Abstract
How many motors move cargos on microtubules inside a cell, and how do they work together to achieve regulated transport? A new study uses an optical trap to investigate the motion of protein-bound beads on the surface of flagella to address these questions and comes up with some intriguing answers.
The study of cytoskeletal molecular motor-driven transport has come a long way. Not so long ago, the focus was on single motors and their properties, but new studies from several groups have highlighted the more complex nature of the transport problem. Multiple motors move cargos and, in many cases, motors of opposite polarity are attached simultaneously, so that a specific cargo can in principle move in either direction [1–5]. This raises the as yet unanswered questions of how do motors function together, and how is net transport controlled? A recent publication [6] in PNAS develops a powerful system — flagellar surface motility — that is amenable to both biophysical and genetic approaches and reveals intriguing similarities and differences with other bi-directional transport systems. Understanding conserved and unique aspects of this system will likely lead to a deeper understanding of intracellular transport.
The absolute number of motors moving a cargo is likely to influence transport — experiments in vitro show that two or three kinesin or dynein motors move cargos much further than one [7,8]. The relative concentration of opposing motors is also important because this can bias transport in either direction. However, it is not so easy to determine the number of engaged and active motors by standard biochemical techniques because some cargo-bound motors may be inactive. Biophysically, one way to do this is by measuring the force required to stop cargos, since, at least for small numbers of motors, motor stall forces are approximately additive [7–9]. Such stalling force measurements are relatively straightforward in vitro where well-characterized polystyrene beads coated with motors are used in buffer. However, calibrated force measurements in vivo are technically challenging because endogenous cargos vary in size and move in cytoplasm of unknown properties.
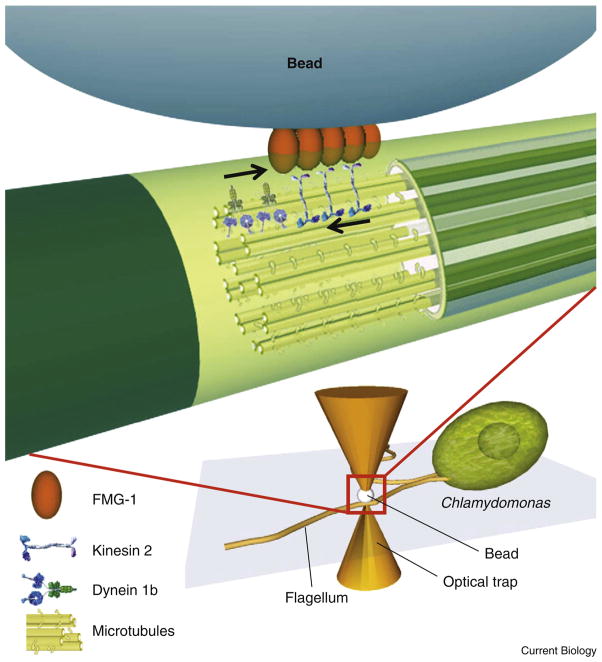
The new system of Laib et al. [6] is clever in that it makes possible such stalling force measurements by combining some of the best aspects of both in vitro and in vivo studies. In short, an intact living Chlamydomonas cell is affixed to a coverslip, and its flagella are immobilized. Then, when a laser trap is used to bring a microsphere (the cargo) into contact with the flagellum (Figure 1), the microsphere binds to the flagellar plasma membrane, specifically to the FMG-1 flagellar membrane protein, and is subsequently transported along the flagellum in either an anterograde or retrograde manner by molecular motors inside the flagellum that are coupled to FMG-1. Thus, the microsphere is in vitro (bead in buffer) but it is expected that it reports on the action of motors in vivo (moving inside the intact flagellum). Once the microsphere binds and starts to move, its position is measured with a laser/ quadrant diode system with very high temporal and spatial resolution.
Figure 1. Schematic of the assay developed by Laib et al. [6] for the study of the transport of FMG-1-bound beads on the surface of a flagellum.
The flagellum of a single Chlamydomonas cell is immobilized on a coverslip, and a polystyrene bead held in an optical trap is lowered onto the flagellum. A cluster of FMG-1 proteins on the flagellar surface is believed to bind to the bead (red box; blown-up region). Back-and-forth surface motion of the bead thus represents FMG-1 motion driven by opposing microtubule motors (cytoplasmic dynein 1b and kinesin 2; arrows indicate direction of motion) within the flagellum. The optical trap exerts a controlled force (‘load’) on the bead (and connected motors), directed towards the trap center and proportional to the bead’s displacement. Thus, the displacement is used to determine the force produced by the motors. Figure prepared with assistance from A. Ramaiya.
To measure the force applied by motors, the bead’s motion (opposed by the optical trap) is monitored; the maximal displacement of the bead from the center of the trap (~80 nm) is then multiplied by the trap stiffness to calculate the maximal force (~60 pN) applied by the motors. The measurements of Laib et al. [6] cannot resolve the forces of single dynein or kinesin motors in this system. Instead, the forces they measure are interpreted to arise from around ten active motors in each direction with an assumption of ~6 pN for both kinesin and dynein motors. These measured forces and the inferred motor numbers are, surprisingly, significantly different from other in vivo force measurements of smaller internal vesicular cargos, such as mitochondria [10] and lipid droplets [11], each of which reported typical forces of less than 10 pN, reflecting fewer motors.
Since previous work found that beads only move a few microns even when no trap was present [12], it was surprising that so many motors appear to move the beads — beads driven by more than three or four motors in vitro move hundreds of microns. Three models were considered to account for these observations. The first hypothesizes a complex of kinesin, dynein and regulatory proteins (similar to a previous suggestion for lipid droplets [13]), able to disengage one set of motors and then rapidly engage the other set. This model appears consistent with all the data. The second, a ‘biased accumulation’ model, hypothesizes that signaling causes the FMG-1 membrane patch to become transiently ‘sticky’ to one set of passing motors; when the signaling changes, the motors detach, and motion in that direction ceases. While formally possible, this model requires a huge flux of moving motors to rapidly bind to the membrane patch (since pauses between reversals of direction only last for hundreds of milliseconds), which seems unlikely. Further, it would be inconsistent with other bi-directionally moving cargos where both sets of motors are bound to the cargo simultaneously [2]. The third hypothesis is related, suggesting that the FMG-1 membrane patch is non-specifically sticky to pre-existing clusters of around ten moving motors, and motion ensues when one such cluster binds stochastically to the patch. This hypothesis also raises issues: what keeps such motors clustered? Wouldn’t clusters interfere with each other? Two clusters of opposing directionality (ten dyneins in one, ten kinesins in the other) seem likely to jam up transport on the microtubule. Finally, why would runs end so rapidly? A cluster of ten motors should move long distances unless it detaches from the cargo, but the attachment appears quite robust — a force of approximately 60 pN is required to stop the cargos. Further work is clearly required to explore these possibilities more fully.
Regardless of the mechanism, the process can disengage one set of many motors (and then re-engage the opposing set) quite rapidly, within hundreds of milliseconds. Further, rapid inactivation of kinesin (via a temperature-sensitive mutant) does not immediately alter minus-end transport: dynein-mediated runs are not longer or more frequent. This observation, combined with the observation of a temporal separation between runs of opposing polarity, supports the hypothesis that the reversal process is able to turn off one set of motors and turn on another set independently. This suggestion is consistent with findings in many other systems (reviewed, for example, in [2]). Importantly, because of the pause between inactivation of one set and activation of the other, the findings from Laib et al. [6] are inconsistent with a recently proposed ‘tug-of-war’ model [3].
While the reversals thus have similarities with other bi-directionally moving cargos, there are also important differences. In addition to the difference in the number of engaged motors, in this system episodes of motion in a particular direction (and hence engagement of opposite polarity motors) are mutually exclusive and are temporally well separated. This is in contrast to vesicular transport, where opposite motors appear to engage in rapid succession (akin to an immediate switching mechanism, without a pause of hundreds of milliseconds), or simultaneously (as in a tug-of-war). The tug-of-war situation has been documented for mitochondria [14] and endosomes [15–17], while immediate switching occurs for lipid droplets [18] and melanosomes [4]. Given the similarities between some aspects of the reversal process for lipid droplets and the flagella–bead system, it is possible that the mechanism underlying the process is actually conserved, but the increased time between excursions in opposite directions results from the increased number of motors that must be engaged or disengaged in the latter case.
These similarities and differences with endogenous cargo motion raise interesting questions: is bead-attached FMG-1 transport really representative of actual transport of this protein? Given the large bead size, is there only one FMG-1 patch per bead? A second possibility is that the flagella–bead motion is unique and different from transport in cytoplasm of cells. However, it seems more likely that there are a number of different types of cargo transport, each adapted to specific requirements; if this is the case, the flagella–bead motion may be a ‘founding member’ of a new class of transport, or it may be an extreme, high-motor member of the lipid droplet motion family. It remains for future work to explore these possibilities.
One very interesting observation, apparently conserved between multiple systems, is that the effect of a perturbation changes over time. The immediate effect of the temperature-sensitive kinesin mutant is to knock-out kinesin function rapidly but leave minus-end transport unaltered. However, over time, all transport ceases. This is interpreted to reflect the need for an anterograde motor to ship retrograde motors back out of the flagellum, but other forms of longer-term feedback are possible. For instance, in the lipid droplet case, similar to the temperature-sensitive effects described above, when a function-blocking anti-kinesin antibody is injected, kinesin function is selectively blocked, and there is net minus-end motion. Further, a kinesin-null mutant also blocks all minus-end motion driven by dynein [11]. However, when kinesin dosage is decreased by 50%, although droplet motion is unaffected from a transport point of view (i.e., the number of moving droplets is unaffected, and their travel distances and velocities are not decreased, and thus any effects cannot be due simply to an inability to come into contact with dynein), the number of engaged motors in both directions is decreased by 50% [11]. Thus, the observed longer-time impairment in the flagella case may also reflect subtle effects or feedback.
It is likely that the activity of opposing motors is regulated using different strategies for different classes of cargo. One of the immediate challenges facing the field is to determine how many such classes there are — is each type of cargo really different, or are there are a few general classes of cargo transport, each with its associated regulation of the underlying motors? At this stage we cannot arrive at a single general model of cargo transport, but the studies here develop an important new system that will help us approach this long-term challenge. Clearly, there will be a lot of back-and-forth before we understand how back-and-forth motion works in the cell.
Contributor Information
Roop Mallik, Email: roop@tifr.res.in.
Steven P. Gross, Email: grosslab@gmail.com.
References
- 1.Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–R537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Gross SP. Hither and yon: a review of bi-directional microtubule-based transport. Phys Biol. 2004;1:R1–R11. doi: 10.1088/1478-3967/1/2/R01. [DOI] [PubMed] [Google Scholar]
- 3.Muller MJ, Klumpp S, Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci USA. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc Natl Acad Sci USA. 1997;94:3720–3725. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laib JA, Marin JA, Bloodgood RA, Guilford WH. The reciprocal coordination and mechanics of molecular motors in living cells. Proc Natl Acad Sci USA. 2009;106:3190–3195. doi: 10.1073/pnas.0809849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci USA. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Kunwar A, Vershinin M, Xu J, Gross SP. Stepping, strain gating, and an unexpected force-velocity curve for multiple-motor-based transport. Curr Biol. 2008;18:1173–1183. doi: 10.1016/j.cub.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashkin A, Schutze K, Dziedzic JM, Euteneuer U, Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990;348:346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- 11.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloodgood RA. Motility occurring in association with the surface of the Chlamydomonas flagellum. J Cell Biol. 1977;75:983–989. doi: 10.1083/jcb.75.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross SP, Welte MA, Block SM, Wieschaus EF. Dynein-mediated cargo transport in vivo. A switch controls travel distance. J Cell Biol. 2000;148:945–956. doi: 10.1083/jcb.148.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennerich A, Schild D. Finite-particle tracking reveals submicroscopic-size changes of mitochondria during transport in mitral cell dendrites. Phys Biol. 2006;3:45–53. doi: 10.1088/1478-3975/3/1/005. [DOI] [PubMed] [Google Scholar]
- 15.Bananis E, Nath S, Gordon K, Satir P, Stockert RJ, Murray JW, Wolkoff AW. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for dynein and kinesin. Mol Biol Cell. 2004;15:3688–3697. doi: 10.1091/mbc.E04-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J Cell Sci. 2003;116:2749–2761. doi: 10.1242/jcs.00478. [DOI] [PubMed] [Google Scholar]
- 17.Pollock N, Koonce MP, de Hostos EL, Vale RD. In vitro microtubule-based organelle transport in wild-type Dictyostelium and cells overexpressing a truncated dynein heavy chain. Cell Motil Cytoskeleton. 1998;40:304–314. doi: 10.1002/(SICI)1097-0169(1998)40:3<304::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Gross SP, Welte MA, Block SM, Wieschaus EF. Coordination of opposite-polarity microtubule motors. J Cell Biol. 2002;156:715–724. doi: 10.1083/jcb.200109047. [DOI] [PMC free article] [PubMed] [Google Scholar]