Abstract
Objective
Oral sodium channel blockers have shown mixed results in randomized controlled trials despite the known importance of sodium channels in generating pain. We hypothesized that differing baseline pain qualities (e.g. “stabbing” vs “dull”) might define specific subgroups responsive to intravenous (IV) lidocaine—a potent sodium channel blocker.
Design
A prospective cohort study of 71 patient with chronic pain suspected of being neuropathic were recruited between January 2003 and July 2007 and underwent lidocaine infusions at Stanford University Hospital in a single-blind nonrandomized fashion. Baseline sensory pain qualities were measured with the Short-Form McGill Pain Questionnaire (SF-MPQ). Pain intensity was measured with a visual analog scale (VAS).
Results
Factor analysis demonstrated two underlying pain quality factors among SF-MPQ sensory items: a heavy pain and a stabbing pain. Baseline heavy pain quality, but not stabbing quality predicted subsequent relief of pain intensity in response to lidocaine. In contrast, these factors did not predict divergent analgesic responses to placebo infusions. In response to each 1 mcg/mL increase in lidocaine plasma level, patients with high heavy pain quality drop their VAS 0.24 (95% CI 0.05–0.43) more points than those with low heavy pain quality (P < 0.013).
Conclusions
“Heavy” pain quality may indentify patients with enhanced lidocaine responsiveness. Pain quality may identify subgroups among patients with suspected neuropathic pain responsive to IV lidocaine. Further investigation is warranted to validate and extend these findings.
Keywords: Lidocaine, Pain, Neuropathic Pain, Pain Quality, Sodium Channel Blockade, Intravenous Lidocaine, McGill Pain Questionnaire, Factor Analysis
Introduction
Neuropathic pain (NP) afflicts 5.5 million Americans [1]. Researchers have called for studies to identify underlying heterogeneities among patients with NP and to test the ability of these factors to identify responders to sodium channel blockade [2].
Pain qualities (e.g. burning, aching, heavy) constitute a readily measurable index of heterogeneity occurring even among patients with identical diagnoses. These differing qualities may reflect differing underlying pain mechanisms and might therefore predict differing response to treatment [3].
Lidocaine is a sodium channel blocker that can produce analgesia with little to no delay in onset when given intravenously to patients with NP [4]. In this study, lidocaine was used as an agent to probe the interaction of pain quality and responsiveness to sodium channel blockade.
We conducted a prospective cohort study to assess if pain quality predicted subsequent analgesia in response to a lidocaine infusion. We hypothesized that differing baseline pain qualities in patients with suspected but not proven NP would differentially and specifically determine the pain intensity reductions in response to intravenous (IV) lidocaine.
Materials and Methods
Design and Setting
We conducted a prospective, cohort study of patients undergoing both saline-placebo infusions and IV lidocaine for suspected neuropathic pain at the Stanford Pain Management Center, a tertiary referral-based pain management center between January 2003 and July 2007. All patients received a saline-placebo infusion and then a lidocaine infusion. Patients were unaware that they were to receive a placebo infusion before receiving their lidocaine infusion. As a result patients were blind to infusion status, but research staff aware of infusion status. The protocol, including the use of deception, was approved by the Stanford University Medical Center institutional review board.
Recruitment
We examined 71 patients with suspected NP of heterogeneous origin (e.g. post-herpetic neuralgia [PHN], post surgical nerve injury, lumbar radiculopathy). This was a convenience sample, and the decision to stop at 71 patients was arbitrary rather than based on a priori power calculations. All patients were recruited from the Pain Management Clinic at Stanford University. There was only one inclusion criteria: neuropathic pain suspected by the board certified pain specialist assessing them based on the presence of allodynia, hyperalgesia, hyperpathia, hyperesthesia, and hypoesthesia. The only exclusion criteria was the presence of co-morbid cardiac disease. All patients provided written informed consent.
Data Collection and Independent Variables
Baseline sensory pain quality measurements were collected immediately before the placebo and lidocaine infusions with the use of the Short-Form McGill Pain Questionnaire (SF-MPQ) [5]. We were specifically interested in the sensory qualities of pain, of which the SF-MPQ measures 11 distinct sensory pain qualities items: throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender, and splitting. Pain intensity was measured using the visual analog scale (VAS) of present pain intensity.
Lidocaine and Placebo Infusions
The cohort first received a placebo-saline infusion while situated identically to how they would receive their lidocaine infusion, with infusions differing only by the substance infused. Patients completed a VAS of pain intensity immediately before and immediately after the placebo infusion. Lidocaine infusions were accomplished immediately following placebo infusion. Lidocaine was infused in a step-wise, computer-controlled paradigm to a targeted plasma level of 5 microgram/mL using a methodology previously developed in our institution [6]. Before (at 0 microgram/mL), at 3 microgram/mL of lidocaine, and at 5 microgram/mL of lidocaine patients completed a VAS of pain intensity. Plasma levels were held at 3 and 5 mcg/mL of lidocaine just long enough to complete the VAS. Computer controlled infusion was accomplished using 2% lidocaine injected by a Harvard 200 infusion pump (Harvard Apparatus; Holliston, MA) driven by STANPUMP software programmed with patient's weight, height, age, and gender. STANPUMP software is openly available at http://www.opentci.org/doku.php?id=code:code. Infusions took approximately one hour. Exact times of infusion steps and amounts infused differed slightly between patients based on the factors used by STANPUMP (weight, height, gender, age) to deliver targeted plasma concentrations. Blood pressure and pulse oximetry, but not EKG, were monitored continuously throughout the infusions. Adverse side effects were collected passively if patients discontinued infusions early, but were otherwise not actively sought.
Statistical Analysis
We used exploratory factor analysis to uncover the number of independent underlying pain qualities the SF-MPQ items were actually measuring, and then used generalized estimating equations to examine whether these baseline qualities determined subsequent change in VAS of pain intensity over time in response to either placebo or lidocaine infusions.
Exploratory factor analysis (with orthogonal rotation) of baseline SF-MPQ sensory items was accomplished using SAS 9.1.3 (Cary, NC) proc factor. A scree plot was used to indicate the number of underlying factors that explained the correlation structure among the SF-MPQ sensory items. We then used the maximum likelihood method to test against the null hypothesis that the factors were sufficient to explain the correlation structure among the McGill pain items. Because the top three items in each factor were independent but the fourth (“throbbing”) was common to both factors, pain quality summary scores were computed by adding only the scores of the top three factor loading SF-MPQ items. This was done because we wished to specifically investigate pain qualities that differentially predicted response to lidocaine.
Longitudinal data analysis was done using generalized estimating equations in SAS 9.1.3 proc genmod using an unstructured correlation structure. We modeled VAS of pain intensity as a function of the pain quality summary scores derived from the factor analysis, lidocaine dose, and the dose*pain quality summary score interaction term. Each of the three measurements of VAS during lidocaine was treated as an independent measurement occurring at a different dose and time. The dose*pain quality summary score was the primary endpoint, as it indicated whether the change in VAS over lidocaine infused differed based on pain quality summary score. For patients who discontinued the infusion, missing data was not imputed, analysis used all available pairs of existing data. Associations were considered statistically significant when P < 0.05. Results were corrected for multiple comparisons using the method of Bonferroni.
Results
Patient Characteristics
We enrolled 71 patients. Patient characteristics are listed in Table 1. All patients provided baseline SF-MPQ data.
Table 1.
Patient characteristics (N = 71)
| Characteristic | Mean | Range |
|---|---|---|
| Age | 47 | 17–82 |
| Gender (% male) | 39 | |
| Baseline pain score | 6.2 | 3–10 |
| Pain duration (years) | 5.2 | 0.3–33 |
| Presumptive diagnosis prior to enrollment | Number | |
| Traumatic peripheral nerve injury | 26 | |
| Back pain with associated radiculopathy: | ||
| Lumbar | 18 | |
| Thoracic | 1 | |
| Cervical | 3 | |
| Complex regional pain syndrome | 4 | |
| Central pain | 4 | |
| Post herpetic neuralgia | 4 | |
| Atypical face pain | 3 | |
| Pelvic pain | 3 | |
| Fibromyalgia | 2 | |
| Other | 3 |
Pain scores are visual analog scores of pain intensity.
One patient discontinued the infusion during the placebo infusion and provided no further VAS information. Two patients discontinued the infusion during the lidocaine infusion between 0 and 3 mcg/mL and six patients discontinued the infusion between 3 and 5 mcg/mL. All discontinuations were due to transient central nervous system (CNS) side effects including dizziness, somnolence, agitation, and (in one case) tearfulness. Side effects were mild and transient, and none required treatment. There were no serious adverse events. Patients who discontinued the infusion did not differ significantly in any baseline variable from those who did not.
Factor Analysis
Factor analysis demonstrated that two factors were sufficient to explain the underlying correlation structure among SF-MPQ sensory items. Null hypothesis test of no common factors was rejected (P < 0.0001); testing against the null hypothesis that more than two factors was needed was not significant (P > 0.33). Factor one is characterized by high factor loading on stabbing, sharp, and shooting sensory items. Factor two is characterized by high factor loading on heavy, gnawing, and aching sensory items (Table 2). “Throbbing” and “splitting” qualities appeared to load equally on both factors. Therefore, only the top three factor items were used to calculate summary scores for each factor to test their discriminative abilities to predict subsequent response to IV lidocaine. For clarity, factor one is hereafter referred to as “stabbing pain quality” and factor two is hereafter referred to as “heavy pain quality.”
Table 2.
Rotated factor loading matrix for the short form McGill pain questionnaire sensory pain quality items
| Factor |
||
|---|---|---|
| Pain Quality Item | I | II |
| Stabbing pain quality factor | ||
| Stabbing | 0.91 | 0.03 |
| Sharp | 0.72 | − 0.10 |
| Shooting | 0.69 | 0.24 |
| Throbbing | 0.53 | 0.45 |
| Splitting | 0.40 | 0.44 |
| Tender | 0.38 | 0.16 |
| Heavy | 0.20 | 0.70 |
| Cramping | 0.20 | 0.33 |
| Aching | 0.13 | 0.62 |
| Gnawing | 0.10 | 0.68 |
| Hot-burning | −0.13 | 0.31 |
| Heavy pain quality factor | ||
| Heavy | 0.20 | 0.70 |
| Gnawing | 0.10 | 0.68 |
| Aching | 0.13 | 0.62 |
| Throbbing | 0.53 | 0.45 |
| Splitting | 0.40 | 0.44 |
| Cramping | 0.20 | 0.33 |
| Hot-Burning | −0.13 | 0.31 |
| Shooting | 0.69 | 0.24 |
| Tender | 0.38 | 0.16 |
| Stabbing | 0.91 | 0.03 |
| Sharp | 0.72 | −0.10 |
Items loading 0.60 and higher are bolded, and we summed to compute factor summary scores for ability to predict lidocaine-induced analgesia.
Pain Quality Prediction of Lidocaine Analgesia
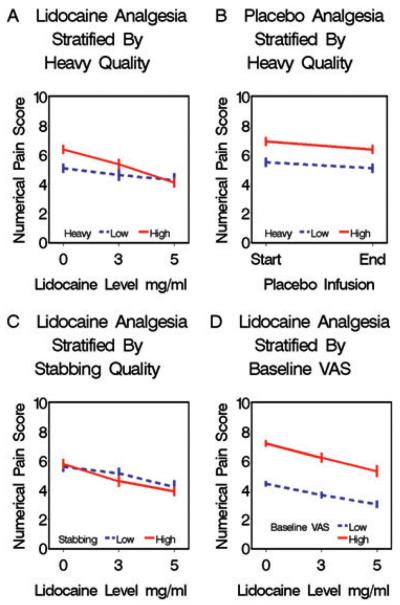
Pain reduction in response to lidocaine is specifically determined by baseline levels of heavy pain quality. Patients with high baseline heavy pain quality experience greater reductions in pain intensity (VAS) during lidocaine infusion than patients with low baseline ratings of heavy pain quality. For each 5 mcg/mL increase in lidocaine plasma level, the high heavy pain quality group drop their VAS 1.2 (95% CI 0.25–2.15 P < 0.013) more points than the low heavy quality group (Figure 1A). Furthermore, the larger reduction in VAS seen among those patients with high heavy pain quality is a lidocaine specific phenomena and did not occur during placebo infusion making regression to the mean an unlikely explanation (Figure 1B). Unlike patients with high baseline heavy pain quality, patients with high baseline stabbing pain quality do not experience greater reductions in pain intensity (VAS) during lidocaine infusion than patients with low baseline ratings of stabbing pain quality (Figure 1C). Thus, heavy pain quality, but not high levels of other pain qualities, identifies patients more likely to have relief with IV lidocaine. This finding is specific to infusion of lidocaine and does not occur with placebo. To further exclude the possibility that the increased reduction in pain intensity (VAS) among those with high baseline heavy quality was a reflection of regression to the mean, we stratified patients according to baseline pain intensity (VAS) ratings (Figure 1D). Unlike patients with high baseline heavy pain quality, patients with higher baseline pain intensity ratings (VAS) do not experience greater reductions in pain intensity during lidocaine infusion than patients with lower baseline ratings of pain intensity.
Figure 1.
Pain reduction in response to lidocaine but not placebo is specifically determined by baseline levels of Heavy pain quality. Each figure shows the same 71 patients stratified differently according to their baseline levels of heavy pain quality (parts A and B), stabbing pain quality (part C), or VAS (part D) at their respective median values. Dose*pain quality summary score interaction terms from generalized estimating equations model and 95% confidence intervals reported. (A) Patients stratified by baseline heavy pain quality ratings during lidocaine infusion. Patients with high baseline heavy pain quality experience greater reductions in pain intensity (VAS) during lidocaine infusion than patients with low baseline heavy pain quality ratings (Slopes differ: interaction term = −0.2428 95% CI [−0.4334 to −0.0521] P < 0.013). (B) Patients stratified by baseline heavy pain quality ratings during placebo infusions. Unlike during lidocaine infusions, patients with high baseline heavy pain quality do not experience greater reductions in pain intensity (VAS) during placebo infusion than patients with low baseline ratings of heavy quality (Slopes do not differ: interaction term = 0.0781 95% CI [−0.3717–0.2154] P < 0.60). (C) Patients stratified by baseline stabbing pain quality ratings during lidocaine infusions. Unlike patients with high baseline heavy pain quality, patients with high baseline stabbing pain quality do not experience greater reductions in pain intensity (VAS) during lidocaine infusion than patients with low baseline ratings of stabbing pain quality (Slopes do not differ: interaction term = −0.0649 95% CI [−0.2635–0.1337] P < 0.52). (D) Patients stratified according to pain intensity (VAS) ratings. Patients with higher baseline pain intensity are not generally more responsive to lidocaine than patients with lower baseline ratings of pain intensity (Slopes do not differ: interaction term = −0.1001 95% CI [−0.2935 to 0.0933] P < 0.31). VAS = visual analog scale.
Discussion
Among patients with suspected neuropathic pain of diverse etiology, factor analysis of the eleven baseline SF-MPQ sensory items reveals two underlying pain domains: A heavy pain quality and a stabbing pain quality. We identified a specific and differential analgesic response to lidocaine based on these pain qualities. Compared with patients with low levels of heavy pain quality, patients with high levels of heavy pain quality had significantly greater decreases in VAS pain intensity scores in response to IV lidocaine but not in response to placebo. Furthermore, this did not occur in patients with higher levels of stabbing pain quality, nor did it occur in those people who simply had greater pain intensity at baseline. In short, this enhanced response to IV lidocaine is both specific to lidocaine and specific to the pain quality described by heavy-gnawing-aching items on the SF-MPQ. To our knowledge this study represents the first time that pain quality has been used in humans to predict response to a specific analgesic intervention.
The factor analysis described here was exploratory and subject to false positive results despite attempts to correct for type II error, and multiple comparisons. Validation of our results with specific hypothesis testing research is required. Existing datasets may readily provide this validation. SF-MPQ data has been collected during clinical trials of numerous agents tested for efficacy in neuropathic pain. Factor analysis of the SF-MPQ sensory items from these data sets can be used to confirm or refute the factor analysis presented here. Such validation could have a significant impact on the development of existing and future medications as treatments for neuropathic pain by enriching the study group for those most likely to be responders. We propose that these results support further investigations of oral sodium channel blockers with an analogous approach seeking to identify underlying heterogeneity that may be marked by differing pain qualities.
This study has several significant methodological limitations that make this study more appropriate for spurring further research rather than drawing any specific scientific conclusions. Infusions were administered in a fixed order to eliminate the potential problem of inadequate washout of lidocaine effects during placebo infusions that would have been encountered if a lidocaine infusion were done first. The unbalanced design we pursued prevented double blinding, and creates the possibility of bias being introduced based on knowledge of the order of treatment. Including randomization in the order of infusion and having a generous washout period (days) would have markedly strengthened the study and should be considered for future investigations in this area aimed at reaching more definite conclusions. Inadequate time for full washout of the placebo effect is possible. Additionally, it should be recognized that pain qualities not included in the SF-MPQ might enhance and strengthen the factor structure and its ability to predict divergent response to differing therapies. Future work would be strengthened by measurement of pain characteristics by instruments whose goal is to specifically identify symptoms generally thought of as being neuropathic in nature such as the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) [7]. This might help validate the presence of neuropathic pain especially in a heterogeneous group such as the one studied here. In addition to addressing the above mentioned problems a more rigorous design should include active capture of side-effects. Finally, the study population contained several participants with provisional diagnoses not thought of as being associated with neuropathic pain (e.g. fibromyalgia) who were referred for lidocaine infusions because the pain specialist assessing them in our clinic noted symptoms consistent with neuropathic pain (e.g. allodynia). Therefore, this study is most applicable to the kind of patients who were included, specifically patients suspected on clinical grounds of having neuropathic pain based on allodynia, hyperalgesia, hyperpathia, hyperesthesia, and hypoesthesia. While this inclusive approach may be the most relevant issue to clinicians making inference about patients in their own practices whom they suspect on clinical grounds of having neuropathic pain, it should be recognized that results might be different in a homogeneous group of PHN or diabetic neuropathy patients.
In conclusion, our results suggest that among a group of patients with clinical suspicion of neuropathic pain of diverse etiology, there is underlying heterogeneity that segregates with measurements of pain quality, and that this heterogeneity is important because it identifies patients with enhanced treatment responsiveness. Further investigation is warranted to validate and extend these findings including to see if these changes persist with prolonged treatment and occur with oral sodium channel blockers.
Acknowledgments
Dr. Carroll was supported by a Foundation for Anesthesia Education and Research Mentored Research Training Grant. Dr. Carroll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was done in the department of Anesthesiology at Stanford University. This work was supported by a mentored research training grant from the Foundation for Anesthesia Education and Research to Dr. Carroll. These results were presented as a poster at the 2008 American Pain Society meeting in Tampa Bay, FL.
Footnotes
The authors report no conflicts of interest.
References
- 1.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65:S66–73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 2.Sindrup SH, Jensen TS. Are sodium channel blockers useless in peripheral neuropathic pain? Pain. 2007;128:6–7. doi: 10.1016/j.pain.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MP. Using pain quality assessment measures for selecting analgesic agents. Clin J Pain. 2006;22:S9–13. doi: 10.1097/01.ajp.0000193829.45571.4f. [DOI] [PubMed] [Google Scholar]
- 4.Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87:7–17. doi: 10.1016/S0304-3959(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 5.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 6.Schnider TW, Gaeta R, Brose W, et al. Derivation and cross-validation of pharmacokinetic parameters for computer-controlled infusion of lidocaine in pain therapy. Anesthesiology. 1996;84:1043–50. doi: 10.1097/00000542-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–57. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]