Abstract
The neural mechanisms underlying the transition from a drug-nondependent to a drug-dependent state remain elusive. Chronic exposure to drugs has been shown to increase brain-derived neurotrophic factor (BDNF) levels in ventral tegmental area (VTA) neurons. BDNF infusions into the VTA potentiate several behavioral effects of drugs, including psychomotor sensitization and cue-induced drug seeking. We found that a single infusion of BDNF into the VTA promotes a shift from a dopamine-independent to a dopamine-dependent opiate reward system, identical to that seen when an opiate-naïve rat becomes dependent and withdrawn. This shift involves a switch in the γ-aminobutyric acid type A (GABAA) receptors of VTA GABAergic neurons, from inhibitory to excitatory signaling.
The ventral tegmental area (VTA) serves as an anatomical locus controlling the switch from an opiate-nondependent to an opiate-dependent state (1, 2). In nondependent rats, opiate reward is mediated by a dopamine-independent neural system, involving the brainstem tegmental pedunculopontine nucleus (TPP) (3). Once chronically exposed to opiates and in a state of withdrawal, opiate reward switches to a dopamine-dependent system (3). It has been observed that the switch between the two motivational systems is due to a switch in γ-aminobutyric acid type A (GABAA) receptor functioning in VTA GABAergic neurons, from an inhibitory to an excitatory signaling state (fig. S1) (2).
Brain-derived neurotrophic factor (BDNF) is capable of producing this change in GABAergic response, from inhibitory to excitatory, as has been observed in the hippocampus during epileptic seizures (4) and in the spinal cord during neuropathic pain (5). BDNF is present in the VTA (6), and its TrkB receptors are present on both GABA (fig. S2) and dopamine VTA neurons (7, 8). Chronic exposure to drugs of abuse increase BDNF levels in VTA neurons (6). Furthermore, BDNF infusions into the VTA dramatically enhance several behavioral effects of drugs, including psychomotor sensitization (6, 9) and drug seeking (6, 10). We hypothesized that, along with the changes in structural plasticity induced by BDNF in VTA dopaminergic neurons (11), increasing BDNF levels in the VTA would induce a switch to a drug-dependent motivational state in drug-nondependent rats due to the effects of BDNF on GABAergic neurons.
First, we examined whether BDNF protein and mRNA levels in the VTA were increased in opiate-dependent rats. Sixteen hours after withdrawal from repeated daily exposure to heroin (0.5 mg/kg, subcutaneously) for 8 days [see supporting online material (SOM)], BDNF protein (F3,37 = 7.63, P < 0.05) and BDNF mRNA (F3,19 = 4.04, P < 0.05) levels in the VTA increased by 150% (P < 0.05) and 193%, respectively, of the control drug-naïve rats (P < 0.05). However, there were no increases in BDNF either when rats received a single injection of heroin (P > 0.05) or 15 days after withdrawal from repeated heroin exposure (P > 0.05) (fig. S3).
To explore whether BDNF alone was sufficient to cause a change in the neurobiological substrates mediating opiate reward, we next performed place conditioning procedures on rats after single bilateral intra-VTA BDNF (0.25 μg each) infusions (10). Place conditioning procedures (12) (see SOM) were performed 3 days after BDNF infusions (10). During training, we administered four alternating injections of intraperitoneal morphine (10 mg/kg) and vehicle to the rats over 8 days. The dopamine receptor antagonist alpha-flupenthixol (0.8 mg/kg) (or its saline vehicle) was injected intraperitoneally 2.5 hours before each conditioning trial. Testing was performed drug-free between 48 and 72 hours after the final conditioning session. After VTA BDNF (n = 10 animals) [but not the saline vehicle phosphate-buffered saline (PBS) (n = 20)] infusion, antagonism of the dopaminergic system blocked the rewarding effects of acute systemic morphine in drug-nondependent rats (F1,59 = 5.3, P < 0.05, interaction of morphine and BDNF treatment). Infusion of BDNF outside of the VTA (due to cannulae misplaced rostral, ventral, or lateral to the VTA) (fig. S4) did not allow the dopamine antagonist to block the rewarding effects of morphine in nondependent rats (t10 = 4.65, P < 0.05, n = 11) (Fig. 1A). It is possible that intra-VTA BDNF modifies the ability of opiates to produce conditioned place preferences, making them easier to block. However, neither BDNF (n = 7) nor PBS (n = 7) alone had any effect on the sizes of the conditioned place preferences produced by morphine [F1,27 = 12.84, P < 0.05, main effect of the morphine treatment only in both BDNF (P < 0.05) and PBS (P < 0.05) groups] (Fig. 1B).
Fig. 1.

Motivational effects of a single intra-VTA BDNF infusion in drug-nondependent rats. (A) Blockade of the dopaminergic system with neuroleptics (alpha-flupenthixol, 0.8 mg/kg) blocked the rewarding effects of morphine (10 mg/kg) in nondependent rats after a single intra-VTA BDNF (0.25 μg) infusion, but not after intra-VTA PBS infusion (*P < 0.05). The same treatment failed to block the rewarding effects of morphine in nondependent rats when BDNF was infused rostral, ventral, or lateral to the VTA because of missed cannulae placements (*P < 0.05). Error bars indicate SE of the mean. (B) Intra-VTA BDNF alone did not affect the sizes of the conditioned place preferences produced by acute morphine administration in drug-nondependent rats (*P < 0.05). (C) BDNF restored the rewarding properties of acute morphine administration in drug-nondependent TPP-lesioned rats (*P < 0.05). (D) Cresyl violet–stained coronal section showing one side of a bilateral TPP lesion and (below) a schematic of the anatomical region from which the section displayed was taken. The arrow indicates the TPP lesion area. (E) Cresyl violet–stained coronal section of a typical bilateral intra-VTA cannula placement.
To investigate whether BDNF in the VTA changes opiate reward from a dopamine-independent (TPP-dependent) to a dopamine-dependent reward system, we examined the effects of intra-VTA BDNF in TPP-lesioned rats (13, 14). In drug-nondependent rats, bilateral TPP (n = 12) [but not sham (n = 14)] lesions blocked the rewarding properties of morphine (F1,51 = 14.29, P < 0.05, interaction of lesion and morphine treatment). However, after BDNF infusion (n = 12), TPP lesions did not block morphine reward (P < 0.05) in drug-nondependent rats, similar to sham-lesioned rats, where morphine also caused reward (P < 0.05) (F1,51 = 47.87, P < 0.05, main effect of morphine only), but opposite to TPP-lesioned rats infused with PBS, where morphine reward was blocked (P < 0.5) (F1,47 = 17.14, P < 0.05, interaction of BDNF and morphine treatment) (Fig. 1C).
To examine whether the GABAA receptors on VTA GABAergic neurons (15) are involved in the change in opiate reward system after intra-VTA BDNF infusion, we explored the effects of intra-VTA infusion of the GABAA receptor antagonist bicuculline. In drug-nondependent rats, intra-VTA bicuculline (50 ng) produced a robust rewarding effect through a non-dopaminergic, TPP-dependent reward system (P < 0.05). However, in drug non-dependent rats infused with intra-VTA BDNF (n = 11) [but not PBS (n = 8)], alpha-flupenthixol now blocked the rewarding effects of bicuculline (P > 0.05) (F1,37 = 5.88, P < 0.05, interaction of BDNF and bicuculline). (Fig. 2A). To study the physiological effects of BDNF on GABAA inhibition of VTA GABA neurons, we evaluated the response of VTA GABA neurons to in situ administration of the GABAA agonist muscimol. Single-unit extracellular recordings of VTA GABAergic neurons (16) (four to five cells from rat) revealed that a shift from inhibitory to excitatory GABAA receptor signaling was observed in a subset of GABAergic neurons. Iontophoretic application of muscimol excited 42% [12 out of 31 (12/31)] of VTA GABAergic neurons in intra-VTA BDNF-infused rats (n = 8) compared with intra-VTA PBS (n = 8) infused controls, where 100% (29/29) of the VTA GABAergic neurons were inhibited by muscimol (Mann-Whitney U test, P < 0.05) (Fig. 2, B and C). This shift is very similar to that seen in a previous study with opiate-dependent rats, where 44% of VTA GABAergic neurons switched to excitatory GABAA receptor signaling (2). In BDNF-treated rats (16.6 ± 8.9%, n = 11), as in control PBS-treated rats (19.5 ± 9.26%, n = 7), the GABAB receptor agonist baclofen was still able to inhibit those GABA neurons that had been excited by muscimol (t17 = 0.216, P > 0.05) (Fig. 2, B and C), suggesting very specific effects of BDNF on GABAA receptor signaling on the VTA GABAergic neurons themselves.
Fig. 2.
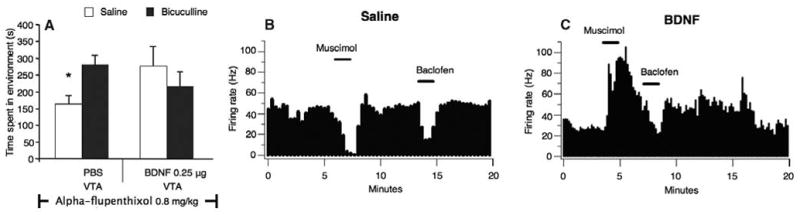
Motivational and electrophysiological effects of a single intra-VTA BDNF infusion in drug-nondependent rats. (A) Blockade of the dopaminergic system with alpha-flupenthixol blocked the rewarding effects of an intra-VTA–administered GABAA receptor antagonist bicuculline (50 ng) in nondependent rats after a single intra-VTA BDNF infusion, but not after intra-VTA PBS infusion (*P < 0.05). (B and C) Single-unit extracellular recordings of VTA GABAergic neurons showing the effects of two current applications of muscimol (+50 nA) and baclofen (+50 nA) on the firing rate of representative VTA GABAergic neurons. In saline-treated rats, muscimol always decreased the firing rate of the VTA GABAergic neurons, whereas in BDNF-treated rats, muscimol enhanced the firing rate. Conversely, in both saline-treated and BDNF-treated rats, baclofen moderately decreased the firing rate of these VTA GABA neurons, as in this example.
The intra-VTA alterations that lead to this transformation are unclear. BDNF may change the anion gradient by reducing the level of the potassium-chloride co-transporter KCC2, so that the GABAergic neuron intracellular chloride concentration increases (6, 17). GABAA receptor activation would then result in anions flooding out of the neuron, making the neuron’s membrane potential more positive, or depolarized, relative to the resting membrane potential. Also, BDNF might elevate intracellular carbonic anhydrase levels, favoring HCO3− efflux and resulting in a depolarizing response in VTA GABAergic neurons (fig. S1) (2).
The present work suggests that BDNF in the VTA induces a transition to a drug-dependent motivational state, a crucial issue in drug addiction research. Other studies have found that BDNF levels within the mesolimbic system progressively increase after cocaine withdrawal (18). Intra-VTA BDNF infusion produces long-lasting enhancement of cocaine seeking (18) and locomotor stimulation (6, 9), and it increases the rewarding effects of cocaine itself (9). Conversely, cocaine-conditioned place preference was reduced in heterozygous BDNF knockout mice (9), and inhibiting BDNF activity reduces amphetamine-induced dopamine release (19). Our findings complement research in animal and clinical studies suggesting that VTA BDNF is implicated in the pathogenesis of drug addiction (20).
Supplementary Material
Acknowledgments
This work was funded by the Canadian Institutes of Health Research. S.C.S. received funding from NIH (grant AA13666).
Footnotes
References and Notes
- 1.Laviolette SR, van der Kooy D. Eur J Neurosci. 2001;13:1009. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- 2.Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Nat Neurosci. 2004;7:160. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- 3.Laviolette SR, Nader K, van der Kooy D. Behav Brain Res. 2002;129:17. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 4.Rivera C, et al. J Cell Biol. 2002;159:747. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coull JA, et al. Nature. 2005;438:1017. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 6.Bolaños CA, Nestler EJ. Neuromol Med. 2004;5:69. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 7.Numan S, Seroogy KB. J Comp Neurol. 1999;403:295. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Hyman C, et al. J Neurosci. 1994;14:335. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horger BA, et al. J Neurosci. 1999;19:4110. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. J Neurosci. 2004;24:1604. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman SE, Malenka RC, Nestler EJ. Annu Rev Neurosci. 2006;29:565. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 12.Bechara A, van der Kooy D. Behav Neurosci. 1992;106:351. doi: 10.1037//0735-7044.106.2.351. [DOI] [PubMed] [Google Scholar]
- 13.Kippin TE, van der Kooy D. Eur J Neurosci. 2003;18:2581. doi: 10.1046/j.1460-9568.2003.02918.x. [DOI] [PubMed] [Google Scholar]
- 14.Vargas-Perez H, et al. Eur J Neurosci. 2007;25:3713. doi: 10.1111/j.1460-9568.2007.05599.x. [DOI] [PubMed] [Google Scholar]
- 15.Laviolette SR, van der Kooy D. Eur J Neurosci. 2004;20:2179. doi: 10.1111/j.1460-9568.2004.03665.x. [DOI] [PubMed] [Google Scholar]
- 16.Steffensen SC, Lee RS, Stobbs SH, Henriksen SJ. Brain Res. 2001;906:190. doi: 10.1016/s0006-8993(01)02581-1. [DOI] [PubMed] [Google Scholar]
- 17.Wake H, et al. J Neurosci. 2007;27:1642. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm JW, et al. J Neurosci. 2003;23:742. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Neuroscience. 2003;119:767. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai SJ. Med Hypotheses. 2007;68:410. doi: 10.1016/j.mehy.2006.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


