Abstract
Previous studies from our laboratory have shown that acute alcohol/ethanol (EtOH) intoxication combined with burn injury suppresses T cell IL-2 and IFN-γ production by inhibiting p38 and ERK activation. Because IL-12 plays a major role in Th1 differentiation and IFN-γ production, we examined whether diminished IL-2 and IFN-γ production after EtOH plus burn injury resulted from a decrease in IL-12. Furthermore, we investigated whether IL-12 utilizes the p38/ERK pathway to modulate T cell IL-2 and IFN-γ production after EtOH and burn injury. Male rats (~250 g) were gavaged with 5 ml of 20% EtOH 4 h before ~12.5% total body surface area burn or sham injury. Rats were sacrificed on day 1 after injury, and mesenteric lymph node T cells were isolated. T cells were stimulated with anti-CD3 in the absence or presence of rIL-12 (10 ng/ml) for 5 min and lysed. Lysates were analyzed for p38/ERK protein and phosphorylation levels using specific Abs and Western blot. In some experiments, T cells were cultured for 48 h with or without the inhibitors of p38 (10 μM SB203580/SB202190) or ERK (50 μM PD98059) to delineate the role of p38 and ERK in IL-12-mediated restoration of IL-2 and IFN-γ. Our findings indicate that IL-12 normalizes both p38 and ERK activation in T cells, but the results obtained using p38 and ERK inhibitors indicate that the restoration of ERK plays a predominant role in IL-12-mediated restoration of T cell IL-2 and IFN-γ production after EtOH and burn injury.
Alcohol/ethanol (EtOH)3 intoxication has been frequently encountered in both intentional and unintentional traumatic injuries (1–5). An estimated 1 million burn injuries are reported every year within the United States, and nearly one-half of these injuries occur under the influence of EtOH. Although the overall impact of EtOH intoxication on postburn pathogenesis remains to be investigated, a few studies suggested that patients who are intoxicated at the time of injury are more susceptible to infection, exhibit higher morbidity, and are more likely to die than patients who are not intoxicated at the time of injury (1–6). Similarly, findings from the experimental settings also indicate that EtOH intoxication before burn injury exacerbates the suppression of host immune defense, increases susceptibility to infection, and decreases survival after burn injury (4, 5, 7–10). Additional findings from our laboratory have demonstrated that EtOH intoxication before burn injury exacerbates the suppression of intestinal Th cell effector responses (7, 8, 10, 11). Furthermore, an increase in the intestinal bacterial translocation was observed following a combined insult of EtOH and burn injury (7, 8, 11). Intestine-derived bacteria and their products may accentuate the inflammatory response resulting in multiple organ dysfunction which is a major cause of death in patients who survive the initial insult of injury (8, 12, 13).
T cell activation and subsequent proliferation precedes a cascade of signaling events (14–16). Several lines of evidence indicate that signals emanating from TCR or other costimulatory receptors converge on MAPKs. MAPK, in turn, phosphorylates and activates various downstream molecules resulting in T cell activation and proliferation. Thus, MAPK plays a central role in T cell activation, proliferation, and subsequent differentiation into Th1 or Th2 (14–16). There are three major MAPK-dependent pathways: ERK; JNK; and p38. ERK is 42 and 44 kDa and is referred to as ERK 1 and 2, respectively. Similarly, JNK is a complex of 46- and 54-kDa proteins. Recent findings indicated that IL-12 which helps T cell differentiation into IFN-γ-producing Th1 cell uses p38 MAPK pathway (17–20). However, most of these findings are based on T cells from healthy mice or individuals, and it remains unclear whether a similar scenario exists under disease or other inflammatory conditions. Recently, we have shown a role of p38 and ERK in suppressed mesenteric lymph node (MLN) T cell IL-2 and IFN-γ production after EtOH intoxication and burn injury (10, 11). Because IL-12 has been shown to play a role in Th-1 differentiation as well as in the production of IFN-γ, we investigated whether the diminished IL-2 and IFN-γ production following EtOH plus burn injury resulted from a decrease in IL-12. Furthermore, we also explored whether IL-12 utilizes p38/ERK pathway(s) to modulate T cell IL-2 and IFN-γ production following EtOH intoxication and burn injury.
Materials and Methods
Animals and reagents
Male Sprague-Dawley rats (225–250 g) were obtained from Charles River Laboratories. Nylon wool was obtained from Polysciences. The reagents for cell culture were obtained from Fisher Scientific. Purified mouse monoclonal anti-rat CD3 Abs were purchased from BD Pharmingen. This Ab preparation contains no azide, and the endotoxin levels are <0.01 ng/μg protein. Con A was obtained from Sigma-Aldrich. LPS was purchased from InvivoGen. Abs to p38 protein, phospho-p38 (Thr180/Tyr182); ERK 1/2 protein; phospho-ERK 1/2 (Thr202/Tyr204), phospho-activating transcription factor (ATF)-2 (Thr71), phospho- megakaryocytic protein tyrosine kinase (MKK) 3/MKK6 (Ser189/207), and other respective secondary Abs were obtained from Cell Signaling Technology. Rabbit polyclonal Ab to β-actin was purchased from Abcam. Inhibitors of p38 (SB202180 and SB203590; SB) and ERK (PD98059; PD) were purchased from EMD Chemicals. The reagents for the SDS-PAGE were obtained from Bio-Rad. Immobilon P membrane (polyvinylidine fluoride) was purchased from Fisher Scientific. Protein molecular weight markers were obtained from Bio-Rad. ELISA kits for IL-2 and IL-12 measurements were obtained from Biosource International, and those for IFN-γ measurement were obtained from R&D Systems.
Rat model of acute EtOH intoxication and burn injury
As reported in our previous studies (7, 21–23), rats were randomly divided into four groups: saline plus sham; EtOH plus sham; saline plus burn; and EtOH plus burn. In EtOH-treated groups, blood EtOH levels equivalent to 90–100 mg/dl were achieved by gavage feeding of 5 ml of 20% EtOH (v/v) in saline which equals to ~3.155 g/kg of body weight (7). We have shown earlier that rats gavaged with 5 ml of 20% EtOH exhibited blood EtOH levels in the range of 170–180 mg/dl within 30 min after the gavage (7). Approximately 70–75% of the circulating EtOH is metabolized within 8 h after the gavage and 100% after 24 h (7). A somewhat similar amount of EtOH was used in many previous studies (24–26). Rats in saline groups were gavaged with 5 ml of saline. Four hours after gavage, rats were anesthetized by i.p. injection of sodium pentobarbital (40 mg/kg of body weight) and transferred into a template, which was fabricated to expose ~12.5% of the total body surface area (TBSA). TBSA was calculated using Meeh’s formula (A = kW2/3) where k (constant factor) = 10 as described by Walker and Mason (27). Animals were then immersed in boiling water (95–97°C) for 10–12 s. Sham-injured rats were subjected to identical anesthesia and immersed in lukewarm water. The animals were dried immediately and resuscitated i.p. with 10 ml of physiological saline. After the recovery from anesthesia, rats were returned to their cages and allowed food and water ad libitum. Rats were sacrificed on day 1 after injury.
All the experiments were conducted in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Alabama (Birmingham, AL) and Loyola University Medical Center (Maywood, IL), Animal Institutional Care and Use Committees.
Flow cytometry for the measurement of MLN T cell, macrophage, and dendritic cell population
On day 1 after injury, rats were anesthetized, and via a midline incision intestine was exposed. MLN were removed aseptically and were gently crushed to prepare single cell suspension in HBSS (Fisher Scientific) supplemented with 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, and 100 μg/ml streptomycin (10, 28). Flow cytometric analysis was performed to determine MLN T cell, macrophage and dendritic cell population. For T cell, MLN mixed cells (1 × 106/100 μl of PBS containing 5% FCS) were incubated with Alexa Fluor 647-labeled anti-rat CD3 (BioLegend), PE/Cy7-labeled anti-rat CD4 (BioLegend), and PerCP-labeled anti-rat CD8 (BD Biosciences) Abs on ice for 1 h in dark. For macrophage and dendritic cells, MLN cells were incubated with R-PE-labeled anti-rat OX-62 (Serotec), Alexa Fluor 647-labeled ED1 (Serotec), and FITC-labeled anti-rat MHC class II (eBioscience) Abs. The cells were washed twice with PBS containing with 5% FCS, resuspended in 0.5 ml of PBS, and analyzed at Loyola University Medical Center FACS Core Facility using a six-color flow cytometer (BD FACSCanto) and FlowJo software (Tree Star). Cells found positive for ED1 and MHC class II were considered as macrophages, whereas cells positive for OX62 and MHC class II were selected as dendritic cells. Similarly, cells appeared positive for CD3 and CD4 or CD3 and CD8 were considered CD4+ and CD8+ T cells, respectively.
Measurement of MLN IL-12 protein and mRNA expression
MLN mixed cells (1 × 107 cells/ml) were resuspended in RPMI 1640 supplemented with 2 mM L-glutamine, 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FCS (10, 28). The cells were cultured in the presence of LPS (1 μg/ml) for 24 h, and supernatants were harvested for the measurement of IL-12 using IL-12p70 ELISA kit.
For mRNA expression, MLN mixed cells (1 × 107 cells/ml) were incubated with LPS (1 μg/ml) for 2 h at 37°C and lysed in TRIzol (Invitrogen). The lysates were analyzed for IL-12p35 and -p40 mRNA expression using IL-12p35 and -p40 primers (Applied Biosystems) as described previously in our study (29). In brief, a 3-μl aliquot of cDNA, 5 μl of TaqMan Universal PCR Master Mix (Applied Biosystems), 0.5 μl of primer and probe (Applied Biosystems), and 1.5 μl of water (total volume, 10 μl) were added to each well of a 384-well plate. Amplification of cDNA was performed on an ABI PRISM 7900HT Sequence Detection System. The 18s primer was purchased from Applied Biosystems and was used as the endogenous control. All the samples were amplified for 1 cycle at 50°C for 2 min and at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 1 min. The expression of the gene within each sample was normalized against 18s. Analysis was performed using SDS version 2.1 software (Applied Biosystems) according to the manufacturer’s instructions.
T cell preparation
On day 1 after injury, MLN were removed aseptically and were gently crushed to prepare single-cell suspension in HBSS. The cell suspension was loaded on to the nylon wool packed columns. These columns were pre-equilibrated with HBSS containing 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin with 100 μg/ml streptomycin, and 5% FCS. The columns containing cells were incubated at 37°C for 50–60 min. T cells were obtained by eluting the columns with 15 ml of HBSS at a flow rate of 1 drop/s (10, 11). T cells thus obtained were found to be ~95% positive for anti-CD3 (30).
Measurement of T cell cytokines
Nylon wool-purified T cells were resuspended at a density of 5 × 106 cells/ml in RPMI 1640 supplemented with 2 mM L-glutamine, 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin with 100 μg/ml streptomycin, and 10% FCS. As described previously (10, 11), 100 ml of T cell suspension were cultured in a 96-well plate with anti-CD3 (wells precoated with 200 μl of a 2-μg/ml anti-CD3 suspension) alone or in the presence of rIL-12 at 37°C and 5% CO2. In some experiments, T cells were cultured with Con A (5 μg/ml) instead of anti-CD3. After 48 h of culture, supernatants were harvested and tested for IL-2 and IFN-γ levels using respective ELISA kits according to the manufacturer’s instructions.
T cell stimulation and lysate preparation
As described previously (10, 11), nylon wool-purified T cells were stimulated with anti-CD3 (1 μg/ml) in the absence or presence of rIL-12. Cells were lysed in a lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 100 mM NaF, 1 mM MgCl2, 10 mM Na4P2O7, 200 μM Na3VO4, 0.5% Triton X-100, and 10% glycerol) on ice for 45 min–1 h. Lysates were centrifuged; supernatants were harvested and stored at −70°C until analysis.
Immunoblotting
For the analysis of protein and phosphorylation, an equal amount of total protein from each T cell lysate preparation was analyzed on SDS-PAGE and transferred to Immobilon polyvinylidine fluoride membranes using a semidry Trans-Blot system (Bio-Rad). The membranes were saturated with blocking buffer (10 mM Tris, 150 mM NaCl, 0.05% Tween 20 supplemented with 5% dry milk) for 2 h at room temperature and incubated with the desired primary Ab (1/1000 dilution) at 4°C overnight. The membranes were washed five times with TBS supplemented with 0.05% Tween 20 (TBST). The membranes were incubated with a secondary Ab conjugated with HRP (1/2000 dilution) for 1 h at room temperature. The membranes were washed five times with TBST, probed using ECL dye (Amersham), and autoradiographed (10, 11).
Reprobing the membranes
Membranes were reprobed for the desired protein after stripping the Abs. For stripping, membranes were incubated with Western Blot Stripping Buffer (Pierce) for 30 min at room temperature. The membranes were washed with TBST, immunoblotted with specific Abs, and autoradiographed (10, 11).
Statistical analysis
The data, wherever applicable, are presented as the mean ± SEM and were analyzed using the ANOVA statistical program and Tukey’s test (Statistical Package for Social Sciences Software Program, version 2.0; Sigma Stat). A value of p < 0.05 between groups was considered statistically significant.
Results
MLN T cells, macrophages, and dendritic cells
We determined the effect of EtOH and/or burn injury on MLN T cells, macrophages, and dendritic cell numbers using fluorochrome-labeled specific Abs and flow cytometry. The results from this experiment as summarized in Table I did not show any significant difference in the percentage of T cell, macrophage, or dendritic cell populations among various experimental groups.
Table I.
Percentage of T cells, macrophages, and dendritic cells in MLN after EtOH intoxication and burn injurya
| Experimental Groups | T Cell (%) |
Macrophage (%)(ED1+MHCII+) | Dendritic Cell (%)(OX62+MHCII+) | ||
|---|---|---|---|---|---|
| CD3+ | CD3+CD4+ | CD3+CD8+ | |||
| Saline + sham | 85.21 ± 1.76 | 50.23 ± 3.53 | 29.23 ± 3.85 | 2.05 ± 0.21 | 2.27 ± 0.34 |
| EtOH + sham | 87.28 ± 2.43 | 56.49 ± 2.89 | 31.31 ± 1.35 | 2.07 ± 0.25 | 2.33 ± 0.06 |
| Saline + burn | 81.76 ± 2.23 | 54.53 ± 1.49 | 29.06 ± 1.72 | 1.76 ± 0.23 | 2.28 ± 0.16 |
| EtOH + burn | 81.97 ± 3.83 | 56.40 ± 3.51 | 28.95 ± 2.07 | 1.72 ± 0.18 | 2.00 ± 0.07 |
MLN harvested from rats on day 1 after injury were processed for single-cell suspension. For T cells, MLN mixed cells (1 × 106/100 μl of PBS) were coincubated with Alexa Fluor 647-labeled anti-rat CD3, PE/Cy7-labeled anti-rat CD4, and PerCP-labeled anti-rat CD8. For macrophage and dendritic cells, cells were coincubated with R-PE-labeled anti-rat OX-62, Alexa Fluor 647-labeled ED1, and FITC-labeled anti-rat MHC class II. The cells were washed and analyzed using a six-color flow cytometer and FlowJo software. Cells found positive for ED1 and MHC class II were considered macrophages, whereas cells positive for OX62 and MHC class II were selected as dendritic cells. Similarly, the CD4+ and CD8+ population represents T cells that appeared positive, respectively, for CD3 and CD4 or CD3 and CD8.
MLN IL-12 production
Previously, we showed a significant decrease in MLN T cell IL-2 and IFN-γ production following EtOH and burn injury (10). Because IL-12 plays a critical role in T cell production of IFN-γ (i.e., Th1 differentiation), we examined whether EtOH and/or burn injury affects the release of IL-12. In our preliminary experiments, we used both mixed and adherent cells (adherent population potentially represents macrophages and dendritic cells) for the measurements of IL-12, and the results were more or less similar in both cell preparation. The results presented in Fig. 1 are obtained from mixed cells. MLN mixed cells were cultured with LPS for 24 h and the supernatants were harvested for IL-12 measurement. The results shown in Fig. 1A clearly indicate that the levels of IL-12 were significantly decreased in cells derived from rats receiving a combined insult of EtOH and burn injury compared with shams. In addition to IL-12 protein, we also measured IL-12 mRNA expression using RT-PCR. IL-12 consists of p35 and p40 subunits, and the expression of each subunit may differ following EtOH and burn injury. Therefore, we determined both p35 and p40 subunits using their respective primers. The results indicate that EtOH combined with burn injury suppresses MLN IL-12p35 gene expression (Fig. 1B). Although there was a trend of a decrease in IL-12p40 subunit, this was not found to be significantly different (Fig. 1C). These findings indicate that acute EtOH intoxication combined with burn injury results in a decreased MLN IL-12 release which in turn may cause a decrease in T cell IL-2 and IFN-γ production. To test this, we examined whether restitution of IL-12 normalizes the T cell IL-2 and IFN-γ production.
FIGURE 1.
MLN IL-12 protein (A) and mRNA (B and C) expression following EtOH intoxication and burn injury. MLN mixed cells (1 × 107 cells/ml) were cultured in the presence of LPS (1 μg/ml) for 24 h, and supernatants were harvested to determine the release of IL-12 (IL-12p70 ELISA kit). For mRNA expression, MLN cells (1 × 107 cells/ml) were cultured in the presence of LPS (1 μg/ml) for 2 h, and IL-12 p35 and p40 mRNA expression was measured using RT-PCR and primer sets available from Applied Biosystems. Values are means ± SEM from four to six animals in each group. *, p < 0.05 compared with other groups.
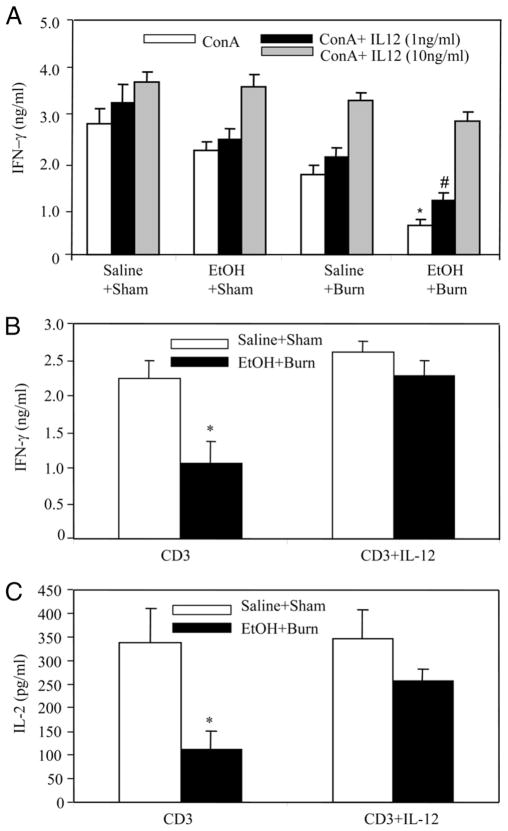
IL-12 restitution in the culture medium normalizes T cell IL-2 and IFN-γ production
A significant decrease in Con A-mediated T cell IFN-γ production was observed after a combined insult of EtOH and burn injury; however, the restitution of IL-12 in the culture medium dose dependently restored IFN-γ production to sham levels (Fig. 2A). T cell IFN-γ production was also measured in response to anti-CD3 stimulation which is a specific mitogen for TCR activation. This and subsequent experiments were performed using 10 ng/ml rIL-12, given that IL-12 at this dose was found to restore IFN-γ to sham levels (Fig. 2A). The results as shown in Fig. 2B indicate that similar to Con A, there was a significant decrease in anti-CD3-mediated T cell IFN-γ production following EtOH and burn injury. Furthermore, the restitution of IL-12 normalized the T cell IFN-γ production after EtOH and burn injury. Moreover, the production of IL-2 was also restored in T cells cultured in the presence of rIL-12 to the levels observed in sham animals (Fig. 2C).
FIGURE 2.
IL-12 restitution prevents the suppression in T cell cytokine production after EtOH intoxication and burn injury. MLN T cells (5 × 106 cells/ml) were cultured in the presence of T cell mitogen Con A (5 μg/ml; A) or plate-bound anti-CD3 (2 μg/ml; B and C) with or without rIL-12. Cells were cultured for 48 h at 37°C, and supernatants were harvested for the measurement of IL-2 and IFN-γ. Values are means ± SEM from six animals in each group. *, p < 0.05 compared with other groups; #, p < 0.05 compared with shams.
IL-12 restitution and T cell p38 and ERK activation
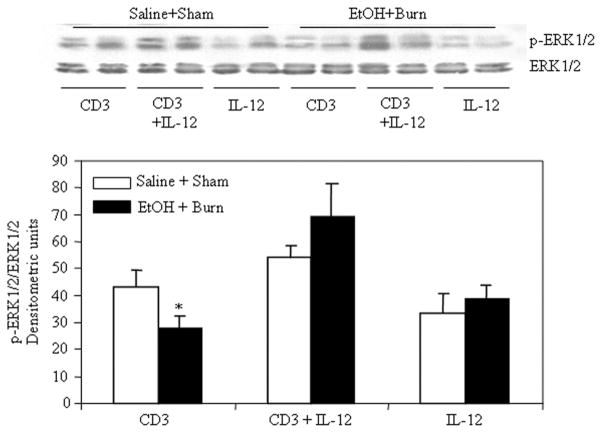
We next examined whether the suppression in MLN T cell p38 and ERK phosphorylation following a combined insult of EtOH intoxication and burn injury is due to a decrease in IL-12. To test this, T cells harvested from sham and EtOH plus burn-injured rats were treated with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs; the results from this experiment are summarized in Figs. 3 and 4. A significant decrease in T cell p38 (Fig. 3) and ERK (Fig. 4) phosphorylation was observed after a combined insult of EtOH intoxication and burn injury compared with shams. The suppression in p38 and ERK 1/2 phosphorylation was prevented in T cells stimulated with anti-CD3 in the presence of rIL-12. There was no demonstrable change in the p38 and ERK 1/2 total protein content in any group. No difference in the p38 and ERK phosphorylation was found in T cell exposed to rIL-12 alone regardless of the injury.
FIGURE 3.

IL-12 restitution prevents the suppression in MLN T cell p38 phosphorylation following EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were treated with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for p38 phosphorylation by Western blot. Blots were stripped and reprobed for p38 total protein contents in various lanes. Blots obtained from four to six animals in each group were analyzed using densitometry. Densitometric values for phosphorylation were normalized to the total protein and are shown as means ± SEM. *, p < 0.05 compared with saline plus shams and CD3 plus IL-12 group.
FIGURE 4.
IL-12 restitution prevents the suppression in MLN T cell ERK phosphorylation following EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were treated with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for ERK phosphorylation by Western blot. Blots were stripped and reprobed for ERK total protein contents in various lanes. Blots obtained from four to six animals in each group were analyzed using densitometry. Densitometric values for phosphorylation were normalized to the total protein and are shown as means ± SEM. *, p < 0.05 compared with saline plus shams and CD3 plus IL-12 group.
These results indicate that IL-12 restores both p38 and ERK phosphorylation, but which of the two is critical in the restoration of IL-2 and IFN-γ production is further investigated in the subsequent experiments.
Effect of p38 and ERK inhibitors on IL-12 restoration of T cell IL-2 and IFN-γ production
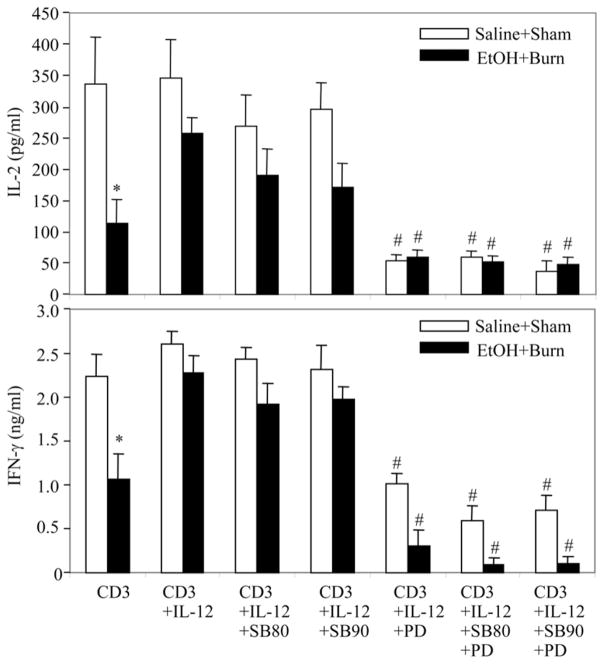
To delineate the role of p38 and ERK, we performed a series of experiments using pharmacological agents that inhibit p38 (10 μM SB203580 or SB202190) and ERK (50 μM PD) activation. The effect of these agents was observed on IL-12 restoration of IL-2 and IFN-γ production. The results from these experiments as shown in Fig. 5 indicate that T cells cultured in the presence of either of the SB p38 inhibitors plus rIL-12 did not exhibit a significant decrease in IL-2 and IFN-γ production. In contrast, T cells cultured in the presence of ERK inhibitor PD plus rIL-12 produced significantly lower levels of IL-2 and IFN-γ than did the T cells cultured in the presence of rIL-12 without PD. The decrease in PD-treated cells was observed in both sham and EtOH plus burn-injured rat T cells. However, the suppression was significantly higher in T cells from EtOH plus burn-injured rat T cells. Addition of both SB and PD simultaneously did not further influence significantly the cytokine response in T cells from either sham or EtOH plus burn-injured rats. These findings suggest that the restoration of ERK rather than p38 alone is critical to IL-12 restoration of T cell IL-2 and IFN-γ production.
FIGURE 5.
Effect of p38 and ERK inhibitors on IL-12-mediated restoration of MLN T cell IL-2 and IFN-γ production following EtOH intoxication and burn injury. MLN T cells (5 × 106 cells/ml) were cultured with anti-CD3 with or without rIL-12 in the presence or absence of the inhibitors of p38 (10 μM SB203580 or SB202190) and/or ERK (50 μM PD98059). Cells were cultured for 48 h at 37°C and supernatants were harvested for the measurement of IL-2 and IFN-γ. Values are means ± SEM from six animals in each group. *, p < 0.05 compared with saline plus shams and CD3 plus IL-12 group; #, p < 0.05 compared with the respective CD3 plus IL-12 group.
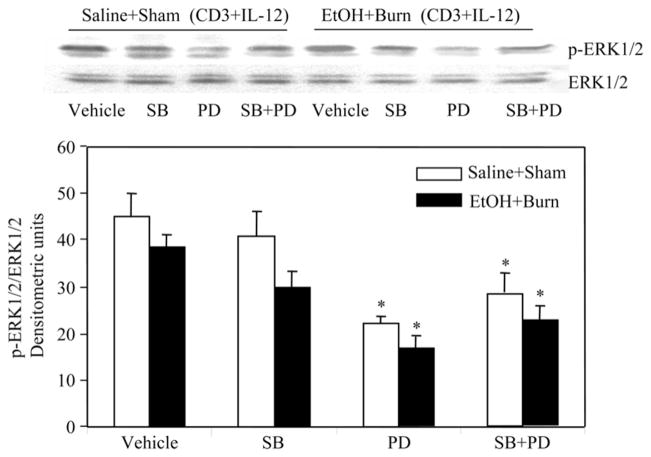
Because some of the recent studies have indicated a role of p38 in IL-12-mediated T cell IFN-γ production or Th1 differentiation (18–20), we performed additional experiments to examine whether SB (SB203580, 10 μM) and PD (50 μM) are indeed inhibiting their respective pathways. The results from these experiments as summarized in Figs. 6 and 7 indicate that p38 inhibitor SB203580 (10 μM) did not significantly affect the phosphorylation of p38 (Fig. 6) and ERK (Fig. 7) in T cells harvested from sham or EtOH plus burn-injured rats. However, as expected, SB203580 has significantly reduced the phosphorylation of ATF-2, which is downstream to p38 (Fig. 8). T cells treated with ERK inhibitor PD (50 μM) before their stimulation with anti-CD3 Abs and rIL-12 significantly diminished ERK phosphorylation (Fig. 7). Furthermore, PD also significantly inhibited p38 phosphorylation in T cells regardless of their source (Fig. 6). These findings confirm that both SB and PD are inhibiting their respective pathways at the doses used in our study. However, the finding that PD inhibits p38 phosphorylation suggests a cross-talk between ERK and the p38 pathway.
FIGURE 6.
Effect of p38 and ERK inhibitors on IL-12-mediated restoration of MLN T cell p38 phosphorylation following EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were pretreated with SB203580 (10 μM) and/or PD98059 (50 μM) for 30 min and then with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for p38 phosphorylation and total protein contents. Blots obtained from four animals in each group were analyzed using densitometry. Densitometric values for p38 phosphorylation were normalized to the total p38 protein and are as means ± SEM.*, p < 0.05 compared with the respective vehicle-treated groups.
FIGURE 7.
Effect of p38 and ERK inhibitors on IL-12-mediated restoration of MLN T cell ERK phosphorylation following EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were pretreated with SB203580 (10 μM) and/or PD98059 (50 μM) for 30 min and then with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for ERK phosphorylation and ERK total protein contents. Blots obtained from four animals in each group were analyzed using densitometry. Densitometric values for ERK phosphorylation were normalized to the total ERK protein and are shown as means ± SEM. *, p < 0.05 compared with respective vehicle-treated groups.
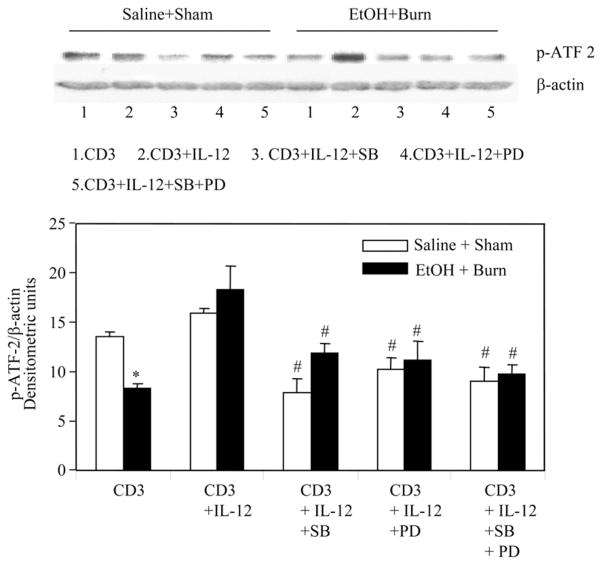
FIGURE 8.
Effect of p38 and ERK inhibitors on IL-12-mediated restoration of MLN T cell ATF-2 phosphorylation following EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were pretreated with SB203580 (10 μM) and/or PD98059 (50 μM) for 30 min and then with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for ATF-2 phosphorylation by Western blot. Blots were stripped and reprobed for β-actin contents in various lanes. Blots obtained from four animals in each group were analyzed using densitometry. Densitometric values for ATF-2 phosphorylation were normalized to β-actin and are shown as means ± SEM. *, p < 0.05 compared with saline plus shams and CD3 plus IL-12 group; #, p < 0.05 compared with respective CD3 plus IL-12 group.
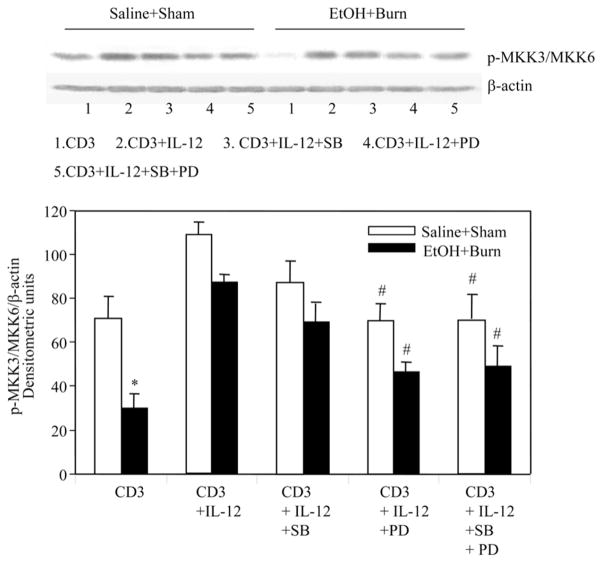
To further characterize the cross-talk between ERK and p38 pathway, we examined p38 up- and downstream signaling molecule MKK and ATF-2. The results from these experiments as shown in Figs. 8 and 9 clearly indicate that PD (50 μM) significantly inhibited phosphorylation of the p38 downstream molecule ATF and the upstream MKK3. However, p38 inhibitor SB203580 has no effect on the phosphorylation of p38 upstream molecules MKK3/MMK6 (Fig. 9). Altogether, these findings indicate a crosstalk between ERK and p38 pathways and that ERK appears to be upstream to p38.
FIGURE 9.
Effect of p38 and ERK inhibitors on IL-12-mediated restoration of MLN T cell MKK3/MKK6 phosphorylation after EtOH intoxication and burn injury. MLN T cells (1 × 107 cells/ml) harvested from various experimental groups were pretreated with SB203580 (10 μM) and/or PD98059 (50 μM) for 30 min and then with rIL-12 (10 ng/ml) 1 h before their stimulation with anti-CD3 Abs (1 μg/ml) for 5 min and lysed. Lysates were analyzed for p38 phosphorylation by Western blot. Blots were stripped and reprobed for β-actin contents in various lanes. Blots obtained from four animals in each group were analyzed using densitometry. Densitometric values for MKK3/MKK6 phosphorylation were normalized to β-actin and are shown as means ± SEM. *, p < 0.05 compared with saline plus shams and CD3 plus IL-12 group; #, p < 0.05 compared with the respective CD3 plus IL-12 group.
Discussion
The results presented in this article clearly indicate that IL-12 restitution prevents the suppression of T cell IL-2 and IFN-γ production following EtOH intoxication and burn injury. We also found that although IL-12 restores both p38 and ERK pathways, restoration of the ERK pathway plays a predominant role in the restoration of T cell IL-2 and IFN-γ production following EtOH and burn injury. Our findings further indicate a cross-talk between p38 and ERK pathways and that ERK appears to be upstream of the p38 pathway. The process of T cell activation is complex and involves several surface receptors. Studies have shown that while an interaction of TCR with Ag or mitogen induces T cell activation, its subsequent proliferation and differentiation into IFN-γ-producing Th1 or IL-4-producing Th2 cells depends on additional signals generated via costimulatory molecules (15, 31–37). For example, IL-12 helps the differentiation of T cell into Th1; IL-10, alternatively, potentiates the differentiation into Th2 (33–37). Previous studies have shown that suppression of Th1 response following burn and other traumatic injuries was often accompanied with a decrease in host resistance and increased susceptibility to infection (17, 38–40). Furthermore, studies have also suggested a role of IL-12 in the decreased Th1 cytokines following burn injury and other disease conditions (17, 38–40). Consistent with these findings, we observed that EtOH intoxication combined with burn injury causes a decrease in MLN IL-12 levels and that IL-12 restitution prevents the suppression of T cell IL-2 and IFN-γ production After EtOH and burn injury. IL-12 is predominantly produced by macrophages/monocytes and dendritic cells; thus, these cells play a major role in T cell differentiation into Th1 and Th2 (41, 42). In addition, physical interaction between macrophages/dendritic and T cells is also required for T cell activation (42–46). Although in the present study, we did not obverse any significant change in macrophage and dendritic cell numbers in MLN following EtOH and burn injury, it is likely that their ability to produce IL-12 is decreased following EtOH and burn injury. This, in turn, may cause a decrease in T cell IL-2 and IFN-γ production after EtOH and burn injury.
We investigated the role of p38 and ERK pathways in IL-12 restoration of T cell IL-2 and IFN-γ following EtOH and burn injury. Our findings indicate that T cells cultured in the presence of p38 inhibitor SB plus rIL-12 did not diminish IL-2 and IFN-γ production. In contrast, T cells cultured in the presence of ERK inhibitor PD plus rIL-12 significantly reduced the release of IL-2 and IFN-γ compared with the T cells cultured in the presence of rIL-12 without PD. Furthermore, the decrease in PD-treated T cell IL-2 and IFN-γ was observed in both sham and EtOH plus burn-injured rat. Although at higher concentrations (25–50 μM) p38 inhibitor significantly suppressed T cell cytokines (data not shown), other studies indicated that the specificity of p38 inhibitor is lost at these higher concentrations. It is likely that at 25 μM or higher, it may influence other MAPK proteins including ERK. Both SB and PD are heavily used in previous studies; however, their concentration is a key to their specificity because these inhibitors are ATP analogs and act by inhibiting the respective kinase activity through competition with ATP at the ATP-binding sites (18, 47, 48). Many previous studies have used SB and PD at concentrations similar to those used in our present study: SB (10 μM); and PD (50 μM). At these concentrations, they are specific to their respective pathways. To confirm whether p38 inhibitor at 10 μM is indeed inhibiting p38 MAPK pathway, we examined the effect of SB on IL-12 restoration of the p38 downstream molecule, ATF. ATF-2 is one of the transcription factors downstream to p38 MAPK (49). Our findings indicate that prior treatment of T cells with p38 inhibitor SB at 10 μM did not affect the phosphorylation of p38, but it significantly inhibited the downstream molecule ATF-2. Thus, this confirms that SB at a concentration of 10 μM does inhibit p38 pathways and further supports the suggestion that the p38 MAPK pathway alone may not be critical in IL-12 restoration of T cell IL-2 and IFN-γ following a combined insult of EtOH and burn injury.
Our findings further indicate that the treatment of cells with ERK inhibitor PD resulted in not only a decrease in ERK phosphorylation but also caused a decrease in p38 phosphorylation. To further characterize the cross-talk between ERK and p38 pathways in T cells following EtOH and burn injury, we examined p38 upstream signaling molecule MAP kinase kinases MKK3/MKK6. Both MKK3 and MKK6 phosphorylate and activate p38 at its activation site threonine-tyrosine sites but do not phosphorylate or activate ERK or JNK (14–16, 49). We found that PD, which inhibits MEK, significantly inhibited the phosphorylation of MKK3/MKK6. Treatment of cells with p38 inhibitor SB as expected did not influence the phosphorylation of MKK3/MKK6. Thus, it is possible that the activation of MEK directly or indirectly via ERK activation modulates p38 pathway. Although our findings did not establish whether the cross-talk between p38 and ERK is at the level of ERK or MEK; it can safely be concluded that ERK pathway plays a predominant role in IL-12-mediated restoration of T cell IL-2 and IFN-γ following EtOH and burn injury. Several other studies have shown the involvement of the ERK pathway in the positive selection of T cells in the thymus and in T cell activation (50). Using transgenic mice or MEK inhibitors, studies suggested that ERK activation is required for Th2 differentiation (15). p38, alternatively, was reported to be selectively activated in mouse Th1 effectors cells (15, 18–20). Although a definitive cause for the observed differences between our and previously published (15, 18–20) studies is not known, it is possible that those differences could result from T cell source that the two studies have utilized. We used freshly isolated T cell from MLNs, whereas the previous studies were performed using established Th1 or Th2 cell lines.
Although our findings suggest the decrease in IL-12 to be a cause for decreased T cell effectors response following EtOH and burn injury, the other potential factors (e.g., IL-10, TGF-β, PGE2, and other inducible NO synthase derivatives) may also play a role in the suppressed T cell responses following EtOH and burn injury. We found a slight but significant decrease in IL-10 in MLN cells after EtOH and burn injury. Furthermore, the ratio of IL-10 to IL-12 was significantly increased in rats receiving a combined insult of EtOH and burn injury as compared with rats receiving either EtOH or burn injury alone (X. Li and M. A. Choudhry, unpublished information). Such an increase in the IL-10:IL-12 ratio following EtOH and burn injury may play a role in T cell differentiation following EtOH and burn injury; however, more studies are needed to confirm this. Additionally, studies have also shown a role of NKT, γδ T, and T regulatory cells in suppressed immune responses following burn injury (51–53). Whether EtOH exposure before burn injury mediates its action by modulating NKT, γδ T, and T regulatory cells, remains to be established. Earlier, we have shown a role of corticosterone in suppressed T cell responses following EtOH and burn injury. More studies are needed to determine whether corticosterone directly suppresses T cell effector response or it is indirectly mediated via IL-12. Thus, more studies are needed to identify the role of these factors in altered T cell effector responses following EtOH intoxication and burn injury.
We also recognize that the present study was performed using a ~12.5% TBSA burn injury which by itself did not produce any deleterious effects on the T cell effector responses on day 1 after injury. Studies have shown that the severity of the postburn pathogenesis is directly proportional to the burn size and that thus burn size is a critical factor in the overall outcome from the injury (40). However, age, gender, and other preclinical manifestation can also influence the outcome of burn patients, especially the patients with small burn injury (54, 55). Likewise, EtOH exposure at the time of burn injury has been shown to further confound postburn pathogenesis (4, 8, 56). Thus, although a smaller burn by itself may not have any deleterious effect on host defense, when combined with existing conditions such as EtOH intoxication, it may become detrimental.
In summary, the findings included in this article indicated a role of IL-12 in suppressed T cell IL-2 and IFN-γ following EtOH and burn injury. We found that IL-12 restitution prevents the suppression of T cell IL-2 and IFN-γ production and that it utilizes the ERK and not the p38 pathway in the restoration of T cell effectors responses. Earlier we have shown that T cell plays an important role in bacterial clearance (7). Thus, any alterations in T cell effectors responses due to a decrease in IL-12 or altered ERK/p38 pathways may impair bacterial clearance leading to their multiplication and accumulation in MLNs. Although the mechanisms by which T cells prevent bacterial translocation are yet to be known, the finding of decreased MLN T cell effector responses (e.g., IL-2 and IFN-γ production) is likely to impair the host’s ability to clear the translocated bacteria, thereby resulting in bacterial accumulation in MLN and other organs of the injured host.
Footnotes
This study is supported by National Institutes of Health Grant R01AA015731.
Abbreviations used in this paper: EtOH, alcohol/ethanol; MLN, mesenteric lymph node; TBST, TBS supplemented with 0.05% Tween 20; SB, SB203580 or SB202190; PD, PD98059; TBSA, total body surface area; MKK, megakaryocytic protein tyrosine kinase; ATF, activating transcription factor.
References
- 1.Choudhry MA, Gamelli RL, Chaudry IH. Alcohol abuse: a major contributing factor to post-burn/trauma immune complications. In: Vincent J-L, editor. 2004 Yearbook of Intensive Care and Emergency Medicine. Springer; New York: 2004. pp. 15–26. [Google Scholar]
- 2.Maier RV. Ethanol abuse and the trauma patient. Surg Infect (Larchmt) 2001;2:133–141. doi: 10.1089/109629601750469456. [DOI] [PubMed] [Google Scholar]
- 3.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Mandrekar P, Verma B, Isaac A, Catalano D. Acute ethanol consumption synergizes with trauma to increase monocyte tumor necrosis factor alpha production late postinjury. J Clin Immunol. 1994;14:340–352. doi: 10.1007/BF01546318. [DOI] [PubMed] [Google Scholar]
- 6.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukocyte Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukocyte Biol. 2006;79:453–462. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Rana SN, Kovacs EJ, Gamelli RL, Chaudry IH, Choudhry MA. Corticosterone suppresses mesenteric lymph node T cells by inhibiting p38/ERK pathway and promotes bacterial translocation after alcohol and burn injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R37–R44. doi: 10.1152/ajpregu.00782.2004. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 14.Cantrell DA. T-cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. J Biol Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- 17.Hedrick MN, Olson CM, Jr, Conze DB, Bates TC, Rincon M, Anguita J. Control of Borrelia burgdorferi-specific CD4+-T-cell effector function by interleukin-12- and T-cell receptor-induced p38 mitogen-activated protein kinase activity. Infect Immun. 2006;74:5713–5717. doi: 10.1128/IAI.00623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JJ, Tripp CS, Russell JH. Regulation and phenotype of an innate Th1 cell: role of cytokines and the p38 kinase pathway. J Immunol. 2003;171:6112–6118. doi: 10.4049/jimmunol.171.11.6112. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Kaplan MH. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-γ expression. J Immunol. 2000;165:1374–1380. doi: 10.4049/jimmunol.165.3.1374. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin 18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2-production in a two-hit model of alcohol intoxication and burn injury. J Immunol. 2008;180:6933–6940. doi: 10.4049/jimmunol.180.10.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute Alcohol intoxication potentiates neutrophil-mediated intestine tissue damage following burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami M, Switzer BR, Herzog SR, Meyer AA. Immune suppression after acute ethanol ingestion and thermal injury. J Surg Res. 1991;51:210–215. doi: 10.1016/0022-4804(91)90096-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Welsh DA, Siggins RW, Bagby GJ, Raasch CE, Happel KI, Nelson S. Acute alcohol intoxication inhibits the lineage-c-kit+Sca-1+ cell response to Escherichia coli bacteremia. J Immunol. 2009;182:1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukocyte Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Szalay L, Choudhry MA, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Mechanism of salutary effects of androstenediol on hepatic function after trauma-hemorrhage: role of endothelial and inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2005;288:G244–G250. doi: 10.1152/ajpgi.00387.2004. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry MA, Uddin S, Sayeed MM. Prostaglandin E2 modulation of p59fyn tyrosine kinase in T lymphocytes during sepsis. J Immunol. 1998;160:929–935. [PubMed] [Google Scholar]
- 31.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 32.Mustelin T, Alonso A, Bottini N, Huynh H, Rahmouni S, Nika K, Louisdit-Sully C, Tautz L, Togo SH, Bruckner S, Mena-Duran AV, al Khouri AM. Protein tyrosine phosphatases in T cell physiology. Mol Immunol. 2004;41:687–700. doi: 10.1016/j.molimm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 34.Neel BG. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- 35.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Cytokines and chemokines in T lymphopoiesis and T-cell effector function. Immunol Today. 2000;21:416–418. doi: 10.1016/s0167-5699(00)01670-4. [DOI] [PubMed] [Google Scholar]
- 37.Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur Cytokine Netw. 2000;11:510–511. [PubMed] [Google Scholar]
- 38.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 39.O’Suilleabhain C, O’Sullivan ST, Kelly JL, Lederer J, Mannick JA, Rodrick ML. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery. 1996;120:290–296. doi: 10.1016/s0039-6060(96)80300-x. [DOI] [PubMed] [Google Scholar]
- 40.Schwacha MG, I, Chaudry H. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 41.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O’Garra A. B7 and interleukin 12 cooperate for proliferation and interferon γ production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 44.McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol. 1999;77:1–10. doi: 10.1046/j.1440-1711.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 45.Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 46.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 47.Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Biol. 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- 48.Wilson KP, McCaffrey PG, Hsiao K, Pazhanisamy S, Galullo V, Bemis GW, Fitzgibbon MJ, Caron PR, Murcko MA, Su MS. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem Biol. 1997;4:423–431. doi: 10.1016/s1074-5521(97)90194-0. [DOI] [PubMed] [Google Scholar]
- 49.Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ, Jr, Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 50.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 51.Ni CN, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 52.Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Taniguchi M, Faunce DE. Injury-induced suppression of effector T cell immunity requires CD1d-positive APCs and CD1d-restricted NKT cells. J Immunol. 2006;177:92–99. doi: 10.4049/jimmunol.177.1.92. [DOI] [PubMed] [Google Scholar]
- 53.Schwacha MG, Ayala A, Chaudry IH. Insights into the role of γδ T lymphocytes in the immunopathogenic response to thermal injury. J Leukocyte Biol. 2000;67:644–650. [PubMed] [Google Scholar]
- 54.McGwin G, Jr, George RL, Cross JM, Reiff DA, Chaudry IH, Rue LW., III Gender differences in mortality following burn injury. Shock. 2002;18:311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2008;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J Burn Care Rehabil. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]