Abstract
Shwachman Diamond Syndrome (SDS) is an inherited bone marrow failure syndrome caused by biallelic SBDS gene mutations. Here we examined SBDS protein levels in human bone marrow. SBDS protein expression was high in neutrophil progenitors, megakaryocytes, plasma cells and osteoblasts. In contrast, SBDS protein levels were low in all hematopoietic cell lineages from patients harboring the common SBDS mutations. We conclude that SBDS protein levels vary widely between specific marrow lineages. Uniformly low SBDS protein expression levels distinguish the majority of SDS patients from controls or other marrow failure syndromes.
Keywords: Shwachman Diamond Syndrome, SBDS, bone marrow failure, neutropenia, immunohistochemistry
INTRODUCTION
Shwachman-Diamond Syndrome (SDS) is an autosomal recessive multi-system syndrome characterized by exocrine pancreatic dysfunction, bone marrow failure, skeletal abnormalities, and leukemia predisposition (Reviewed in [1]). Neutropenia, defined as a neutrophil count less than 1,500 × 109/L, is a common feature of SDS. Abnormal neutrophil chemotaxis has also been noted. Thrombocytopenia and anemia may also develop and a subset of patients progress to severe aplastic anemia. Immunologic abnormalities, such as low IgG or IgG subclasses, low numbers of circulating B cells, impaired B-lymphocyte proliferation and lack of specific antibody production against protein and polysaccharide antigens, have been described for some SDS patients [2].
Mutations in the Shwachman-Bodian-Diamond Syndrome (SBDS) gene are found in over 90% of patients with SDS. The SBDS gene encodes a highly conserved novel protein that is implicated in ribosomal biogenesis [3, 4], actin cytoskeletal function [5], and mitotic spindle stabilization [6]. The majority of mutant alleles (74%) associated with SDS are the result of gene conversion events with an adjacent pseudogene and lead to a marked decrease in SBDS expression. The remaining pathogenic alleles include frameshift and, rarely, missense changes [7].
We hypothesized that SBDS protein expression patterns may provide insights into the hematologic phenotype of SDS. To this end, we characterized the SBDS expression patterns across different cell lineages in human bone marrow. We further compared SBDS protein expression patterns in SDS patients harboring the common SBDS mutations to those in normal controls and patients with other marrow failure syndromes.
METHODS
Patient Selection
Bone marrow biopsy specimens from controls and patients with SDS and other marrow failure syndromes were obtained from the pathology archives at three institutions: Children’s Hospital Boston, Seattle Children’s Hospital, and Seattle Cancer Care Alliance. Control samples were obtained from patients with solid tumors who had normal BM findings on initial diagnostic staging work-ups. When available, multiple, longitudinal samples from patients were assayed to ascertain the reproducibility of the assay. Biallelic SBDS mutations were found in 9 of 10 SDS patients. SBDS sequence data was not available for the tenth patient, who died prior to the advent of SBDS sequencing but had exocrine pancreatic insufficiency and BM failure requiring hematopoietic stem cell transplant.
Immunohistochemical Staining
The polyclonal antibody against SBDS was previously described [8]. Immunohistochemical staining of formalin- or Bouin’s-fixed, paraffin-embedded tissue was optimized using a Ventana Discovery XT automated immunohistochemistry slide processing platform. Following the Closed Loop Assay Development (CLAD) protocol (Ventana Medical Systems, Tucson, AZ), SBDS visualization was optimized using the OmniMap DAB anti-Rabbit (HRP) detection kit (Ventana Medical Systems, Tucson, AZ). Appropriate positive, negative, and no primary antibody controls were included in each staining run.
Data Collection and Analysis
SBDS expression was scored independently by two hematopathologists in a blinded fashion on a scale from 0 to 3. In order to scale the immunohistochemical staining, a set of between three and five control samples was evaluated for each fixation/tissue processing method and the staining intensity of the strongest control samples in the megakaryocytic lineage was defined as a score of "3". The absence of detectable staining was set as a score of “0”. Scores of “1” and “2” were assigned to minimal detectable staining and intermediate staining, respectively (Supplemental Figure 1). Rare cases with discordant scores were jointly evaluated and a consensus score was assigned. In order to insure both general immunoreactivity and to confirm the presence and location of myeloid precursors, several SDS marrows were stained with myeloperoxidase and showed appropriate reactivity (Supplemental Figure 2). The individual scores from the above four cell lineages were then averaged to form a mean score for each specimen.
RESULTS AND DISCUSSION
SBDS expression levels vary between different marrow lineages
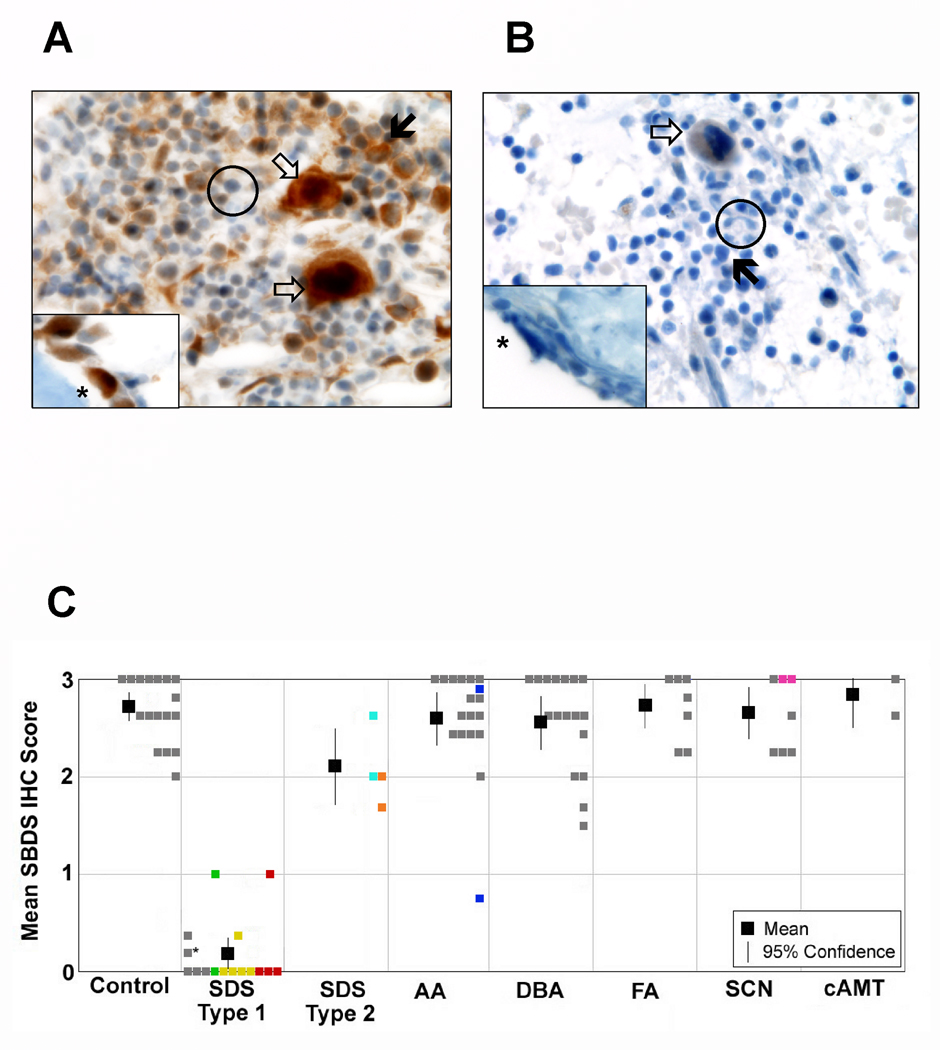
We first examined SBDS expression patterns in bone marrow biopsies from control marrows (Figure 1A). We observed that SBDS protein levels were not homogeneous within the bone marrow but varied widely between different marrow lineages. SBDS was highly expressed in early neutrophil precursors, plasma cells, megakaryocytes and osteoblasts. Early neutrophilic precursors exhibited high levels of SBDS protein in a cytoplasmic and nuclear distribution. In contrast, SBDS expression was weak in mature neutrophils, consistent with the early, but not late, myelopoiesis defects observed in SDS models [9,10].
Figure 1.
SBDS protein expression in the bone marrow. A. Immunohistochemical staining for SBDS in a representative control BM biopsy (100X). B. Immunohistochemical staining for SBDS in a BM biopsy from a representative patient with SDS (100x). In both A and B, open arrow: megakaryocyte; closed arrow: neutrophil precursor; asterick: osteoblast; black circle: neutrophil. C. Mean SBDS immunohistochemical staining scored on a scale of 0 (weak) to 3 (strong). Grey boxes denote individual patient scores. Colored boxes represent multiple specimens from the same patient. Asterisk denotes the SDS patient without a confirmed mutation in SBDS. The mean immunohistochemical score for each diagnostic group is indicated by the black box and the 95% confidence interval is shown with the vertical bar.
Interestingly, the cell types with high SBDS protein expression reflected the SDS clinical phenotype. Neutropenia is the most common peripheral cytopenia in these patients. Platelet counts may also be reduced in these patients. The high SBDS protein levels in osteoblasts was striking since skeletal abnormalities such as delayed secondary ossification centers, metaphyseal dysostosis, and low turn-over osteopenia, are frequent in SDS patients [11–13]. Osteoblasts also constitute an important component of the hematopoietic niche, and stromal cell defects have been reported in patients with SDS [14]. Plasma cells produce antibodies, and low immunoglobulin levels have been observed in patients with SDS. SBDS has been implicated in ribosome biogenesis, and neutrophils, plasma cells, and pancreatic acinar cells all share a high synthetic demand to produce large quantities of proteins for secretion.
SBDS protein levels were low in lymphocytes and virtually undetectable in erythroid precursors. Current evidence does not support a role for SBDS in lymphopoiesis since mice stably engrafted with SBDS RNAi-transduced cells demonstrated normal percentages of early and mature B-lymphocytes subsets in bone marrow and normal B lymphopoiesis, despite decreased number of circulating B cells [10]. Isolated anemia is uncommon in patients with SDS so it is possible that SBDS plays a limited role in terminal erythroid differentiation. This is intriguing since mutations in genes encoding ribosomal components are associated with Diamond-Blackfan anemia, which is an inherited marrow failure syndrome characterized by red cell aplasia. Thus, the clinical effects of ribosomal perturbations emanating from SBDS loss differ from those resulting from ribosomal protein deficiency.
SBDS expression is diminished in SDS patients bearing the common SBDS mutations
We next compared SBDS protein levels in bone marrow biopsies from controls versus SDS patients. In contrast to specimens from normal controls, samples from SDS patients with common SBDS mutations that result in decreased protein expression stained weakly for the SBDS protein in all marrow cell lineages (Figure 1). Sample immunoreactivity as well as the presence and location of myeloid precursors was confirmed in several cases with staining for myeloperoxidase (Supplemental Figure 2). When serial, longitudinal marrow samples were available from an individual SDS patient, staining intensity was consistently diminished across all samples. The SBDS mutations found in the majority of SDS patients [7], particularly the IVS2+2T>C and 183-184TA>CT(K62X) mutations, result in minimal levels of SBDS protein expression [8,15]. Indeed, based on the marked reduction of SBDS protein expression levels, the majority of SDS patients could be easily distinguished from controls (Figure 1C).
Two siblings with SDS in this cohort each carried one gene conversion mutation allele (IVS2+2 T>C) and one missense mutation allele (R169C). The R169C mutation has been previously reported to result in intermediate levels of SBDS protein expression [8]. The marrow specimens from these patients also demonstrated SBDS protein levels that overlapped with the lower range of the control samples (Figure 1C).
SBDS expression in patients with other marrow failure syndromes
To determine whether decreased SBDS expression in the bone marrow is specific to SDS, we evaluated SBDS protein levels in marrow samples from patients with idiopathic aplastic anemia, Fanconi anemia, Diamond-Blackfan anemia, severe congenital neutropenia, and congenital amegakaryocytic thrombocytopenia (Table 1). SBDS protein levels from individual samples are represented in Figure 1C. We found that SBDS IHC staining was similar to that of controls in all cases. Bone marrow samples from other marrow failure syndrome patients were readily distinguished from those of the majority of SDS patients based on the differences in SBDS protein levels.
Table I.
Distribution of patients and specimens by diagnosis
| Diagnosis | No. of patients | No. of specimens assayed |
|---|---|---|
| Control | 17 | 17 |
| Shwachman-Diamond Syndrome - Common SBDS mutations - Missense SBDS mutations - Molecular sequencing not done |
10 7 2 1 |
20 15 4 1 |
| Aplastic anemia | 17 | 17 |
| Diamond-Blackfan anemia | 17 | 17 |
| Fanconi anemia | 7 | 7 |
| Severe congenital neutropenia | 6 | 7 |
| Congenital amegakaryocytic thrombocytopenia | 2 | 2 |
We also examined archived marrow samples from patients carrying the diagnosis of idiopathic aplastic anemia. Generally, the bone marrow samples from aplastic anemia patients expressed high levels of SBDS protein similar to that of controls, except for one case, which showed reduced staining for SBDS protein (Figure 1C). This patient did not display any other clinical features of SDS, and staining of a subsequent marrow specimen, showed robust staining for SBDS (Supplemental Figure 3). This may represent either a single technical failure of the assay or a transient decrease in expression of SBDS in this patient; in either case, it underscores the need for assay results to be interpreted in conjunction with clinical and additional laboratory findings.
Our results demonstrate that SBDS protein expression in the bone marrow exhibited lineage-specific patterns in a manner reflecting the SDS clinical phenotype. Further functional studies of the role of SBDS in early myelopoiesis, thrombopoiesis, osteoblasts and plasma cell function are ongoing. SDS is currently diagnosed by SBDS gene sequencing. The majority of SDS patients have SBDS mutations resulting from a gene conversion event with the adjacent pseudogene [7]. This is problematic in that when the gene conversion spans a large region extending beyond the borders of the primers, the mutated allele may be missed by exon-directed sequencing methodologies [16]. Furthermore, rapid diagnosis of SDS is crucial when marrow aplasia, myelodysplastic syndrome, or leukemia is the presenting clinical manifestation since studies have shown that SDS patients experience increased treatment-related toxicities with standard-intensity chemotherapy and hematopoietic stem cell transplant regimens [17]. This study demonstrates that immunohistochemical staining for SBDS can readily distinguish individuals carrying the common SBDS mutations that result in markedly diminished protein expression. As this assay will not identify patients carrying rare missense mutations in SDS, follow-up molecular sequencing would be necessary for patients with a high index of suspicion. This method may provide a useful rapid screen to identify patients with a high probability of having SDS. Validation of these findings in a larger, prospective study is warranted.
Supplementary Material
Representative immunohistochemical staining in megakaryocytes for SBDS. A. Staining assigned a score of “0”: no detectable staining. B. Staining assigned a score of “1”: minimal detectable staining. C. Staining assigned a score of “2”: intermediate staining. D. Staining assigned a score of “3”: strongest staining.
Representative immunohistochemical staining for myeloperoxidase in an SDS patient and an aplastic anemia patient. A. Immunohistochemical staining for SBDS in an aplastic anemia patient. B. Immunohistochemical staining for myeloperoxidase in the same patient as in (A). C. Immunohistochemical staining for SBDS in an SDS patient. D. Immunohistochemical staining for myeloperoxidase in the same patient as in (C). MPO: myeloperoxidase.
Discordant immunohistochemical staining results in one patient with aplastic anemia. A. Immunohistochemical staining for SBDS in the bone marrow biopsy included in this analysis, showing only weak staining. B. Immunohistochemical staining for SBDS in a subsequent bone marrow biopsy from the same patient showing robust staining.
ACKNOWLEDGMENTS
This project was funded by grants from the National Institutes of Health (R01 HL079582), V Foundation, and Seattle Children’s Hospital Endowment Fund to A.S. NIH training grants supported T.E.W. (T32CA009351, Department of Pediatric Oncology, Fred Hutchinson Cancer Research Center) and M.H.H (T32HL076115-04, Department of Pathology, Children's Hospital Boston).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
References
- 1.Burroughs L, Woolfrey A, Shimamura A. Shwachman-Diamond syndrome: a review of the clinical presentation, molecular pathogenesis, diagnosis, and treatment. Hematology/oncology clinics of North America. 2009;23(2):233–248. doi: 10.1016/j.hoc.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dror Y, Ginzberg H, Dalal I, et al. Immune function in patients with Shwachman-Diamond syndrome. Br J Haematol. 2001;114(3):712–717. doi: 10.1046/j.1365-2141.2001.02996.x. [DOI] [PubMed] [Google Scholar]
- 3.Menne TF, Goyenechea B, Sanchez-Puig N, et al. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nature genetics. 2007;39(4):486–495. doi: 10.1038/ng1994. [DOI] [PubMed] [Google Scholar]
- 4.Ganapathi KA, Austin KM, Lee CS, et al. The human Shwachman-Diamond syndrome protein, SBDS, associates with ribosomal RNA. Blood. 2007;110(5):1458–1465. doi: 10.1182/blood-2007-02-075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orelio C, Kuijpers TW. Shwachman-Diamond syndrome neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. Haematologica. 2009;94(3):409–413. doi: 10.3324/haematol.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin KM, Gupta ML, Coats SA, et al. Mitotic spindle destabilization and genomic instability in Shwachman-Diamond syndrome. The Journal of clinical investigation. 2008;118(4):1511–1518. doi: 10.1172/JCI33764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boocock GR, Morrison JA, Popovic M, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nature genetics. 2003;33(1):97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- 8.Austin KM, Leary RJ, Shimamura A. The Shwachman-Diamond SBDS protein localizes to the nucleolus. Blood. 2005;106(4):1253–1258. doi: 10.1182/blood-2005-02-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Fujimura K, Toga H, et al. Shwachman-Diamond syndrome is not necessary for the terminal maturation of neutrophils but is important for maintaining viability of granulocyte precursors. Exp Hematol. 2007;35(4):579–586. doi: 10.1016/j.exphem.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Rawls A, Gregory A, Wolosznek J, et al. Lentiviral-mediated RNAi inhibition of Sbds in murine hematopoietic progenitors impairs their hematopoietic potential. Blood. 2007;110(7):2414–2422. doi: 10.1182/blood-2006-03-007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makitie O, Ellis L, Durie PR, et al. Skeletal phenotype in patients with Shwachman-Diamond syndrome and mutations in SBDS. Clin Genet. 2004;65(2):101–112. doi: 10.1111/j.0009-9163.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 12.Toiviainen-Salo S, Mayranpaa MK, Durie PR, et al. Shwachman-Diamond syndrome is associated with low-turnover osteoporosis. Bone. 2007;41(6):965–972. doi: 10.1016/j.bone.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura G, Nakashima E, Hirose Y, et al. The Shwachman-Bodian-Diamond syndrome gene mutations cause a neonatal form of spondylometaphysial dysplasia (SMD) resembling SMD Sedaghatian type. J Med Genet. 2007;44(4):e73. doi: 10.1136/jmg.2006.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dror Y, Freedman MH. Shwachman-Diamond syndrome: An inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood. 1999;94(9):3048–3054. [PubMed] [Google Scholar]
- 15.Woloszynek JR, Rothbaum RJ, Rawls AS, et al. Mutations of the SBDS gene are present in most patients with Shwachman-Diamond syndrome. Blood. 2004;104(12):3588–3590. doi: 10.1182/blood-2004-04-1516. [DOI] [PubMed] [Google Scholar]
- 16.Nicolis E, Bonizzato A, Assael BM, et al. Identification of novel mutations in patients with Shwachman-Diamond syndrome. Hum Mutat. 2005;25(4):410. doi: 10.1002/humu.9324. [DOI] [PubMed] [Google Scholar]
- 17.Bhatla D, Davies SM, Shenoy S, et al. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone Marrow Transplant. 2008 doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative immunohistochemical staining in megakaryocytes for SBDS. A. Staining assigned a score of “0”: no detectable staining. B. Staining assigned a score of “1”: minimal detectable staining. C. Staining assigned a score of “2”: intermediate staining. D. Staining assigned a score of “3”: strongest staining.
Representative immunohistochemical staining for myeloperoxidase in an SDS patient and an aplastic anemia patient. A. Immunohistochemical staining for SBDS in an aplastic anemia patient. B. Immunohistochemical staining for myeloperoxidase in the same patient as in (A). C. Immunohistochemical staining for SBDS in an SDS patient. D. Immunohistochemical staining for myeloperoxidase in the same patient as in (C). MPO: myeloperoxidase.
Discordant immunohistochemical staining results in one patient with aplastic anemia. A. Immunohistochemical staining for SBDS in the bone marrow biopsy included in this analysis, showing only weak staining. B. Immunohistochemical staining for SBDS in a subsequent bone marrow biopsy from the same patient showing robust staining.