Abstract
Chronic respiratory disorders such as asthma are believed to be associated with adverse cardiovascular events. We hypothesize that asthmatic inflammation translates into systemic inflammation and alters vascular responses where adenosine (AD) plays an important role. Therefore, this study investigated the effects of aerosolized AD, used to elevate lung AD levels, on vascular reactivity and inflammation in our allergic mouse model of asthma. Balb/c mice were divided into four groups: control (Con), Con + aerosolized AD (Con + AD), allergen sensitized and challenged (Sen), and Sen + aerosolized AD (Sen + AD). The animals were sensitized with ragweed (200 μg ip) on days 1 and 6, followed by 1% ragweed aerosol challenges from days 11 to 13. On day 14, the Con + AD and Sen + AD groups received a single AD aerosol challenge (6 mg/ml) for 2 min, followed by the collection of the aorta and plasma on day 15. Organ bath experiments showed concentration-dependent aortic relaxations to AD in the Con and Con + AD groups, which were impaired in the Sen and Sen + AD groups. Real-time PCR data showed changes in aortic AD receptors (ARs), with the expression of A1ARs upregulated, whereas the expression of A2ARs and endothelial nitric oxide synthase genes were downregulated, resulting in an impairment of vasorelaxation in the Sen and Sen + AD groups. The A1AR antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) reversed the impairment in vasorelaxation observed in the Sen and Sen + AD groups, whereas the A2BAR antagonist alloxazine inhibited vasorelaxation in all groups. Allergen challenge caused systemic inflammation in allergic mice, with AD aerosol further enhancing it as determined by the inflammatory cytokines profile in plasma. In conclusion, asthmatic mice showed altered vascular reactivity and systemic inflammation, with AD aerosol further exacerbating these effects.
Keywords: allergen, systemic inflammation
ASTHMA, A CHRONIC LUNG DISEASE affecting 10% of the population in North America, is characterized by the perpetuation and amplification of inflammation in the airways and systemic circulation (4, 5). Over the past two decades, adenosine has been increasingly implicated in the pathophysiology of asthma. Chronic cellular stress, inflammation, and tissue damage observed in asthmatic airways are associated with increases in adenosine levels (11). The inhalation of adenosine leads to bronchoconstriction in patients with asthma as opposed to normal subjects who show no response to adenosine (9). The elevation in lung adenosine levels observed in adenosine deaminase (ADA)-deficient mice led to severe pulmonary inflammation, airway hyperreactivity, and airway remodeling (3, 7), suggesting that a chronic elevation of adenosine levels can lead to the promotion of lung inflammation.
Several epidemiological studies have reported that people suffering from chronic respiratory diseases such as asthma are at an increased risk for developing cardiovascular complications (10, 20, 22, 42). Systemic inflammation in these patients is believed to be a consequence of airway inflammation (21, 30), which may be one of the reasons for altered cardiovascular parameters. Some recent studies have also reported enhanced systemic inflammation, myocardial ischemia-reperfusion injury, and neutrophil recruitment to the myocardium in animal models of allergic asthma (18, 19). However, no study so far has attempted to establish a link between airway inflammation, vascular reactivity, and systemic inflammation, especially in relation to adenosine.
Adenosine has a well-established role in the control of vascular tone, and its effects are exerted through the activation of four different adenosine receptor (AR) subtypes: A1, A2A, A2B, and A3 (39). A1- and A3ARs have been shown to be involved in vasoconstriction (17, 37, 40, 41), whereas A2A- and A2BARs cause vasorelaxation of the aorta (1, 16, 23). However, the modulation of the expression of aortic ARs in response to the allergen and adenosine aerosol challenge as an added insult in a murine model of asthma has not been previously studied.
Allergen has been shown to cause the release of adenosine among other inflammatory mediators from allergic lung and activated leukocytes (26, 27). Adenosine generated in allergic lungs may be involved in the release of further chemotactic and inflammatory mediators in the lung and systemic circulation by acting on its receptors present on different cells including mast cells, eosinophils, neutrophils, and other inflammatory cells (12, 13, 31, 38). This has been confirmed by recent observations where experimentally induced temporary elevations in lung adenosine levels through the inhalation of adenosine (resulting from a breakdown of adenosine 5′-monophosphate) have been shown to cause an increase in the infiltration of eosinophils in patients with asthma (44). A recent study from our laboratory has also shown an increased release of inflammatory cell markers in the lung and plasma after the inhalation of adenosine aerosol in allergic mice (14). These studies thus suggest that adenosine inhalation amplifies allergen-induced airway inflammation in the lungs.
Therefore, this study was undertaken to investigate the effects of inhaled adenosine on vascular reactivity and systemic inflammation using our murine model of asthma. Our data suggest that asthmatic mice have altered peripheral vascular reactivity and systemic inflammation, with inhaled adenosine further exacerbating these effects.
MATERIALS AND METHODS
Animals
Balb/c mice, 8–10-wk-old males, were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The animals were maintained on a ragweed-free diet. All experimental animals used in this study were handled under a protocol approved by the Institutional Animal Care and Use Committee of West Virginia University.
Animal sensitization
Sensitization was performed according to the protocol described earlier from this laboratory (13–15, 29, 34). This model of allergic asthma has been shown to develop airway inflammation and airway hyperreactivity to methacholine. The study comprised four groups of animals: 1) control (Con), 2) Con challenged with 6 mg/ml of adenosine aerosol for 2 min on day 14 (Con + AD), 3) allergen sensitized and challenged (Sen), and 4) Sen further challenged with 6 mg/ml of adenosine aerosol for 2 min on day 14 (Sen + AD). Mice were sensitized on days 1 and 6 with intraperitoneal injections of ragweed allergen (Greer, Lenoir, NC), 200 μg/dose with 200 μl Imject Alum (Pierce, Rockford, IL). Nonsensitized Con animals received only the Imject Alum with the same volumes. Ten days after sensitization, the mice were placed in a Plexiglas chamber and challenged with 1% aerosolized ragweed or with 0.9% saline as a control, using an ultrasonic nebulizer (DeVilbiss, Somerset, PA) for 20 min both in the morning and afternoon for 3 consecutive days. The aerosolization of allergen was performed at a flow rate of 2 ml/min, and the aerosol particles had a median aerodynamic diameter of <4 μm (DeVilbiss).
Acute elevations in lung adenosine levels were produced experimentally by adenosine inhalation (6 mg/ml for 2 min on day 14) to the Sen + AD group to further enhance allergen-induced effects (the control for this group was Con + AD). This dose was chosen based on previous studies from our laboratory in this model (13, 14). Adenosine inhalation in this model has been shown to enhance allergen-induced airway inflammation and airway hyperreactivity to adenosine [or its analog 5′-(N-ethylcarboxamido)adenosine (NECA)] (13–15). ADA-deficient mice, having sustained and chronic elevations in lung adenosine levels, have also been shown to have similar features (7). We chose 24-h post-adenosine inhalation for our current studies based on an earlier study from our laboratory, which showed maximum eosinophilic inflammation in airways after 24 h of adenosine inhalation (13), which is a hallmark of asthmatic inflammation. The Con and Sen groups received only saline on day 14. Twenty-four hours after the last challenge, the animals were euthanized for collection of the aorta and blood for further experiments.
Preparation of isolated mouse aorta and isometric force measurement
Mice were euthanized by anesthesia with pentabarbitol sodium (65 mg/kg ip) followed by thoracotomy and the removal of the aorta, which was then cut transversely into 3 to 4-mm rings. The rings were mounted vertically between two stainless steel wire hooks and then suspended in 10-ml organ baths containing Krebs-Henseleit buffer. The Krebs-Henseleit buffer (pH 7.4), containing (in mM) 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2, was maintained at 37°C with continuous bubbling of 95% O2-5% CO2. For the measurement of isometric force response, the aortic rings were equilibrated for 90 min with a resting force of 1 g and a change of the bathing solution at 15-min intervals. The resting force of 1 g has been used earlier in our laboratory (1, 41). At the end of the equilibration period, the tissues were contracted with 50 mM KCl to check the contractility of individual aortic rings twice, which were washed out with Krebs-Henseleit buffer. The aortic rings were then constricted with phenylephrine (PE; 10−7 M) to obtain a steady contraction, and changes in tension were monitored continuously with a fixed-range precision force transducer (TSD 125C; Biopac) connected to a differential amplifier (DA 100B; Biopac). The data were recorded using the MP100 Biopac digital acquisition system and analyzed using Acknowledge 3.5.7 software (Biopac).
Contraction/relaxation experiments
After equilibration, the responsiveness and stability of individual rings were checked by successive administration of a submaximally effective concentration of PE (10−7 M). The integrity of the vascular endothelium was assessed pharmacologically by acetylcholine (10−7 M) to produce the relaxation of PE-precontracted rings. The aortic rings were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began.
Experimental protocol
The concentration-response curves (CRCs) for adenosine (10−11–10−4 M) were run parallel in aortic rings from all the groups. In all cases, adenosine was added to yield the next higher concentration only when the response to the earlier dose reached a steady state. In experiments where the effects of an antagonist were measured, it was added 30 min before the contraction of the tissue with PE and was present throughout the experiments.
Contraction/relaxation responses were expressed as a percentage of increase/decrease in the contraction with respect to PE (alone) in response to each concentration of agonist used.
Real-time PCR: A1-, A2A-, A2B-, and A3AR and endothelial nitric oxide synthase genes expression
The aortic tissues from all experimental groups were processed for total RNA isolation using the TRIzol reagent from Life Technologies/Invitrogen, followed by DNase treatment to eliminate potential genomic DNA contamination as described recently by our laboratory (1). This was followed by a conversion of 0.5 μg of total RNA into cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) in a total volume of 100 μl. Real-time PCR was then performed using an ABI PRISM 7300 detection system (Applied Biosystems) using Taqman Universal Mastermix (Applied Biosystems, Branchburg, NJ) according to the instructions of the manufacturer. The reaction volume (25 μl) consisted of (in μl) 12.5 2× Taqman Universal Mastermix, 1 cDNA, and 1.25 20× 6-carboxy-fluorescein-labeled Taqman gene expression assay master mix solution. For the real-time PCR of the concerned genes [A1-, A2A-, A2B-, and A3AR and endothelial nitric oxide synthase (eNOS)], the Taqman inventoried assays-on-demand gene expression products (assay identifications for A3-, A2B-, A2A-, and A1AR and eNOS, respectively, are Mm00802076_m1, Mm00839292_m1, Mm00802075_m1, Mm01308023_m1, and Mm00435204_m1) were purchased from Applied Biosystems. 18S ribosomal RNA was used as an endogenous control. The fold difference in the expression of target cDNA was determined using the comparative cycle threshold method as described earlier (25).
Assessment of systemic inflammation
Mice were euthanized by pentobarbital sodium (65 mg/ml ip), followed by the collection of blood by cardiac puncture in heparinized syringes. The blood collected from different groups was centrifuged at 800 g for 10 min at 4°C, and the resulting plasma was used for cytokine and C-reactive protein (CRP) assays.
Multiplex cytokine assay
The multiplex cytokine assay of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), TNF-α, IFN-γ, granulocyte (G)-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein-1 (MCP-1), keratinocyte-derived chemokine, regulated on activation, normal T cell expressed and secreted (RANTES), IL-9, IL-13, IL-1α, IL-7, IL-15, IL-17, interferon-γ inducible protein (IP)-10, and G-CSF in the plasma was measured by a commercial kit from Linco Research (St. Charles, MO) using the Luminex 200 system (Luminex, Austin, TX), which is a multianalyte bioassay detection system capable of performing up to 100 assays simultaneously in a single microtiter plate well. This system uses polystyrene microspheres internally dyed with red and infrared fluorophores that can be individually identified. The fluorescent microspheres were coated with capturing antibodies specific for different chemokines. The chemokine-captured beads, after incubation with the sample, were then mixed with phycoerythrin-conjugated detection antibodies to form immune complexes. Following incubation, washing, and the acquisition of fluorescence data, the concentration results were generated in graphical format using the standard curve generated for each cytokine. Results were expressed in picograms per milliliter.
CRP assay
CRP was measured in plasma with a mouse high-sensitive ELISA kit according to the instruction manual (Kamiya Biomedical, Seattle, WA). This kit specifically detects mouse CRP. Results were expressed in nanograms per milliliter.
Drugs used
Acetylcholine, adenosine, and PE were dissolved in distilled water. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) and 2,4-dioxobenzo[g] pteridine (alloxazine) were dissolved in 100% DMSO as a 10 mM stock solution. The final concentration of DMSO in organ bath (10 ml) had no effect by itself on the aortic rings (1). Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals (St. Louis, MO).
Statistical analysis
The data were expressed as means ± SE. Comparisons among different groups were analyzed by ANOVA, followed by Tukey’s multiple comparison test/Bonferroni selected pair test. Comparison between two groups was assessed by an unpaired t-test. A P value of <0.05 was considered as the level of significance for all statistical tests. All the statistical analyses were performed using the GraphPad Prism statistical package.
RESULTS
Systemic inflammation in control, allergic, and adenosine-aerosolized allergic mice
Table 1 shows the multiplex cytokine profile in all the groups. Allergen challenge significantly increased plasma levels of inflammatory cytokines IL-5, IL-6, IL-13, and MCP-1 in sensitized (Sen) compared with control (Con) mice. Allergen-sensitized and challenged mice exposed to adenosine aerosol (Sen + AD) had the highest level of the proinflammatory cytokines including IL-1β, IL-5, IL-13, MCP-1, MIP-1α, and TNF-α compared with that of other groups. Other cytokines in the panel were either undetectable or not different among the groups (data not shown). In addition, the cytokine profile was also studied in the Con + AD group. Cytokine levels were not different between this group of animals aerosolized with adenosine compared with the Con group, suggesting that aerosolized adenosine in the absence of allergic inflammation had no effect on the inflammatory cytokine profile. CRP was also measured as an important systemic inflammatory marker. Plasma CRP levels significantly increased in the Sen compared with the Con group, with a further increase in the Sen + AD compared with the Sen group. Again, there was no difference between the Con and Con + AD groups in CRP levels. These data show that sensitized mice challenged with allergen have systemic inflammation and that aerosolized adenosine further enhances the inflammation in these mice. Earlier studies from our laboratory have also shown no difference in the lung inflammation of control mice aerosolized with adenosine (13, 14).
Table 1.
Systemic inflammation
| Inflammatory Marker | Con | Con + AD | Sen | Sen + AD |
|---|---|---|---|---|
| IL-1β, pg/ml | 3.46±0.21 | 3.29±0.09 | 3.2±0.06 | 24.08±10.32*† |
| IL-5, pg/ml | 3.35±0.14 | 4.19±0.67 | 8.22±1.65* | 11.12±1.50* |
| IL-6, pg/ml | 10.42±2.754 | 12.90±3.79 | 60.47±17.63* | 43.85±10.08* |
| IL-13, pg/ml | 27.44±5.57 | 33.19±7.60 | 45.41±9.91* | 170.5±49.97*† |
| MCP-1, pg/ml | 37.26±4.80 | 39.91±4.59 | 56.48±7.26* | 119.6±29.01*† |
| MIP-1α, pg/ml | 51.06±4.69 | 54.36±8.13 | 53.38±12.47 | 165.0±31.57*† |
| TNF-α, pg/ml | 6.66±1.03 | 5.52±0.360 | 6.25±0.230 | 14.22±3.60*† |
| C-reactive protein, ng/ml | 13.75±0.69 | 14.51±0.67 | 18.18±1.83* | 24.42±2.42*† |
Values are means ± SE; n = 5–8 mice. Systemic inflammation as assessed by levels of inflammatory cytokines in plasma of control (Con), adenosine-aerosolized control (Con + AD), allergic (Sen), and adenosine-aerosolized allergic (Sen + AD) mice.
P < 0.05 compared with Con;
P < 0.05 compared with Sen.
MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α.
Vascular reactivity in control, allergic, and allergic + adenosine groups
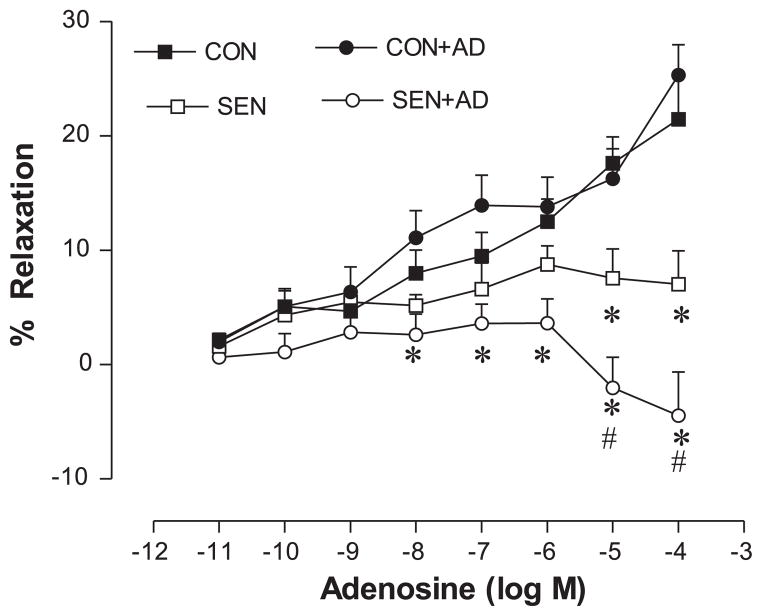
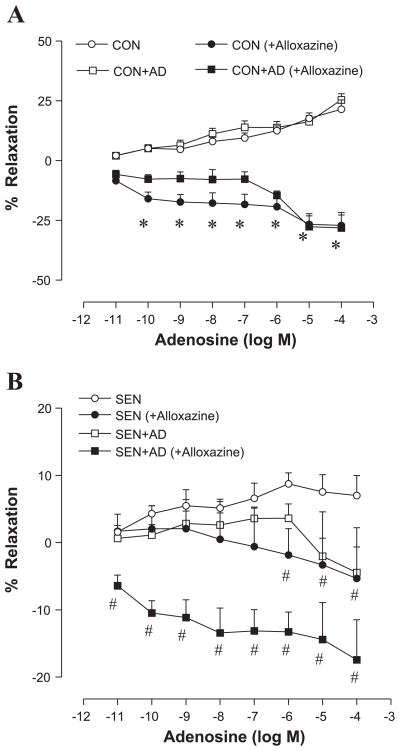
The aortic relaxation/contraction responses to adenosine were studied in each of the following four groups: Con, Con + AD, Sen, and Sen + AD. Organ bath data showed a concentration-dependent relaxation response to adenosine in the Con aorta (Fig. 1). The maximum relaxation produced by adenosine (10−4 M) was 21% and 25% in the Con and Con + AD groups, respectively. There was no difference in the vasorelaxation between the Con and Con + AD groups. Adenosine-induced aortic relaxations were impaired in the Sen aorta with a maximum relaxation of 7%. The impairment of vasorelaxation response to adenosine observed in the Sen group was further aggravated in the Sen + AD aorta. Adenosine failed to elicit any relaxation response in the Sen + AD group; however, it produced a contractile response (−5%) at higher doses. These data show that allergen sensitization and challenge altered the vascular reactivity, with adenosine aerosol further exacerbating it in allergic mice.
Fig. 1.
Concentration-response curves for adenosine-mediated relaxation/contraction in aorta of control (Con), adenosine-aerosolized control (Con + AD), allergic (Sen), and adenosine-aerosolized allergic (Sen + AD) mice. Values are expressed as means ± SE (n = 8 mice). On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with Con/Con + AD; #P < 0.05 compared with Sen.
Endothelial function and eNOS expression in control, allergic, and allergic + adenosine groups
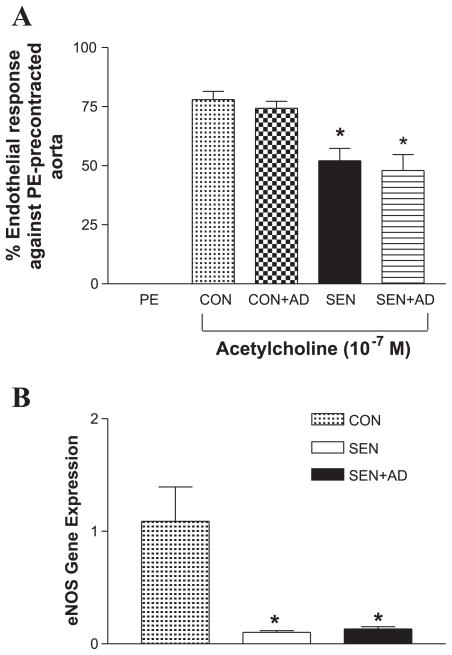
The Sen and Sen + AD groups had significantly lower (~50% relaxation) endothelial response to acetylcholine (Fig. 2A) compared with that of the respective controls (~75% relaxation in controls). Real-time PCR data also showed that the expression of eNOS in the aorta was significantly decreased in the Sen and Sen + AD groups compared with the Con group (Fig. 2B). These data show that allergen challenge probably led to dysfunctional endothelium in the Sen and Sen + AD groups.
Fig. 2.
Endothelial responses in Con, Con + AD, Sen, and Sen + AD mice. A: effect of acetylcholine (10−7 M) on phenylephrine (PE)-precontracted aorta (n = 8 mice). B: expression of endothelial nitric oxide synthase by real-time PCR (n = 4 mice). For gene expression by comparative cycle threshold (CT) method using real-time PCR, first column was made as the calibrator against which all other groups were compared. Values are expressed as means ± SE. *P < 0.05 compared with Con/Con + AD.
With the aerosolization of adenosine, it is difficult to assess the exact amount of adenosine reaching the lungs. However, the dose we have chosen is based on previous work from our laboratory (13, 14). This dose of adenosine (6 mg/ml) has no systemic effects, which has been shown by the lack of any systemic inflammation, improvement in vascular reactivity, and endothelial response in the Con + AD compared with the Con group. We also know that the half-life for adenosine is less than a minute. In fact, this was the reason why adenosine was chosen and not an adenosine analog. Most of the systemic effects of this dose would be gone within minutes. Therefore, it is unlikely that the effects of adenosine in this study are due to any hemodynamic changes in these animals.
AR gene expression in control, allergic, and allergic + adenosine groups
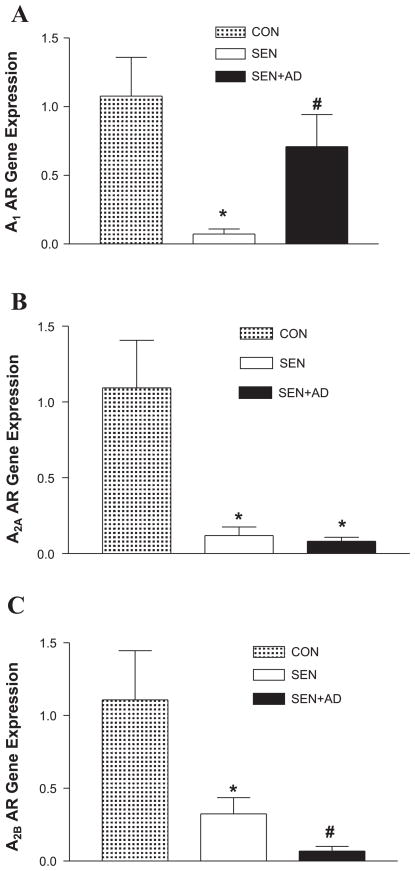
To gain further insight into the pattern of the expression of ARs and their involvement in the impairment of vasorelaxation observed in the Sen and Sen + AD groups, we first measured the aortic gene expression of all ARs before conducting organ bath experiments. Real-time PCR data showed that A1- (Fig. 3A), A2A- (Fig. 3B), and A2BAR (Fig. 3C) expression was decreased in the Sen compared with the Con group, whereas A3AR expression remained unaffected (data not shown). The expression of A1AR increased significantly in the Sen + AD compared with the Sen group, whereas A2A- and A2BARs decreased further; however, only the decrease in A2BAR expression reached statistical significance. These data show that an alteration in AR expression is responsible for the impairment of vasorelaxation observed in the Sen and Sen + AD groups.
Fig. 3.
Expression of A1 (A), A2A (B), and A2B (C) adenosine receptors (ARs) by real-time PCR in mice aorta of Con, Sen, and Sen + AD mice. For gene expression by comparative CT method using real-time PCR, first column was made as the calibrator against which all other groups were compared. Values are expressed as means ± SE (n = 4 mice). *P < 0.05 compared with Con; #P < 0.05 compared with Sen.
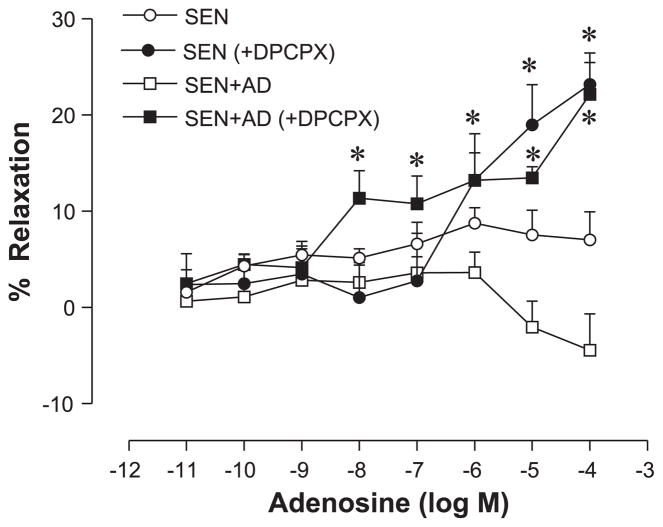
Effect of specific A1- (DPCPX) and A2BAR (alloxazine) antagonists on vascular reactivity in control, allergic, and allergic + adenosine groups
Based on real-time PCR data, we used the A1AR antagonist to reverse the impairment observed in the Sen and Sen + AD groups since A1AR activation has been shown to cause vasoconstriction in earlier studies from our laboratory (41). Treatment of the aorta with the A1AR antagonist DPCPX (10−5 M) before CRC with adenosine resulted in a reversal of impaired vasorelaxation in the Sen and Sen + AD groups (Fig. 4). DPCPX shifted the CRC to adenosine toward the left in the Sen and Sen + AD groups, with the vasorelaxation observed in these groups being similar to the Con group (Fig. 1). A concentration-dependent relaxation was seen in Con animals (Con and Con + AD), which was not affected by DPCPX except at the highest concentration of adenosine that was significant (data not shown).
Fig. 4.
Effect of a specific A1AR antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 10−5 M), on adenosine-mediated relaxation/contraction in aorta of Sen and Sen + AD mice. Values are expressed as means ± SE (n = 8 mice). On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with Sen/Sen + AD.
We expected the inhibition of vasorelaxation by the blocking of A2BAR since it is considered to be involved in the relaxation of the aorta (1, 16). The pretreatment of the aorta with the A2BAR antagonist alloxazine (10−4 M) before CRC with adenosine resulted in the inhibition of vasorelaxation observed in the Con and Con + AD aorta. Alloxazine shifted the CRC to adenosine toward the right, indicating a contraction in the Con and Con + AD aorta (Fig. 5A). Impaired vasorelaxation observed in the Sen and Sen + AD groups was also affected by prior treatment of the aorta with alloxazine (Fig. 5B), with a further rightward shift of their respective CRCs. These data suggest that A1- and A2BARs have a significant role in the impairment of vascular reactivity observed in the Sen and Sen + AD groups. DPCPX and alloxazine are selective inhibitors of A1- and A2BAR, respectively, and have been used by others and our laboratory in earlier studies (1, 35, 41).
Fig. 5.
Effect of A2BAR antagonist alloxazine (10−4 M) on adenosine-mediated relaxation/contraction in aorta of Con (A), Sen (B), and Sen + AD mice. Values are expressed as means ± SE (n = 4–8 mice). On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with Con/Con + AD; #P < 0.05 compared with Sen/Sen + AD.
DISCUSSION
The principal finding of the present study is that allergic mice had impaired vasorelaxation to adenosine and systemic inflammation, both of which were further aggravated after acute adenosine aerosol challenge in these mice. The AR expression profile was also altered in allergic mice and those aerosolized with adenosine after allergen challenge, which resulted in an impairment of vasorelaxation in these mice. Endothelium was found to be dysfunctional in both of these groups, since they had a decrease in aortic eNOS expression and endothelial relaxation to acetylcholine. The A1AR antagonist DPCPX attenuated the impairment in vasorelaxation observed in allergic mice and those subjected to adenosine aerosol after allergen challenge, whereas the A2BAR antagonist alloxazine impaired the vasorelaxation further in these groups, supporting a role for A2BAR in aortic relaxation.
Airway inflammation is associated with chronic elevations in lung adenosine levels that may be due to the release of adenosine among other inflammatory mediators from sensitized lung and activated leukocytes (11, 26, 27). This is also experimentally supported by chronically increasing lung adenosine levels by knocking out ADA, an enzyme responsible for adenosine breakdown or temporarily by the inhalation of adenosine aerosol, as in this study. Mice deficient in the ADA enzyme develop severe pulmonary inflammation, airway hyperreactivity, and airway remodeling in association with the elevated levels of adenosine in the lungs (3, 7). Adenosine inhalation to the allergic lungs has also been shown to increase further airway responsiveness and inflammatory mediators (12–14, 44), suggesting an important role for adenosine in the amplification of lung inflammation.
Systemic inflammation has also been reported earlier in humans with asthma and animal models of asthma (18, 21). Hazarika et al. (19) recently showed an increase in neutrophils and eosinophils in the bronchoalveolar lavage and blood in a murine model of asthma. Systemic inflammatory markers have also been shown to be elevated in patients with asthma and in the allergic mouse model of asthma after the adenosine aerosol challenge performed earlier by our laboratory (12, 13). We further extended these observations in the present study to investigate the cytokine profile in the systemic circulation in our murine model of asthma. We found that allergic mice exposed to acute adenosine aerosol had increased systemic inflammation since several proinflammatory cytokines levels (IL-1β, IL-5, IL-13, MCP-1, MIP-1α, and TNF-α) were found to be highest in this group. A recent report from this laboratory using this model of asthma has also shown increased activities of eosinophilic peroxidase, myeloperoxidase, and β-hexosaminidase not only in the lung but also in the systemic circulation of allergic mice exposed to adenosine aerosol (13). Adenosine aerosol in humans with asthma has also been shown to cause an increased release of neutrophil chemotactic factor in serum (12). These studies suggest that adenosine enhances the release of systemic inflammatory mediators from sensitized inflammatory cells. Our murine model of asthma, therefore, was appropriate to study the inflammatory effects of adenosine in combination with the allergen on vascular reactivity.
Asthma has been associated with an increased incidence of cardiovascular disorders (10, 20, 22, 42); however, there is little information regarding the role of asthmatic inflammation in the development of cardiovascular disease, especially in relation to the vascular effects of adenosine. In the present study, adenosine produced a concentration-dependent vasorelaxation in control mice, which was impaired in allergic mice. Vascular reactivity to adenosine was further altered in the aorta of allergic mice that had received aerosolized adenosine. These data implicated a role for ARs in the impairment of the vasorelaxation response in allergic mice and those challenged with adenosine aerosol after allergen. We have found that allergic animals subjected to theophylline inhalation before adenosine aerosol challenge have improved vasorelaxation to NECA in the aorta (unpublished data). This further supports a role for adenosine in impaired vasorelaxation.
Because the direct effects of adenosine on hemodynamic parameters (blood pressure, oxygen saturations, and heart rate) were not assessed, it is possible that there could be adenosine-mediated effects on the vasculature independent of inflammation in the allergic group (Sen + AD). The exact cause of why adenosine inhalation blunted aortic vasorelaxation to adenosine in sensitized and challenged mice, but not in control mice, is unclear. However, because the Con + AD group did not show a change in inflammatory markers and vasorelaxation to adenosine, it is likely that changes observed in the vasculature were due to allergen and adenosine in sensitized mice. Further studies are needed to completely assess the effects of adenosine on hemodynamic parameters in this model of asthma.
To gain further insight into the AR responsible for the alteration in vascular reactivity, changes in the expression of ARs in allergen and adenosine-aerosolized allergic mice were measured. Real-time PCR data showed a downregulation of A1-, A2A-, and A2BARs in allergen-sensitized and challenged mice, the net result possibly being the impaired vasorelaxation observed in this group since it is known that A2A and A2BAR are involved in vasorelaxation (1, 16). A2A- and A2BAR expression decreased further, and the A1AR expression increased in allergic mice exposed to adenosine aerosol compared with allergic mice, leading to aortic contraction in this group. This effect could be due to the combined net result of an increase in the A1AR and further decrease in the expression of A2ARs since the former is involved in vasoconstriction and the latter in vasorelaxation, as shown in earlier studies from others and our laboratory (1, 23, 41).
Real-time PCR studies of ARs prompted us to look further into the roles of A1- and A2BAR on vascular responses. The A2BAR antagonist alloxazine blocked the vasorelaxation in controls and caused a further impairment in allergic mice and those exposed to adenosine aerosol after allergen challenge, suggesting that adenosine-mediated vasorelaxation could mainly be via the A2BAR. The A1AR antagonist DPCPX reversed the impaired vasorelaxation in allergic mice and those exposed to adenosine aerosol after allergen challenge, suggesting that the A1AR has an important role in vasoconstriction, especially when the vasorelaxant A2BAR is downregulated. In an unpublished observation from our laboratory, pretreatment of the aorta with selective the A2A antagonist SCH-58261 showed a decrease in NECA (nonselective analog of adenosine)-induced aortic relaxation in control mice. However, SCH-58261 pretreatment did not have any significant effect on altered aortic relaxation to NECA observed in the Sen and Sen + AD groups, suggesting a limited role in vascular reactivity for the A2AAR in this particular experimental model. In conclusion, the data further support the involvement of the A1AR in contraction and the A2BAR in the relaxation of the mice aorta in our model of allergic asthma.
Endothelium serves as an important regulator of vascular function and homeostasis by releasing mediators such as nitric oxide and prostaglandins (28); however, its role in relation to allergen exposure and adenosine challenge has not been explored. Allergen exposure probably contributed to the impairment in endothelial responses observed in allergic and adenosine-aerosolized allergic mice since eNOS expression and vasorelaxation to acetylcholine were decreased equally in both of these groups. The exact cause of this dysfunction is not known, but it could be possibly due to an increase in systemic inflammatory cytokines since they are reported to suppress eNOS and nitric oxide production (6, 24).
Endothelial dysfunction has been reported earlier after an exposure to particulate matter in the aorta of mice (8). Nurkiewicz et al. (32) have also shown an impairment of endothelium-dependent arteriolar dilation in rat spinotrapezius muscle upon inhalation of particulate matter. Endothelial dysfunction caused by allergen exposure could be somewhat similar to that of particulate matter exposure in many ways. First, allergen particle size is in the same range as that of particulate matter. Second, particulate matter such as the allergen is also known to cause lung inflammation, which spills over in systemic circulation, leading to endothelial dysfunction and other cardiovascular complications (2, 33). It is also reported to be associated with pulmonary as well as cardiovascular disorders (36).
The fact that endothelial responses to acetylcholine and eNOS expression were almost equal in allergic mice and those exposed to adenosine aerosol after allergen challenge, whereas vasorelaxation responses to adenosine were different between these groups, could be due to two reasons. First, it could be due to the further increase in several proinflammatory cytokines found in allergic mice exposed to adenosine aerosol since some of these cytokines are reported to directly alter vascular function (43). Second, it could be due to the increased A1- and decreased A2BAR expression favoring aortic contraction in allergic mice exposed to adenosine aerosol. Further studies are required to delineate the role of each AR in the alteration of vascular responses using specific AR knockout mice.
In conclusion, our findings provide evidence for the first time that there are alterations in systemic parameters (inflammation, lower endothelial response, and impaired vasorelaxation to adenosine) as a result of allergen exposure and adenosine aerosol challenge in sensitized mice. Therefore, therapeutic strategies designed to attenuate lung inflammation should also focus on the prevention of systemic inflammation and harmful systemic side effects. This strategy may be beneficial in lowering the incidence of adverse cardiovascular events in patients with asthma.
Acknowledgments
We thank the Center for Interdisciplinary Research in Cardiovascular Sciences at West Virginia University for the use of their core research facilities and equipment (Luminex 200 system).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-027339.
References
- 1.Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol. 2007;292:H719–H725. doi: 10.1152/ajpheart.00593.2006. [DOI] [PubMed] [Google Scholar]
- 2.Bai N, Khazaei M, van Eeden SF, Laher I. The pharmacology of particulate matter air pollution-induced cardiovascular dysfunction. Pharmacol Ther. 2007;113:16–29. doi: 10.1016/j.pharmthera.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn MR, Kellems RE. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv Immunol. 2005;86:1–41. doi: 10.1016/S0065-2776(04)86001-2. [DOI] [PubMed] [Google Scholar]
- 4.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 6.Cardaropoli S, Silvagno F, Morra E, Pescarmona GP, Todros T. Infectious and inflammatory stimuli decrease endothelial nitric oxide synthase activity in vitro. J Hypertens. 2003;21:2103–2110. doi: 10.1097/00004872-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- 8.Cozzi E, Hazarika S, Stallings HW, Cascio WE, 3rd, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2006;291:H894–H903. doi: 10.1152/ajpheart.01362.2005. [DOI] [PubMed] [Google Scholar]
- 9.Cushley MJ, Tattersfield AE, Holgate ST. Adenosine-induced broncho-constriction in asthma. Antagonism by inhaled theophylline. Am Rev Respir Dis. 1984;129:380–384. doi: 10.1164/arrd.1984.129.3.380. [DOI] [PubMed] [Google Scholar]
- 10.Drislane FW, Samuels MA, Kozakewich H, Schoen FJ, Strunk RC. Myocardial contraction band lesions in patients with fatal asthma: possible neurocardiologic mechanisms. Am Rev Respir Dis. 1987;135:498–501. doi: 10.1164/arrd.1987.135.2.498. [DOI] [PubMed] [Google Scholar]
- 11.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Driver AG, Kukoly CA, Metzger WJ, Mustafa SJ. Bronchial challenge with adenosine causes the release of serum neutrophil chemotactic factor in asthma. Am Rev Respir Dis. 1991;143:1002–1007. doi: 10.1164/ajrccm/143.5_Pt_1.1002. [DOI] [PubMed] [Google Scholar]
- 13.Fan M, Jamal Mustafa S. Role of adenosine in airway inflammation in an allergic mouse model of asthma. Int Immunopharmacol. 2006;6:36–45. doi: 10.1016/j.intimp.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Fan M, Mustafa SJ. Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pulm Pharmacol. 2002;15:147–155. doi: 10.1006/pupt.2001.0329. [DOI] [PubMed] [Google Scholar]
- 15.Fan M, Qin W, Mustafa SJ. Characterization of adenosine receptor(s) involved in adenosine-induced bronchoconstriction in an allergic mouse model. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1012–L1019. doi: 10.1152/ajplung.00353.2002. [DOI] [PubMed] [Google Scholar]
- 16.Grbovic L, Radenkovic M. Analysis of adenosine vascular effect in isolated rat aorta: possible role of Na+/K+ -ATPase. Pharmacol Toxicol. 2003;92:265–271. doi: 10.1034/j.1600-0773.2003.920603.x. [DOI] [PubMed] [Google Scholar]
- 17.Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol. 2003;14:2457–2465. doi: 10.1097/01.asn.0000086474.80845.25. [DOI] [PubMed] [Google Scholar]
- 18.Hazarika S, Van Scott MR, Lust RM. Myocardial ischemia-reperfusion injury is enhanced in a model of systemic allergy and asthma. Am J Physiol Heart Circ Physiol. 2004;286:H1720–H1725. doi: 10.1152/ajpheart.01064.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hazarika S, Van Scott MR, Lust RM. Severity of myocardial injury following ischemia-reperfusion is increased in a mouse model of allergic asthma. Am J Physiol Heart Circ Physiol. 2007;292:H572–H579. doi: 10.1152/ajpheart.01361.2005. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–748. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 21.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol. 2002;89:381–385. doi: 10.1016/S1081-1206(10)62039-X. [DOI] [PubMed] [Google Scholar]
- 22.Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521–2526. doi: 10.1001/archinte.165.21.2521. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CD, Hourani SM, Long CJ, Collis MG. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol. 1994;25:1381–1387. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Roumeliotis N, Sawamura T, Renier G. C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ Res. 2004;95:877–883. doi: 10.1161/01.RES.0000147309.54227.42. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Mann JS, Holgate ST, Renwick AG, Cushley MJ. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol. 1986;61:1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- 27.Mann JS, Renwick AG, Holgate ST. Release of adenosine and its metabolites from activated human leucocytes. Clin Sci (Lond) 1986;70:461–468. doi: 10.1042/cs0700461. [DOI] [PubMed] [Google Scholar]
- 28.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- 29.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A2B adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 30.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 31.Nadeem A, Mustafa SJ. Adenosine receptor antagonists and asthma. Drug Discov Today. 2006;3:269–275. [Google Scholar]
- 32.Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oldenburg PJ, Mustafa SJ. Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther. 2005;313:319–324. doi: 10.1124/jpet.104.071720. [DOI] [PubMed] [Google Scholar]
- 35.Rose’Meyer RB, Harrison GJ, Headrick JP. Enhanced adenosine A2B mediated coronary response in reserpinised rat heart. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:266–273. doi: 10.1007/s00210-002-0678-z. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, Dybing E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25:559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd RK, Linden J, Duling BR. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res. 1996;78:627–634. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- 38.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533:77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 39.Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91:133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 40.Talukder MA, Morrison RR, Jacobson MA, Jacobson KA, Ledent C, Mustafa SJ. Targeted deletion of adenosine A3 receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol. 2002;282:H2183–H2189. doi: 10.1152/ajpheart.00964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411–H1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 42.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol. 1996;25:617–620. doi: 10.1093/ije/25.3.617. [DOI] [PubMed] [Google Scholar]
- 43.Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17:225–233. doi: 10.1016/j.cytogfr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Van den Berge M, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Provocation with adenosine 5′-monophosphate, but not methacholine, induces sputum eosinophilia. Clin Exp Allergy. 2004;34:71–76. doi: 10.1111/j.1365-2222.2004.01832.x. [DOI] [PubMed] [Google Scholar]