Abstract
The interactive effects of physiological reactivity and social support on children's memory were examined. Four- to 6-year-olds completed a laboratory protocol during which autonomic responses and salivary cortisol were measured. Memory was assessed shortly afterward and 2 weeks later. During the second interview, children were questioned by a supportive or nonsupportive interviewer. Few significant relations emerged between reactivity and children's short-term memory. Following a 2-week delay, cortisol reactivity was associated with poorer memory and autonomic reactivity was associated with increased accuracy among children questioned in a supportive manner but decreased accuracy among children questioned in a nonsupportive manner. Results question traditional conceptualizations of reactivity as a risk factor and instead suggest that reactivity may only confer risk in certain environmental contexts.
Researchers in developmental psychobiology, child development, and behavioral pediatrics have increasingly acknowledged that exaggerated, prolonged physiological responses to stress and challenge put children at risk for a variety of problems in childhood, including physical and mental disorders, poor emotion regulation, and cognitive impairments (Johnston-Brooks, Lewis, Evans, & Whalen, 1998; Porges, 1997; Raine, Venables, & Mednick, 1997; Scarpa, 1997). Despite a seeming universal acceptance of direct links between physiological reactivity and problems, several studies have failed to support such linkages. Inconsistencies in findings have led several researchers to reconceptualize reactivity not as a risk factor but instead as a response proclivity that, under certain circumstances, confers increased risk, and in other circumstances, serves a functional or even protective role (e.g., Boyce et al., 1995; Davis, Donzella, Krueger, & Gunnar, 1999; Quas, Murowchick, Bensadoun, & Boyce, 2002). Specifically, reactivity is perceived as a form of biological sensitivity to environmental or contextual influences. The purpose of this study was to provide an experimental test of this conceptualization in relation to cognitive outcomes.
In the study, we examined the activation of the stress response system and social context as joint predictors of children's memory for a mildly stressful event. Our primary goals were to assess: (a) whether children with exaggerated physiological stress responses (i.e., reactive children) evince poorer memory than children with small to moderate physiological responses, and (b) whether children's physiological reactivity interacts with the supportiveness of an interview context to affect their memory.
Physiological Reactivity in Childhood
Physiological reactivity has been measured using parameters reflecting autonomic nervous system activation, such as heart rate, respiratory sinus arrhythmia (RSA, an index of parasympathetic influence on the cardiac cycle), and pre-ejection period (PEP, an index of sympathetic influence on the cardiac cycle), and parameters reflecting hypothalamic-pituitary-adrenal (HPA) axis activation, most commonly, salivary cortisol. Although the magnitude and duration of autonomic and HPA responses to stress vary considerably across children, individual children's general response proclivities are relatively stable and consistent across situations and over time, at least when measured across 1- to 2-year periods (Bar-Haim, Marshall, & Fox, 2000; Kagan, Snidman, Arcus, & Reznick, 1994; Marshall & Stevenson-Hinde, 1998; Murphy, Alpert, & Walker, 1991). Additionally, despite the autonomic and HPA systems directly influencing one another's activities, they operate independently, and reactivity in one system does not necessarily predict reactivity in the other (Bauer, Quas, & Boyce, 2002; Cacioppo, Uchino, & Berntson, 1994). Thus, it is important to include multiple measures of reactivity to determine the independent, unique association of each with specific outcomes.
Increased autonomic reactivity has been associated with a range of problems, including social inhibition, internalizing symptoms, poor attention, respiratory illness, and poor immune functioning (e.g., Boyce et al., 1995; Boyce et al., 2001; Fabes, Eisenberg, & Eisenbud, 1993; Kagan et al., 1994; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). Increased HPA reactivity has also been linked to behavior problems, especially internalizing symptoms, and impaired cognitive functioning (Hefflinger & Newcomer, 2001; Kagan, Reznick, & Snidman, 1988; Scarpa, 1997; Schmidt, Fox, Rubin, & Sternberg, 1997). Explanations for these associations traditionally focus on costs associated with chronic arousal: Reactive individuals, including children, need to expend effort regulating their exaggerated physiological arousal at the expense of attending to other important internal (e.g., physical) and external information (e.g., Matthews & Woodall, 1988; McEwen & Seeman, 1999; Porges, 1992; Strelau & Eliasz, 1994). The demands of this self-regulation require considerable resources, which over time lead to a state of increased “allostatic load” (e.g., McEwen, 1998; McEwen & Stellar, 1993; Sterling & Eyer, 1988), or chronic, taxing overarousal.
Not all studies, however, have revealed direct or consistent associations between reactivity and adverse outcomes (e.g., Boyce et al., 1995; Eisenberg, Fabes, Murphy, Karbon, Smith, & Maszk, 1996; Gannon, Banks, Shelton, & Luchetta, 1989; Lundberg, Rasch, & Westermark, 1991-1992), and a few studies have revealed that heightened reactivity is associated with reduced risk under some circumstances. For instance, Boyce et al. (1995) examined respiratory illness and autonomic reactivity in 3- to 6-year-olds. Consistent with traditional views of reactivity and risk, greater heart rate reactivity was associated with increased frequency of respiratory illnesses, but only when reactivity was combined with high levels of family stress. Greater reactivity, in combination with low levels of family stress, was associated with fewer illnesses. Also, Gunnar, Tout, de Haan, Pierce, and Stansbury (1997) found high levels of morning cortisol during the first few weeks of a new school year in outgoing and extroverted preschoolers, but high levels of salivary cortisol later in the year in the less outgoing and socially competent preschoolers. Thus, cortisol reactivity was associated with adaptive behavior (sociability) in some children and maladaptive behavior (inhibition) in others.
In an attempt to synthesize contradictory findings, Boyce and colleagues (e.g., Boyce & Ellis, 2002; Ellis, Essex, & Boyce, 2002) proposed an interactive model of physiological reactivity and environment as joint predictors of adverse outcomes in childhood. The researchers conceptualized physiological reactivity as an evolutionary-based biological marker of children's sensitivity to social and environmental influences, with physiologically reactive children being more sensitive than nonreactive children. When the environment is stressful, high reactivity would serve as a risk factor (see also Belsky, 1995; Strelau & Eliasz, 1994). However, when the environment is nurturing and supportive, high reactivity would enhance children's ability to attend to and engage in the environment, thus potentially leading to associated benefits.
Although Boyce and colleagues' (Boyce & Ellis, 2002; Ellis et al., 2002) conceptualization offers a parsimonious explanation for many conflicting findings in the literature, it has yet to be tested experimentally. Previous studies to which the model has been applied were naturalistic in design. General environmental characteristics (e.g., levels of parent-reported family conflict) were examined in relation to reactivity and outcomes. It is possible that children's physiological reactivity was a product of or caused by the environment (e.g., Ellis et al., 2002; Gunnar, 1998; Hertsgaard, Gunnar, Erickson, & Nachmias, 1995; Kamarck, Manuck, & Jennings, 1990; Meyer, Chrousos, & Gold, 2001) rather than an independent trait of children. Or, the adverse social contexts may have directly led to the outcomes (e.g., Chen, Matthews, & Boyce, 2002; Harold & Conger, 1997; Salzinger, Feldman, Ng-Mak, Mojica, & Stockhammer, 2001; Vandell & Corasaniti, 1990). Finally, the children were not randomly assigned to high- or low-stress social environments. Other factors (e.g., temperament) may have contributed to the evident associations among reactivity, context, and outcomes in the naturalistic studies. An experimental test of the interaction between reactivity and context, a test yet to be conducted, could provide further support for this emerging conceptualization.
Physiological Reactivity and Memory
Memory is a particularly amenable outcome to study in an experimental paradigm involving physiological reactivity and context. Insofar as reactive children do not attend well to external information under stress, their encoding, and hence memory, should be adversely affected. Indeed, studies have reported negative relations between physiological arousal and attention in children (e.g., Richards & Casey, 1991; Suess, Porges, & Plude, 1994). Reactive children may also be too aroused at retrieval to conduct an adequate memory search or be unwilling or unable to cooperate with an interviewer. These latter situations may be particularly salient when children are asked to retrieve stressful information or when the retrieval context is itself stressful or challenging.
Few studies have directly examined the relations between children's autonomic stress responses and memory (e.g., Chen, Zeltzer, Craske, & Katz, 2000; Peters, 1991). In a study of children's memory for a video of a child receiving an inoculation, Bugental, Blue, Cortez, Fleck, and Rodriguez (1992) found that increases in 5-year-olds' heart rate while watching the video were negatively related to their memory. Stein and Boyce (1995) examined children's memory for a fire alarm incident (the same procedure employed here) and found that children with high heart rates across a series of challenging tasks evinced poorer fire alarm memory shortly afterward than did children with consistently low heart rates. Both research teams interpreted their findings as suggesting that children who exhibited large physiological reactions did not attend and hence encode the event as well as did nonreactive children, an interpretation that is consistent with theories concerning reactivity, limited resources, and poor regulation abilities (Calkins & Fox, 2002; McEwen, 1998; McEwen & Stellar, 1993; Porges, 1997). However, in the studies, the memory interviews took place shortly after the to-be-remembered events. The reactive children may have still been too aroused to devote adequate resources to conducting a memory search. Or they may not have been sufficiently comfortable with the interviewer to describe the event, even if they remembered it. Additionally, Bugental et al. measured children's heart rate only during the video. To identify reactive children, it is necessary to assess physiological responses to a range of stimuli (Boyce et al., 2001) and compare the pattern of responses with children's memory. Finally, in Stein and Boyce's study, only children extreme (high and low) in heart rate reactivity were examined. By identifying reactive and nonreactive children based on multiple physiological indexes, extending the delay between the to-be-remembered event and memory test, and ensuring children are comfortable with an interviewer, it is possible to determine, more definitely, how autonomic reactivity relates to children's memory for a mild stressor.
Unlike the paucity of research concerning autonomic reactivity and memory, studies have examined relations between cortisol and memory, although few studies have included children. In nonhuman animals and human adults, mild to moderate levels of adrenocortical activation during or shortly after a stressor often enhance cognitive performance, including memory (e.g., Moffat & Hampson, 1996; Roozendaal & McGaugh, 1996), but chronically high levels of adrenocortical activation are associated with poor attention and memory (e.g., Kelly & Hayslip, 2000; Lupien, Gillin, & Hauger, 1999; Newcomer et al., 1999; see Golier & Yehuda, 1998). These nonlinear relations are thought to be a product of differential activation of mineralocorticoid versus glucocorticoid receptors (MRs and GRs, respectively). Low-circulating glucocorticoids (i.e., cortisol in humans) primarily activate MRs, which facilitate memory, whereas high-circulating glucocorticoids activate GRs, which impair memory (Sapolsky, Romero, & Munck, 2000).
In one of the few studies of cortisol and children's event memory, Merritt, Ornstein, and Spicker (1994) assessed salivary cortisol before and after a highly distressing medical procedure took place. Adjusted cortisol, computed by comparing pre- and postprocedure cortisol levels with a control measure taken at the same time on a different day, was unrelated to memory shortly or several weeks after the procedure. Of note, the small sample size (N = 17) could have affected the ability to detect meaningful associations. Also, it is unclear how the adjusted cortisol levels were computed, making it difficult to interpret the results. Nonsignificant findings were also reported in a study of children's memory for lumbar punctures (Chen et al., 2000). Again, however, the results are difficult to interpret because the procedure was administered at variable times of day and some children received medication before the treatment, medication that could affect physiology and memory. Given findings from nonhuman animals suggesting relations between cortisol and memory, further studies in this area are needed.
Social Support, Reactivity, and Memory
One method of varying the stressfulness of social context is by manipulating the support individuals receive during a task. High social support, operationalized either globally (e.g., number of close friends) or situationally (e.g., interviewer demeanor), is beneficial to physical and emotional health, relationship quality, and academic and cognitive performance (e.g., Burleson, Albrecht, Goldsmith, & Sarason, 1994; Cohen & Wills, 1985; Sarason, Sarason, & Pierce, 1990). High support also reduces physiological arousal and enhances memory performance.
In studies of social support and physiological reactivity, support is manipulated by having a friend present versus absent or having a confederate behave supportively (e.g., by maintaining an open body posture, smiling, nodding in agreement, appearing interested) versus nonsupportively (e.g., Burleson et al., 1994; Gerin, Milner, Chawla, & Pickering, 1995; Hilmert, Kulik, & Christenfeld, 2002). Although some variable results have emerged, high support, especially when the confederate is female, reduces physiological arousal. Indeed, reduced arousal has been proposed as a primary mechanism underlying the benefits of social support on health (e.g., see Uchino, Cacioppo, & Kiecolt-Glaser, 1996).
In studies of children's memory (e.g., Carter, Bottoms, & Levine, 1996; Goodman, Bottoms, Schwartz-Kenney, & Rudy, 1991), social support is typically manipulated through interviewer demeanor. High-support interviewers maintain eye contact, sit close to and facing children, build rapport, and provide positive feedback. Low-support interviewers either act neutrally or in a serious, cold manner (e.g., by not maintaining eye contact, not smiling, not facing children, and talking in a monotone voice). Children often provide more accurate responses and make fewer errors to yes – no, short-answer, and misleading (embedded false suppositions) questions when interviewed in a supportive rather than nonsupportive manner (e.g., Carter et al., 1996; Davis & Bottoms, 2002).
A few studies have failed to find any effects of interviewer-provided versus withdrawn support on adults' reactivity (Edens, Larkin, & Abel, 1992; see Lepore, 1998) or children's memory (e.g., Imhoff & Baker-Ward, 1999). One explanation for the variable results is that individuals differ in the degree to which they are affected by interviewer demeanor (Davis & Bottoms, 2002; Hilmert, Christenfeld, & Kulik, 2002). Of direct relevance to the present study, insofar as physiological reactivity reflects a form of increased sensitivity to environmental context, reactive children may be more susceptible than nonreactive children to poor performance when questioned by an unsupportive, emotionally unavailable interviewer. The discrepancy in performance should be greatest in response to direct and misleading questions because providing the correct response requires children to contradict an interviewer, a demand that may increase stress already felt by reactive children in the challenging social context.
Present Study
In this study, 4- to 6-year-olds came to a research laboratory on two occasions. During the second session, children completed a series of challenging tasks and experienced a brief fire alarm incident during which measures of autonomic responses and salivary cortisol were collected. Shortly after the alarm, children were interviewed about what happened, allowing us to examine the relations between reactivity and memory for the mild stressor. Following a 2-week delay, children's memory for the previous session was examined. The interviewer behaved in either a warm, supportive, and emotionally available manner or a serious, cold manner, with physiologically reactive and nonreactive children equally distributed across the interview conditions. By manipulating the interviewer's demeanor, we were able to determine whether physiological reactivity interacts with interviewer supportiveness to affect children's memory.
Our first two hypotheses concerned children's first-session fire alarm memory. In Hypothesis 1, consistent with studies of heart rate reactivity and memory (e.g., Bugental et al., 1992; Peters, 1991; Stein & Boyce, 1995), we expected autonomically reactive children to evince poorer memory than nonreactive children when questioned shortly after the fire alarm. In Hypothesis 2, because the alarm was not highly distressing, but instead was a mildly arousing stressor, we expected that increased cortisol would be associated with better memory, consistent with the notion that MR binding facilitates memory and with findings from studies involving nonhuman animals (e.g., Roozendaal & McGaugh, 1996).
The remaining hypotheses concerned children's second-session performance, when the social support manipulation was introduced. In Hypothesis 3, children questioned by a supportive interviewer were expected to be more accurate than children questioned by a nonsupportive interviewer, as reported in prior studies of social support and memory (e.g., Carter et al., 1996; Davis & Bottoms, 2002). In Hypothesis 4, memory was expected to improve and errors were expected to decrease with age in both interviews (e.g., Leichtman & Ceci, 1994; Quas & Schaaf, 2002). In Hypothesis 5, autonomic reactivity was expected to interact with social support to affect memory such that reactive children questioned by the nonsupportive interviewer were expected to perform more poorly than all other children. Furthermore, although the reactive children's performance was expected to improve in the high-support condition, because of their hypothesized encoding limitations (Stein & Boyce, 1995), their performance was not expected to be comparable to the nonreactive children's performance. Finally, in Hypothesis 6, greater cortisol reactivity was expected to be associated with poorer memory in the nonsupportive condition. However, in the supportive condition, greater cortisol reactivity was expected to be associated with enhanced memory.
Method
Participants
Sixty-three children (33 boys and 30 girls) served as participants in Session 1. Their age ranged from 48 to 82 months (M = 63 months, SD = 10.50). Children were from diverse ethnic backgrounds, where 71% were Caucasian, 5% were African American, 13% were Asian American, 8% were Latino, and 2% were other ethnicities. Families were recruited from newspaper ads, at local day care facilities, and by word of mouth. Parents were fairly well educated; with 44% reporting to have a professional degree or graduate training. None of the children had a chronic disease or respiratory illness, or mental health problems, and none was on medication at the time of the study.
Fifty-seven children (M age = 62.23 months; 31 males) completed Session 2. Of the initial 63 children, 2 children's data were not included because their memory interview was substantially different, and 4 children did not complete Session 2 (1 did not wish to answer questions, 1 did not answer questions coherently, and 2 families did not return in a reasonable time). The 6 children did not differ significantly in age or autonomic and cortisol responses from the other children.
Questionnaires
Demographic questionnaire
A brief demographic questionnaire collected information about children's family background (e.g., parents' education and income, children's ethnicity).
Session 1 fire alarm memory interview
The fire alarm memory interview followed a funnel approach commonly used in memory and suggestibility studies (e.g., Poole & Lindsay, 1995; Quas & Schaaf, 2002). It began with two general, free-recall prompts asking what happened when the alarm sounded (“Tell me what happened”; “Tell me everything you remember”). Next were nine direct, nonleading questions probing for event details: Three required correct yes responses, three required correct no responses, and three required short-answer responses. None of the questions was misleading (i.e., suggested incorrect responses).
Session 2 memory interview
The second-session memory interview also followed a funnel approach. The free-recall question, “Tell me everything about what happened the last time you came to the research laboratory,” was followed by three prompts to obtain additional narrative detail (e.g., “Tell me everything that happened”). Next were 41 direct questions. Twenty-three were nonleading in that they did not suggest an incorrect response (e.g., “Did they put stickers in the middle of your chest?” [yes] “Did you get to put the whipped cream in your cup?” [no] “Why did the alarm make all that noise?”), and 18 were misleading. They suggested an incorrect response or included a false supposition (e.g., “You took your shirt off so they could put the stickers on you, didn't you?” when the child did not take his or her shirt off; “Why did the man get in trouble?” when he did not). Within the nonleading and misleading questions, responses included approximately equal numbers of yes, no, and open-ended, short-answer correct answers. Of note, for 5 children, the direct questions differed slightly from those asked of the larger sample. Only the 23 questions that were identical across both groups were included in the computation of the 5 children's proportion scores.
Procedure
All procedures were approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley. Interested parents were contacted by phone and screened for eligibility (e.g., the child's health). A convenient time for the first session was then scheduled, with the restriction that the session take place during the afternoon to minimize diurnal effects on basal cortisol levels (Carrion et al., 2002; Gunnar, Morison, Chisholm, & Schuder, 2001).
When families arrived for the initial session, the study was described in detail, and the parent's consent and child's assent were secured. An initial saliva sample was then collected from the child. Following collection, the sample was frozen until shipment for analysis.
While parents completed questionnaires, the child was escorted to an adjacent room. An electrocardiograph (Minnesota Impedence Cardiograph, Model 304B) was introduced. Disposable spot electrodes were placed on the child's right clavicle, right lower abdomen, left rib, and left lower abdomen (for the ground) to obtain electrocardiographic (ECG) data. Additionally, two inner electrodes were placed at the suprasternal notch and xiphoid process, and two outer electrodes were placed on the fourth cervical and ninth thoracic vertebra to obtain impedance data (see Alkon et al., 2003, for procedure details). Once the equipment was in place, the child was left with a single, unfamiliar male researcher to complete a standardized laboratory protocol (termed reactivity protocol; Boyce et al., 2001).
The protocol began and ended with 3-min neutral stories read to the child. Between the stories were five challenging tasks and a brief rest. The tasks lasted between 1 and 3 min, were designed to be ecologically valid challenges for 4- to 8-year-olds, and have been shown in past research to identify individual differences in children's physiological stress responses (Alkon et al., 2003; Boyce et al., 1995; Boyce et al., 2001). The first task, a social interview adapted from the Gesell School Readiness Screening Test (Ilg, Ames, Haines, & Gillespie, 1978), involved the researcher asking questions about the child, his or her family, and school. The second task consisted of a digit-recall test, obtained from the Kaufman Assessment Battery for Children (Kaufman & Kaufman, 1983). The child repeated increasingly difficult sets of digits read by the researcher. This was followed by a brief 1-min rest period during which the child sat quietly. For the third task, the researcher asked the child to identify an unknown substance (lemon juice) placed on the anterior tongue (Kagan & Snidman, 1991). The fourth and fifth tasks consisted of the child watching two video clips. In one video, designed to elicit fear, a boy appeared frightened by a thunderstorm. In the other video, designed to elicit sadness, a girl appeared sad because her pet died (Eisenberg et al., 1988). The stories were included to obtain resting cardiovascular data, and the tasks (social interview, digit span, unfamiliar substance taste, fear and sad videos) were included to obtain cardiovascular data under conditions of mild stress.
After the second story, the second saliva sample was collected (delay between the cortisol samplings M = 50 min, range = 39 – 70 min). Next, the fire alarm incident took place, based on a procedure developed by Stein and Boyce (1995). The researcher explained that they were going to make hot chocolate and then proceeded to make the hot chocolate. When the child saw steam coming from the kettle, the alarm sounded. The researcher acted surprised and excitedly stated that the alarm is sounding. The researcher explained that there was no fire; instead, the steam set off the alarm. He next went through specific actions to stop the alarm, which sounded for 20 s. He then finished making hot chocolate.
Shortly after the alarm, a familiar female researcher entered and stated, “I heard the alarm. Is everything OK?” She then asked the Session 1 fire alarm interview questions. To ensure that the child answered the questions, the male researcher left. After the questions, the male researcher returned, and female researcher left. The session ended with the child drinking the hot chocolate, the electrodes being removed, and the child and parent being thanked. The entire session was videotaped.
Between the first and second sessions, RSA was scored to identify physiologically reactive children and assign them to support conditions, thereby ensuring that a sufficient number of highly reactive children were interviewed in both the high- and low-support manner. RSA, as opposed to heart rate, pre-ejection period, or cortisol, was relied on for this preliminary scoring for several reasons. First, unlike heart rate, RSA is a relatively pure measure of parasympathetic influence on the cardiac cycle. Second, parasympathetic reactivity has been studied more extensively in childhood than sympathetic reactivity and has been linked to emotionality and emotion regulation (e.g., Porges et al., 1996). Third, cortisol had to be assayed in a single batch following data collection, whereas the RSA could be scored as data were collected. Finally, RSA data were available from a larger sample (N = 72, Alkon et al., 2003) to which these children's data could be compared.
To score RSA, data were edited for movement noise with a custom software package (Cacioppo et al., 1994) by raters trained and reliable on sample cases, r > .90. RSA was estimated using the natural logarithm of the variance of high-frequency heart period within the frequency bandpass associated with respiration. Mean RSA was computed for each 1-min epoch and then averaged for each story and task.
Because researchers have conceptualized reactivity as a multidimensional response pattern across a range of stimuli, an index was computed based on children's story and task RSA. Included in this index were the following four scores: (a) task mean (the average of each child's responses across the five challenging tasks), (b) task standard deviation (the within-child response standard deviation across the five tasks), (c) recovery (the child's RSA during the digit span minus RSA during the rest period), and (d) slope (the coefficient of each child's five RSA task scores regressed on epoch, that is, the change across the tasks in the child's RSA). Children's four scores were standardized by comparing them with means from children of the same age and gender in a separate study (Alkon et al., 2003). (Means for the present study were not available as data collection was ongoing. It was thus necessary to compare this sample's scores with those from a previous study to create standardized scores.) Consistent with prior research using similar scores to assess reactivity using a multidimensional approach (Boyce et al., 2001), larger RSA task means, standard deviations, and slopes reflect greater parasympathetic tone (i.e., decreased reactivity), whereas larger recovery scores reflect more parasympathetic withdrawal or increased reactivity. The four standardized scores were summed into a single index, with higher values reflecting greater reactivity (i.e., negative task mean, standard deviation, and slope scores were added to the RSA recovery scores; Boyce et al., 2001).
The second session took place after approximately 2 weeks (M = 16 days, range = 11 – 26 days). Upon arrival, the social support manipulation was explained to parents, and their written consent was secured. Parents were not informed of the manipulation before the session to preclude them from preparing their child for the possibility of a serious, professional interviewer. All parents consented to the interviews. Parents were not with their child but were able to observe the interview through a two-way mirror. The child's assent to be interviewed was obtained, and the interviews were videotaped.
Before the start of the second session, reactive and nonreactive children, defined as those above or below the mean on the preliminary RSA reactivity index, respectively, were randomly assigned to the high- or low-interviewer-support condition. This was done so that a sufficiently large number of reactive children would be interviewed in each condition. Interviews took place in an unfamiliar room. The child was escorted to the room by a known female researcher where the interviewer was waiting. The interviewer had not met the child before, was not present during the child's first session, and was blind to the child's reactivity and study hypotheses. Each interviewer conducted an approximately equal number of high- and low-support interviews, with a total of six interviewers assisting with data collection.
The social support manipulations were based on theory and research concerning interpersonal communication and dyadic interactions (Andersen, 1985; Mehrabian, 1969) and followed that employed by Davis and Bottoms (2002). In the low-support condition, the child sat to the side of and 3 ft from the interviewer. The interviewer, dressed in formal attire (e.g., pressed dark pants, a white shirt, and dark jacket) was reviewing paperwork when the child entered. She did not speak for 2 min, after which she explained that she needed to ask the child some questions about the last time the child visited the laboratory, that the child should answer accurately, but that the child could say “I don't know” if the child forgot an answer. The researcher then proceeded with the memory interview. She asked the questions in a monotone voice, did not smile or maintain eye contact with the child, and did not face the child during the interview. After asking two thirds of the questions, she stood for the remainder of the interviewer. The interviewer repeated the child's responses, but provided no feedback about the child's performance.
In the high-support interview condition, the interviewer sat facing the child and built rapport for 2 min by asking scripted questions. She then explained that she wanted to ask the child questions about the last time the child came to the laboratory, that the child should answer the questions as best as possible, but that the child could say “I don't know” if the child forgot an answer. The interviewer asked the memory questions, throughout which she used considerable vocal intonation, maintained eye contact, smiled, and provided verbal encouragement (e.g., “You're doing a great job!”) at prescribed times.
Immediately after the interview, the interviewer left. A female researcher entered and asked the child a brief set of manipulation check questions. She showed the child a picture depicting four stick-figure faces: one with a large smile (1), one with a slight smile (2), one with a slight frown (3), and one with a large frown (4). A second picture was also shown that substituted the frowning with angry faces (i.e., eyebrows burrowed, jagged mouth). The left-to-right order of faces was counterbalanced. The researcher asked the child to point to the face that showed how the interviewer felt. Children in the high-support condition pointed to significantly happier faces (Ms = 1.46, 1.25 for the sad and angry faces, respectively) than did children in the low-support condition (Ms = 2.28 and 2.09, ts > 3.33, ps < .01) on a scale ranging from 1 (very positive face) to 4 (very negative). Although the questions required children to speculate about the interviewer's internal state, the faces did not perfectly depict the emotional unavailability of the nonsupportive interviewer, and some children were reticent to pick unhappy faces (they pointed to happy faces before the questions were asked), the support manipulation was effective and noticeable to children.
Following the manipulation check, children were debriefed regarding the interviewer's behavior and importance of telling the truth. Children in the low-support condition were told that the interviewer had to be serious during the interview but was relaxed now that the interview was over. The interviewer talked with children briefly and explained how hard it was to be serious. Memory errors were also corrected at this time. Children and parents were thanked, parents received an honorarium, and children received toys.
Coding
Physiological reactivity variables
Three final autonomic reactivity scores were computed from the task and story RSA (editing and coding described previously) and PEP scores. To create the PEP scores, coders, reliably trained on sample cases (r > .90), inspected beat-by-beat data and removed movement artifact using custom software (Cacioppo et al., 1994). PEP corresponds to isovolumetric contraction during the cardiac cycle and is quantified as the time (ms) between the onset of ventricular depolarization and the onset of left ventricular ejection. The sympathetic nervous system directly affects this phase of the cardiac cycle, and heightened sympathetic activation shortens this cardiac phase with corresponding lower PEP scores. Both RSA and PEP scores were ensemble averaged by minute, averaged across task minutes, and then averaged across all the tasks and resting periods. One child's PEP data could not be scored because of partial equipment failure, leaving a total of 62 participants in PEP analyses.
The first and second of the three final autonomic scores were difference scores, one for RSA and one for PEP, computed by subtracting the average of each child's responses during the two neutral stories from their average responses across the five tasks. Although standardized residuals (obtained from regressing a task score on a baseline score) or other adjusted scores can be computed to take into account baseline physiological levels when computing change scores, difference scores are the most commonly used measures in studies of children's and adults' physiological reactivity (e.g., Allen & Matthews, 1997; Manuck, Kasprowicz, & Muldoon, 1990) and were thus included in the present study. Our results did not differ substantially whether raw or adjusted values were included. For both PEP and RSA difference scores, lower scores reflect greater reactivity (i.e., heightened sympathetic activation and increased parasympathetic withdrawal).
The third score was computed because of recent arguments that reactivity is a multidimensional response profile across indexes of autonomic responses (e.g., Berntson, Cacioppo, & Quigley, 1991; Salomon, Matthews, & Allen, 2000). Using scoring procedures described by Boyce et al. (2001), a single, multidimensional, autonomic composite score was created. PEP task means, standard deviation, recovery, and slope scores were calculated in a manner similar to that described for RSA (see the previous discussion). For PEP, larger task mean, standard deviation, and slope scores correspond to diminished sympathetic tone or decreased overall arousal, whereas larger recovery scores correspond to increased sympathetic reactivity. Next, both the RSA and PEP scores (the task mean, standard deviation, slope, and recovery) were standardized so that all measures were on the same scale. The standardized RSA task mean, standard deviation, recovery, and slope scores described previously were recomputed after data collection had finished using this sample's mean scores (the preliminary scores had been created using the means from another sample). Children's scores remained virtually identical to those created for the preliminary RSA reactivity index. The autonomic index was created by averaging children's negative task means, standard deviations, slopes, and positive recoveries (for both RSA and PEP). The highest scores reflect concurrent parasympathetic withdrawal and sympathetic activation.
The preprotocol and postprotocol cortisol samples were frozen following collection and later shipped on dry ice to the University of Minnesota to be assayed in a single batch. Each sample was assayed twice using an Amersham International Amerlex cortisol RIA kit. Because of a change in the analysis procedure requiring additional saliva, some children's cortisol data could not be assayed, leaving a total of 47 children with preprotocol and 49 children with postprotocol cortisol data. Because cortisol levels are elevated between 10 and 30 min following exposure to a stressor (Kirschbaum & Hellhammer, 1994), the preprotocol values represent children's HPA axis activity upon arrival at the laboratory, and the postprotocol values represent HPA axis activity during the first part of the reactivity protocol. Difference scores (n = 46) were computed by subtracting children's preprotocol from their postprotocol level. Three cortisol scores (preprotocol, postprotocol, and difference scores) were of interest in the study.
Memory coding
Children's free-recall responses in both interviews were scored for units of information using a system employed in previous studies (e.g., Poole & Lindsay, 1995; Quas & Schaaf, 2002). Units were defined as any agent, action, and object independently stated by the child. Units were scored as either correct or incorrect. For instance, the statement, “A fire alarm sounded,” received two correct units, one each for “fire alarm” and “sounded.” Children rarely provided incorrect information in either interview (Ms = 1.00 in both interviews), and incorrect units are not considered further.
Children's direct question responses in both interviews were coded as correct, incorrect, do not know, or unscorable. Proportions were created by summing responses and dividing them by the number of questions asked. In the fire alarm interview, virtually no child provided do-not-know or unscorable responses (response proportion means were less than 1%). Thus, correct and incorrect proportions summed to approximately 1.0, and only correct proportions are reported. In the second interview, children provided do-not-know responses, and the correct and incorrect responses were not the inverse of each other. Correct, incorrect, and do-not-know responses were thus analyzed. Unscorable responses (e.g., incomprehensible answers) constituted 1.5% of children's answers and are not considered further. Also, because our predictions did not distinguish between nonleading and misleading questions, they were collapsed into one “direct question” category for the Session 2 memory interview. Analyses conducted separately for nonleading versus misleading questions revealed patterns identical to those reported in the text for do-not-know proportions. When correct responses were examined, the findings were stronger for misleading than for nonleading questions.
Across memory measures, two independent raters scored 12 children's responses (equal number of boys and girls in each interview condition). Proportion agreement ranged from .87 to .96. Discrepancies were discussed and resolved, and the remaining data were coded by one rater.
Results
Preliminary analyses were conducted to identify potential confounds. Hypotheses concerning reactivity and children's fire alarm memory were tested using correlations. Hypotheses concerning reactivity and context as joint predictors of children's protocol memory were tested using regressions.
Preliminary Analyses
Potential confounds included the time of day of the cortisol collections, the delay between the first and second cortisol samplings, the delay between the two sessions, and child gender. Correlations failed to reveal any significant associations between children's pre- and post-protocol cortisol levels or difference scores and the time of the first cortisol collection or the delay in minutes between the two samplings (rs < .12). Additionally, the day delay between sessions was unrelated to children's age, the autonomic reactivity scores (PEP and RSA difference score, autonomic reactivity index), the cortisol scores, social support condition, or memory performance (rs = −.21 to .18). No differences emerged in the age of children assigned to the high- versus low-support conditions, t(55) = .02. Girls and boys did not differ on any of the physiological or memory variables (ts = −1.95 to 1.81, dfs = 44 to 61). Thus, gender is not considered further.
Age, Reactivity, and Fire Alarm Memory
The first goal of the study was to investigate the relations between autonomic and HPA reactivity and children's memory for the mildly stressful fire alarm incident. Means and standard deviations for the physiological and fire alarm memory variables are presented in Table 1.
Table 1. Means (Standard Deviations in Parentheses) of Children's Physiological Reactivity Scores, Fire Alarm Session I Memory Performance, and Protocol Session II Memory Performance.
| High support | Low support | Final sample | |
|---|---|---|---|
| Autonomic scores | N = 26 | N = 27 – 28 | N = 62 – 63 |
| PEP difference | 0.43 (1.72) | −0.13 (1.70) | 0.01 (1.74) |
| RSA difference | 0.05 (0.25) | 0.04 (0.43) | 0.05 (0.35) |
| Reactivity index | 0.04 (0.66) | −0.10 (0.64) | 0.03 (0.70) |
| Cortisol scores | N = 15 – 16 | N = 24 – 26 | N = 46 – 49 |
| Cortisol Time 1 | 0.11 (0.07) | 0.10 (0.07) | 0.11 (0.07) |
| Cortisol Time 2 | 0.07 (0.04) | 0.07 (0.05) | 0.07 (0.05) |
| Cortisol difference | −0.04 (0.06) | −0.03 (0.06) | −0.03 (0.06) |
| Session 1 fire alarm memory interview | N = 28 | N = 29 | N = 62 |
| Units of correct information | 9.59 (8.27) | 8.18 (6.76) | 8.65 (7.45) |
| Direct question correct | 0.85 (0.18) | 0.82 (0.18) | 0.84 (0.18) |
| Session 2 memory interview | N = 28 | N = 29 | N = 57 |
| Units of correct information | 12.35 (9.11) | 12.07 (8.61) | 12.21 (8.78) |
| Direct question correct | 0.67 (0.11) | 0.67 (0.12) | 0.67 (0.12) |
| Direct question errors | 0.23 (0.09) | 0.24 (0.10) | 0.24 (0.10) |
| Direct question do not know | 0.08 (0.07) | 0.08 (0.07) | 0.08 (0.07) |
Note. PEP = pre-ejection period; RSA = respiratory sinus arrhythmia. For PEP and RSA difference scores, greater values reflect lower reactivity. The autonomic reactivity index was a composite score, with high scores reflecting the combination of high sympathetic activation and parasympathetic withdrawal. The sample numbers for some of the high- and low-support condition means do not sum to the final sample number because the social support manipulation was introduced at the outset of the second session, and 6 children who have data during the first session were not included in the second session. Also, 1 child was missing PEP data because of equipment failure, and several children's cortisol samples could not be assayed because of insufficient saliva to conduct duplicate assays. One child did not answer the fire alarm memory questions.
Before analyzing the relations between reactivity and memory, age effects and the relations among the reactivity measures were examined. Correlations revealed that increasing age was associated with lower pretask cortisol levels, r(47) = −.30, p < .05. Additionally, as expected, age was positively related to the amount of correct narrative detail children provided in free recall, r(62) = .39, p < .01. Given that age was related to reactivity and memory, it was covaried in subsequent analyses.
Among the reactivity measures, correlations (age partialed) revealed that lower PEP difference scores (indicative of greater reactivity) were associated with higher preprotocol cortisol levels (r = −.29), and lower RSA difference scores (also indicative of greater reactivity) were associated with larger cortisol difference scores (r = −.32). Thus, to some extent, greater PEP and RSA reactivity levels were related to greater cortisol reactivity. Additionally, as would be expected, children's pre- and post-protocol salivary cortisol levels were relatively strongly related (r = .53), and preprotocol cortisol was strongly and negatively related to the cortisol difference scores (r = −.66, all dfs = 43, ps < .05).
We hypothesized that autonomic reactivity would be negatively and cortisol reactivity would be positively related to children's fire alarm memory. Correlations between the reactivity and memory scores (age partialed) are presented in Table 2. Consistent with our hypothesis, lower RSA difference scores, which are indicative of increased parasympathetic reactivity, were associated with providing fewer correct responses to the direct questions. Neither the PEP difference score nor the autonomic reactivity composite was significantly related to children's memory. Finally, although nonsignificant, higher pre- and post-protocol cortisol levels were marginally associated with higher proportion correct responses to the direct questions.
Table 2. Correlations (Age Partialed) Between Children's Physiological Reactivity Scores and Fire Alarm Session 1 Memory Performance (Autonomic dfs = 60 or 61; Cortisol dfs = 36 to 39).
| Free-recall correct units | Direct question proportion correct | |
|---|---|---|
| Autonomic scores | ||
| PEP difference | .11 | .05 |
| RSA difference | −.11 | .29* |
| Reactivity index | .03 | .12 |
| Cortisol scores | ||
| Time 1 | .03 | .27† |
| Time 2 | .06 | .25† |
| Cortisol difference | −.01 | −.08 |
Note. PEP = pre-ejection period, RSA = respiratory sinus arrhythmia. For PEP and RSA difference scores, greater values reflect lower reactivity. The reactivity index is a composite score, with higher values corresponding to greater sympathetic activation and parasympathetic withdrawal.
p < .10.
p < .05.
In summary, our hypotheses were partially supported. Consistent with previous research, increased parasympathetic reactivity was associated with poorer performance, and increased HPA reactivity was associated with enhanced performance.
Age, Reactivity, Social Support, and Memory
In the second session, children were interviewed by either a supportive or nonsupportive interviewer about what happened during the first session. We hypothesized that autonomically and HPA reactive children would be more susceptible to negative memory performance when questioned by a nonsupportive interviewer than would nonreactive children. That is, we expected children's reactivity to interact with interviewer-provided social support to affect their memory. We also expected performance to improve with age and an overall benefit of social support on performance. Mean reactivity and memory performance in the high- and low-support conditions are presented in Table 1.
We first conducted bivariate correlations among children's age, reactivity, and second-session memory performance. As hypothesized, with age, children provided more units of information in free recall (r = .26) and a higher proportion of correct responses to direct questions (r = .36); increasing age was also associated with providing fewer incorrect responses to direct questions (r = −.32, all ns = 57, ps < .05). With age controlled statistically, higher preprotocol cortisol levels were associated with enhanced free-recall performance (r = .40) and with fewer do-not-know responses to direct questions (r = −.33); in addition, larger cortisol difference scores, or increased cortisol reactivity, were related to a lower proportion of correct responses and a higher proportion of do-not-know responses to direct questions (rs = −.30 and .32, respectively, all dfs = 39, ps < .05). Finally, two correlations approached significance: autonomic reactivity and proportion do-not-know responses to direct questions, r(54) = −.23, and cortisol difference scores and free-recall correct units, r(39) = .27, ps < .10.
Thus, high cortisol levels in terms of absolute preprotocol values enhanced memory, whereas larger increases in cortisol during the protocol inhibited memory. We next sought to investigate the extent to which reactivity interacted with social support to affect children's performance.
Four hierarchical regressions were conducted. The four Session 2 memory variables (free-recall units correct and direct question proportion correct, incorrect, and do-not-know responses) were entered as separate dependent measures. Children's age, the autonomic reactivity index, and interviewer support were entered on the first step, and interaction between reactivity and social support was entered on the second step. The autonomic reactivity index was included rather than the RSA or PEP difference scores because the index reflects a multidimensional response profile across the parasympathetic and sympathetic systems. Similar regressions were then conducted substituting cortisol difference scores for the autonomic reactivity index (the two reactivity measures could not be entered concurrently because of the small sample size and missing cortisol data). Variables were centered before their inclusion.
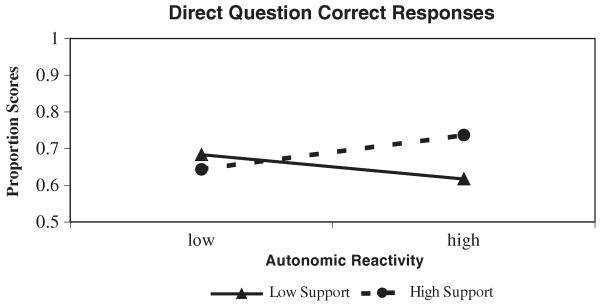
The models examining children's proportion correct and do-not-know responses to direct questions were significant, Fs (4, 52) = 3.76 and 3.39, respectively, ps < .05, and explained 22% and 21% of the variance, respectively. When correct proportions were examined, age was positively associated with correct responses. The interaction between autonomic reactivity and support condition was also significant (betas are presented in Table 3). A plot of the interaction (Figure 1) revealed the hypothesized interaction: In the high-support condition, autonomic reactivity was positively associated with correct responses, whereas in the low-support condition, autonomic reactivity was negatively related to correct responses.
Table 3. Regressions Beta Weights Predicting Children's Session 2 Memory Performance From Their Age, Autonomic Reactivity, and Support Condition.
| Free-recall correct units | Direct question proportion | |||
|---|---|---|---|---|
| Correct | Incorrect | Do not know | ||
| Step 1 | β | β | ||
| Children's age | – | .31* | – | −.02 |
| Autonomic reactivity | – | .08 | – | −.27* |
| Support condition (1 = high, 2 = low) | – | −.02 | – | −.10 |
| R2 | .13 | .07 | ||
| Step 2 | β | β | ||
| Reactivity × Support interaction | – | −.30* | – | .37** |
| ΔR2 | .09 | .14 | ||
Note. β = standardized betas. High scores on the autonomic index reflect a combination of high-sympathetic activation and parasympathetic withdrawal.
p < .05.
p < .01.
Figure 1.
The significant Autonomic Reactivity × Social Support interaction predicting children's correct responses to direct questions.
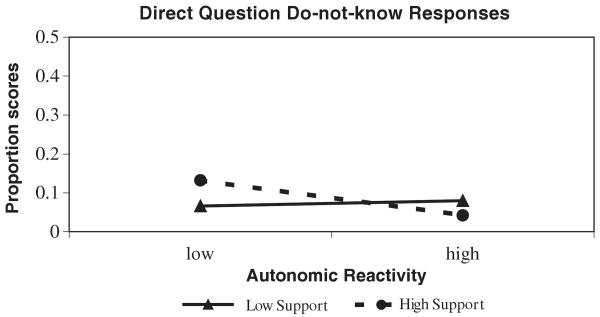
When children's do-not-know responses were examined, a significant main effect of autonomic reactivity emerged, with greater reactivity being associated with decreases in do-not know responses. However, a significant interaction revealed that this pattern was only evident among children questioned by a supportive interviewer (see also Table 3). As is evident in Figure 2, the most reactive children provided the fewest do-not-know responses to the supportive interviewer, and it is surprising, the least reactive children provided the most do-not-know responses to the supportive interviewer. When the interviewer was nonsupportive, reactivity was largely unrelated to children's do-not-know responses.
Figure 2.
The significant Autonomic Reactivity × Social Support interaction predicting children's do-not-know responses to direct questions.
Next, regressions including children's cortisol difference scores were conducted. Although none of the models was significant, the model predicting proportion correct responses to direct questions approached significance, F(4, 37) = 2.52, p < .06, and explained 21% of the overall variance. Age was positively associated with correct responses (β = .38, p < .05). Additionally, larger cortisol difference scores were moderately associated with lower proportion of correct responses (β = −.31, p = .06).
Summary
Together, the analyses provide tentative support for the hypothesized interactions between reactivity and social context, at least for autonomic reactivity. Under nonsupportive interview conditions, autonomically reactive children provided the fewest correct responses. Under supportive interview conditions, autonomically reactive children provided the fewest do-not-know responses. Unexpectedly, lower reactivity was associated with opposite pattern: Nonreactive children fared slightly worse in the supportive condition, at least in terms of providing do-not-know responses.
Discussion
The overarching goal of the study was to provide an experimental test of physiological reactivity as a form of biological sensitivity to social context. Also of interest were the more general relations between physiological reactivity and memory for a mildly stressful experience following a relatively short and somewhat longer delay. Results revealed some direct relations between children's autonomic and cortisol reactivity and memory. Results also revealed interactive effects of autonomic reactivity and social context predicting children's memory. We now discuss the implications of these results for a broader understanding of physiological reactivity in childhood.
Reactivity and Memory
Several results were consistent with findings from previous studies of reactivity and memory (Bugental et al., 1992; Stein & Boyce, 1995). Poorer memory performance during the first-session fire alarm interview was evident among parasympathetically reactive children. Perhaps, as suggested by previous researchers, the autonomically reactive children were too aroused during the laboratory tasks and fire alarm to attend to and encode the event sufficiently, and lack of encoding would contribute to poorer memory (see Kail, 1990). However, we believe that such an explanation does not adequately account for the general pattern of results. For one, significant relations between parasympathetic reactivity and memory were not evident in children's free-recall responses. Free recall is often considered a purer measure of memory than responses to closed-ended or direct questions, which are more easily affected by social acquiescence and response biases (Fivush, Peterson, & Schwarzmueller, 2002; Leichtman & Ceci, 1994). Insofar as reactive children had not encoded the alarm adequately, their free-recall performance should have suffered. Also, although the second-session memory interview concerned the entire protocol rather than only the fire alarm (which was the focus of the first interview), a subset of the second-session questions concerned the alarm. Similar findings should thus have emerged (i.e., negative associations between reactivity and memory) if reactive children had not encoded the alarm or first session sufficiently. Instead, in the second session, autonomic reactivity was associated with fewer do-not-know responses. Also, the reactive children outperformed the nonreactive children when questioned by a supportive interviewer. If reactivity led to encoding difficulties, the reactive children should not have performed better, even in the high-support condition. Therefore, either an unwillingness to talk or inability to retrieve fire alarm details appeared to have contributed to the reactive children's poor initial performance. In future research, the inclusion of non-event-related questions as control items may help distinguish whether reactive children are generally unwilling to answer questions or have trouble specifically with retrieving event-related details.
With regard to HPA reactivity and memory, correlations revealed that heightened cortisol levels at the outset of the laboratory session were associated with enhanced memory, but larger cortisol difference scores were associated with poorer memory. The children with high initial cortisol levels may have been reacting to the anticipation of coming to a new environment, and these high cortisol levels may have activated neural structures involved in memory consolidation and enhanced retention. The children who exhibited larger cortisol increases during the protocol, however, may be those traditionally considered reactive. They may have had difficulty regulating their arousal during protocol, leading to difficulties attending to the ongoing event. Such an interpretation is consistent with Sapolsky et al.'s (2000) proposal that basal glucocorticoids can facilitate memory formation through effects on MRs in the hippocampus. However, long-term heightened stress-response glucocorticoids can impair memory formation through effects on hippocampal GRs. In general, continued research is needed concerning when and how children's basal cortisol versus cortisol responses, both in terms of GRs and MRs, relate to memory for personal experiences.
Reactivity, Social Support, and Memory
Our study provides experimental support for the conceptualization of reactivity as a form of biological sensitivity to social context. Exaggerated autonomic reactivity was associated with enhanced performance in the high-support condition, as indexed by more correct and fewer do-not-know responses to direct questions, but inhibited performance in the low-support condition, as indexed by fewer correct responses. Obviously, this study is not the first to propose or uncover interactions between individual and environmental characteristics (e.g., Brofenbrenner & Ceci, 1994; Chess & Thomas, 1991; Lerner & Lerner, 1994; Plomin, 1996). However, our study is the first to test, using an experimental paradigm, the extent to which physiological reactivity per se interacts with the supportiveness of an immediate interview context to affect children's event memory. Furthermore, our results are consistent with and extend results of studies concerning reactivity and general social environment (e.g., Boyce et al., 1995; Gunnar et al., 1997) as joint predictors of adverse outcomes. In prior studies, social context was not manipulated experimentally, precluding causal interpretations of results. High-stress environments were identified using questionnaires about parents' marital relationships or family conflict. In this study, the stressfulness of the immediate social context was manipulated using interviewer behavior. Despite the “stress” of the emotionally unavailable interviewer being short lived, situationally based, and particularly mild, the fact that the effects on memory performance were similar to the effects of general social stress on health and behavior is noteworthy. Furthermore, because the regressions suggested that the most reactive children performed better than the least reactive children when questioned in a supportive manner, reactivity does not appear to impede children's cognitive or at least memory abilities. Instead, reactive children's performance may only suffer when the social context is demanding, unsupportive, or emotionally cold.
We found it surprising that a trend suggested that children especially low in reactivity provided a higher proportion of do-not-know responses when questioned by a supportive rather than nonsupportive interviewer. Previous studies of effects of interviewer-provided versus interviewer-withdrawn social support on children's memory and suggestibility have not found adverse effects of high support, although some studies have failed to uncover effects of high versus low interviewer support (e.g., Imhoff & Baker-Ward, 1999). Perhaps children particularly low in reactivity were not sufficiently aroused in the high-support interview condition to put forth effort on the memory task. As Yerkes and Dodson (1908) and others (Christianson, 1992; Easterbrook, 1959) have outlined, moderate levels of arousal may facilitate performance. The nonreactive children may have required some arousal to perform the task, and they received it in the nonsupportive condition. It is important that the results only emerged with do-not-know responses. Our interpretation is thus speculative until further research confirms whether children low in autonomic reactivity are generally better at answering questions posed by a serious rather than supportive interviewer.
As a final note, the hypothesized Reactivity × Social Context interaction only emerged when autonomic arousal was measured. Studies of autonomic reactivity and cognition in childhood are rare, especially studies that include measures of social context and parasympathetic, sympathetic, and HPA reactivity, making it difficult to speculate as to why our findings were limited to autonomic reactivity. Some insight, however, may be gleaned from a consideration of each system's functions. In response to perceived stress, a virtually instantaneous but short-lived signal is sent by sympathetic fibers to target organs throughout the body, allowing fast, diffuse actions characteristic of the sympathetic system (Cannon, 1914, 1929/1953). Thus, the sympathetic system is highly dependent on environmental input for activation, and it responds quickly to that input. The parasympathetic nervous system also reacts quickly following exposure to stress, although the system's function is to restore and maintain resting physiology. In contrast, responses of the HPA system, which involve release of glucocorticoids into the blood, take longer to emerge than autonomic, especially sympathetic, responses (Sapolsky et al., 2000). The delay between stress exposure and the appearance of cortisol in saliva may make cortisol levels not as noticeably altered in studies involving minor variations of a single social context as are autonomic values. Although challenging, it will be necessary in the future to include measures of multiple systems across a range of stressors to determine each system's relations to context and adverse cognitive outcomes (Cacioppo, Berntson, Sheridan, & McClintock, 2000).
Limitations and Conclusions
The present study represents an important, but only a first, step in the study of physiological reactivity, social context, and memory. Some caution in interpreting the results is warranted. First, the sample was small. Although we detected several predicted and meaningful results, additional findings approached significance. More robust associations may have been uncovered in a larger sample. Also, physiological reactivity was not assessed at retrieval. We speculate that the low-support condition was arousing to children, especially those considered physiologically reactive. Such a pattern has been found in studies with adults (e.g., Lepore, Allen, & Evans, 1993), but we are only beginning to investigate this possibility in children. In one study, for example, we are examining children's physiological responses at encoding as well as retrieval to determine the extent to which a low-support interviewer is arousing to children and the way arousal at retrieval relates to children's memory (e.g., Quas, 2003). Finally, the reactivity protocol was designed to identify individual differences in children's physiological stress responses. As in previous studies (e.g., Alkon et al., 2003), children's performance in our study varied considerably, allowing us to examine how this variability related to memory. However, some children were not physiologically aroused during the protocol. It will be necessary in future research to investigate how children's physiological reactions to other types of stressors relate to social context and performance on both memory and nonmemory tasks.
In closing, although children who exhibit large or prolonged physiological responses to stress are routinely labeled at risk for physical and mental health disorders, social problems, and cognitive difficulties, this label needs to be qualified, at least as it pertains to cognitive difficulties. The extent to which reactivity serves as a risk factor for cognitive or memory impairments may depend on the context in which children's abilities are assessed (e.g., situationally supportive vs. nonsupportive interview), the physiological system being studied (e.g., autonomic vs. HPA), and the type of memory being measured (e.g., free recall vs. recognition). Theoretical models of reactivity in childhood, experimental methods assessing reactivity, and measures of cognitive outcomes must all become more complex to understand more fully if and when reactivity in childhood is a risk factor for adverse outcomes and how to target interventions to help reactive children who are at risk adjust better to their increased sensitivity.
Acknowledgments
This study was supported by the MacArthur Foundation Research Network on Psychopathology and Development. We wish to thank Abbey Alkon, Jason DeCaro, Holly Eibs, Monique Ewig, Lauren Goldstein, and Mimi Wolff for their assistance during the project. Gratitude is also expressed to the families who elected to take part in the project and the undergraduate research assistants who helped with data collection.
Contributor Information
Jodi A. Quas, Department of Psychology and Social Behavior, University of California, Irvine
Amy Bauer, Department of Psychiatry, Massachusetts General and McLean Hospital.
W. Thomas Boyce, School of Public Health and Institute of Human Development, University of California, Berkeley.
References
- Alkon AA, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Allen M, Matthews K. Hemodynamic responses to laboratory stressors in children and adolescences: The influence of age, race, and gender. Psychophysiology. 1997;34:329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Andersen PA. Nonverbal immediacy in interpersonal communication. In: Siegman AW, Feldstein S, editors. Multichannel integrations of nonverbal behavior. Hillsdale, NJ: Erlbaum; 1985. pp. 1–36. [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: Advantages of a multi-system approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Belsky J. Expanding the ecology of human development: An evolutionary perspective. In: Moen P, Elder GH Jr, Luscher K, editors. Examining lives in context: Perspectives on the ecology of human development. Washington, DC: American Psychological Association; 1995. pp. 545–561. [Google Scholar]
- Berntson G, Cacioppo J, Quigley K. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, et al. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: An evolutionary-developmental theory of the origins and functions of stress reactivity. 2002 doi: 10.1017/s0954579405050145. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas JA, Alkon A, Smider N, Essex M, Kupfer D. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brofenbrenner U, Ceci SJ. Nature – nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Blue J, Cortez V, Fleck K, Rodriguez A. The influence of witnessed affect on information processing in children. Child Development. 1992;63:774–786. [PubMed] [Google Scholar]
- Burleson BR, Albrecht TL, Goldsmith DJ, Sarason IG. Introduction: The communication of social support. In: Burleson BR, Albrecht TL, Sarason IG, editors. Communication of social support. Thousand Oaks, CA: Sage; 1994. pp. xi–xxx. [Google Scholar]
- Cacioppo JT, Berntson GG, Sheridan JF, McClintock MK. Multilevel integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychological Bulletin. 2000;126:829–843. doi: 10.1037/0033-2909.126.6.829. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and pre-ejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development & Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The interrelations of emotions as suggested by recent physiological researches. American Journal of Psychology. 1914;25:256–282. [Google Scholar]
- Cannon WB. Bodily changes in pain, hunger, fear, and rage; an account of recent researches into the function of emotional excitement. 2nd. Boston: Branford; 1953. Original work published 1929. [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Carter CA, Bottoms BL, Levine M. Linguistic and socio-emotional influences on the accuracy of children's reports. Law and Human Behavior. 1996;20:335–358. [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: How and why do these relationship change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chen E, Zeltzer LK, Craske MG, Katz ER. Children's memories for painful cancer treatment procedures: Implications for distress. Child Development. 2000;71:933–947. doi: 10.1111/1467-8624.00200. [DOI] [PubMed] [Google Scholar]
- Chess S, Thomas A. Temperament and the concept of goodness-of-fit. In: Strelau J, Angleitner A, editors. Explorations in temperament: International perspectives on theory and measurement. New York: Plenum Press; 1991. pp. 15–28. [Google Scholar]
- Christianson SA. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Davis SL, Bottoms BL. The effects of social support on children's eyewitness reports: A test of the underlying mechanism. Law & Human Behavior. 2002;26:185–215. doi: 10.1023/a:1014692009941. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol responses in relation to child temperament. Developmental Psychobiology. 1999;35:188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Edens JL, Larkin KT, Abel JL. The effect of social support and physical touch on cardiovascular reactions to mental stress. Journal of Psychosomatic Research. 1992;36:371–381. doi: 10.1016/0022-3999(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Bustamante D, Mathy RM, Miller PA, Lindholm E. Differentiation of vicariously induced emotional reactions in children. Developmental Psychology. 1988;24:237–246. [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Karbon M, Smith M, Maszk P. The relations of children's dispositional empathy-related responding to their emotionality, regulation, and social functioning. Developmental Psychology. 1996;32:195–209. [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: Tests of an evolutionary-developmental hypothesis. 2002 doi: 10.1017/s0954579405050157. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Eisenbud L. Behavioral and physiological correlates of children's reactions to others in distress. Developmental Psychology. 1993;29:655–663. [Google Scholar]
- Fivush R, Peterson C, Schwarzmueller A. Questions and answers: The credibility of child witnesses in the context of specific questioning techniques. In: Eisen M, Quas J, Goodman G, editors. Memory and suggestibility in the forensic interview. Mahwah, NJ: Erlbaum; 2002. pp. 331–354. [Google Scholar]
- Gannon L, Banks J, Shelton D, Luchetta T. The mediating effects of psychophysiological reactivity and recovery on the relationship between environmental stress and illness. Journal of Psychosomatic Research. 1989;33:167–175. doi: 10.1016/0022-3999(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Gerin W, Milner D, Chawla S, Pickering TG. Social support as a moderator of cardiovascular reactivity in women: A test of the direct effects and buffering hypotheses. Psychosomatic Medicine. 1995;57:16–22. doi: 10.1097/00006842-199501000-00003. [DOI] [PubMed] [Google Scholar]
- Golier J, Yehuda R. Neuroendocrine activity and memory-related impairments in posttraumatic stress disorder. Development & Psychopathology. 1998;10:857–869. doi: 10.1017/s0954579498001904. [DOI] [PubMed] [Google Scholar]
- Goodman GS, Bottoms B, Schwartz-Kenney B, Rudy L. Children's testimony about a stressful event: Improving children's reports. Journal of Narrative and Life History. 1991;7:69–99. [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: Potential effects on the developing human brain. Preventive Medicine. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Harold GT, Conger R. Marital conflict and adolescent distress: The role of adolescent wariness. Child Development. 1997;68:333–350. doi: 10.1111/j.1467-8624.1997.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Hefflinger AK, Newcomer JW. Glucocorticoid effects on memory function over the human life span. Development & Psychopathology. 2001;13:491–513. doi: 10.1017/s0954579401003054. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar MR, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Hilmert CJ, Christenfeld N, Kulik JA. Audience status moderates the effects of social support and self-efficacy on cardiovascular reactivity during public speaking. Annals of Behavioral Medicine. 2002;24:122–131. doi: 10.1207/S15324796ABM2402_09. [DOI] [PubMed] [Google Scholar]
- Hilmert CJ, Kulik JA, Christenfeld N. The varied impact of social support on cardiovascular reactivity. Basic & Applied Social Psychology. 2002;24:229–240. [Google Scholar]
- Ilg F, Ames L, Haines J, Gillespie C. Gesell School Readiness Test. New York: Harper & Row; 1978. [Google Scholar]
- Imhoff MC, Baker-Ward L. Preschoolers' suggestibility: Effects of developmentally appropriate language and interviewer supportiveness. Journal of Applied Developmental Psychology. 1999;20:407–429. [Google Scholar]
- Johnston-Brooks CH, Lewis MA, Evans GW, Whalen CK. Chronic stress and illness in children: The role of allostatic load. Psychosomatic Medicine. 1998;60:597–603. doi: 10.1097/00006842-199809000-00015. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Kagan J, Snidman N, Arcus D, Reznick SJ. Galen's prophecy. New York: Basic Books; 1994. [Google Scholar]
- Kail R. The development of memory in children. 3rd. New York: Freeman; 1990. [Google Scholar]
- Kamarck TW, Manuck SB, Jennings JR. Social support reduces cardiovascular reactivity to psychological challenge: A laboratory model. Psychosomatic Medicine. 1990;52:42–58. doi: 10.1097/00006842-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- Kelly KS, Hayslip B., Jr Gains in fluid ability performance and their relationship to cortisol. Experimental Aging Research. 2000;26:153–157. doi: 10.1080/036107300243614. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Leichtman M, Ceci SJ. The effects of stereotypes and suggestions on preschoolers' reports. Developmental Psychology. 1994;31:568–578. [Google Scholar]
- Lepore SJ. Problems and prospects for the social support-reactivity hypothesis. Annals of Behavioral Medicine. 1998;20:257–269. doi: 10.1007/BF02886375. [DOI] [PubMed] [Google Scholar]
- Lepore SJ, Allen KM, Evans GW. Social support lowers cardiovascular reactivity to an acute stressor. Psychosomatic Medicine. 1993;55:518–524. doi: 10.1097/00006842-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Lerner JV, Lerner RM. Explorations of the goodness-of-fit model in early adolescence. In: Carrey WB, McDevitt SC, editors. Prevention and early intervention: individual differences as risk factors for the mental health of children: A festschrift for Stella Chess and Alexander Thomas. Philadelphia: Brunner/Mazel; 1994. pp. 161–169. [Google Scholar]
- Lundberg U, Rasch B, Westermark O. Physiological reactivity and Type A behavior in preschool children: A longitudinal study. Behavioral Medicine. 1991-1992;17:149–157. doi: 10.1080/08964289.1991.9935166. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kasprowicz AL, Muldoon MF. Behaviorally-evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Annals of Behavioral Medicine. 1990;12:17–29. [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Developmental Psychobiology. 1998;33:283–292. [PubMed] [Google Scholar]
- Matthews KA, Woodall KL. Childhood origins of overt Type A behaviors and cardiovascular reactivity to behavioral stressors. Annals of Behavioral Medicine. 1988;10:71–77. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. In: Adler NE, Marmot M, editors. Socioeconomic status and health in industrial nations: Social, psychological, and biological pathways. New York: New York Academy of Sciences; 1999. pp. 30–47. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Mehrabian D. Some referents and measures of nonverbal behavior. Behavioral Research Methods and Instruments. 1969;1:213–217. [Google Scholar]
- Merritt KA, Ornstein PA, Spicker B. Children's memory for a salient medical procedure: Implications for testimony. Pediatrics. 1994;94:17–23. [PubMed] [Google Scholar]
- Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system: A life span perspective. Developmental Psychopathology. 2001;13:565–580. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: Possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Alpert BS, Walker SS. Whether to measure change from baseline or absolute level in studies of children's cardiovascular reactivity: A two-year follow-up. Journal of Behavioral Medicine. 1991;14:409–419. doi: 10.1007/BF00845116. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Peters DP. The influence of stress and arousal on the child witness. In: Doris J, editor. The suggestibility of children's recollections. Washington, DC: American Psychological Association; 1991. pp. 60–76. [Google Scholar]
- Plomin R. Beyond nature versus nurture. In: Hall LL, editor. Genetics and mental illness: Evolving issues for research and society. New York: Plenum Press; 1996. pp. 29–50. [Google Scholar]
- Poole D, Lindsay DS. Interviewing preschoolers: Effects of nonsuggestive techniques, parental coaching, and leading questions on reports of nonexperienced events. Journal of Experimental Child Psychology. 1995;60:129–154. [Google Scholar]
- Porges SW. Autonomic regulation and attention. In: Campbell B, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Erlbaum; 1992. pp. 201–223. [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. In: Carter CS, Lederhendler I, Kirkpatrick B, editors. The integrative neurobiology of affiliation. New York: New York Academy of Sciences; 1997. pp. 62–77. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Quas JA. Children's memory for mild stressors: Combined influence of child and contextual factors. Paper presented at the biennial conference of the Cognitive Development Society; Park City, UT. 2003. Oct, [Google Scholar]
- Quas JA, Murowchick E, Bensadoun J, Boyce WT. Predictors of children's cortisol activation during the transition to kindergarten. Journal of Developmental and Behavioral Pediatrics. 2002;23:304–313. doi: 10.1097/00004703-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Quas JA, Schaaf JM. Children's memories of experienced and nonexperienced events following repeated interviews. Journal of Experimental Child Psychology. 2002;83:304–338. doi: 10.1016/s0022-0965(02)00150-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning & Memory. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Salomon K, Matthews K, Allen M. Patterns of parasympathetic and sympathetic reactivity in a sample of children and adolescence. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Salzinger S, Feldman RS, Ng-Mak DS, Mojica E, Stockhammer T. Effects of physical abuse on children's social and affective status: A model of cognitive and behavioral processes explaining the association. Development and Psychopathology. 2001;13:805–825. [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarason BR, Sarason IG, Pierce GR, editors. Social support: An interactional view. New York: Wiley; 1990. [Google Scholar]
- Scarpa A. Aggression in physically abused children: The interactive role of emotion regulation. In: Raine A, Brennan PA, Farrington D, Mednick SA, editors. Biosocial bases of violence. New York: Plenum Press; 1997. pp. 341–343. [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Stein N, Boyce T. The role of physiological reactivity in attending to, remembering, and responding to an emotional event. In: Children's memory for emotional and traumatic events; Symposium conducted at the Society for Research in Child Development Meetings; Indianapolis, IN. 1995. Apr, Chairs. [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. New York: Wiley; 1988. pp. 629–649. [Google Scholar]
- Strelau J, Eliasz A. Temperament risk factors for Type A behavior pattern in adolescents. In: Carey WB, McDevitt S, editors. Prevention and early intervention: Individual differences as risk factors for the mental health of children. A festschrift for Stella Chess and Alexander Thomas. New York: Brunner/Mazel; 1994. pp. 42–49. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Vandell DL, Corasaniti MA. Childcare and the family: Complex contributors to child development. In: McCartney K, editor. Child care and maternal employment: A social ecological approach. San Francisco: Jossey-Bass; 1990. pp. 23–37. [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology of Psychology. 1908;18:459–482. [Google Scholar]