Abstract
Studies that seek to associate reduced human health with exposure to occupational and environmental aerosols are often hampered by limitations in the exposure assessment process. One limitation involves the measured exposure metric itself. Current methods for personal exposure assessment are designed to estimate the aspiration of aerosol into the human body. Since a large proportion of inhaled aerosol is subsequently exhaled, a portion of the aspirated aerosol will not contribute to the dose. This leads to variable exposure misclassification (for heterogenous exposures) and increased uncertainty in health effect associations. Alternatively, a metric for respiratory deposition would provide a more physiologically relevant estimate of risk. To address this challenge, we have developed a method to estimate the deposition of aerosol in the human respiratory tract using a sampler engineered from polyurethane foam. Using a semi-empirical model based on inertial, gravitational, and diffusional particle deposition, a foam was engineered to mimic aerosol total deposition in the human respiratory tract. The sampler is comprised of commercially available foam with fiber diameter = 49.5 μm (equivalent to industry standard 100 PPI foam) of 8 cm thickness operating at a face velocity of 1.3 m s−1. Additionally, the foam sampler yields a relatively low-pressure drop, independent of aerosol loading, providing uniform particle collection efficiency over time.
Keywords: aerosols, exposure estimation, gravimetric analysis
INTRODUCTION
The ability of particulate matter air pollution to reach specific regions of the respiratory tract has long been recognized to depend on particle size. Regulatory institutions (ACGIH, 1985; CEN, 1993; ISO, 1995) have developed ‘penetration curves’ that define the fraction of particles, as a function of particle diameter, that can penetrate to different regions of the respiratory tract: inhalable (nose and mouth, see Fig. 1), thoracic (bronchial region), or respirable (alveolar region of lung). Personal aerosol samplers have since been designed to mimic these penetration curves to estimate the risk posed to humans working in proximity to various aerosol hazards. Size-selective sampling designs include the use of cyclones, diffusion batteries, and personal filter samplers with specially designed inlets, such as the Institute of Occupational Medicine (IOM), button sampler, and 37 mm cassette. Such samplers (inhalable, thoracic, and respirable), therefore, estimate the aspiration of aerosol into different regions of the respiratory tract. However, aerosol aspiration fractions can be a factor of 2–6 higher than both regional or total deposition fractions, due in large part to particle exhalation (Hodgkins et al., 1991). Both aspiration and deposition depend on particle size; however, the two phenomena are not linearly correlated. Consequently, the extent of exposure misclassification resulting from the use of an aspiration metric, or ‘penetration-based’ sampler, is highly variable and depends predominantly on the aerosol size distribution.
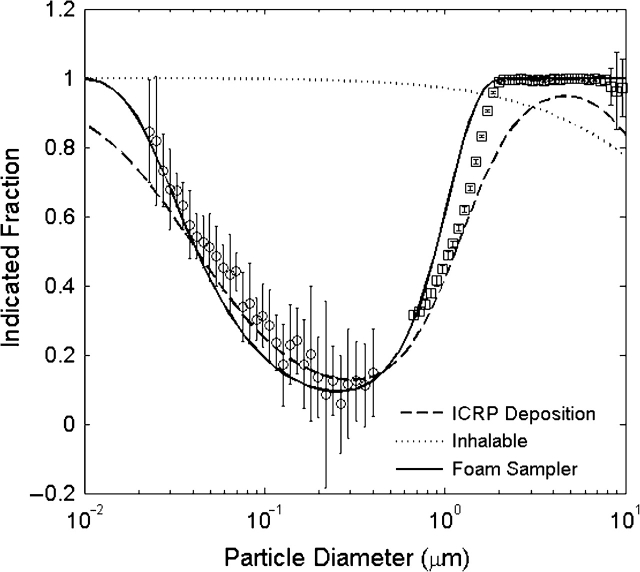
Fig. 1.
Particle deposition fraction for aerosol aspirated in the foam sampler compared to the ICRP total deposition for equivalent unit density spheres within the human respiratory tract. The inhalable fraction is shown for comparison (ACGIH, 1985). The solid line represents data [equation (1)]; the symbols represent experimental data. Error bars represent one standard deviation.
Johnson and Esmen (2004) noted that aspiration metrics are becoming more inadequate with the current trend for reduced aerosol exposure in the workplace. This is because changes in aerosol size distribution can cause dramatic changes in dose and, as such, there is not a constant proportion between aspiration and deposition. In fact, Esmen and Johnson (2002) found that dose estimation based on exposure measurement was only accurate within an order of magnitude. These observations led Johnson and Esmen (2004) to conclude that the ideal solution would be the development of a deposition-based sampler, but they reasoned that such a sampler was not likely to be achieved any time soon. Instead, they suggested that full aerosol size distributions should be measured in conjunction with penetration-based exposure estimates. However, repeated measurements of full aerosol size distributions are time and resource intensive.
Researchers have discussed the concept of a ‘deposition-based’ aerosol sampler, noting that although penetration-based samplers introduce an inherent ambiguity into exposure assessment, deposition-based samplers would simulate the size-specific deposition of inhaled aerosol. Thus, a sampler based on total (or regional) deposition would appear to provide improved information on which to base hazard evaluations (Soderholm and McCawley, 1990). However, history shows that the development of penetration-based samplers was instead pursued because of gaps in the knowledge base pertaining to specific particle deposition in the respiratory tract and to a lack of technology to mimic such deposition; penetration-based samplers were easier to design and manufacture (Soderholm and McCawley, 1990). Today, however, particle deposition patterns are well documented for both healthy and at-risk populations (EPA US, 2004).
A sampler that estimates respiratory deposition would provide a better indication of dose than commonly used aspiration-based samplers and, therefore, should provide a better framework for assessing the risk for exposed workers. Kuo et al. (2005) developed a size-selective sampling pre-separator to mimic the International Commission on Radiological Protection (ICRP, 1994) convention for total deposition in a healthy, human respiratory tract for aerosol with aerodynamic diameter (dae) < 0.8 μm. However, the foam pre-separator for this device removes virtually all particles with dae > 0.8 μm. Thus, their technique greatly underestimates the contribution from supermicron particles. Those authors suggested that since the number concentration of micrometer-sized aerosol was generally orders of magnitude lower than those of submicron aerosol, this might be a negligible flaw, if number is of interest. However, in many occupational settings, aerosol mass (as opposed to number) concentration is the preferred metric for estimating risk. Because the mass of a single 1 μm particle is equal to 1000 particles of 0.1 μm diameter (assuming similar shape and density), the underestimation of supermicron aerosol will significantly impact this sampler's ability to estimate deposition and, ultimately, risk.
In this work, we employed a semi-empirical model to inform the design of an aerosol sampler made from porous, polyurethane foam that can reproduce the size-specific deposition of aerosol in the human respiratory tract. We have used the ICRP Deposition Model for the average of adult males and females under light exercise and nose breathing conditions. Hereafter, we will refer to the deposition model run under these conditions as the ‘ICRP model’. We then verified our design with laboratory tests of sampler performance and reliability.
METHODS
Sampler design
Vincent et al. (1993) developed a semi-empirical model that predicts gravitational and inertial deposition of aerosol within foam plugs. Clark et al. (2009) extended this model to account for diffusive deposition for particle diameters between 0.01 and 10 μm. This model describes the penetration, P, of aerosol through a foam plug as:
 |
(1) |
where t is the thickness of the foam plug (in mm), df is the diameter of a typical foam fiber (in μm) and St, Ng, and Pe represent the Stokes number, the gravitational settling number, and the Peclet number, respectively. They are quantified by the following equations:
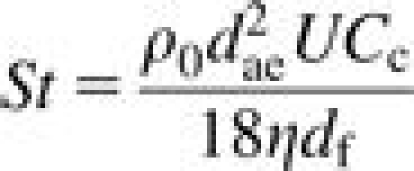 |
(2) |
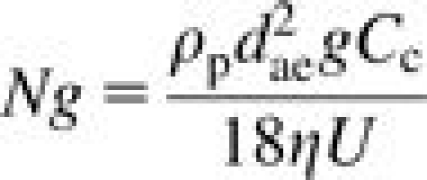 |
(3) |
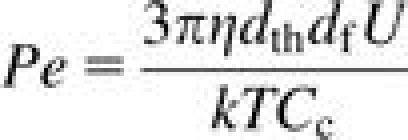 |
(4) |
where dae is the particle aerodynamic diameter, dth is the thermodynamic diameter, ρ0 is standard density, η is the viscosity of air, U is the face velocity of air through the foam, Cc is the Cunningham slip correction factor, df is the foam fiber diameter, g is the acceleration due to gravity, k is Boltzmann's constant, and T is temperature in Kelvin. All units are in SI. Each number describes the tendency of a particle to deposit within the foam due to inertial impaction (St), gravity (Ng), or diffusion (Pe). The thermodynamic equivalent diameter is related to the aerodynamic diameter via the particle density (ρp) and the dynamic shape factor (χ) (Bailey and Roy, 1994):
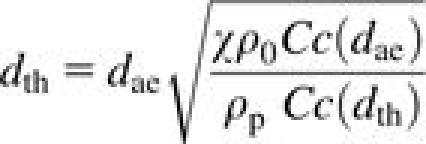 |
(5) |
The thermodynamic diameter is used in the computation of the Peclet number as this diameter more properly characterizes particle deposition by diffusional mechanisms (as opposed to the aerodynamic diameter for both gravitational and inertial mechanisms). The coefficients in equation (1) were determined from a set of aerosol penetration measurements through foam plugs of varying dimension and porosity. Details on the procedure for measuring penetration fraction are available in Clark et al. (2009). Briefly, a foam plug was securely fit within a tube housing and aerosol was passed through the foam to determine the efficiency of particle penetration as a function of size. The penetration fraction for a given particle size, Pi, was calculated by:
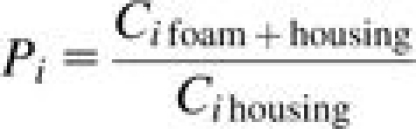 |
(6) |
where is the aerosol number concentration after passing through the tubing section without foam, and is the aerosol number concentration after passing through the tubing section with foam in place; all values are specific to ‘i’, which denotes the particle size of interest. Particles with 0.011 ≤ dae ≤ 1.08 μm were sized and counted in 44 size channels with a sequential mobility particle sizer (SMPS, GRIMM Technologies, Inc.), consisting of a differential mobility analyzer and a condensation particle counter. Particles with 0.523 ≤ dae ≤ 19.81 μm were sized and counted with a TSI Aerodynamic Particle Sizer (APS, Model 3321) and binned into 52 size channels. The SMPS measures mobility diameter. These diameters were converted to aerodynamic diameter for particle diameters > 0.5 μm using the procedure described by Clark et al. (2009) and thermodynamic diameter for smaller perticle diameters. The APS measures aerodynamic diameter directly, so no corrections were performed on the data.
Foam plugs are typically employed as size-selective pre-separators (Vincent et al., 1993; Chen et al., 1998; Kuo et al., 2005) allowing only a certain fraction of aerosol to penetrate to a downstream filter for subsequent collection. Examples include foam inserts in samplers such as an insert for the IOM (SKC Ltd, Dorset, UK) or for use in an asbestos sampling cowl (Chen et al., 1998) for respirable fraction sampling. As pre-selectors, the foam was generally not intended for analysis in these samplers. The CIP10-T (Fabries et al., 1998) used foam in the sampling cup with a size-selective inlet for thoracic fraction collection. Analysis of the CIP10-T sampler involved weighing the entire sampling cup along with the foam. Fabries et al. (1998) however noted that the foam in the sampling cup was not 100% efficient at capturing the small particles penetrating through the sampling head.
Instead, we sought to design a foam with a deposition efficiency (where deposition, D, is simply 1-P) similar to that of the ICRP model for total deposition in the human respiratory tract. As such, the foam itself could function simultaneously as the size selector and the collection substrate. The model described by equation (1) was inverted using a routine optimization algorithm (Matlab, Mathworks Inc., Natick, MA, USA) to provide foam characteristics necessary to best match the ICRP deposition curve using a least squares approach. This process yielded foam parameters (t, df, and U) that produce a deposition curve similar to that for the human respiratory tract.
Sampler evaluation
Polyurethane foams are amenable to gravimetric analysis for the determination of aerosol mass concentration by weighing the foam before and after sampling. However, typical polyurethane foam can contain semi-volatile contaminants as a result of the manufacturing process. Therefore, all plugs were baked at ∼115°C for at least 12 h prior to testing. All plugs were stored in a clean chamber within the weighing room prior to use. The relative humidity (RH) in the weighing room was not controlled during experimentation, but was continuously monitored and remained <50% RH during our studies. Foam plugs are also susceptible to static charging and require neutralization on a Polonium strip for at least 30 s prior to weighing. Clean, unbaked mixed cellulose ester (MCE) filters were also weighed for comparison. The filters were neutralized on a Polonium strip for at least 15 s prior to weighing and were stored in the same equilibration chamber as the foam plugs. A Mettler-Toledo analytical microbalance (model MX5) accurate to ±1 μg was used for all weighings.
Several experiments were conducted with the engineered foam to assess performance and reliability. First, particle deposition efficiency was tested using high molecular weight oil aerosol following the procedure described above (Clark et al., 2009). Second, the pressure drop across the foam was measured at the model-determined face velocity for both clean plugs and plugs subjected to variable liquid (oleic acid) and solid (sodium chloride) aerosol loadings up to 20 and 8 mg, respectively. Finally, foam plugs were examined for stability of laboratory and clean air blanks and the adsorption of water vapor. A laboratory blank is a foam plug that is repeatedly weighed on consecutive days while remaining unused. The laboratory blank mass was determined for 12 foam plugs repeatedly over a 7-day period. The mean for each plug (using all measurements) was removed to normalize the weights between separate plugs. A clean air blank is a foam plug that is weighed before and after particle-free air is pulled through the foam for a pre-determined amount of time (6.5 h). To determine the adsorption of water vapor, humidified (50 and 85% RH), particle-free air was pulled through the plugs for 1 h. Plugs were weighed immediately after exposure and then returned to the equilibration chamber. Plugs were weighed again the following morning to determine the removal of adsorbed water.
RESULTS
Validation of engineered foam
The best agreement with the ICRP deposition curve was found for a plug of foam with df = 49.5 μm that is 8 cm thick operating with a face velocity of 1.3 m s−1. The diameter of the foam plug can be scaled to conform to any desired flow rate. For example, a plug with a 0.8 cm diameter will operate at a flow rate of 4 l min-1. The ICRP model for total deposition in the human respiratory tract (dashed line) is compared to the deposition predicted by our model for the engineered foam (solid line) and to experimental deposition data from the SMPS (circles) and APS (squares) in Fig. 1. The data are plotted for unit density spheres. Due to differences in deposition mechanism, this can be interpreted as the thermodynamic diameter for particle diameters at which the diffusional force dominates (e.g. <0.5 μm) and the aerodynamic diameter for larger sizes where the inertial/gravitational force dominates, if the density or shape factor of the particles is not equal to unity. There was good agreement between the modeled and measured aerosol deposition within the foam, particularly for diameters between 0.05 and 2.0 μm. However, the true collection by a sampler using the engineered foam will need to account for the aspiration efficiency of the foam housing, necessitating wind tunnel studies beyond the scope of the present study. The size of the error bars in Fig. 1 is due primarily to particle counting statistics associated with the test setup, especially in the submicron range and, to a lesser extent, due to variability in replicate foams (whose fiber diameter and porosity vary by <10% between plugs (Vincent et al., 1993; Clark et al., 2009). The deviation between the engineered foam and the ICRP model for particles with diameters >7 μm was due to the reduced inhalability of such particle sizes. Although the foam will collect particles with diameter >7 μm with 100% efficiency, the aspiration efficiency of most personal samplers in this size range is generally <100% and often <50%. The aspiration efficiency of a sampler intending to use this foam will need to be known to determine the accuracy of estimated deposition fractions for larger particles.
Reliability of foam
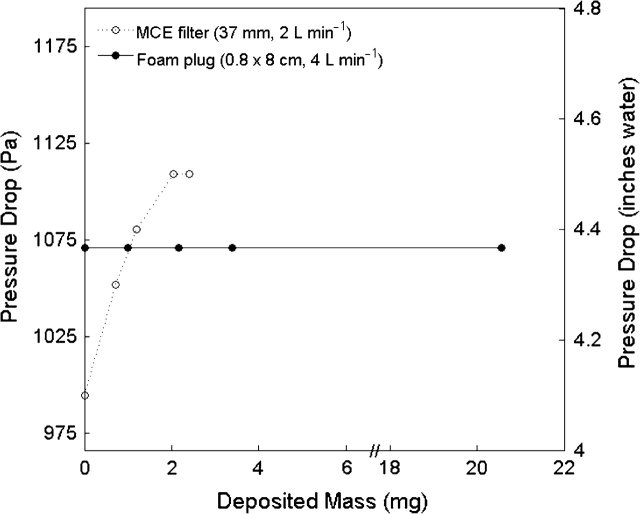
The pressure drop through the engineered foam as a function of aerosol loading is presented in Fig. 2. The pressure drop through the engineered foam was independent of aerosol loading, for masses up to 20.0 mg. The pressure drop across a typical filter substrate (MCE) is shown for reference and was found to have substantial increase with particle loading.
Fig. 2.
Pressure drop through the engineered foam plug and traditional filter media (MCE) as a function of aerosol mass loading.
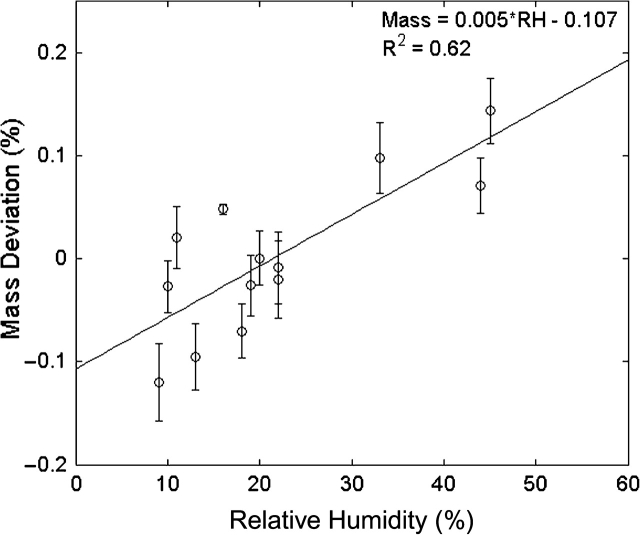
Repeated weighing of laboratory blanks resulted in large day to day variability that was linearly correlated with ambient RH. Despite measurements being performed during the winter in Colorado, where the daytime RH is often <30%, large variations in measured mass were observed. The mean-removed change in laboratory blank mass versus ambient RH for the foam is presented in Fig. 3. Results are shown in terms of percent change in mass, since the diameter of the foam can be scaled to any desired flow rate. Although the percentage change is very small, the deviations are substantial compared to masses commonly collected for air sampling measurements. The following discussion is appropriate for a foam sampler scaled to operate at 4 l min−1. The standard deviation (σ) in foam plug mass across all measurements (and RH levels) was found to be much larger than for other typical sampling media; the pooled σ = 111 μg (coefficient of variation, CV ∼0.08%) as compared to pooled σ = 2.9 μg for 37 mm MCE filters (CV ∼0.007%) measured six times over 10 days. The measurement error observed for the MCE filters is in good agreement with Hanninen et al. (2002) who found a standard deviation of 3.4 μg (CV ∼0.003%) for 37 mm Teflon filters. The average mass change of the foam plug varied significantly (P < 0.002) for periods when the ambient RH change was >1% between weighings. Within measurement, variability was considerably smaller though, median σ = 4.0 μg. Fabries et al. (1998) also found high variability in foam sampled mass with a standard 95% confidence interval around a collected mass of ±0.4 mg. They found a limit of detection for an 8-h sampling duration operating at 7.0 l min−1 of 0.3 mg m−3.
Fig. 3.
Mass of foam plug laboratory blanks as a function of ambient RH present in the weighing environment. Error bars indicate one standard deviation in the measurement.
When sampling dry, particle-free air, for 6.5 h the pre-baked foam plugs did not show any appreciable increase or decrease of mass. However, upon exposure to elevated RH, the foam plugs experienced a substantial increase in mass (0.05–0.2% increase in mass) due to adsorbed water vapor. After a 24 h equilibration at ambient conditions, the change in foam mass was linearly correlated with ambient RH, as shown in Fig. 3, indicating that adsorbed water can be removed upon return to a lower RH environment.
DISCUSSION
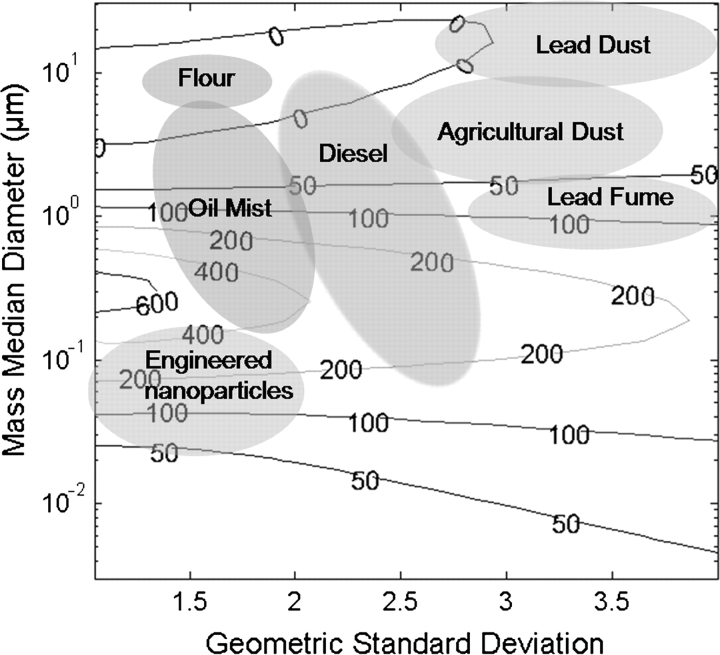
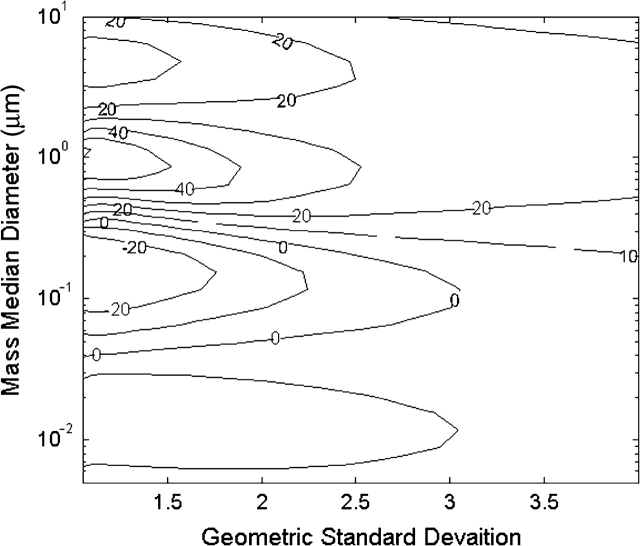
To illustrate the issue of exposure misclassification, we developed a bias map for inhaled mass (using the inhalable aerosol curve) versus total deposited mass for a typical personal sampler, such as the IOM, operating under myriad aerosol size distributions. The mass of aerosol collected by the IOM for 550 lognormal size distributions with mass median diameters (MMDs) from 0.005 to 20 μm and geometric standard deviations (GSDs) between 1 and 4 was calculated and compared to the convention for total aerosol deposition within the human respiratory tract according to the ICRP model (ICRP, 1994). All particles were assumed to be unit density spheres. The percent differences between the aerosol mass measured by the IOM and the mass depositing within the respiratory tract are plotted in Fig. 4 as isopleths (hence, ‘bias map’) as a function of the MMD and the GSD of the aerosol size distribution. Overlaid on the Figure are shaded regions that represent common aerosol size distributions reported in the literature.
Fig. 4.
Theoretical bias map for the error between aspiration (ideal inhalable sampler) and total respiratory deposition (ICRP model) for varying particle size distributions of unit density spheres. Numbered lines represent isopleths of the percent overestimation of mass deposited in the human lung as measured with an inhalable sampler. Ovals represent particle size ranges reported in the literature for several occupational aerosol types.
For many occupational aerosols, the mass collected by the IOM greatly overestimates the mass that actually deposits within the respiratory tract. Integrating over the entire figure, the average bias was +108% with a maximum bias of +670%. The estimated exposure according to the inhalable sampler is at least twice that of the respiratory deposition for MMD ∼0.1–1 and GSD ∼1–3. This exemplifies the difficulty in relating inhalability (i.e. aspiration) with deposition, dose, and risk. Due to the large MMD associated with some occupational exposures, such as lead dust (Spear et al., 1997), flour from baking industries (Laurière et al., 2008), and agricultural dust (Lee et al., 2006), the risk associated with these aerosols may be adequately represented by the IOM sampler. However, other aerosols such as oil mist (Chen et al., 2007), diesel exhaust (Lin et al., 2008), and lead fume (Spear et al., 1998) can be a factor of two to four overestimated using the IOM sampler. Recently, there has also been a growing concern about the potential risks of exposure to engineered nanoparticles. The size distribution of C60 fullerene nanoparticles and their agglomerates, shown in the bottom left corner of Fig. 4 (Gupta et al., 2007), suggests this hazard would also be overestimated. However, because of the varying nature of these aerosol size distributions, the correlation between aspiration and risk is never quite clear.
In Fig. 5, a similar bias map for the mass concentration captured by the engineered foam versus total respiratory-deposited mass is presented. In comparison to the bias shown for an inhalable sampler in Fig. 4, the engineered foam provides much better agreement with the deposited aerosol fraction, with an average bias over the same field of +17%. The maximum bias occurs for near-monodisperse aerosols (GSD <1.5), which are rarely encountered outside the laboratory. Consequently, we believe the foam sampler will provide a reasonable estimate of the respiratory-deposited fraction of airborne particulate matter, erring on the conservative (overestimation of risk) when possible.
Fig. 5.
Theoretical bias map for the error between estimated deposition (developed sampler) and assumed deposition (ICRP model) in the human respiratory tract for varying particle size distributions of unit density spheres.
Figure 5 allows a user of our developed foam sampler to predict its bias, given a priori knowledge of the aerosol size distribution. However, other factors should also be considered for sampling system development. The pressure drop through the sampling media is a main consideration when selecting a pump for operation. The increasing pressure drop across a typical filter substrate (Fig. 2) with increasing particle loading means that filter substrates require more advanced pumps that self-adjust to maintain the correct flow. The engineered foam showed a constant pressure drop, even for very large loadings, much higher than would be sampled during a typical work shift. If a sampler operates at 4 l min−1, the ambient particle mass concentration would need to be >10 mg m−3 during an 8-h work shift to collect 20 mg of aerosol mass, which is greater than typical occupational concentrations and their associated exposure limits. Because foam pressure drop is independent of aerosol loading, a simple, inexpensive pump can likely operate a foam sampler without the need for automated flow control. Such pumps may provide a significant cost savings over contemporary flow-regulated devices. In addition, a constant pressure drop as a function of aerosol loading implies that foam performance should remain constant throughout a sampling period. Linnainmaa et al. (2008) found that the cutoff diameter of the IOM aerosol sampler using the manufacturer's respirable foam inserts was reduced by ∼1 μm for dust loadings of 5–7 mg. Chen et al. (1999) found that while their respirable foam sampler was consistent with the International Organization for Standardization respirable convention for supermicron liquid aerosols, solid aerosol with dae > 5 μm showed appreciable penetration through the foam, likely due to particle bounce. On the contrary, we have verified that loadings of up to 20 mg for a liquid aerosol (8 mg for a solid aerosol) have no affect on particle deposition efficiency in the engineered foam, by virtue of an APS and SMPS measurement similar to those used for Fig. 1 (data not shown). The engineered foam used here is twice as thick as those suggested by Chen et al. (1999), therefore we expect that particle bounce has been minimized. However, the length of our foam (8 cm) requires the design of a housing capable of capturing an inhalable fraction while mounted within the workers breathing zone. Such a design will be the focus of future work.
The artifacts associated with gravimetric analysis of sampling filters are well documented (Willeke and Baron, 1993) and are applicable to polyurethane foam. First, as with many filter types, the foam media adsorbed water vapor, which can contribute to gravimetric measurement bias when the RH is elevated during sampling. Linnainmaa et al. (2008) also found that the mass of polyurethane foam increased substantially over a matter of minutes upon exposure to elevated RH (in the absence of air flow). Gravimetric analysis using filter media often account for this bias by placing the filters in a controlled RH environment for a defined period of time before and after sampling. Our measurements suggest this procedure, if the RH is very carefully controlled, will be sufficient to minimize this bias for the engineered foam as well. Filter weighing facilities that contain carefully sealed, temperature, and RH controlled rooms are likely necessary for accurate gravimetric analysis with foam plugs due to the strong dependence of mass on ambient RH. Other semi-volatile species can bias the mass collection on foam, albeit at an order of magnitude less than for water vapor. Further studies are needed to determine the biases associated with such species.
Polyurethane foam can also be used for other types of chemical trace analysis. Protocols have been developed to examine the concentration of trace metals captured on polyurethane foam (Dillner et al., 2007). Dillner et al. (2007) performed trace metal analysis by inductively coupled plasma mass spectrometry for 29 species with molecular weights ranging from sodium to uranium. The foam was first pre-cleaned using a procedure specific for extraction of inorganic species. Plugs were soaked in Alconox detergent and then rinsed with deionized water, followed by sonication in isopropanol then dichloromethane. Plugs were then dried in a vacuum dessicator. After air sampling, the foam may be digested, allowing for near complete recovery of metals. To digest the foam it was mixed with 16 N HNO3, 30% H2O2, 12 N HCl, and HF and placed in a temperature-controlled microwave that ramps the temperature to 180°C. Dillner et al. (2007) found the average recovery of digested spiked samples to be 98.8%. In addition to good recovery, this procedure generated blank values of the foam that were 25 ng or less for 27 of the 29 metal elements. For many elements, the method limits of detection when the foam was used as the collection substrate were lower than for traditional sampling on Teflon filters (Dillner et al., 2007).
The foam sampler can also be used for identification and quantification of polycyclic aromatic hydrocarbons (PAHs). Maddalena et al. (1998) used foam specially produced without additives like biocides or antioxidants and further pre-cleaned it by rinsing with deionized water and sonicating with methanol and dichloromethane. After air sampling, those authors inserted the foam directly into the syringe barrel for a simple flow through extraction to the gas chromatograph with a quadrupole mass selective detector. Maddalena et al. (1998) found that the recovery of foam spiked with 18 PAHs ranged from 85 to 132% when acetone and hexane were used as the solvent; half of the studied PAH species had recoveries of 100 ± 3%. Blank values for the pre-cleaned foam were below the level of detection for all compounds except naphthalene (91 ng per sample), phenanthrene (65 ng per sample), acenaphthene (33 ng per sample), and fluorene (25 ng per sample). Using foam, this method precludes the need for time-consuming extractions requiring expensive equipment and instrumentation.
CONCLUSIONS
Recognizing the discrepancy between inhaled and deposited aerosol, we have engineered a foam for use as an sampling substrate with properties that provide an accurate, yet conservative estimate of aerosol deposition in the human respiratory tract. The polyurethane foam functions as both the size selector and collection substrate, providing a more physiologically relevant estimate of aerosol hazard than aspiration-based samplers. Future work may find foam plugs with properties that can provide, either individually or potentially in series, an estimate of the deposition in specific regions of the respiratory tract. Given the low and consistent pressure drop across the foam, we expect that this sampler can be operated with a simple, inexpensive flow system that is both compact and lightweight. The foam provides a functional substrate for both gravimetric and trace chemical analyses.
FUNDING
National Institute for Occupational Safety and Health (#5R03OH009248); a Pilot Project Award from the Mountain and Plains Education and Research Center.
References
- ACGIH. Particle size-selective sampling in the workplace: report of the ACGIH technical committee on air sampling procedures. Cincinnati, Ohio: ACGIH; 1985. [Google Scholar]
- Bailey MR, Roy M. Annexe E. Clearance of particles from the respiratory tract. In: Smith H, editor. ICRP Publication 66 Human Respiratory Tract Model for Radiological Protection. Oxford: UK: Elsevier Science, Ltd.; 1994. p. 301. [Google Scholar]
- CEN. Size fraction definitions for measurement of airborne particles. Brussels, Belgium: European Standards Committee; 1993. [Google Scholar]
- Chen MR, Tsai PJ, Chang CC, et al. Particle size distributions of oil mists in workplace atmospheres and their exposure concentrations to workers in a fastener manufacturing industry. J Hazard Mater. 2007;146:393–8. doi: 10.1016/j.jhazmat.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lai CY, Shih TS, et al. Development of respirable aerosol samplers using porous foams. Am Ind Hyg Assoc J. 1998;59:766–73. doi: 10.1080/15428119891010938. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lai CY, Shih TS, et al. Laboratory performance comparison of respirable samplers. Am Ind Hyg Assoc J. 1999;60:601–11. [Google Scholar]
- Clark P, Koehler KA, Volckens J. An improved model for particle deposition in porous foams. J Aerosol Sci. 2009;40:563–72. [Google Scholar]
- Dillner AM, Shafer MM, Schauer JJ. A novel method using polyurethane foam (PUF) substrates to determine trace element concentrations in size-segregated atmospheric particulate matter on short time scales. Aerosol Sci Tech. 2007;41:75–85. [Google Scholar]
- EPA US. Air quality criteria for particulate matter. 2004;Vol. II (Office of Research and Development, ed): National Center for Environmental Assessment. [Google Scholar]
- Esmen NA, Johnson DL. The variability of delivered dose of aerosols with the same respirable concentration but different size distributions. Ann Occup Hyg. 2002;46:401–7. doi: 10.1093/annhyg/mef046. [DOI] [PubMed] [Google Scholar]
- Fabries JF, Gorner P, Kauffer E, et al. Personal thoracic CIP10-T sampler and its static version CATHIA-T. Ann Occup Hyg. 1998;42:453–65. doi: 10.1016/s0003-4878(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Gupta A, Forsythe WC, Clark ML, et al. Generation of C-60 nanoparticle aerosol in high mass concentrations. J Aerosol Sci. 2007;38:592–603. [Google Scholar]
- Hanninen OO, Koistinen KJ, Kousa A, et al. Quantitative analysis of environmental factors in differential weighing of blank Teflon filters. J Air Waste Manage Assoc. 2002;52:134–9. doi: 10.1080/10473289.2002.10470772. [DOI] [PubMed] [Google Scholar]
- Hodgkins DG, Robins TG, Hinkamp DL, et al. The effect of airborne lead particle-size on worker blood-lead levels—an empirical-study of battery workers. J Occup Environ Med. 1991;33:1265–73. [PubMed] [Google Scholar]
- ICRP. Human respiratory tract model for radiological protection. Oxford UK: Elsevier Science, Ltd; 1994. [Google Scholar]
- ISO. Geneva: International Standards Organization; 1995. Air quality—particle size fraction definitions for health-related sampling. [Google Scholar]
- Johnson DL, Esmen NA. Method-induced misclassification for a respirable dust sampled using ISO/ACGIH/CEN criteria. Ann Occup Hyg. 2004;48:13–20. doi: 10.1093/annhyg/meg082. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Huang SH, Shih TS, et al. Development of a size-selective inlet-simulating ICRP lung deposition fraction. Aerosol Sci Tech. 2005;39:437–43. [Google Scholar]
- Lee SA, Adhikari A, Grinshpun SA, et al. Personal exposure to airborne dust and microorganisms in agricultural environments. J Occup Environ Hyg. 2006;3:118–30. doi: 10.1080/15459620500524607. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lee CF, Fang T. Characterization of particle size distribution from diesel engines fueled with palm-biodiesel blends and paraffinic fuel blends. Atmos Environ. 2008;42:1133–43. [Google Scholar]
- Linnainmaa M, Laitinen J, Leskinen A, et al. Laboratory and field testing of sampling methods for inhalable and respirable dust. J Occup Environ Hyg. 2008;5:28–35. doi: 10.1080/15459620701763723. [DOI] [PubMed] [Google Scholar]
- Maddalena RL, McKone TE, Kado NY. Simple and rapid extraction of polycyclic aromatic hydrocarbons collected on polyurethane foam adsorbent. Atmos Environ. 1998;32:2497–503. [Google Scholar]
- Lauriere M, Gorner P, Bouchez-Mahiout I, et al. Physical and biochemical properties of airborne flour particles involved in occupational asthma. Ann Occup Hyg. 2008;52:727–37. doi: 10.1093/annhyg/men061. [DOI] [PubMed] [Google Scholar]
- Soderholm SC, McCawley MA. Should dust samplers mimic human lung deposition? Appl Occup Environ Hyg. 1990;5:829–35. [Google Scholar]
- Spear TM, Svee W, Vincent JH, et al. Chemical speciation of lead dust associated with primary lead smelting. Environ Health Perspect. 1998;106:565–71. doi: 10.1289/ehp.98106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear TM, Werner MA, Bootland J, et al. Comparison of methods for personal sampling of inhalable and total lead and cadmium-containing aerosols in a primary lead smelter. Am Ind Hyg Assoc J. 1997;58:893–9. doi: 10.1080/15428119791012243. [DOI] [PubMed] [Google Scholar]
- Vincent JH, Aitken RJ, Mark D. Porous plastic foam filtration media—penetration characteristics and applications in particle size-selective sampling. J Aerosol Sci. 1993;24:929–44. [Google Scholar]
- Willeke K, Baron P. Aerosol measurement: principles, techniques, and applications. New York, NY: John Wiley & Sons; 1993. [Google Scholar]