Abstract
The aim of this study was to evaluate the effects of cocaine on γ-aminobutyric acid (GABA) and dopamine (DA) neurons in the ventral tegmental area (VTA). Utilizing single-unit recordings in vivo, microelectrophoretic administration of DA enhanced the firing rate of VTA GABA neurons via D2/D3 DA receptor activation. Lower doses of intravenous cocaine (0.25–0.5 mg/kg), or the DA transporter (DAT) blocker methamphetamine, enhanced VTA GABA neuron firing rate via D2/D3 receptor activation. Higher doses of cocaine (1.0–2.0 mg/kg) inhibited their firing rate, which was not sensitive to the D2/D3 antagonist eticlopride. The voltage-sensitive sodium channel (VSSC) blocker lidocaine inhibited the firing rate of VTA GABA neurons at all doses tested (0.25–2.0 mg/kg). Cocaine or lidocaine reduced VTA GABA neuron spike discharges induced by stimulation of the internal capsule (ICPSDs) at dose levels 0.25–2 mg/kg (IC50 1.2 mg/kg). There was no effect of DA or methamphetamine on ICPSDs, or of DA antagonists on cocaine inhibition of ICPSDs. In VTA GABA neurons in vitro, cocaine reduced (IC50 13 μm) current-evoked spikes and TTX-sensitive sodium currents in a use-dependent manner. In VTA DA neurons, cocaine reduced IPSCs (IC50 13 μm), increased IPSC paired-pulse facilitation and decreased spontaneous IPSC frequency, without affecting miniature IPSC frequency or amplitude. These findings suggest that cocaine acts on GABA neurons to reduce activity-dependent GABA release on DA neurons in the VTA, and that cocaine's use-dependent blockade of VTA GABA neuron VSSCs may synergize with its DAT inhibiting properties to enhance mesolimbic DA transmission implicated in cocaine reinforcement.
Keywords: cocaine, dopamine, GABA, sodium current, ventral tegmental area
Introduction
The mesocorticolimbic dopamine (DA) system originating in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAcc) has been implicated in motivated behaviors, various types of reward and the habit-forming actions of addictive drugs including cocaine (for review see Wise, 2004). The prevailing view is that cocaine's locomotor and reinforcing properties (Roberts et al., 1980; Koob et al., 1994) are mediated primarily by enhancement of extracellular DA release (Hurd et al., 1989; Pettit & Justice, 1989, 1991; Wise et al., 1995; Hemby et al., 1997) via inhibition of the DA transporter (DAT; Kuhar et al., 1991; Kuhar, 1992; Woolverton & Johnson, 1992). Cocaine-induced cellular and molecular reshaping of this system may contribute to learned reinforcement (for review see Jones & Bonci, 2005). The potency of psychostimulants as positive reinforcers being correlated to their DAT-binding affinity (Ritz et al., 1987; Bergman et al., 1989; Wilcox et al., 1999), and cocaine's high affinity for the DAT [IC50 = 0.3–0.8 μm; (Rothman et al., 2001)], support this view.
Other high-affinity targets for cocaine include voltage-sensitive sodium channels (VSSCs; Gawin & Ellinwood, 1988). It is well established that local anesthetics, including cocaine, are use-dependent blockers of VSSCs (Strichartz, 1976; Bean et al., 1983; Postma & Catterall, 1984; O'Leary & Chahine, 2002). Although cocaine's affinity for VSSCs is lower than that for monoamine transporters (IC50 = 14–17 μm; Ritz et al., 1987; Gifford & Johnson, 1992), peak brain (2–6 min) cocaine levels of 2, 6, 9 and 26 μm can be obtained from single intravenous reinforcing doses of 0.1, 0.25, 0.5 and 1 mg/kg, respectively (Fowler et al., 1998). Indeed, much higher levels of cocaine would be obtained by self-administration, given that response intervals at these doses are typically shorter than the elimination kinetics of cocaine (Pettit et al., 1990; Pan et al., 1991). The studies demonstrating acute and chronic cocaine-induced synaptic plasticity in rodent VTA DA neurons utilize a 15 mg/kg intraperitoneal dose (Ungless et al., 2001; Liu et al., 2005), corresponding to peak brain concentrations of at least 15 and 25 μm, respectively (Pan et al., 1991). Recently, it has been hypothesized that the reinforcing properties of cocaine might involve combined or opposing effects at both the DAT and VSSCs (Kiyatkin & Leon Brown, 2006).
As repeated high-dose cocaine exposure induces LTP in VTA DA neurons via a reduction in γ-aminobutyric acid (GABA)-mediated inhibition, a possible role for VTA GABA neurons in cocaine-induced plasticity has emerged (Liu et al., 2005). We have identified a homogeneous population of GABA neurons in the VTA which may serve to inhibit DA neurons (Steffensen et al., 1998), and whose firing rate and afferent-evoked responses are enhanced by DA (Stobbs et al., 2004; Lassen et al., 2007). We hypothesized that cocaine would enhance GABA neuron firing rate and evoked discharges at low reinforcing doses due to its DAT inhibiting properties, but at higher reinforcing doses its use-dependent VSSC blocking effect would inhibit VTA GABA neurons, leading to DA neuron disinhibition.
Materials and methods
Animal subjects
Rats were housed two to a cage from the time of weaning [postnatal day(P)25], with ad libitum access to food and water. The room temperature was controlled (22–25°C) and maintained on a reverse 12-h light–dark cycle (lights off at 08.00 and on at 20.00 h). Animal care, maintenance and experimental procedures were in accordance with the Brigham Young University Animal Research Committee and met or exceeded National Institutes of Health guidelines for the care and use of laboratory animals.
Single-unit recordings in anesthetized rats
Extracellular potentials in isoflurane (1%)-anesthetized adult 250–400 g male Wistar rats (Charles River Laboratory, Hollister, CA, USA) were recorded with a single 3.0 m NaCl-filled micropipette (1–3 MΩ; 1–2 μm inside diameter), cemented 10–20 μm distal to a four-barrel micropipette (20–60 MΩ resistance), and amplified and filtered with a MultiClamp 700A programmable amplifier (Axon Instruments, Union City, CA, USA). Microelectrode assemblies were oriented into the VTA [from bregma: 5.6–6.5 posterior, 0.5–1.0 lateral and 7.0–8.5 ventral (V)] with a piezoelectric inchworm microdrive (Burleigh, Fishers, NY, USA). Single-unit activity was filtered at 0.3–10 kHz (−3dB) and displayed on Tektronix 2200 digital oscilloscopes. Square-wave constant-current pulses (50–1000 μA, 0.15 ms duration, average frequency 0.1 Hz) were generated by an IsoFlex constant-current isolation unit controlled by a Master-8 Pulse Generator (AMPI, Israel), or by computer. The internal capsule (IC; from bregma: −1.5 AP, 2.5–3.0 ML, 5.0–6.5 V) was stimulated with insulated bipolar stainless steel electrodes. Extracellularly recorded action potentials (minimum 5 : 1 signal-to-noise ratio) were discriminated with WPI-121 (Sarasota, FL, USA) spike analyzers and converted to computer-level pulses.
Characterization of VTA GABA neurons in vivo
All neurons classified as VTA GABA neurons in vivo were located in the VTA, met the criteria established in previous studies for spike waveform characteristics and response to IC stimulation (Steffensen et al., 1998; Stobbs et al., 2004; Allison et al., 2006), and often were activated and spike-coupled by microelectrophoretic DA (Stobbs et al., 2004). Presumed VTA GABA neurons were characterized by short-duration (< 200 μs; measured at half-peak amplitude of the spike), initially negative-going, nonbursting spikes, and were identified by the following IC stimulation criteria (Steffensen et al., 1998): short latency (i.e. 2–5 ms) antidromic or orthodromic activation via single stimulation of the IC, and multiple spiking following high-frequency (10 pulses, 200 Hz) stimulation of the IC [internal-capsule evoked poststimulus spike discharges (ICPSDs); Steffensen et al., 1998; Stobbs et al., 2004; Allison et al., 2006; Lassen et al., 2007]. In all studies, stimulation was performed at a level that produced 50% maximum VTA GABA neuron ICPSDs. This was accomplished by determining the current needed to produce the maximum number of ICPSDs at 200 Hz and 10 pulses, and then adjusting the stimulus intensity until 50% ICPSDs were achieved.
Single-unit recordings in vivo
Single-unit potentials, discriminated spikes and stimulation events in vivo were captured by National Instrument's NB-MIO-16 digital I/O and counter/timer data acquisition boards (Austin, TX, USA) and processed by customized National Instruments LabVIEW software in Macintosh-type computers. Potentials were digitized at 20 kHz and 12-bit voltage resolution. For single-unit activity, all spikes were captured by computer and time-stamped. Spontaneous firing rates were determined on- and off-line by calculating the number of events over a 5-min epoch, typically 5 min before and at specific intervals after drug injection. Peristimulus and interval–spike histograms were generated off-line using IGOR Pro (WaveMetrics, Lake Oswego, OR, USA) analysis of the time-stamped data. The duration (ms) and extent (number of events per bin) of poststimulus permutation of ICPSDs was determined by rectangular integration at specific time points on the peristimulus spike histogram using IGOR Pro analysis software. The minimum bin width for peristimulus spike histograms was 1.0 ms and the number of bins was 1000. These parameters allow for detection of all phases of pre- and poststimulus spike activity.
Drug preparation and administration in vivo
Cocaine hydrochloride, cocaine methiodide, DA, lidocaine hydrochloride and methamphetamine hydrochloride were dissolved in 0.9% saline and administered intravenously through an indwelling jugular catheter. Given the transient duration of effects of cocaine and lidocaine on VTA GABA neuron firing rate and ICPSDs, dose–response studies were performed for these two drugs, as well as for cocaine methiodide, in the same rats with a 40-min interval between successive doses and by randomizing the sequence of dose levels, while dose–response studies for amphetamine, whose effects on firing rate were more prolonged, were performed in separate rats. For in situ microelectrophoretic application of drugs in the VTA, DA (10 mm), eticlopride hydrochloride (1.0 mm), SCH23390 hydrochloride (1.0 mm), SKF38393 hydrochloride (1.0 mm) and quinpirole hydrochloride (1 mm) were dissolved in distilled water and iontophoresed by current injection (25–100 nA) which was regulated by Medical Systems BH-2 iontophoretic pump and balance unit modules. For systemic drug studies on VTA GABA neuron responses, all drugs including cocaine were administered intravenously through a jugular catheter. For pharmacology studies on the effects of cocaine on VTA GABA neuron responses, drugs or saline were administered 10 min before cocaine. All drugs except cocaine were obtained from Sigma Chemical (St Louis, MO, USA). Cocaine was a gift from NIDA.
Preparation of brain slices
Wistar rats (P21–45) were anesthetized with ketamine (60 mg/kg) and decapitated. The brains were quickly dissected and sectioned in ice-cold artificial cerebrospinal fluid (ACSF), bubbled with 95% O2 and 5% CO2. This cutting solution consisted of (in mm): sucrose, 220; KCl, 3; NaH2PO4, 1.25; NaH2CO3, 25; MgSO4, 12; glucose, 10; CaCl2, 0.2; and ketamine, 0.4. VTA-targeted horizontal slices (∼200 μm thick) were immediately placed into an incubation chamber containing normal ACSF at 34–35°C, bubbled with 95% O2 and 5% CO2 at 36° consisting of (in mm): NaCl, 124; KCl, 3; NaH2PO4, 1.25; NaHCO3, 26; glucose, 12; MgSO4, 1.5; and CaCl2, 2 (pH 7.3), and allowed to incubate for at least 45 min prior to being transferred to a recording chamber. Once transferred to a recording chamber with continuous normal ACSF flow (2.0 mL/min) maintained at 34–35°C throughout the experiment, the slices where then allowed to settle for an additional 15–30 min before recordings began. These incubation and settling periods allowed cells to recover and stabilize while ketamine was washed out of the tissue. Cells were visualized with either a Nikon Eclipse FN1 or E600FN microscope in the transmitted de Sénarmont differential interference contrast/infrared configuration.
Whole-cell recordings in vitro
Electrodes pulled from borosilicate glass capillary tubes were filled with one of two types of pipette solutions. For inhibitory postsynaptic current (IPSCs), the pipette solution consisted of (in mm): KCl, 128; NaCl, 20; CaCl2, 0.3; MgCl2, 1.2; HEPES, 10; EGTA, 1; Mg-ATP, 2; Na-GTP, 0.25; and QX314, 4.5 (pH 7.3). For voltage waveform and current-evoked spiking experiments the pipette solution consisted of (in mm): K-gluconate, 115; NaCl, 9; KCl, 25; HEPES, 10; EGTA, 0.2; MgCl2, 1.2; Na-ATP, 3; and Na-GTP, 1, and had resistances of 2–4 MΩ. Series resistance (Ra) was typically 10–20 MΩ and input resistance (Rm) typically 300–400 MΩ, were continuously monitored with a 10-mV voltage step delivered at 0.1 Hz throughout each experiment and only experiments that maintained stable Ra and Rm (< 15% change) were included in this study. IPSCs were filtered at 2 kHz while voltage waveform-generated currents and current-driven spikes were filtered at 6 kHz using an Axon Instruments Multiclamp 700A or 700B amplifier and digitized at 5–20 kHz, respectively, using an Axon 1440A digitizer, and collected and analyzed using pClamp10 and Igor Pro (Wavemetrics) software packages. Evoked and spontaneous(s) IPSCs were recorded in the presence of 100 μm D-L 2-amino-5-phosphonopentanoic acid (APV), 30 μm 6-cyano-23-dihydroxy-7-nitro-quinoxaline (CNQX) and 100 nm eticlopride to block N-methyl-d-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and DA D2-mediated synaptic currents (Bonci & Williams, 1997), respectively. Miniature IPSCs (mIPSCs) were isolated from all other spontaneous IPSCs by addition of 0.5 μm tetrodotoxin (TTX). To evoke IPSCs, cells were stimulated at 0.1 Hz with a stainless steel–platinum/iridium concentric bipolar stimulating electrode placed ∼100 μm rostral to the recording electrode. Evoked IPSCs were inward at the holding potential of −70 mV and were completely blocked by picrotoxin (100 μm). Evoked IPSC amplitudes were calculated by taking the difference between the 1.0-ms window around the peak and the 5.0-ms baseline window immediately preceding the stimulation artifact. Spontaneous IPSC activity amplitude and frequency were calculated similarly for both sIPSCs and mIPSCs; the average amplitude or frequency during a 2-min period 8–10 min following drug were normalized to the average amplitude or frequency from a 2-min window prior to drug.
Characterization of neuron types in vitro
Traditionally, neurons have been classified as either primary (DAergic) or secondary (GABAergic) based on electrophysiological and pharmacological properties (Grace & Onn, 1989; Johnson & North, 1992; Cameron et al., 1997; Ungless et al., 2004). Despite extensive research, no single best electrophysiological characteristic has been identified to conclusively distinguish VTA DA neurons from other neurons in the VTA. The most widely accepted electrophysiological method to distinguish DA neurons from non-DA neurons has been the presence of a noncation specific inward rectifying current (Ih). However, not all Ih(+) neurons stain positive for tyrosine hydroxylase (TH), a molecular marker specific to DA neurons, while Ih(-) cells appear to stain negative for TH (Johnson & North, 1992; Allison et al., 2006; Margolis et al., 2006). The combination of several electrophysiological characteristics, depending on experiment type, were used to distinguish putative DA from putative non-DA neurons in this study. Specifically, neurons that exhibited a modest noncation specific inward rectifying current (Ih) in combination with spike accommodation, low input resistance, higher spike threshold and shorter spike duration were assumed to be DA neurons. Neurons that exhibited a complete lack of Ih in combination with high input resistance, lack of spike accommodation and low spike threshold were assumed to be nondopminergic (or putative GABA neurons; Johnson & North, 1992; Allison et al., 2006; Margolis et al., 2006). A recent anatomical study has demonstrated the presence of glutamatergic neurons in the VTA and surrounding areas (Yamaguchi et al., 2007). However, this subpopulation of VTA cells remains uncharacterized and the highest concentration of vesicular transport-2 mRNA is in the rostral medial aspects of the VTA, with the lowest density in the parabrachial pigmental area (PBP). Most of the in vitro recordings in this study were conducted in the PBP in order to maintain uniformity with previous work. To provide additional assurance that the characterization criteria described above effectively discriminates VTA DA neurons from non-DA neurons (putative GABA neurons), we performed a separate set of experiments to link these electrophysiological criteria to the presence or absence of TH mRNA, a marker for DA neurons. This was accomplished using quantitative real-time PCR.
Single-cell quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Following electrophysiological characterization, putative VTA GABA neurons and putative DA neurons in mature rats were aspirated under visual observation by application of suction attached to the recording pipette, and were immediately added to an RT reaction mixture. The iScript cDNA synthesis kit (Bio-Rad) was used for a total volume of 10 μL per reaction. Reactions were run at 25°C for 10 min, 42°C for 60 min and 95°C for 5 min in a PTC-200 thermal cycler (MJ Research Inc., Watertown, MA, USA). Reactions were then stored at −20°C until running the PCR. A pre-amplification round of multiplex PCR was performed by adding iTAQ Supermix with ROX (Bio-Rad) and a cocktail of primers to the completed RT reaction, for a final volume of 50 μL. The reactions were held at 94°C for 30 s then cycled 20 times. Each cycle consisted of: 92°C for 15 s, 60°C for 20 s and 72°C for 30 s. One-microlitre samples of the initial multiplex PCR were then used as substrate for each reaction in the subsequent real-time quantitative PCR. Real-time quantitative PCR using gene specific primers with FAM-TAMRA TaqMan® probes (Applied Biosystems; TH plus primer: CTTCCAGTACAAGCACGGTGAA, TH minus primer: AGCGTGACATATA-CCTCCTTCCA, and TH probe: CCC-CATGTGGAATACACAGCGGAAGAG; D2 plus primer: CGCAG AAAGCTCTCCCAGCAGA, D2 minus primer: GACTGGTGGGA TGTTGCAATCACA and D2 probe: CCATTGTTCTCGGTGTGT TCA; 18s plus primer: GTGCATGGCCGTTCTTAGTTG, minus 18s primer: GCCACTTGTCCCTCTAAGAAGTTG, and 18s probe: TGGAGCGATTTGTCTGGTTAATTCCGATAAC) were performed using the iTaq Supermix with ROX (Bio-Rad) with an iCycler IQ (Bio-Rad) real-time PCR System. Samples were amplified in triplicate, together with a negative control for each subunit (an ACSF-only aspiration taken from the brain slice recording chamber when the cells were aspirated). The amplification protocol was 50°C for 2 min, 95°C for 5 min, then 50 cycles of 95°C for 15 s, 60°C for 20 s and 72°C for 30 s. Cycle threshold (Ct) values were calculated automatically by the iCycler IQ software, with threshold values set between 5 and 20. Normalized expression was calculated using the 2−ΔΔCT method as described in (Livak & Schmittgen, 2001).
Voltage waveform command
In order to mimic in VTA GABA neurons recorded in vitro the frequency and underlying ionic currents linked to high frequency spontaneous spiking and current-evoked postsynaptic discharges elicited from VTA GABA neurons recorded in vivo a ‘typical’ train of high frequency current-evoked spikes was used as a ‘voltage waveform command.’ The voltage waveform command was derived from a recording of current-evoked spikes obtained in current-clamp mode with a spiking frequency of 172 Hz evoked at 250 pA. This voltage waveform command was used due to the length of time required to generate a voltage-clamp protocol from a recorded current-clamp waveform for each neuron (Llinas et al., 1982; Do & Bean, 2003; Enomoto et al., 2006). The resulting data obtained in voltage-clamp mode was a train of whole-current events with inward TTX-sensitive (0.5 μm) currents that followed the voltage waveform command closely. In order to subtract passive membrane and capacitative currents from the ionic current trace acquired from the voltage waveform (baseline −55 mV) the same waveform was applied sub-threshold (−120 mV), and the resulting current trace was subtracted from the current trace acquired at −55 mV. Three measurements were then obtained from the remaining current trace: (i) the amplitude of the first inward current event; (ii) the average of all, or total inward current; and (iii) the average total inward current of the last third (25 current events) or plateau phase of the trace. Currents are presented as a percentage of control 8 min following cocaine application to the ACSF. Due to space-clamp problems common to slice preparations, no measurements of inward current kinetics were made. The voltage waveform command protocol utilized in this study was designed to clamp frequency, while imitating natural spike dynamics. We found that when Ra and Rm remained stable, and the time between the applications of the voltage waveform command protocol was not less than 2 min, waveform-generated current amplitudes remained constant and exhibited little sign of run-down.
Statistical analyses
For in vivo and in vitro studies, the results for control and drug treatment groups were derived from calculations performed on VTA GABA neuron spontaneous firing rate, ICPSDs, current-evoked spikes, sodium currents, DA neuron evoked and spontaneous IPSCs, and mean normalized expression differences of TH and D2 mRNA. A paired two-sample for means t-test was performed to determine statistical significance for all measures. Welch's correction was applied to RT-PCR data due to unequal variances. All results except for paired-pulse are presented in the text as the mean ± SEM and the t-distribution variance for a two-tailed level of confidence of 95%. Paired-pulse results are presented as one-tailed 95% confidence intervals. Results in figures are presented as mean ± SEM; P < 0.05 was taken as indicating statistical significance. Analysis software included Microsoft Excel and Igor Pro (Wavemetrics) Stat Pak. Figures were constructed with Igor Pro software.
Results
Effects of systemic cocaine, cocaine methiodide, dopamine, lidocaine and methamphetamine on VTA GABA neuron firing rate in vivo
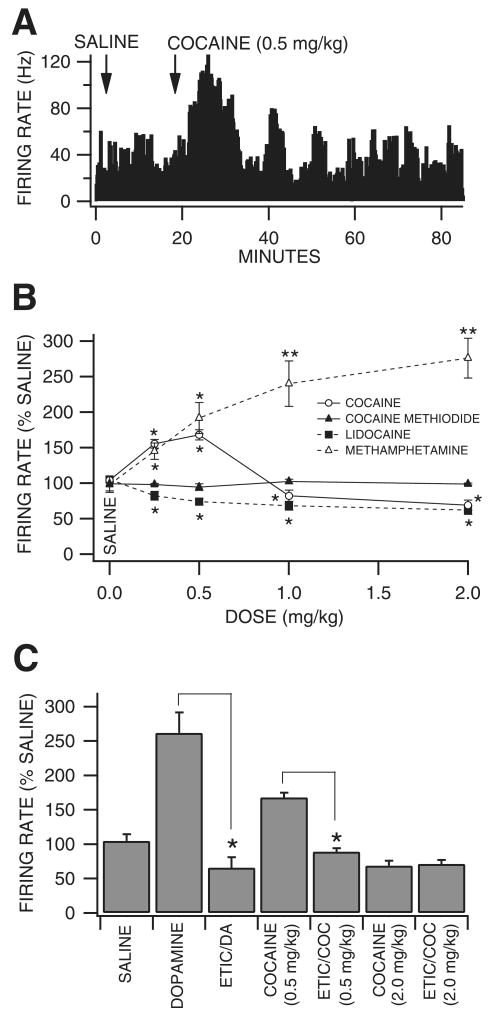
Fifty-three VTA GABA neurons were tested in vivo for their sensitivity to intravenous administration of cocaine, cocaine methiodide, DA, lidocaine or methamphetamine. Initially, we hypothesized that reinforcing doses of cocaine would enhance VTA GABA neuron firing rate in a manner similar to microelectrophoretic DA (Stobbs et al., 2004), given its DAT-inhibiting properties. However, unexpectedly, cocaine (0.25–2 mg/kg) had somewhat variable but significant dose-dependent effects on firing rate. Cocaine significantly increased VTA GABA neuron firing at doses at or below 0.5 mg/kg and significantly decreased firing rate at doses > 0.5 mg/kg (Fig. 1A and B; 0.25 mg/kg: +56 ± 6.1%, P < 0.01, t(2,7) = 2.3; 0.5 mg/kg: +68 ± 6.9%, P < 0.01, t(2,9) = 2.2; 1.0 mg/kg: −18 ± 8%, P < 0.05, t(2,24) = 2.0; 2.0 mg/kg: −31 ± 7.2%, P < 0.05, t(2,7) = 2.3; mean saline firing rate, 31.4 ± 2.84 Hz; n = 25) compared to saline control. In order to determine whether cocaine's well-known peripheral cardiovascular pressor effects (Schindler et al., 1995) or temperature effects (Kiyatkin & Leon Brown, 2006) might have contributed to the effects of cocaine on VTA GABA neuron firing rate, we evaluated the effects of intravenous DA, which has well-known pressor effects, and cocaine methiodide, which does not cross the blood-brain barrier but has cocaine's peripheral temperature and blood pressure effects (Shriver & Long, 1971; Kiyatkin & Leon Brown, 2006). Intravenous administration of DA (3 mg/kg) increased mean arterial pressure (223%) and respiratory rate, and induced piloerection, but had no effect on VTA GABA neuron firing rate (P > 0.05, t(2,4) = 2.7; n = 5). Intravenous administration of cocaine methiodide did not significantly alter firing rate at any dose level tested (Fig. 1B; 0.25 mg/kg: −1 ± 9%, P > 0.24, t(2,4) = 2.7; 0.5 mg/kg: −2.2 ± 1.3%, P > 0.05, t(2,4) = 2.7; 1.0 mg/kg: +5.5 ± 4.5%, P > 0. 05, t(2,4) = 2.7; 2.0 mg/kg: −1.3 ± 1.3%, P > 0.05, t(2,4) = 2.7; mean saline firing rate, 26.3 ± 4.73; n = 5) compared to saline control. Intravenous administration of lidocaine, a VSSC blocker with negligible DAT activity, significantly decreased VTA GABA neuron firing rate at all doses tested (Fig. 1B; 0.25 mg/kg: −18 ± 5.8%, P < 0.05, t(2,8) = 2.3; 0.5 mg/kg: −26%, P < 0.05, t(2,6) = 2.4; 1.0 mg/kg: −32 ± 6%, P < 0.05, t(2,8) = 2.3; 2.0 mg/kg: −38 ± 5.8%, P < 0.01, t(2,6) = 2.4; mean saline firing rate, 26.3 ± 4.73 Hz; n = 9) compared to saline control. Intravenous administration of methamphetamine, a DAT inhibitor with negligible VSSC activity, significantly increased VTA GABA neuron firing rate at all dose levels tested (Fig. 1B; 0.25 mg/kg: +45 ± 11.6%, P < 0.05, t(2,4) = 2.7; 0.5 mg/kg: +92 ± 22%, P < 0.05, t(2,4) = 2.7; 1.0 mg/kg: +140 ± 32%, P < 0.05, t(2,4) = 2.7; 2.0 mg/kg: +176 ± 28%, P < 0.05, t(2,4) = 2.7; mean saline firing rate, 22.2 ± 3.93 Hz; n = 9) compared to saline control.
Fig. 1.
Dopamine pharmacology of the effects of cocaine on VTA GABA neuron firing rate in vivo. (A) The ratemeter record shows the effects of saline and 0.5 mg/kg cocaine on a representative VTA GABA neuron with a baseline rate of ∼32 Hz. At this dose level, cocaine enhanced the firing rate of this neuron. (B) Cocaine significantly enhanced VTA GABA neuron firing rate at dose levels ≤ 0.5 mg/kg and modestly, but significantly, reduced firing rate at dose levels 1–2 mg/kg. Cocaine methiodide, which elicits cocaine's peripheral but not central effects, did not significantly alter firing rate at any dose level. Lidocaine significantly reduced firing rates at all cocaine dose levels. Methamphetamine significantly enhanced firing rate at all dose levels. *P < 0.05 and **P < 0.01. (C) Compared to saline microelectrophoresis, DA significantly increased firing rate 162% and microelectrophoretic application of the D2 antagonist eticlopride blocked DA activation. Intravenous administration of 1.0 mg/kg eticlopride blocked low-dose cocaine activation of firing rate, but not higher dose cocaine inhibition of firing rate. *P < 0.001 compared to saline control.
Dopamine pharmacology of the effects of cocaine on VTA GABA neuron firing rate in vivo
Of the forty-eight VTA GABA neurons tested in vivo for sensitivity to microelectrophoretically applied DA, 46% of them were activated by DA (Stobbs et al., 2004; Lassen et al., 2007). We evaluated the DA pharmacology of 22 of these neurons. Microelectrophoretic application of DA (Fig. 1C; +50 nA; P < 0.001, t(2,21) = 5.0; n = 22), or the D2 agonist quinpirole (+50 nA; P < 0.001, t(2,14) = 4.31; n = 15; data not shown) significantly increased the firing rate of VTA GABA neurons 162 and 140% compared to saline control (mean saline firing rate 27.3 ± 4.1 Hz; +50-nA ejection current). Neither the D1 agonist SKF38393 nor the D1 antagonist SCH23390 (+50 nA; P > 0.05, n = 4) significantly altered the firing rate of VTA GABA neurons compared to saline control (data not shown). In situ microelectrophoretic administration of the D2/D3 antagonist eticlopride blocked DA activation of firing rate, and i.v. administration of eticlopride (1.0 mg/kg) blocked low-dose (0.5 mg/kg) i.v. cocaine activation, but not higher dose i.v. cocaine inhibition, of VTA GABA neuron firing rate (P < 0.05; n = 6; Fig. 1C).
Effects of systemic cocaine, cocaine methiodide, dopamine, lidocaine and methamphetamine on VTA GABA neuron evoked spikes in vivo
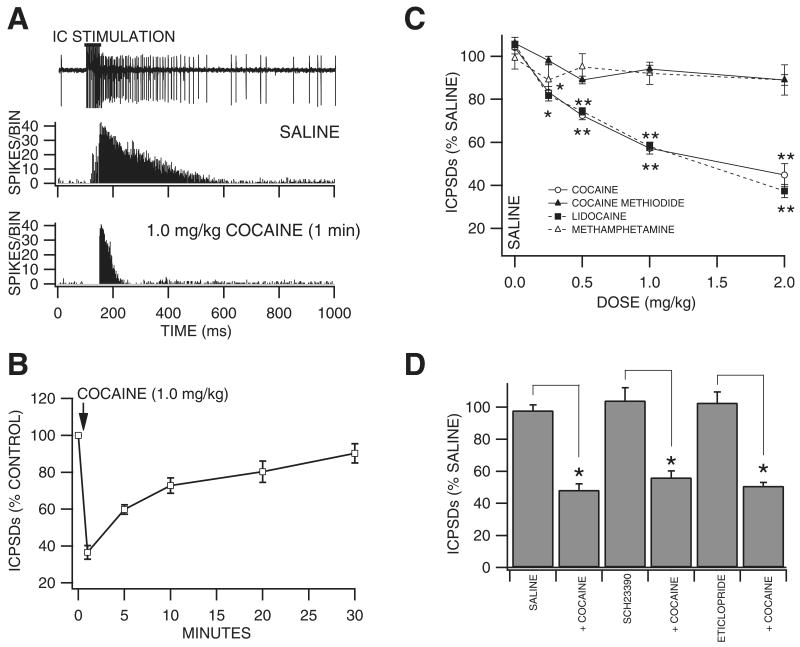
Brief, high frequency stimulation of the internal capsule (IC) results in multiple poststimulus VTA GABA neuron spike discharges (ICPSDs; Fig. 2A; Steffensen et al., 1998). We studied the effects of intravenous cocaine on ICPSDs obtained in 53 VTA GABA neurons and compared its effects to those of cocaine methiodide, DA, lidocaine and methamphetamine. Figure 2A shows the effects of 1.0 mg/kg cocaine on ICPSDs obtained from a representative VTA GABA neuron. While saline had no effect, this dose of cocaine markedly reduced VTA GABA neuron ICPSDs to ∼50–60% 1 min after injection. The time course of cocaine inhibition at the 1.0 mg/kg dose was characterized by rapid and pronounced inhibition at 1 min with recovery in 30 min (Fig. 2B; n = 5). The effects of cocaine on VTA GABA neuron ICPSDs was tested at dose levels 0.25–2.0 mg/kg (Fig. 2C), and significantly decreased ICPSDs at doses at or above 0.25 mg/kg (0.25 mg/kg: −16.7 ± 2.7%, P < 0.005, t(2,5) = 2.5; 0.5 mg/kg; −27.5 ± 1.9%, P < 0.002, t(2,5) = 2.5; 1.0 mg/kg: −42.8 ± 2.7%, P < 0.002, t(2,5) = 2.5; 2.0 mg/kg: −55.3 ± 5.3%, P < 0.0004, t(2,5) = 2.5; mean 50% maximum saline ICPSDs, 128.5 ± 30.7; n = 6 each) compared to saline control. The IC50 for cocaine on ICPSDs was ∼1.2 mg/kg, based on linear interpolation from the dose–response curve (r2 = 0.86). As with firing rate, in order to determine whether cocaine's well-known cardiovascular pressor effects or temperature effects might contribute to cocaine reduction of VTA GABA neuron ICPSDs, we evaluated the effects of intravenous DA and cocaine methiodide. Intravenous administration of DA (3 mg/kg) had no effect on VTA GABA neuron ICPSDs (P > 0.05, n = 4). Similarly, intravenous administration of cocaine methiodide did not significantly affect ICPSDs at any dose level tested (Fig. 2C; 0.25 mg/kg: −2 ± 2%, P < 0.24, t(2,5) = 2.5; 0.5 mg/kg: −11 ± 2.7% P < 0.18, t(2,5) = 2.5; 1.0 mg/kg: −6 ± 1.7%, P < 0.105, t(2,5) = 2.5; 2.0 mg/kg: −10.1 ± 3.5%, P < 0.11, t(2,5) = 2.5; mean 50% maximum saline ICPSDs, 121.5 ± 4.2; n = 6) compared to saline control. As local DA markedly enhanced firing rate (Fig. 1; Stobbs et al., 2004), we evaluated the effects of local application of DA on ICPSDs. Compared to saline control, microelectrophoretic application of DA (+ 50 nA) did not significantly alter ICPSDs (P > 0.5; mean 50% maximum saline ICPSDs, 60.6 ± 4.4; n = 21 each). Intravenous administration of lidocaine markedly decreased ICPSDs at all doses tested (Fig. 2C; 0.25 mg/kg: −18.3 ± 2.4%, P < 0.005, t(2,5) = 2.5; 0.5 mg/kg: −25.6 ± 1.7%, P < 0.0002, t(2,5) = 2.5; 1.0 mg/kg: −41.9 ± 2%, P < 0.0001, t(2,5) = 2.5; 2.0 mg/kg: −62.6 ± 3.1%, P < 0.00003, t(2,5) = 2.5; mean 50% maximum saline ICPSDs, 136.9 ± 14.3; n = 6) compared to saline control. The IC50 for lidocaine on ICPSDs was ∼1.2 mg/kg, based on linear interpolation from the dose–response curve (r2 = 0.91). Intravenous administration of methamphetamine did not significantly alter VTA GABA neuron ICPSDs (Fig. 2C; 0.25 mg/kg: −10 ± 5%, P > 0.05, t(2,4) = 2.7; 0.5 mg/kg: −5 ± 6.2%, P > 0.05, t(2,3) = 3.1; 1.0 mg/kg: −8 ± 5.2%, P > 0.05, t(2,3) = 3.1; mean 50% maximum saline ICPSDs, 74.9 ± 9.5; n = 15) compared to saline control.
Fig. 2.
Cocaine inhibition of VTA GABA neuron ICPSDs in vivo: time course and comparison to dopamine agonists, VSSC blockers and DAT inhibitors. (A) The inset shows a 1.0-s trace of a representative VTA GABA neuron spike in association with brief (50 ms), high frequency (200 Hz) stimulation of the IC. The horizontal bar above the stimulus artifacts indicates the time of the stimulus train. VTA GABA neurons evince multiple spike discharges for hundreds of ms following IC stimulation. These are termed IC-induced poststimulus spike discharges, or ICPSDs. The peristimulus spike histogram (PSH) below the trace shows the average of 12 IC stimulation trials on ICPSDs obtained from this representative VTA GABA neuron following i.v. saline. The stimulus artifacts in the PSH are omitted to illustrate spikes only. The bottom PSH trace shows the effects of 1.0 mg/kg i.v. cocaine on ICPSDs in this same neuron 1.0 min after cocaine injection. Note that cocaine markedly decreased VTA GABA neuron ICPSDs at this dose level. (B) This graph summarizes the time course of cocaine inhibition of VTA GABA neuron ICPSDs at the 1.0 mg/kg dose level. Note the marked and pronounced inhibition of ICPSDs at 1 min with recovery in 30 min (n = 5). (C) Cocaine significantly reduced ICPSDs at all dose levels tested, cocaine methiodide did not significantly alter them at any dose level, lidocaine, similar to cocaine, significantly reduced them at all dose levels tested, and methamphetamine did not significantly alter them at any dose level. *P < 0.005 and **P < 0.0005. (D) This graph summarizes the effects of DA receptor antagonists on i.v. cocaine inhibition of VTA GABA neuron ICPSDs at 1.0 min after injection. The ability of 1.0 mg/kg cocaine to reduce ICPSDs was not affected by a dose of saline administered 10 min prior to cocaine. Intravenous administration of the D1/D5 receptor antagonist SCH23390 had no effect on ICPSDs, nor on the ability of 1.0 mg/kg cocaine to reduce them when administered 10 min prior to cocaine (n = 5). Similarly, i.v. administration of the D2/D3 receptor antagonist eticlopride had no effect on ICPSDs, nor on the ability of 1.0 mg/kg cocaine to reduce them when administered 10 min prior to cocaine (n = 5). *P < 0.005.
Dopamine pharmacology of the effects of cocaine on VTA GABA neuron evoked spikes in vivo
In order to evaluate the role of DA in mediating cocaine inhibition of VTA GABA ICPSDs we tested the effects of select DA receptor subtype antagonists on cocaine inhibition of VTA GABA neuron ICPSDs at the 1.0-mg/kg dose level 1 min after injection, and compared to i.v. saline (Fig. 2D). Intravenous administration of the D1/D5 DA receptor antagonist SCH23390 (1.0 mg/kg) had no effect on VTA GABA neuron ICPSDs (104.2 ± 7.9%; P > 0.05; n = 5), and did not alter cocaine's ability to reduce ICPSDs at the 1.0 mg/kg dose level (56.2 ± 4.0%) when administered 10 min after injection of SCH23390. Similarly, i.v. administration of the D2/D3 antagonist eticlopride (1.0 mg/kg) had no effect on VTA GABA neuron ICPSDs (102.7 ± 6.6%; P > 0.05; n = 5), and did not alter cocaine's ability to reduce ICPSDs (51 ± 2.1%) when administered 10 min after injection of eticlopride.
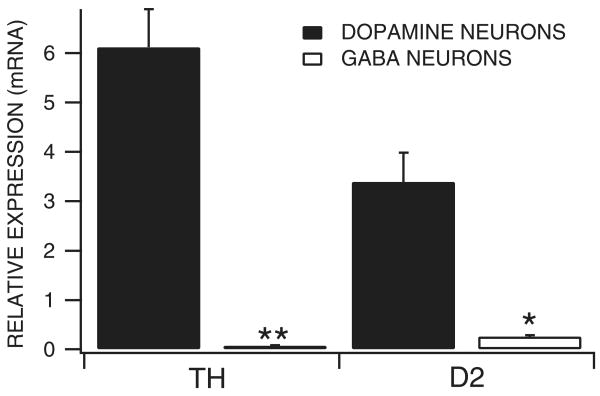
Expression of select genes in dopamine vs. GABA neurons in mature rats: quantitative single-cell RT-PCR
In order to further evaluate the role of D2 receptors in DA activation of VTA GABA neurons, we evaluated the quantitative expression of D2 receptor gene products in these neurons. In addition, in order to validate the electrophysiological criteria used to distinguish VTA neurons, we compared the expression of TH in VTA GABA and DA neurons. Figure 3 summarizes the differences between VTA DA and GABA neurons for the expression of TH and D2 mRNA transcripts in adult male rats. TH expression in DA neurons was significantly greater than in GABA neurons (P = 0.0002, t(1,23) = 4.5; mean DA neuron TH expression 6.12 ± 0.77, and mean GABA neuron TH expression 0.07 ± 0.01; n = 33). The cycle threshold for 18s, the housekeeping gene that was used for the quantification of the relative expression of each of the gene products, was not significantly different between DA and GABA neurons (P = 0.35, t(1,31) = 0.95; mean DA cell 18s CT, 12.53 ± 0.18 and mean GABA cell 18s CT, 12.87 ± 0.34). The expression of D2 was significantly greater in DA neurons than GABA neurons (P = 0.019; mean DA cell D2 expression, 3.39 ± 0.59 and mean GABA cell D2 expression, 0.26 ± 0.03; n = 29). The cycle threshold for 18s was not significantly different between groups (P = 0.82, t(1,27) = 0.23; mean DA cell 18s CT = 12.53 ± 0.18 and mean GABA cell 18s CT = 12.63 ± 0.35).
Fig. 3.
Expression of TH and D2 receptors in dopamine vs. GABA neurons. Single-cell quantitative RT-PCR was performed on neurons in the VTA of adult male rats. Both DA and GABA neurons expressed TH and D2 transcripts. However, the expression of TH and D2 receptors was much greater in DA neurons than in GABA neurons. **P < 0.0002 and *P < 0.02.
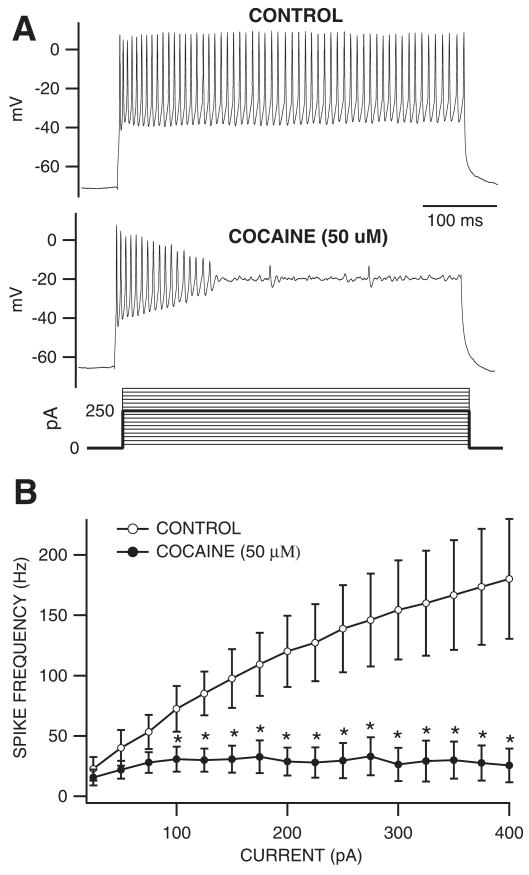
Effects of cocaine on current-evoked VTA GABA neuron spikes in vitro
Based on in vivo studies, cocaine did not appear to be acting through DA to reduce VTA GABA neuron firing rate or ICPSDs. Thus, we tested the effect of cocaine on VTA GABA neuron current-evoked spiking in vitro to determine whether cocaine might reduce spiking similarly to ICPSDs in vivo. VTA GABA neurons displayed an average spiking frequency of 22.8 ± 9.9 Hz at threshold (25 pA) and 180 ± 49.7 Hz at the maximum current step (400 pA). Not surprisingly, 50 μm cocaine markedly reduced spiking at current levels of 100–400 pA (85.7 ± 13.8 Hz, P < 0.05, t(2,4) = 2.8 at 400 pA; Fig. 4A and B). As cocaine reduced current-evoked spikes in vitro similarly to ICPSDs in vivo we postulated that cocaine was blocking ICPSDs and current-evoked spikes, and reducing firing rate via its VSSC-blocking properties. To provide evidence to support this assumption, we next examined the effects of cocaine on VTA GABA neuron sodium currents.
Fig. 4.
Cocaine reduced current-evoked spiking of VTA GABA neurons in vitro. (A) These are representative current-clamp traces of current-evoked VTA GABA neuron spikes recorded in the horizontal midbrain slice preparation. Note the lack of spike accommodation characteristic of VTA GABA neurons (Allison et al., 2006). Superfusion of cocaine (50 μm) markedly reduced spiking at this level of depolarization (250 pA; darker line of the current steps that were performed). (B) This graph summarizes the effects of 50 μm cocaine on the frequency of current-evoked spiking of VTA GABA neurons at all current steps tested (25–400 pA steps shown in A). Superfusion of cocaine significantly reduced spiking at current steps 100–400 pA in all cells tested. *P < 0.03 at each point.
Effects of cocaine on VTA GABA neuron sodium currents in vitro
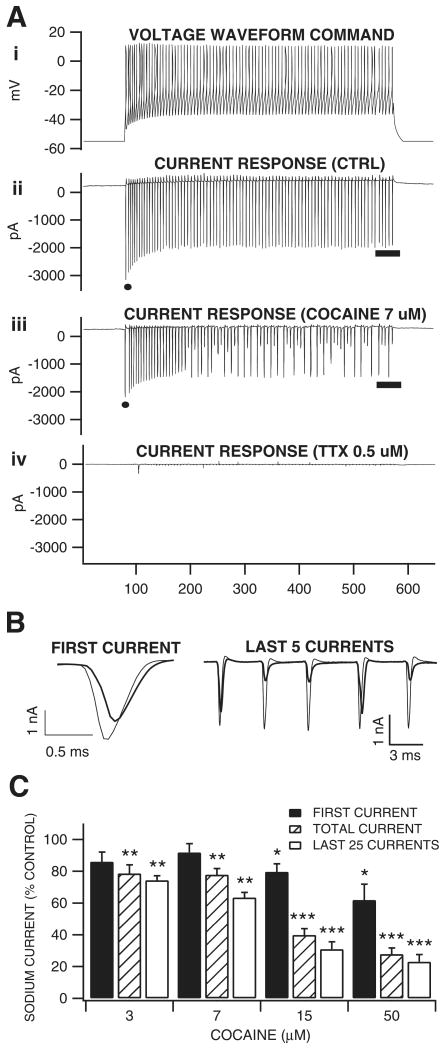
Cocaine appeared to exert greater inhibition on the plateau than on the initial phase of ICPSDs in vivo (see Fig. 2A) and current-evoked spiking in vitro (see Fig. 4A). This use-dependent inhibition suggested that cocaine was acting via the well-established mechanism of preferentially binding to the sodium channel in the open, inactivated conformational state (Strichartz, 1976; Bean et al., 1983; Postma & Catterall, 1984; O'Leary & Chahine, 2002). To confirm that cocaine was inhibiting ICPSDs and current-evoked spiking by blocking VSSCs in a use-dependent manner, we tested the effect of cocaine on VTA GABA neuron whole-currents elicited by a voltage waveform command protocol (Llinas et al., 1982; Do & Bean, 2003; Enomoto et al., 2006). The voltage waveform command protocol was created from a typical voltage trace obtained in current clamp mode (Fig. 5, Ai) of a VTA GABA neuron driven to fire at 172 Hz, a frequency consistent with what we have previously determined for ICPSDs in vivo (Lassen et al., 2007) at 50% maximum stimulus level, and at submaximum current levels for spikes driven in current-clamp mode. This current-clamp derived voltage waveform command protocol has the advantage of clamping both spike dynamics and frequency compared to a sequence of square waves. All inward current events obtained using the voltage waveform command were abolished by TTX (0.5 μm), indicating that they were sodium currents. Figure 5 (Aii–iv) illustrates the effects of cocaine (7 μm) and TTX (0.5 μm) on sodium currents evoked by the voltage waveform command in a typical VTA GABA neuron. We postulated that if cocaine was blocking VSSCs in a use-dependent manner, suggested by the effect observed on current-evoked spikes, cocaine would have its greatest effect during the plateau phase of the waveform trace. We therefore measured cocaine's effect on the amplitude of the first inward current event, the mean of all or total inward current, and the mean inward current of the last 25 events: the plateau phase of the trace (Fig. 5B and C). The superimposed traces of the first current event and last five current events in Fig. 5B taken from the raw traces in Fig. 5A illustrate this. Cocaine was tested at concentration levels of 3, 7, 15 and 50 μm and significantly reduced VTA GABA neuron TTX-sensitive sodium currents in a concentration-dependent manner, exerting its greatest VSSC-blocking effect at each dose on the plateau phase of the trace (percentage reduction in plateau phase inward current: 3 μm, 25.8 ± 2.9%, P < 0.001, t(2,4) = 2.7; 7 μm, 36.6 ± 3.3%, P < 0.01, t(2,3) = 3.1; 15 μm, 69.1 ± 4.6%, P < 0.001, t(2,3) = 3.1; 50 μm, 76.9 ± 4.5%; P < 0.0001, t(2,5) = 2.5; Fig. 5C). Cocaine's IC50 on sodium currents in VTA GABA neurons was calculated to be 13 μm, using a least-squares fit line to the points generated from 3, 7 and 15 μm, (r2 = 0.90, y = −3.1x + 90.4).
Fig. 5.
Concentration-dependent and use-dependent effects of cocaine on sodium currents in VTA GABA neurons in vitro. (Ai) This graph shows the ‘voltage waveform command’ applied to VTA GABA neurons in voltage-clamp mode to elicit TTX-sensitive sodium currents (Aii–iv). (Aii) Control current response, (Aiii) the effect of cocaine (7 μm) after 8 min, and (Aiv) the effect of 0.5 μm TTX showing abolition of sodium currents. (B) Superimposed traces of the first current events (taken from points indicated by filled circles in Aii and iii) and last five current events (taken from point indicated by line on current traces Aii and iii) of the control and cocaine effect. (C) This graph summarizes the dose-dependent effects of cocaine on the first sodium current, the total of all currents and the last 25 currents induced by the voltage waveform command. All concentrations of cocaine significantly reduced the total current and last 25 sodium current events. Only 15 and 50 μm significantly reduced the first current event. *P < 0.05, **P < 0.01 and ***P < 0.001.
Effects of cocaine on VTA DA neuron evoked and spontaneous IPSCs in vitro
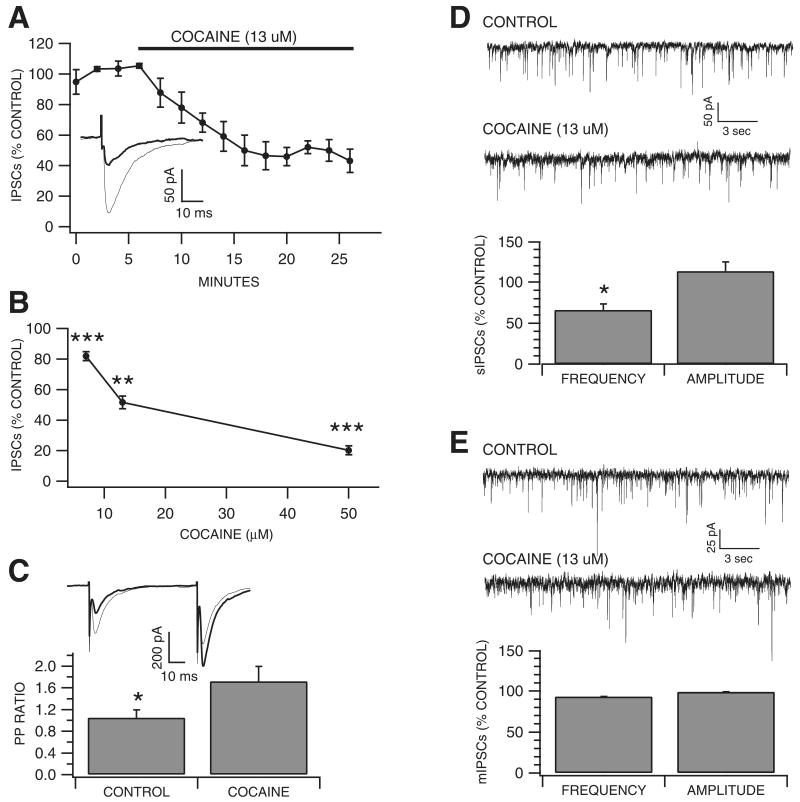
As cocaine inhibited putative VTA GABA neuron sodium currents as well as current-evoked spikes in vitro, and local circuit GABA neurons are presumed to inhibit VTA DA neurons, we evaluated the effects of cocaine (7, 13 and 50 μm) on DA neuron IPSCs evoked by local stimulation. The concentrations tested were based on the assumption that if cocaine blocked evoked IPSCs, the IC50 for this effect would be similar to the IC50 of cocaine's blocking effects on putative VTA GABA neuron waveform-evoked sodium currents (13 μm). Indeed, cocaine significantly reduced evoked VTA DA neuron IPSCs within 8–10 min (Fig. 6A) at all concentrations tested, with an IC50 of 13 μm (7 μm, 18 ± 2.9%, P < 0.001, t(2,3) = 4.18; 13 μm, 48.3 ± 4.3%, P < 0.01, t(2,3) = 3.18; 50 μm, 79.7 ± 2.9%; P < 0.001, t(2,3) = 3.18; Fig. 6B). As it has been shown that changes in transmitter release affect the paired-pulse ratio (Khazipov et al., 1995; Mennerick & Zorumski, 1995; Salin et al., 1996; Steffensen et al., 1999) we examined the IPSC paired-pulse ratio at 50 ms to determine whether cocaine's reduction of evoked IPSCs was correlated to a change in the probability of transmitter release. Calculating the paired-pulse ratio using the test/conditioning IPSC peak amplitude ratio (i.e. IPSC2/IPSC1), 13 μm cocaine increased the DA neuron IPSC paired-pulse ratio from 1.16 ± 0.11 to 1.94 ± 0.19 (P = 0.006, t(2,4) = 2.44; Fig. 6C), suggesting cocaine was acting pre-synaptically and decreasing the probability of GABA release. To further investigate the effects of cocaine on GABAergic synapses on VTA DA neurons, and to confirm whether cocaine was acting pre-synaptically or postsynaptically, we examined the effects of cocaine on spontaneous action potential-dependent GABAergic IPSCs (sIPSCs). Cocaine (13 μM) reduced sIPSC frequency by 33.7 ± 7.2% (P = 0.018, t(2,3) = 3.18; Fig. 6D), but did not significantly affect sIPSC amplitude (control, 37 ± 10.2 pA; n = 4; cocaine, 40.4 ± 9.1 pA; n = 4; P = 0.31; Fig. 6D). Finally, in order to determine whether cocaine was reducing the action potential-dependent or -independent components of sIPSC activity, or both, we sought to isolate this effect by examining the action potential-independent, TTX-insensitive or mIPSC component alone. Cocaine did not significantly affect mIPSC frequency or amplitude (frequency: control, 2.78 ± 0.48 Hz; n = 5; cocaine, 2.61 ± 0.46 Hz; n = 5; P = 0.3; amplitude: control, 33.8 ± 2.3 pA; n = 5; cocaine, 33.4 ± 2.7 pA; n = 5; P = 0.88; Fig. 6E), suggesting that cocaine was acting pre-synaptically as a VSSC blocker to reduce the action potential-dependent component of spontaneous IPSC activity.
Fig. 6.
Cocaine reduces evoked and spontaneous GABA inhibitory synaptic transmission to VTA DA neurons in vitro. (A) This graph shows the time-course for the effects of cocaine (at the IC50 for inhibition of VTA GABA neuron sodium current) on VTA DA IPSCs. The inset shows representative superimposed traces of IPSCs (each trace is an average of 12 sweeps collected at 0.1 Hz) obtained in a DA neuron before (lighter trace) and 10 to 12 min after cocaine (darker trace). (B) Cocaine significantly decreased DA IPSCs at 7, 13 and 50 μm. (C) The inset shows superimposed representative traces of IPSCs (each is an average of 12 sweeps collected at 0.1 Hz) before (lighter trace) and 10 to 12 min after cocaine (darker trace). Cocaine increased the paired-pulse ratio of DA neuron IPSCs. (D) These insets show the effects of cocaine on spontaneous IPSCs (sIPSCs) recorded in a representative DA neuron. Cocaine (13 μm) significantly reduced the frequency, but not amplitude, of DA neuron sIPSCs. (E) These insets show the effects of cocaine on mIPSCs recorded in a representative DA neuron after treatment with TTX. Cocaine (13 μm) had no effect on frequency or amplitude of TTX-insensitive miniature IPSCs. *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
We have previously demonstrated that DA enhances the firing rate of VTA GABA neurons (Stobbs et al., 2004). Here we ascribe this enhancement to mediation by D2/D3 receptor activation. This activation may occur directly via D2 receptors located on GABA neurons, as has been observed in substantia nigra (Ruffieux & Schultz, 1981; Waszczak & Walters, 1986; Sesack et al., 1994), or indirectly, through some unknown mechanism involving D2/D3 autoreceptors on DA neurons (for review see Adell & Artigas, 2004). Accordingly, we evaluated the quantitative mRNA expression of D2 receptors in VTA neurons. Both DA and GABA neurons expressed D2 receptor mRNA, providing molecular support for our DA pharmacology studies that GABA neurons are excited by DA. Although D2 excitatory effects have been the subject of controversy (Waddington, 1997), D2 receptors have been demonstrated in GABA neurons in substantia nigra (Ruffieux & Schultz, 1981; Waszczak & Walters, 1986; Sesack et al., 1994), suggesting at least a role for D2 receptors in DA modulation of midbrain GABA neurons. Cocaine enhanced VTA GABA neuron firing rate at low doses (0.25–0.5 mg/kg) but inhibited it at higher doses (1.0–2.0 mg/kg), while the DAT inhibitor methamphetamine had only excitatory effects on VTA GABA neuron firing rate across all doses. The enhancement of firing rate by cocaine was not due to its peripheral effects as neither systemic cocaine methiodide, which does not cross the blood brain barrier but retains all of cocaine's peripheral effects on blood pressure and temperature, nor systemic DA, which has similar effects on blood pressure, affected VTA GABA neuron firing rate. The VSSC blocker lidocaine had only inhibitory effects on VTA GABA neuron firing rate. While D2 receptor antagonists blocked low-dose cocaine activation of VTA GABA neurons, they had no effect on cocaine's ability to inhibit firing rate at higher doses, suggesting that cocaine enhances the VTA GABA neuron firing rate at low doses via its central effects on DA release and D2 receptor activation by its DAT-inhibiting properties but at higher doses it inhibits the firing rate by its central VSSC blocking properties.
Cocaine significantly reduced ICPSDs, a putative physiological index of electrical coupling (Allison et al., 2006) that is sensitive to NMDA agonists and antagonists (Steffensen et al., 1998; Stobbs et al., 2004), ethanol (Stobbs et al., 2004) and connexin-36 gap junction (GJ) blockers (Allison et al., 2006). The number of ICPSDs is a monotonic function of frequency, stimulus intensity and pulse number (Lassen et al., 2007), rats will self-stimulate the IC, and the number of ICPSDs is directly proportional to responding for brain stimulation reward (Lassen et al., 2007). Neither local DA nor systemic methamphetamine had any significant effect on ICPSDs, evincing a lack of involvement of DA in cocaine reduction of ICPSDs, and distinguishing them mechanistically from spontaneous activity. Cocaine methiodide had no effect on ICPSDs, suggesting that cocaine's peripheral actions were not responsible for its inhibitory effects. In support of the lack of DA involvement in the effects of cocaine on ICPSDs, systemic administration of D1/D5 or D2/D3 antagonists had no effect on cocaine reduction in ICPSDs. Lidocaine markedly reduced ICPSDs, nearly identically to cocaine. Thus, while ICPSDs depend on activation of NMDA receptors (which may induce oscillations by acting as current amplifiers due to their voltage dependence; Kuznetsov et al., 2006), and appear to require electrical synaptic connectivity (Stobbs et al., 2004; Allison et al., 2006), cocaine appears to be reducing ICPSDs via block of VSSCs, and does not appear to affect them through DA neurotransmission.
While the characterization of VTA neurons in vitro is problematic, it seems VTA GABA and DA neurons each possess unique electrophysiological characteristics that may aid in distinguishing them (for overview see Margolis et al., 2006). In an effort to reconcile these electrophysiological characteristics with a post hoc test that might aid in distinguishing GABA from DA neurons, we examined the expression of TH mRNA in a separate set of adult male rats. Surprisingly, TH mRNA was detected in both putative DA (Ih positive, low input resistance, spike accomodating) and putative GABA (Ih negative, high input resistance, no spike accommodation) neurons in the VTA. However, TH was expressed 143× more in putative DA than putative GABA neurons, suggesting this small amount of TH mRNA detected in GABA neurons might simply be contamination-related background noise associated with the harvesting of cells from the slice. There is evidence, however, that TH protein is present in GABA neurons (Klink et al., 2001; Olson & Nestler, 2007). In spite of the possible disparity between detection of mRNA transcripts and the presence of encoded protein, and some persisting vagaries regarding how the VTA is defined anatomically (Ikemoto, 2007), this RT-PCR data supports the idea that VTA neurons can be distinguished somewhat reliably based on certain electophysiological characteristics in some areas of the VTA.
Based on cocaine's reduction of ICPSDs and firing rate in vivo, and current evoked spikes in vitro, we performed voltage-clamp experiments in the midbrain slice preparation to evaluate the effects of cocaine on sodium current in VTA GABA neurons. As VTA GABA neurons lack spike accommodation, very high-frequency (∼ 200 Hz) sodium currents could be studied in vitro and these might emulate the high-frequency spiking obtained in current clamp in vitro (i.e., current-evoked spiking) and in vivo (i.e., ICPSDs). A voltage waveform command (Llinas et al., 1982; Do & Bean, 2003; Enomoto et al., 2006) was created from a typical recording of current-evoked spiking at 172 Hz, a frequency observed in ICPSDs in vivo (Fig. 2; Lassen et al., 2007) and current-evoked spikes in vitro (Fig. 4). The inward currents obtained from this voltage waveform command were abolished by TTX, indicating they were sodium currents. Cocaine suppressed sodium currents with an IC50 of 13 μm, a concentration consistent with its reinforcing properties (Fowler et al., 1998; Kiyatkin & Leon Brown, 2006). Of the three measurements of sodium currents (first current, total current and last 25 currents), cocaine exerted its greatest effect on the amplitude of the last 25 currents or plateau phase of sodium currents, confirming that cocaine was acting in a use-dependent manner on VSSCs (Strichartz, 1976; Bean et al., 1983; Postma & Catterall, 1984; O'Leary & Chahine, 2002). While cocaine's anesthetic effects are not specific to GABA neurons, the use-dependent nature of cocaine's VSSC actions would exert a greater effect on relatively fast-firing, wide-bandwidth GABA neurons than DA neurons. VTA GABA neurons have baseline firing rates that are more than ten times higher than those of DA neurons, even in unanesthetized rats (Lee et al., 2001), which would render them more vulnerable to cocaine's use-dependent VSSC blocking properties.
The prevailing view is that VTA GABA neurons may serve to inhibit VTA DA neurons locally. Given the sensitivity of VTA GABA neurons to cocaine's VSSC-blocking properties, we reasoned that cocaine may disinhibit VTA DA neurons and sought to determine the effects of cocaine on GABA receptor-mediated evoked and spontaneous IPSCs in VTA DA neurons. As somatodendritic release of DA elicits D2 receptor-mediated IPSCs in VTA DA neurons (Beckstead et al., 2004), we examined DA IPSCs in the presence of the D2/D3 receptor antagonist eticlopride. To our knowledge, we are the first to demonstrate that cocaine can reduce VTA DA evoked IPSCs at reinforcing dose levels (IC50 13 μm). This same concentration significantly increased the IPSC paired-pulse ratio, suggesting cocaine acts to decrease the probability of GABA release (Khazipov et al., 1995; Mennerick & Zorumski, 1995; Salin et al., 1996; Steffensen et al., 1999). Additionally, 13 μm cocaine reduced action potential-dependent sIPSC frequency, but not amplitude, and did not reduce TTX-insensitive action potential-independent mIPSC frequency or amplitude. Taken together, these observations support the idea that cocaine is acting pre-synaptically through its VSSC-blocking properties on VTA GABA neurons to reduce activity-dependent GABA release in the VTA. This concept is supported by the fact that lidocaine (500 μm) is used to pharmacologically isolate mIPSCs (Melis et al., 2002). Given that acute intravenous injections of cocaine at 1.0 mg/kg or higher can lead to extracellular cocaine concentrations < 25 μm in the brain (Pan et al., 1991), it is somewhat surprising that lidocaine has not been used as a control in in vitro studies wherein cellular and molecular changes in VTA DA neurons have been attributed to high doses of cocaine (Kalivas & Alesdatter, 1993; Ungless et al., 2001; Sarti et al., 2007).
Conceivably, the effects of cocaine in the mesolimbic system are concentration-dependent: a low-dose cocaine effect that enhances DA levels via DAT inhibition at the terminals of DA neurons, while moderate to higher dose level effects of cocaine may be mediated through both its DAT-inhibiting and VSSC-inhibiting properties. These actions would affect the midbrain regions containing DA neuron cell bodies, and their projection areas such as the nucleus accumbens, with cocaine's VSSC-inhibiting properties preferentially affecting fast-firing GABA neurons. In support of the data of this study, and our hypothesis that DA neurons may not be as sensitive as GABA neurons to cocaine's VSSC-blocking effects, Shi et al. (2004) demonstrated that cocaine (1.0 mg/kg) enhancement of DA neuron firing rate, bursting activity and low-frequency oscillations in vivo were unmasked by blocking DA neuron D2 autoreceptor inhibition. In that study, cocaine may have acted to increase DA neuron firing rate and bursting activity by reducing local GABA neuron inhibition when DA neuron autoreceptor inhibition was blocked. Additionally, procaine, which has VSSC-blocking actions and minimal DAT-inhibiting properties (Wilcox et al., 1999), slightly increased DA neuron firing rate in the VTA (Einhorn et al., 1988). We have previously demonstrated that chloral hydrate anesthesia, which was used in the Einhorn et al. study, abolishes GABA neuron activity (Lee et al., 2001), suggesting that the DA firing rate might have been enhanced even more if this anesthetic agent hadn't markedly reduced GABA inhibition. Finally, facilitators of GABA neurotransmission significantly attenuate cocaine-induced locomotor sensitization, stereotypy and reinforcement (Gardner et al., 2002), providing behavioral support for a role for GABA in cocaine reinforcement. Thus, cocaine's inhibition of GABA neuron VSSCs might synergize with its DAT blocking actions to enhance DA neurotransmission over that produced by its DAT inhibitory properties alone. Cocaine's net effect on DA neuron activity in the VTA might result from a combination of cocaine inhibition of DA neurons by D2 autoreceptor activation (Einhorn et al., 1988), and disinhibition of DA neurons via GABA neuron inhibition by virtue of its VSSC-blocking actions.
Working within the existing view that GABA neurons only function to regulate DA neurons might be too restrictive a model for drug reinforcement and reward. Indeed, we have shown previously that this population of GABA neurons connect to cortex (Steffensen et al., 1998), may be involved in cortical activation (Lee et al., 2001) and may form part of a larger network of GABA neurons containing μ-opioid receptors (Steffensen et al., 2006) that are coupled by electrical synapses via GJs (Lassen et al., 2007). Although the following is highly speculative, VTA GABA neurons may act independently of DA neurons to mediate the hedonic properties of cocaine and other drugs, as there is an emerging view that DA codes for the preparatory aspects of behavior (‘wanting’), while brain opioids seem to mediate the perception of the hedonic properties of rewards (‘the liking’); for recent review see Barbano & Cador (2007).
These in vivo and in vitro findings establish the physiological relevancy and mechanism of action for the effects of cocaine on VTA GABA neurons and GABA inhibition of DA neurons. We have shown that cocaine inhibits VTA GABA neuron excitability in vivo as well as sodium currents and inhibitory synaptic potentials in DA neurons in vitro at dose levels known to be reinforcing, providing compelling mechanistic evidence that cocaine is acting through its VSSC-blocking effects to disinhibit VTA DA neurons. By virtue of their proximity to midbrain DA neurons, wide dynamic range, widespread axonal distribution, potential electrical coupling via GJs, dependency on corticotegmental glutamatergic NMDA receptor-mediated input and sensitivity to DA, the VTA GABA neurons are in a critical position to regulate mesocorticolimbic DA neurotransmission. Although highly speculative at this time it is possible that, along with VSSCs, GJ-mediated communication between VTA GABA neurons may be sensitive to cocaine. Modulation of GJ neurotransmission by DA is well-established in the retina and has been implicated in other regions of the brain containing DA (for review see Weiler et al., 2000), and GJ connexin proteins have been shown to be altered in the brain following cocaine self-administration (Bennett et al., 1999). Thus, while the role of VTA GABA neurons in mediating alcohol and opiate reinforcement is gaining wider acceptance, this study suggests a potential role for these neurons in cocaine reinforcement as well.
Acknowledgments
This work was supported by PHS grant AA13666 to SCS. We wish to thank Dr Sterling N. Sudweeks at BYU for material support.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- DA
dopamine
- DAT
dopamine transporter
- GABA
γ-aminobutyric acid
- GJ
gap junction
- IC
internal capsule
- ICPSD
IC-evoked poststimulus spike discharges
- IPSC
inhibitory postsynaptic current
- mIPSC
miniature IPSC
- NAcc
nucleus accumbens
- NMDA
N-methyl-d-aspartate
- P
postnatal day
- RT-PCR
reverse transcription–polymerase chain reaction
- sIPSC
spontaneous IPSC
- TH
tyrosine hydroxylase
- TTX
tetrodotoxin
- VSSC
voltage-sensitive sodium channel
- VTA
ventral tegmental area
References
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SH, Steffensen SC. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse (New York, NY) 2006;60:20–31. doi: 10.1002/syn.20272. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bennett SA, Arnold JM, Chen J, Stenger J, Paul DL, Roberts DC. Long-term changes in connexin32 gap junction protein and mRNA expression following cocaine self-administration in rats. Eur J Neurosci. 1999;11:3329–3338. doi: 10.1046/j.1460-9568.1999.00752.x. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron. 2003;39:109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci. 2006;26:3412–3422. doi: 10.1523/JNEUROSCI.5274-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse (New York, NY) 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Schiffer WK, Horan BA, Highfield D, Dewey SL, Brodie JD, Ashby CR., Jr Gamma-vinyl GABA, an irreversible inhibitor of GABA transaminase, alters the acquisition and expression of cocaine-induced sensitization in male rats. Synapse. 2002;46:240–250. doi: 10.1002/syn.10138. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Johnson KM. Comparison of the role of local anesthetic properties with dopamine uptake blockade in the inhibition of striatal and nucleus accumbens [3H]acetylcholine release by cocaine. J Pharmacol Exp Ther. 1992;263:757–761. [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol (Lond) 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors. J Neurophysiol. 1995;74:2126–2137. doi: 10.1152/jn.1995.74.5.2126. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Leon Brown P. The role of peripheral and central sodium channels in mediating brain temperature fluctuations induced by intravenous cocaine. Brain Res. 2006;1117:38–53. doi: 10.1016/j.brainres.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Exaerde AK, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr. 1994;145:1–18. [PubMed] [Google Scholar]
- Kuhar MJ. Molecular pharmacology of cocaine: a dopamine hypothesis and its implications. Ciba Found Symp. 1992;166:81–89. doi: 10.1002/9780470514245.ch6. discussion 89-95. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AS, Kopell NJ, Wilson CJ. Transient high-frequency firing in a coupled-oscillator model of the mesencephalic dopaminergic neuron. J Neurophysiol. 2006;95:932–947. doi: 10.1152/jn.00691.2004. [DOI] [PubMed] [Google Scholar]
- Lassen MB, Brown JE, Stobbs SH, Gunderson SH, Maes L, Valenzuela CF, Ray AP, Henriksen SJ, Steffensen SC. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res. 2007;1156:46–58. doi: 10.1016/j.brainres.2007.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Steffensen SC, Henriksen SJ. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J Neurosci. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Simon SM. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci USA. 1982;79:2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol. 1995;488(Pt 1):85–101. doi: 10.1113/jphysiol.1995.sp020948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary ME, Chahine M. Cocaine binds to a common site on open and inactivated human heart (Na(v)1.5) sodium channels. J Physiol. 2002;541:701–716. doi: 10.1113/jphysiol.2001.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse (New York, NY) 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Pan HT, Menacherry S, Justice JB., Jr Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem. 1991;56:1299–1306. doi: 10.1111/j.1471-4159.1991.tb11425.x. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB., Jr Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Postma SW, Catterall WA. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984;25:219–227. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science (New York, NY) 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following intraventricular 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;24:881–887. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse (New York, NY) 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ruffieux A, Schultz W. Influence of dopamine on pars reticulata neurones of substantia nigra. J Physiol (Paris) 1981;77:63–69. [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Tella SR, Erzouki HK, Goldberg SR. Pharmacological mechanisms in cocaine's cardiovascular effects. Drug Alcohol Depend. 1995;37:183–191. doi: 10.1016/0376-8716(94)01083-w. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Shriver DA, Long JP. A pharmacologic comparison of some quaternary derivatives of cocaine. Arch Int Pharmacodyn Ther. 1971;189:198–208. [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Henriksen SJ, Wilson MC. Transgenic rescue of SNAP-25 restores dopamine-modulated synaptic transmission in the coloboma mutant. Brain Res. 1999;847:186–195. doi: 10.1016/s0006-8993(99)02023-5. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202:139–151. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves NMDA receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Strichartz G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology. 1976;45:421–441. doi: 10.1097/00000542-197610000-00012. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science (New York, NY) 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Waddington JL. Do D2-like dopamine receptors mediate neuronal excitation or inhibition: some functional-behavioural implications. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997;17:111–112. [PubMed] [Google Scholar]
- Waszczak BL, Walters JR. Endogenous dopamine can modulate inhibition of substantia nigra pars reticulata neurons elicited by GABA iontophoresis or striatal stimulation. J Neurosci. 1986;6:120–126. doi: 10.1523/JNEUROSCI.06-01-00120.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Pottek M, He S, Vaney DI. Modulation of coupling between retinal horizontal cells by retinoic acid and endogenous dopamine. Brain Res Brain Res Rev. 2000;32:121–129. doi: 10.1016/s0165-0173(99)00071-5. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. Eur J Pharmacol. 1999;367:175–181. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]