), or the combination of NF-Y, Sp1 and Sp3 antibodies (lane 5). In C, lanes from the same blot have been rearranged for clarity.
2.8 Chromatin immunoprecipitation (ChIP)
ChIP assays used the same antibodies to acetyl histone H3 and H4 and NF-YA (Santa Cruz Biotechnology, Santa Cruz, CA) as described above for western blotting. Normal rabbit IgG was used as a control. ChIP assays were performed following the protocol recommended by the manufacturer (Upstate, Charlottesville, VA). The recovered DNA was resuspended in 30 μl of water and used as templates in Real-time PCR. Real-time PCR was done using the ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, California). The primers used in PCR to amplify ferritin H promoter region that is close to TSA responsive element are: sense, 5′-AAT GGG AGC CGA ATC AGG ATC A - 3′, antisense, 5′-TCT CTG TGC CCG TTT AGT GGA GTT - 3′. The control primers to amplify ferritin H promoter about 2500bp upstream TSA responsive element are: sense, 5′-AAA GCT GGG AGT GCA GAG ACA AGA -3′, antisense: 5′-CCA AAG TGC TGG GAT TAC AGG CAT - 3′. Primers for Endothelial Nitric-oxide Synthase (eNOS) promoter are: sense, 5′-ACC AGG GGG TCA TAA AGG TC - 3′, antisense, 5′-GGG GAG GTG AAG GAG AGA - 3′[22].
3. Results
3.1 HDAC inhibitors induce ferritin H mRNA and protein
We first tested whether HDAC inhibitors can induce ferritin H expression. HeLa cells were treated with various concentrations of the HDAC inhibitor tricostatin A (TSA) for 24 hours, and effects on ferritin H expression were analyzed. As seen in Fig 1A, Northern Blot analysis demonstrated that TSA significantly upregulates ferritin H mRNA in a dose-dependent manner (Fig 1A, upper panel); similar results were obtained using real time RT-PCR (Fig 1C). To assess the effect of TSA on ferritin H at the protein level, western blot analysis was performed. As shown in Fig. 1A (middle panel), TSA also induced ferritin H protein subunit in a dose-dependent manner. Butyrate, a less potent HDAC inhibitor, similarly induced ferritin H mRNA and protein (Fig 1B), as has previously been shown in mouse fibroblasts [23]. Since ferritin consists of 2 subunits, H and L, we performed similar experiments to determine the effect of TSA on ferritin L. We observed no induction of ferritin L expression after treatment with TSA at the protein level (Fig 1A bottom panel) or mRNA level (Fig 1C).
Figure 1. Ferritin H induction by HDAC inhibitors, TSA and Butyrate.
A, HeLa cells were treated with vehicle alone (0) or various concentrations of TSA for 24 hours. Ferritin H mRNA was determined by Northern Blot. β-actin mRNA was used as the loading control. Ferritin H, ferritin L and GAPDH (loading control) protein level was determined by Western Blot (middle and bottom panel). B, After treatment of HeLa cells with various dose of butyrate for 24 hours, Ferritin H and β-actin mRNA was determined by Northern Blot (upper panel). Western blot assay was performed to determine ferritin H and GAPDH protein level (bottom panel). Shown is a typical experiment; similar results were obtained in 3 independent experiments. C, Hela cells were treated as described in A and real-time RT-PCR was performed to determine ferritin H and ferritin L mRNA level. Shown are means and standard deviations of 4 independent experiments..
To verify that the induction of ferritin H by HDAC inhibitors is not a cell-type specific, we assessed ferritin H expression after TSA and butyrate treatment in various cancer cell lines using real-time RT-PCR and western blot. Ferritin H mRNA was induced approximately 1.8 fold in both HepG2 hepatocellular carcinoma cells and LNCaP prostate cancer cells at 100ng/ml TSA, an effect comparable to the 2.5 fold induction seen in Hela cells at this dose (data not shown). The most striking induction was seen in PC-3 prostate cancer cells, which demonstrated a 6 to 11-fold increase in ferritin H mRNA in response to TSA and butyrate, respectively, with a comparable increase in ferritin H protein (Fig. 2). Similar to results obtained in HeLa cells, no increase in ferritin L mRNA was seen in PC-3 cells following treatment with TSA (not shown). Thus, selective induction of ferritin H by HDAC inhibitors is observed in multiple cancer cell types. We also treated normal cells (primary human mammary epithelial cells) under comparable conditions (24 hours, TSA from 50 to 150ng/ml) to test whether TSA would have a similar effect on non-cancer cells. We observed a very modest increase in ferritin H mRNA by RT-PCR in TSA-treated cells (relative mRNA level was 1.2 ± 0.1 fold relative to that of untreated cells at 150 ng/ml TSA). Although only one primary epithelial cell type was assessed, these results suggest that ferritin H induction may be preferentially observed in cancer cells.
Figure 2. Induction of Ferritin H in PC-3 cells.
PC-3 cells were treated with vehicle alone (control), TSA (100ng/ml), or butyrate (5mM) for 24 hours. A. Ferritin H mRNA was measured by real-time RT-PCR. Means and standard deviations of 4 independent experiments are shown. B. Ferritin H protein was measured by western blot. Shown is a typical experiment, similar results were obtained in 3 independent experiments.
3.2 TSA increases ferritin H transcription
HDAC inhibitors are generally thought to act at the transcriptional level to induce gene expression [13]. However, HDAC inhibitors have also been reported to increase gene expression at a posttranscriptional level by increasing mRNA stability [24]. To determine whether the regulation by HDAC inhibitors of ferritin H mRNA abundance resulted from a change in ferritin H gene transcription, ferritin H primary transcripts were monitored by real-time RT-PCR using primers complementary to intronic sequences. Because introns are rapidly removed from hnRNA during splicing, this method provides a measure of transcription initiation rate, similar to classic nuclear run-on assays [25–27]. HeLa cells were treated with either vehicle or 200ng/ml TSA for 6 hours. Intron 2 was amplified and measured using quantitative RT-PCR. As shown in Fig 3, TSA treatment caused a 5-fold increase in the ferritin H primary transcript level, indicating that HDAC inhibitors induce ferritin H at a transcriptional level.
3.3 Identification of the TSA responsive element in the ferritin H promoter
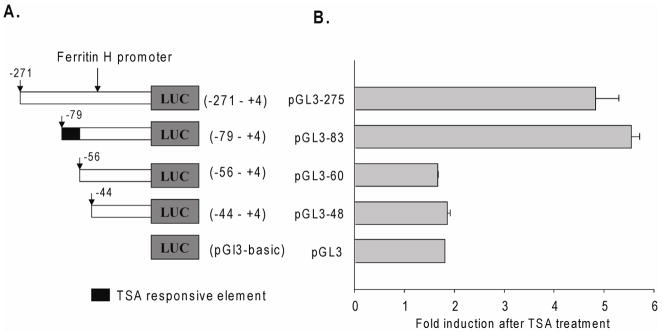
We next characterized the regions and cis-acting elements of the ferritin H promoter that are required for HDAC inhibitor-mediated activation of ferritin H transcription. Serial deletions of ferritin H promoter-luciferase reporter constructs were prepared (Fig. 4A) and transiently transfected into HeLa cells. pRL-beta-actin constructs were cotransfected as an internal control. Eight hours after transfection, cells were treated with 100ng/ml TSA or vehicle alone for 16 hours, and luciferase activities were measured. Addition of TSA to transfected cells increased luciferase activity 4.5–5.5 fold for promoter constructs pGL3-275 and pGL3-83. (Fig. 4B). A similar fold induction was obtained using longer ferritin H promoter (1.2kb -luciferase constructs [data not shown]). However, promoter constructs pGL3-60 and pGL3-48 showed reduced levels of induction, similar to the fold induction of pGL3-basic by TSA. The modest effect of TSA on the pGL3-basic vector observed in these experiments has been previously reported [15, 28], and likely represents an effect on the plasmid backbone. These findings implicate the −79 to −56 region of the ferritin H promoter as a TSA responsive element critical for optimal induction by HDAC inhibitors. To confirm the generality of these results, we performed similar experiments in PC-3 cells. These experiments demonstrated that the −79 to −56 region of the ferritin H promoter also mediates transcriptional induction of ferritin H in PC3 cells (data not shown).
Figure 4. Identification of TSA response elements in the ferritin H promoter.
A, 5′ deletion constructs of the ferritin H promoter. Different lengths of ferritin H promoter sequence were inserted into pGL3-basic vector. B, Ferritin H promoter-luciferase constructs were transiently transfected into HeLa cells. Eight hours after transfection, cells were treated with 100ng/ml TSA for 16 hours. For each construct, luciferase activity in the absence of TSA treatment was set to one, and fold induction after TSA treatment was calculated. Co-transfection with pRL-βactin was used to control for transfection efficiency. Means and standard deviations of 4 independent experiments are shown.
3.4 NF-Y, Sp1 and Sp3 bind to the TSA responsive element of the ferritin H promoter
The sequence of the TSA responsive element (TRE) that we identified in the ferritin H promoter includes GGGCGG and an inverted CCAAT box (Fig. 5A). GGGCGG is a potential binding site for Sp1 and Sp3 while an inverted CCAAT box is a potential binding site for several transcription factors, including NF-Y. To determine whether these transcription factors bind to the TSA responsive element, we performed electrophoretic mobility shift assays (EMSA). Nuclear extracts from HeLa cells bound the TRE (Fig. 5B, lane1). When 25- and 50-fold unlabeled TRE was used as the competitor (Fig 5B, lane 2 and 3), the band was completely competed away, indicating that the band is specific. To determine whether Sp1, Sp3 and NF-Y bind to TRE, we first performed competition assays using Sp1 and NF-Y consensus sequences as the competitors (Fig. 5C). 25- and 50-fold unlabeled Sp1 oligonucleotides diminished the intensity of the band (Fig. 5C, lane 2, 3), which suggests that Sp1/Sp3 bind to TRE sequence. Similarly, 25- and 50-fold unlabeled NF-Y oligonucleotides competed with the band (Fig. 5C, lane 4, 5), indicating that NF-Y binds to the TRE. Using both Sp1 and NF-Y oligonucleotides as the competitors, the band was completely competed away (Fig. 5C, lane 6).
To further confirm that these transcription factors bind to the TRE, we performed supershift assays (Fig 5D). Addition of Sp1, Sp3 or NF-Y antibody diminished the band (Fig 5D, lane 2, 3, and 4), while addition of these three antibodies together totally abolished the binding activity. These results indicate that the transcription factors Sp1, Sp3 and NF-Y bind the TRE region of the ferritin H promoter.
3.5 Both Sp1 site and inverted CCAAT site contribute to the activation of the ferritin H promoter by TSA
Since EMSAs indicated that Sp1/3 and NF-Y bind to the TSA responsive element of the ferritin H promoter, we next determined whether the Sp1 site or NF-Y site (the inverted CCAAT) or both are required for activation of the ferritin H promoter by TSA. We generated specific mutations known to disrupt binding Sp1 and/or NF-Y in ferritin H promoter-luciferase constructs by site-directed mutagenesis. Mutated sequences are indicated in Fig. 6A. Cells were transfected, treated with TSA, and luciferase assays were performed as described in Fig. 4. As shown in Fig 6B, mutations of either the Sp1 site or the inverted CCAAT box attenuated TSA-mediated promoter activity, with mutation of the CCAAT site causing particularly marked reduction of TSA-dependent induction. Double mutations of the Sp1 site and the inverted CCAAT box (pGL3-83-DM) virtually abolished TSA-dependent induction of ferritin H promoter (Fig. 6B) as well as binding activity in EMSA assays (not shown). These results indicate that both the Sp1 site and the inverted CCAAT site are involved in TSA-mediated ferritin H promoter induction.
Figure 6. Both NF-Y and Sp1 site mediate ferritin H promoter induction by TSA.
A, the sequence of TSA responsive element in the ferritin H promoter. Mutated sequences are indicated by asterisks, with the substituted sequences presented above. Sp1 site, CCAAT site, or both sites were mutated in pGL3-83 construct. B, Transfection, drug treatment and luciferase assay were performed as described in Fig. 4. Means and standard deviations of 4 independent experiments are shown.
3.6 TSA does not increase association of acetylated histones H4 or H3 to the ferritin H promoter, but does increase recruitment of NF-Y to the ferritin H promoter in vivo
A major mechanism by which HDAC inhibitors modulate gene transcription is via the accumulation of acetylated histones [8]. Since acetylation of histones H4 and H3 are in particular associated with actively transcribed chromatin [29] we first used ChIP analysis to examine the effect of TSA on the association of acetylated histone H4 with the ferritin H gene promoter. Hela cells were treated with or without TSA for 6 h and chromatin fragments were prepared and immunoprecipitated with antibody to acetylated histone H4. Normal rabbit IgG was used as a control. DNA from the immunoprecipitates was isolated, and real-time PCR was performed using ferritin H promoter primers proximal to the TSA responsive element. Surprisingly, we found that TSA did not increase, but rather slightly decreased, the acetylation of histones H4 in the ferritin H promoter region (Fig 7A, left panel). Similarly, ChIP experiments using antibody to acetylated histone H3 demonstrated a similar decrease in the association of acetylation of histone H3 to the ferritin H promoter after TSA treatment (Fig 7B). To confirm our assay conditions, we amplified the eNOS promoter as a positive control, since TSA is known to induce histone acetylation at the eNOS promoter in Hela cells [22]. As shown in Fig 7A (right panel), histone acetylation in eNOS promoter increased about 3 fold after TSA treatment. Treatment of cells with TSA for a longer time period (18 hours) did not increase the association of acetylated H3 and H4 to ferritin H promoter (data not shown). To confirm that histone acetylation had indeed occurred under our conditions, western blot analysis of TSA-treated cells using antibodies to acetylated histones H3 and H4 was performed. As shown in Fig 7C, both H3 and H4 were acetylated following TSA treatment, as expected. We also used ChIP assays to analyze the association of acetylated histone H4 to the ferritin H promoter in PC3 cells following treatment with TSA. Consistent with results obtained with Hela cells, we saw no increase in the association of acetylated histone to ferritin promoter in TSA-treated PC3 cells (data not shown), despite the dramatic increase in ferritin H expression seen in these cells following TSA treatment (Fig. 2). Thus the TSA-dependent increase in ferritin H expression occurs without increasing acetylation of histones H3 or H4 associated with the ferritin H promoter.
Figure 7. TSA does not increase acetylation of histones H4 or H3 at the ferritin H promoter.
A and B, Hela cells were treated with either vehicle (control) or 100ng/ml TSA for 6 hours and a ChIP assay was performed using acetylated H4 antibody (A) and acetylated H3 antibody (B). Amplification was performed using primers proximal to the TSA-responsive element of ferritin H or eNOS. eNOS is a positive control. Values shown are relative percentage to input. The data represent means and standard deviations of 3 independent drug treatment and chromatin preparations. C, Hela cells were treated with the same condition as A, nuclear extract was harvested and western blot was performed to determine acetylated H3 and H4 level. Ponceau S staining was used as a loading control.
Although histone acetylation is the primary mechanism of action of HDAC inhibitors, a histone acetylation-independent mechanism involving the enhanced recruitment of Sp1 to the promoter has been implicated in the induction of 5-lipoxygenase by TSA [15]. Since NF-Y plays a dominant role in TSA-mediated induction of ferritin H (Fig. 6), we tested whether TSA enhanced the recruitment of NF-Y to the ferritin H promoter using a ChIP assay for NF-YA, the regulatory subunit of NF-Y [30]. As shown in Fig. 8A, association of NF-Y to the TSA-responsive element of the ferritin H promoter increased 3–4 fold following TSA treatment. To confirm the specificity of the CHIP assay, we also used another set of primers as negative controls to amplify a ferritin H promoter region 2500 bp upstream of the TSA responsive element. As shown in Fig. 8B, these experiments revealed that there was no non-specific increase in association of NF-Y to DNA following TSA treatment. Since NF-YA acetylation by p300 has been shown to prevent its ubiquitylation and moderately enhance its stabilization in mouse muscle cells [30], we next performed western blots to test whether NF-YA levels increased after TSA treatment. As shown in Fig 8C, TSA enhanced NF-YA recruitment to the ferritin H promoter without increasing levels of the NF-YA protein.
Figure 8. TSA enhances NF-Y recruitment to the ferritin H promoter.
(A) Hela cells were treated with either vehicle (control) or 100ng/ml TSA for 6 hours and a ChIP assay was performed using NF-YA antibody. Amplification was performed using primers proximal to the TSA-responsive element of ferritin H. (B) A ChIP assay was performed using a non-specific region of the ferritin H promoter located ~2500 bp distal to the TSA-responsive element (control ferritin H promoter). Values shown are relative percentage to input. The data represent means and standard deviations of 3–4 independent drug treatment and chromatin preparations. (C). Nuclear extracts were harvested from cells treated as in (A), and a western blot was performed to determine NF-YA levels. Ponceau S staining was used as a loading control.
4. Discussion
In this report, we demonstrate that the H subunit of ferritin, an iron storage protein, is induced by histone deacetylase inhibitors at transcriptional level via Sp1 and NF-Y sites in the ferritin H promoter.
HDAC inhibitors transcriptionally activate the ferritin H promoter by engaging elements of the human ferritin H promoter approximately 60 nucleotides 5′ of the transcriptional start site, a region known to be important in the transcriptional control of the human ferritin H gene. For example, two cis-acting elements in the proximal promoter of human ferritin H gene have previously been identified: a distal GC box which contains a potential Sp1/Sp3 binding site (−132 to −109 bp from the transcription start), and a proximal site containing an inverted CCAAT box (−65 to −45 bp) [31]. The latter is recognized by a protein complex composed of the trimeric transcription factor NF-Y, p300 (a member of HAT family) and p300/CBP-associated factor (pCAF)[32] which binds NF-Y [33]. cAMP signaling stimulates ferritin H transcription by enhancing the formation of this complex, while E1A represses ferritin H transcription by inhibiting NFY-p300 complex formation [32]. Our observation that ferritin H transcription is upregulated by HDAC inhibitors led us to a detailed analysis of the human ferritin H promoter, and uncovered a ~30-bp element containing an inverted CCAAT box and Sp1 binding site (different from the Sp1/Sp3 site described above, Fig. 5A) required for maximal induction of ferritin H by HDAC inhibitors. Electrophoretic mobility shift assays confirmed that Sp1, Sp3 and NF-Y bind to the TSA responsive sequence. Using nuclear extracts from Friend leukemia cells, others have also shown binding of NF-Y to this element, although binding of SP1/3 was not observed in Friend cells [34].
Our results demonstrate the complexity of ferritin induction by HDAC inhibitors: both the Sp1 binding site and inverted CCAAT box are required for optimal induction of the ferritin H promoter by HDAC inhibitors, since mutations of the Sp1 binding site and/or inverted CCAAT box both reduced the ability of TSA to induce ferritin H promoter activity. Sp1 and/or NF-Y binding sites have also been shown to contribute to the regulation of other critical growth regulatory genes by HDAC inhibitors. For example, activation of transforming growth factor β type II receptor by HDAC inhibitors requires both a Sp1 site and a NF-Y binding site [35]. In other genes, either SP1/3 or NF-Y, but not both, are required. For example, in p21 [36], INK4d [37], tyrosine hydroxylase [38] and hTERT [39], Sp1/Sp3-binding sites are required for HDAC inhibitor-mediated activation, while NF-Y-binding sites (inverted CCAAT boxes) rather than Sp1 sites mediate the activation of genes for Growth-Differentiation Factor 11 (Gdf11)[40], multidrug resistance 1 (MDR1)[41], and GADD45 [42].
Ferritin H induction may contribute to HDAC inhibitor-mediated apoptosis. Iron is essential to cell growth, and limiting iron availability through treatment of cells with iron chelators can cause cell cycle arrest and apoptosis [43]. Similarly, induction of p53 induces an increase in ferritin H, which may contribute to p53-dependent growth arrest [44]. Further, cells that overexpress ferritin H exhibit an iron-deficient phenotype and reduced cell growth [5]. We speculate that HDAC inhibitor-mediated overexpression of ferritin H might act as an endogenous iron chelator to induce iron deficiency, thus contributing to the anti-cancer activity of HDAC inhibitors.
ChIP results indicate that HDAC inhibitors increase ferritin H transcription via a novel mechanism involving increased recruitment of NF-YA to the ferritin H promoter (Fig. 8). To explain the transcriptional induction of ferritin H by HDAC inhibitors, we initially considered a model in which Sp1, Sp3 and NF-Y bind to the proximal region of ferritin H promoter (−80 –50 bp) and serve as a scaffold to recruit HDACs and HATs to the promoter, as has been reported for other genes [45–47]. In this scenario, inhibition of HDACs by HDAC inhibitors would disrupt the association between these transcription factors and HDACs, permitting increased interaction of these transcription factors with HATs (e.g. p300), resulting in increased histone acetylation and increased ferritin H transcription. However, ChIP assays showed that association of acetylated histones with the ferritin H promoter is not increased after HDAC inhibitor treatment (Fig. 7). Rather, HDAC inhibitors increased the recruitment of NF-YA to the ferritin H promoter (Fig. 8). Others have previously observed that HDAC inhibitors can increase recruitment of Sp1/3 to the 5-lipoxygenase promoter [15].
The mechanism by which HDAC inhibitors increase recruitment of transcription factors to various promoters is unknown. One possibility is that HDAC treatment leads to transcriptional induction of the transcription factors themselves. However, in our study, we saw no increase in the net level of NF-YA following TSA treatment as measured by western blotting (Fig. 8), arguing that HDAC inhibitors do not increase levels of ferritin H through indirect stimulation of NF-YA transcription. Another possibility is that HDAC inhibitors lead to increased acetylation of transcription factors, a mechanism that enhances the activity of some transcription factors, such as NFκB [48]. NF-YA has indeed been shown to be susceptible to acetylation by p300 in vitro [30]. Further, in mouse muscle cells, some but not all lysine residues that were acetylated by p300 in vitro were identified as targets for ubiquitination [30], suggesting that acetylation of selected residues may stabilize NF-YA from degradation. However, our finding that NF-YA levels do not increase following treatment with TSA also argues against this model, and suggests that interference with ubiquitination is not a major mechanism underlying our observation that HDAC inhibitors increase recruitment of NF-YA to the ferritin H promoter in human cancer cells. Thus, we favor a model in which the predominant effect of HDAC inhibitors on the ferritin H promoter are acetylation events that enhance binding of NF-YA to the promoter or to transcriptional co-activators associated with the promoter as well as enhance the activity of NF-YA itself, and that these combined events lead to ferritin H induction. Further experiments will be required to clarify the detailed mechanism(s) underlying enhanced binding of NF-YA to the ferritin H promoter following treatment with HDAC inhibitors.
Acknowledgments
This work was supported in part by Public Health Service grant R37DK42412 from the NIDDK (FMT).
The abbreviations used are
- HDAC inhibitors
histone deacetylase inhibitors
- TSA
trichostatin A
- CHIP
Chromatin immunoprecipitation
- TRE
TSA responsive element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 2.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 4.Picard V, Renaudie F, Porcher C, Hentze MW, Grandchamp B, Beaumont C. Overexpression of the ferritin H subunit in cultured erythroid cells changes the intracellular iron distribution. Blood. 1996;87:2057–2064. [PubMed] [Google Scholar]
- 5.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. The Journal of biological chemistry. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 6.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Iglesias O, Ruiz-Llorente L, Sanchez-Martinez R, Garcia L, Zambrano A, Aranda A. Histone deacetylase inhibitors: mechanism of action and therapeutic use in cancer. Clin Transl Oncol. 2008;10:395–398. doi: 10.1007/s12094-008-0221-x. [DOI] [PubMed] [Google Scholar]
- 8.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 9.Villar-Garea A, Esteller M. Histone deacetylase inhibitors: understanding a new wave of anticancer agents. Int J Cancer. 2004;112:171–178. doi: 10.1002/ijc.20372. [DOI] [PubMed] [Google Scholar]
- 10.Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X, Marks PA. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 12.Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- 13.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–18. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 14.Glick RD, Swendeman SL, Coffey DC, Rifkind RA, Marks PA, Richon VM, La Quaglia MP. Hybrid polar histone deacetylase inhibitor induces apoptosis and CD95/CD95 ligand expression in human neuroblastoma. Cancer Res. 1999;59:4392–4399. [PubMed] [Google Scholar]
- 15.Schnur N, Seuter S, Katryniok C, Radmark O, Steinhilber D. The histone deacetylase inhibitor trichostatin A mediates upregulation of 5-lipoxygenase promoter activity by recruitment of Sp1 to distinct GC-boxes. Biochim Biophys Acta. 2007;1771:1271–1282. doi: 10.1016/j.bbalip.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen CS, Weng SC, Tseng PH, Lin HP, Chen CS. Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. The Journal of biological chemistry. 2005;280:38879–38887. doi: 10.1074/jbc.M505733200. [DOI] [PubMed] [Google Scholar]
- 17.Imanishi D, Yamamoto K, Tsushima H, Miyazaki Y, Kuriyama K, Tomonaga M, Matsuyama T. Identification of a novel cytokine response element in the human IFN regulatory factor-1 gene promoter. J Immunol. 2000;165:3907–3916. doi: 10.4049/jimmunol.165.7.3907. [DOI] [PubMed] [Google Scholar]
- 18.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Di X, D’Agostino RB, Jr, Torti SV, Torti FM. Excess capacity of the iron regulatory protein system. The Journal of biological chemistry. 2007;282:24650–24659. doi: 10.1074/jbc.M703167200. [DOI] [PubMed] [Google Scholar]
- 20.Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. The Journal of biological chemistry. 2003;278:2361–2369. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- 21.Jiao Y, Wilkinson Jt, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free radical biology & medicine. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Gan Y, Shen YH, Wang J, Wang X, Utama B, Wang J, Wang XL. Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. The Journal of biological chemistry. 2005;280:16467–16475. doi: 10.1074/jbc.M412960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji Y, Moran E, Torti SV, Torti FM. Transcriptional regulation of the mouse ferritin H gene. Involvement of p300/CBP adaptor proteins in FER-1 enhancer activity. J Biol Chem. 1999;274:7501–7507. doi: 10.1074/jbc.274.11.7501. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch CL, Bonham K. Histone deacetylase inhibitors regulate p21WAF1 gene expression at the post-transcriptional level in HepG2 cells. FEBS Lett. 2004;570:37–40. doi: 10.1016/j.febslet.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci U S A. 1989;86:9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley MN, Zhou L, Smale ST. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol Cell Biol. 2003;23:4841–4858. doi: 10.1128/MCB.23.14.4841-4858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Taniura S, Kamitani H, Watanabe T, Eling TE. Transcriptional regulation of cyclooxygenase-1 by histone deacetylase inhibitors in normal human astrocyte cells. The Journal of biological chemistry. 2002;277:16823–16830. doi: 10.1074/jbc.M200527200. [DOI] [PubMed] [Google Scholar]
- 29.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nature reviews. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 30.Manni I, Caretti G, Artuso S, Gurtner A, Emiliozzi V, Sacchi A, Mantovani R, Piaggio G. Posttranslational regulation of NF-YA modulates NF-Y transcriptional activity. Mol Biol Cell. 2008;19:5203–5213. doi: 10.1091/mbc.E08-03-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevilacqua MA, Giordano M, D’Agostino P, Santoro C, Cimino F, Costanzo F. Promoter for the human ferritin heavy chain-encoding gene (FERH): structural and functional characterization. Gene. 1992;111:255–260. doi: 10.1016/0378-1119(92)90696-m. [DOI] [PubMed] [Google Scholar]
- 32.Bevilacqua MA, Faniello MC, Quaresima B, Tiano MT, Giuliano P, Feliciello A, Avvedimento VE, Cimino F, Costanzo F. A common mechanism underlying the E1A repression and the cAMP stimulation of the H ferritin transcription. J Biol Chem. 1997;272:20736–20741. doi: 10.1074/jbc.272.33.20736. [DOI] [PubMed] [Google Scholar]
- 33.Faniello MC, Bevilacqua MA, Condorelli G, de Crombrugghe B, Maity SN, Avvedimento VE, Cimino F, Costanzo F. The B subunit of the CAAT-binding factor NFY binds the central segment of the Co-activator p300. J Biol Chem. 1999;274:7623–7626. doi: 10.1074/jbc.274.12.7623. [DOI] [PubMed] [Google Scholar]
- 34.Marziali G, Perrotti E, Ilari R, Testa U, Coccia EM, Battistini A. Transcriptional regulation of the ferritin heavy-chain gene: the activity of the CCAAT binding factor NF-Y is modulated in heme-treated Friend leukemia cells and during monocyte-to-macrophage differentiation. Mol Cell Biol. 1997;17:1387–1395. doi: 10.1128/mcb.17.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao S, Venkatasubbarao K, Li S, Freeman JW. Requirement of a specific Sp1 site for histone deacetylase-mediated repression of transforming growth factor beta Type II receptor expression in human pancreatic cancer cells. Cancer Res. 2003;63:2624–2630. [PubMed] [Google Scholar]
- 36.Sowa Y, Orita T, Hiranabe-Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Ann N Y Acad Sci. 1999;886:195–199. doi: 10.1111/j.1749-6632.1999.tb09415.x. [DOI] [PubMed] [Google Scholar]
- 37.Yokota T, Matsuzaki Y, Miyazawa K, Zindy F, Roussel MF, Sakai T. Histone deacetylase inhibitors activate INK4d gene through Sp1 site in its promoter. Oncogene. 2004;23:5340–5349. doi: 10.1038/sj.onc.1207689. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Park JS, Hong SJ, Woo MS, Kim SY, Kim KS. Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. Biochem Biophys Res Commun. 2003;312:950–957. doi: 10.1016/j.bbrc.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Won J, Yim J, Kim TK. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. The Journal of biological chemistry. 2002;277:38230–38238. doi: 10.1074/jbc.M206064200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Wharton W, Yuan Z, Tsai SC, Olashaw N, Seto E. Activation of the growth-differentiation factor 11 gene by the histone deacetylase (HDAC) inhibitor trichostatin A and repression by HDAC3. Mol Cell Biol. 2004;24:5106–5118. doi: 10.1128/MCB.24.12.5106-5118.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirose T, Sowa Y, Takahashi S, Saito S, Yasuda C, Shindo N, Furuichi K, Sakai T. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene. 2003;22:7762–7773. doi: 10.1038/sj.onc.1207091. [DOI] [PubMed] [Google Scholar]
- 43.Greene BT, Thorburn J, Willingham MC, Thorburn A, Planalp RP, Brechbiel MW, Jennings-Gee J, Wilkinson Jt, Torti FM, Torti SV. Activation of caspase pathways during iron chelator-mediated apoptosis. J Biol Chem. 2002;277:25568–25575. doi: 10.1074/jbc.M110345200. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Wang W, Tsuji Y, Torti SV, Torti FM. Post-transcriptional modulation of iron homeostasis during p53-dependent growth arrest. The Journal of biological chemistry. 2008;283:33911–33918. doi: 10.1074/jbc.M806432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doetzlhofer A, Rotheneder H, Lagger G, Koranda M, Kurtev V, Brosch G, Wintersberger E, Seiser C. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol Cell Biol. 1999;19:5504–5511. doi: 10.1128/mcb.19.8.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun JM, Chen HY, Moniwa M, Litchfield DW, Seto E, Davie JR. The transcriptional repressor Sp3 is associated with CK2-phosphorylated histone deacetylase 2. The Journal of biological chemistry. 2002;277:35783–35786. doi: 10.1074/jbc.C200378200. [DOI] [PubMed] [Google Scholar]
- 47.Peng Y, Jahroudi N. The NFY transcription factor inhibits von Willebrand factor promoter activation in non-endothelial cells through recruitment of histone deacetylases. The Journal of biological chemistry. 2003;278:8385–8394. doi: 10.1074/jbc.M213156200. [DOI] [PubMed] [Google Scholar]
- 48.Quivy V, Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochemical pharmacology. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]