Abstract
Background
Inguinal hernia repair is associated with a high incidence of chronic postsurgical pain. This pain may be caused by injury to the iliohypogastric, ilioinguinal, or genitofemoral nerves. It is often difficult to identify the specific source of the pain, in part, because these nerves are derived from overlapping nerve roots and closely colocalize in the area of surgery. It is therefore technically difficult to selectively block these nerves individually proximal to the site of surgical injury. In particular, the genitofemoral nerve is retroperitoneal before entering the inguinal canal, a position that puts anterior approaches to the proximal nerve at risk of transgressing into the peritoneum. We report a computed tomography (CT)-guided transpsoas technique to selectively block the genitofemoral nerve for both diagnostic and therapeutic purposes while avoiding injury to the nearby ureter and intestines.
Case
A 39-year-old woman with chronic lancinating right groin pain after inguinal hernia repair underwent multiple pharmacologic interventions and invasive procedures without relief. Using CT and Stimuplex nerve stimulator guidance, the genitofemoral nerve was localized on the anterior surface of the psoas muscle and a diagnostic block with local anesthetic block was performed. The patient had immediate relief of her symptoms for 36 hours, confirming the diagnosis of genitofemoral neuralgia. She subsequently underwent CT-guided radiofrequency and phenol ablation of the genitofemoral nerve but has not achieved long-term analgesia.
Conclusion
CT-guided transpsoas genitofemoral nerve block is a viable option for safely and selectively blocking the genitofemoral nerve for diagnostic or therapeutic purposes proximal to injury caused by inguinal surgery.
Keywords: Acute Pain, Chronic Pain, Pain Medicine, Pain Management, Pain Disorder, Ablation, Burning Pain, Interventional, Nerve Block, Nerve Conduction
Introduction
Inguinal hernia repair is frequently complicated by debilitating chronic postoperative pain [1,2]. These patients pose a challenge in both diagnosis and treatment [3]. Injuries to the ilioinguinal nerve, iliohypogastric nerve, and/or genital branch of the genitofemoral nerve are well-recognized causes of post-herniorrhaphy pain [4,5]. It is often difficult, however, to identify the specific nerve injury that is giving rise to a patient's post-herniorrhaphy neuritic symptoms because these nerves are derived from overlapping nerve roots and closely colocalize in the area of surgery. Nonetheless, distinguishing among ilioinguinal, iliohypogastric, and genitofemoral nerves as the origin of persistent pain following herniorrhaphy is important in order to appropriately direct invasive treatments such as surgery [5], stimulation [6], or percutaneous pulsed radiofrequency [7,8].
Ectopic impulses responsible for neuropathic pain reach the central nervous system by propagating proximally from the site of nerve injury [9]. Consequently, blockade of nerve impulse proximal to the site of injury by injection of local anesthetic will block pain-generating ectopic impulses arising more distally and provide temporary relief; furthermore, this specifically identifies the nerve playing a role in the patient's pain. By contrast, application of local anesthetic distal to the site of injury may not block ectopic impulses arising more proximally at the site of injury, and may not yield relief even if applied to the correct nerve. Therefore, to best identify a specific nerve as the putative source of a given pain, it is desirable that the nerve be blocked proximal to the point of nerve injury. Unfortunately, the classic approach to blocking the genitofemoral nerve is to block it at the pubic tubercle; this point is distal to the presumed site of injury during herniorrhaphy, typically in the inguinal canal or at the deep inguinal ring. A major difficulty in attempting to block the genitofemoral nerve proximal to its usual site of injury, however, is that it is retroperitoneal prior to entering the inguinal canal. Given this difficulty, we explored the use of computed tomography (CT) guidance to safely perform transpsoas genitofemoral nerve block.
The Case
A 36-year-old woman presented to our pain management clinic, a tertiary referral center, with a chief complaint of right groin pain for the past 13 years following an inguinal hernia repair. The patient was bedbound the majority of the day due to severe spontaneous and activity-evoked pain. She had undergone multiple courses of physical therapy, occupational therapy, cognitive behavioral therapy, and medication trials including opioids, anticonvulsants, and antidepressants without significant relief. She also underwent a trial of peripheral nerve field stimulation with a peripheral stimulating lead placed subcutaneously in the inguinal region by a neurosurgeon, but this was discontinued due to worsening of the groin pain. She was started on a course of duloxetine 120 mg daily and pregabalin 100 mg tid, and she was allowed to continue meperidine started by an outside doctor on a scheduled basis. Despite 2 weeks on our inpatient biobehavioral unit for multidisciplinary care including coordinated intensive cognitive behavioral therapy, physical therapy, and medical optimization, she remained functionally bedbound upon discharge, and reported pain scores ranging from 6/10 to 9/10. The patient had been particularly limited by movement-evoked pain every time she extended her hip on the side of the previous herniorrhaphy. This activity-evoked pain was identified as a significant barrier to our functional restoration approach.
She was subsequently readmitted to our institution for diagnostic CT-guided genitofemoral nerve block. Using CT guidance and with the patient in prone position, a 6-inch Stimuplex (B. Braun Medical Inc., Bethlehem, PA) electrical stimulating needle was advanced perpendicular to the skin just superior to the right L4 transverse process. After several passes, stimulation radiating to the groin and upper thigh was achieved at less than 0.4 V. Three milliliters of 0.5% bupivicaine with Omnipaque 240 (GE Healthcare Waukesha, WI) was injected. In the recovery room, she displayed evidence of sensory loss in the distribution of the genitofemoral nerve. The patient subsequently reported that her pain improved from 8/10 to 3/10 for 3 days; during this time, she was observed in the hospital to be significantly more physically active, including walking up and down stairs, an activity that she could not do prior to the procedure. Her pain gradually returned to baseline after 3 days and she was scheduled to return for a neuroablative procedure.
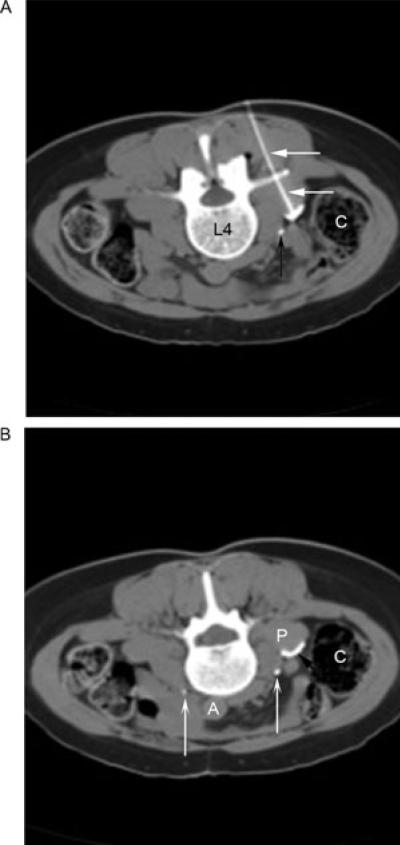
On the date of her procedure, the patient was placed prone on the CT scanner table. Omnipaque 240 was injected intravenously to opacify the ureter. CT guidance was then used to localize the entry point for needle insertion just superior to the L4 transverse process. A 22-gauge 10 cm radiofrequency needle was then inserted and directed toward the anterior surface of the psoas muscle. At the anterior surface of the psoas, electrical stimulation at 50 Hz was applied. Following several needle passes during which the needle was redirected slightly medial and then slightly lateral, the patient achieved sensory stimulation radiating to the groin at 0.35 V at 50 Hz, and two radiofrequency lesions were made at 80°C for 90 seconds each. After the radiofrequency ablation, 0.5 mL of a 5 mL premixed solution containing 25% Omnipaque and 7% phenol was injected and was seen to outline the anterior surface of the psoas muscle (Figure 1A). Although the patient developed numbness in the genitofemoral distribution, she unfortunately continues to experience limited motion, and her use of pain medication is undiminished leading us to characterize the neurolytic block as a therapeutic failure.
Figure 1.
(A) An axial computed tomography (CT) image in soft tissue window with the patient in prone position demonstrates the needle (straight white arrows) being advanced under CT and stimulating guidance until sensory stimulation at 50 Hz was achieved at 0.35 V. Concave black arrow, right ureter. C = ascending colon; L4 = fourth lumbar vertebral body. (B) A later axial CT image in soft tissue window demonstrates 0.5 mL of Omnipaque with phenol spreading (straight black arrow) on the anterior surface of the psoas muscle (P), but remaining medial and posterior to the ureter (concave white arrows, right and left ureters) and lumbar sympathetic chain, posterior and medial to the colon (C), and anterior to the lumbar nerve roots. A = aorta.
Discussion
Injury to the genitofemoral nerve may present with pain radiating from the lower abdomen to the anterior thigh and labia majora in women and the scrotum in men. Although the course of this nerve and its branches is similar in males and females, anatomic studies suggest that wide variability among individuals exists, with as few as 37% of nerves following a traditionally described path [10]. The genitofemoral nerve emerges as a confluence of fibers from the first and second lumbar nerve roots. These fibers then traverse the psoas muscle to emerge and descend on its anterior surface. The genitofemoral nerve then divides into genital and femoral branches at a variable distance proximal to the inguinal ligament. In males, the genital branch passes through the internal inguinal ring and travels with the spermatic cord to supply motor fibers to the cremaster muscle and sensory fibers to the scrotum. In females, the genital branch accompanies the round ligament to innervate the labia majora [11]. The femoral branch is located caudad and lateral to the genital branch and travels on the anterior surface of the external iliac artery under the inguinal ligament to supply the skin of the mid-anterior thigh [12].
Differentiation of nerve pain related to genitofemoral vs ilioinguinal neuropathy can be a diagnostic challenge, as the territories of these nerves overlap. Additionally, distal communications may exist between the ilioinguinal and iliohypogastric nerves, further obscuring the cause of a patient's pain [13]. A single report exists of trying to block the genitofemoral nerve using a transpsoas technique [14]. This author used fluoroscopy to identify the L3-L4 vertebral space and advance a needle using a “double loss of resistance” technique to identify first the anterior surface of the quadratus lumborum and then the anterior surface of the psoas. The author highlighted the drawbacks of such a technique as 1) failure to appreciate the loss of resistance at the anterior surface of the psoas may lead to peritoneal entry, and 2) reduced specificity of the block created by the likelihood of inadvertently anesthetizing the lumbar sympathetic chain due to the large volume of injectate needed to ensure adequate spread to the genitofemoral nerve. To these risks should be added the possibility of injury to the ureter, a complication that has been reported following a similar transpsoas approach to the lumbar sympathetic chain [15]. To accomplish our selective block of the genitofemoral nerve on the anterior surface of the psoas muscle, we used an insulated electrically stimulating needle in combination with CT guidance to 1) ensure correct needle placement depth so that the tip was at the anterior surface of the psoas where the genitofemoral nerve is to be found, and 2) avoid the ureter on the anterior surface of the psoas and peritoneum just anterior to that surface. Identification of the ureter was facilitated by the administration of intravenous (IV) contrast. The genitofemoral nerve itself is too small to be identified on CT scan. In order to place the needle tip sufficiently close to the genitofemoral nerve to obtain Stimuplex-induced paresthesias in the genitofemoral distribution (thereby ensuring a low dose of injectate will be sufficient to block the nerve), several needle passes adjusting the placement of the needle tip medially and laterally on the anterior surface of the psoas are needed to place the needle. This highlights the importance of using CT guidance to avoid the ureter and peritoneum. Furthermore, use of CT and Stimuplex guidance allowed the needle tip to be placed close enough to the genitofemoral nerve that a large volume of injectate was not needed to anesthetize the nerve. Use of low volumes of injectate limits spread on the anterior surface of the psoas muscle and should help to avoid anesthetizing the nearby lumbar sympathetic chain.
The CT-guided transpsoas technique described here, it is hoped, may reduce the risk of injury to the ureter or intestines posed by previously described blind techniques. However, it should be anticipated that these issues remain risks, as well as the possibility of retroperitoneal or psoas hematoma. This is particularly true given the possibility of multiple needle passes through the psoas muscle.
Unfortunately, our patient failed to experience durable pain resolution with percutaneous neurolysis despite evidence of sensory loss of the genitofemoral nerve. This outcome highlights the hazard of interpreting diagnostic blocks as being prognostic for future neurolytic injections. We had particularly hoped that by moving the site of nerve injury from the inguinal scar to a less disturbed retroperitoneal location (at the site of the neurolytic block), we might allow the patient to have less movement-evoked pain, and thereby improve activity. The patient's continued pain in the setting of loss of sensation in the distribution of the genitofemoral nerve (and her only-partial relief following the diagnostic block) suggests that she had pain on the basis of more than simple genitofemoral nerve injury. In this regard, tethering of the iliohypogastric, ilioinguinal, or genitofemoral nerves in scar tissue or hernia repair mesh may be an important part of the pathophysiology that requires surgical exploration [16]. Tethering of the nerves in this context may involve neuropathic, nociceptive, and inflammatory elements not amenable to the neurolysis of a single nerve [17].
Other practitioners should note the potential hazards in attempting to predict the outcome of a neurolysis based on a single diagnostic block as in this case. Given the risks of deafferentation-type pain following a neurolytic block in a noncancer patient, practitioners may find it advisable to repeat diagnostic injections at least once. Practitioners should also consider temporary but possibly long lasting alternatives such as pulsed radiofrequency or cryotherapy. Advocates of these techniques have reported durable relief ostensibly with less risk of neuroma formation, and less risk of deafferentation compared with neurodestructive techniques [18].
Our technique allows for reliable identification of the anterior surface of the psoas muscle and the traversing ureter, as well as the colon. This in turn allows sequential passage of a stimulating needle without fear of intestinal or ureteral puncture until stimulation results in paresthesias in the genitofemoral distribution. Following this, a low-volume injection can be done with contrast to ensure minimal spreading to other nerves such as the lumbosacral plexus or lumbar sympathetic chain. This precise CT-guided technique allows for diagnostic injections as well as definitive neurolysis at a location more proximal than those previously described, and hence at a level where blockade of the genitofemoral nerve can be readily distinguished from that of the ilioinguinal nerve.
Alternative image-guided approaches may also prove useful in these cases. Magnetic resonance neurography [19] is becoming a steadily more reliable manner of imaging nerves [20], and may in the future allow sufficient visualization of the genitofemoral nerve on the surface of the psoas muscle to make multiple passes of the needle through the psoas to find the nerve unnecessary. Ultrasound imaging may also prove useful. The authors have identified two recent reports of ultrasound being used to approach branches of the genitofemoral nerve. Both of these approaches used an anterior approach and blocked either the genital branch [18] or femoral branch [21] distal-to-deep inguinal ring where these nerves are in close proximity to branches of the ilioinguinal nerve. While we were not able to identify reports of an ultrasound-guided transpsoas technique to block the genitofemoral nerve in the proximal location described in this report, we expect that such an approach might be feasible. If so, ultrasound might prove the more desirable technique as it may be more readily available and may cost less than CT guidance. The possible additional costs of doing such a procedure under CT should be considered. For other procedures in which needle placement is image guided, CT has been estimated to be up to twice as costly as when performed under ultrasound guidance [22]. Both of these techniques can be expected to be significantly more costly than traditional landmark-based techniques.
To our knowledge, this report is the second to describe a transpsoas approach to the genitofemoral nerve, and the first to describe using CT guidance to enhance the specificity and safety of approaching the genitofemoral nerve at this desirable proximal location. We hope that the described technique will offer a new and safe approach to the many pain specialists dealing with post-herniorrhaphy pain.
References
- 1.Mui WL-M, Ng CSH, Fung TM-K, et al. Prophylactic ilioinguinal neurectomy in open inguinal hernia repair: A double-blind randomized controlled trial. Ann Surg. 2006;244(1):27–33. doi: 10.1097/01.sla.0000217691.81562.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picchio M, Palimento D, Attanasio U, Matarazzo PF, Bambini C, Caliendo A. Randomized controlled trial of preservation or elective division of ilioinguinal nerve on open inguinal hernia repair with polypropylene mesh. Arch Surg. 2004;139(7):755–8. doi: 10.1001/archsurg.139.7.755. discussion 759. [DOI] [PubMed] [Google Scholar]
- 3.Chambers WA, Krukowski Z, King P, Smith WCS, Bruce J, Poobalan A. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain. 2003;19(1):48–54. doi: 10.1097/00002508-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Aasvang EK, Brandsborg B, Christensen B, Jensen TS, Kehlet H. Neurophysiological characterization of postherniotomy pain. Pain. 2008;137(1):173–81. doi: 10.1016/j.pain.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Amid PK. Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: Triple neurectomy with proximal end implantation. Hernia. 2004;8(4):343–9. doi: 10.1007/s10029-004-0247-0. [DOI] [PubMed] [Google Scholar]
- 6.Rauchwerger J, Giordano J, Rozen D, Kent J, Greenspan J, Closson C-W. On the therapeutic viability of peripheral nerve stimulation for ilioinguinal neuralgia: Putative mechanisms and possible utility. Pain Pract. 2008;8(2):138–43. doi: 10.1111/j.1533-2500.2007.00174.x. [DOI] [PubMed] [Google Scholar]
- 7.Rozen D, Parvez U. Pulsed radiofrequency of lumbar nerve roots for treatment of chronic inguinal herniorraphy pain. Pain Physician. 2006;9(2):153–6. [PubMed] [Google Scholar]
- 8.Mitra R, Zeighami A, Mackey S. Pulsed radiofrequency for the treatment of chronic ilioinguinal neuropathy. Hernia. 2007;11(4):369–71. doi: 10.1007/s10029-007-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aurilio C, Pota V, Pace M, Passavanti M, Barbarisi M. Ionic channels and neuropathic pain: Physiopathology and applications. J Cell Physiol. 2008;215(1):8–14. doi: 10.1002/jcp.21280. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189(6):1574–8. doi: 10.1016/s0002-9378(03)00934-7. discussion 1578. [DOI] [PubMed] [Google Scholar]
- 11.Hollingshead WH, Rosse C. Textbook of Anatomy. 4th edition Harper & Row; Philadelphia: 1985. [Google Scholar]
- 12.Clemente CD. Anatomy: A Regional Atlas of the Human Body. 4th edition Williams & Wilkins; Baltimore, MD: 1997. [Google Scholar]
- 13.Rab M, Ebmer J, Dellon AL. Anatomic variability of the ilioinguinal and genitofemoral nerve: Implications for the treatment of groin pain. Plast Reconstr Surg. 2001;108(6):1618–23. doi: 10.1097/00006534-200111000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Hartrick CT. Genitofemoral nerve block: A transpsoas technique. Reg Anesth. 1994;19(6):432–3. [PubMed] [Google Scholar]
- 15.Kumsar SK, Dirim A. Iatrogenic ureteral injury due to lumbar sympathetic block. Scand J Urol Nephrol. 2008;42(4):395–6. doi: 10.1080/00365590801966911. [DOI] [PubMed] [Google Scholar]
- 16.Delikoukos S, Fafoulakis F, Christodoulidis G, Theodoropoulos T, Hatzitheofilou C. Re-operation due to severe late-onset persisting groin pain following anterior inguinal hernia repair with mesh. Hernia. 2008;12:593–5. doi: 10.1007/s10029-008-0392-y. [DOI] [PubMed] [Google Scholar]
- 17.Miller JP, Acar F, Kaimaktchiev VB, Gultekin SH, Burchiel KJ. Pathology of ilioinguinal neuropathy produced by mesh entrapment: Case report and literature review. Hernia. 2008;12:213–16. doi: 10.1007/s10029-007-0290-8. [DOI] [PubMed] [Google Scholar]
- 18.Campos N, Chiles J, Plunkett A. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009;12:997–1000. [PubMed] [Google Scholar]
- 19.Filler AG, Kliot M, Winn HR, et al. Magnetic resonance neurography. The Lancet. 1993;341:659–61. doi: 10.1016/0140-6736(93)90422-d. [DOI] [PubMed] [Google Scholar]
- 20.Filler A. Magnetic resonance neurography and diffusion tensor imaging: Origins, history, and clinical impact of the first 50,000 cases with an assessment of efficacy and utility in a prospective 5000-patient study group. Neurosurgery. 2009;65:29–43. doi: 10.1227/01.NEU.0000351279.78110.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng PWH, Tumber P. Ultrasound-guided interventional procedures for patients with chronic pelvic pain—A description of techniques and review of literature. Pain Physician. 2008;11:215–24. [PubMed] [Google Scholar]
- 22.Kliewer M, Sheafor D, Paulson E, Helsper R, Hertzberg B, Nelson R. Percutaneous liver biopsy: A cost-benefit analysis comparing sonographic and CT guidance. Am J Roentgenol. 1999;173:1199–202. doi: 10.2214/ajr.173.5.10541088. [DOI] [PubMed] [Google Scholar]