Abstract
The choroid of the eye is primarily a vascular structure supplying the outer retina. It has several unusual features: It contains large membrane-lined lacunae, which, at least in birds, function as part of the lymphatic drainage of the eye and which can change their volume dramatically, thereby changing the thickness of the choroid as much as four-fold over a few days (much less in primates). It contains non-vascular smooth muscle cells, especially behind the fovea, the contraction of which may thin the choroid, thereby opposing the thickening caused by expansion of the lacunae. It has intrinsic choroidal neurons, also mostly behind the central retina, which may control these muscles and may modulate choroidal blood-flow as well. These neurons receive sympathetic, parasympathetic and nitrergic innervation.
The choroid has several functions: Its vasculature is the major supply for the outer retina; impairment of the flow of oxygen from choroid to retina may cause Age-Related Macular Degeneration. The choroidal blood flow, which is as great as in any other organ, may also cool and warm the retina. In addition to its vascular functions, the choroid contains secretory cells, probably involved in modulation of vascularization and in growth of the sclera. Finally, the dramatic changes in choroidal thickness move the retina forward and back, bringing the photoreceptors into the plane of focus, a function demonstrated by the thinning of the choroid that occurs when the focal plane is moved back by the wearing of negative lenses, and, conversely, by the thickening that occurs when positive lenses are worn.
In addition to focusing the eye, more slowly than accommodation and more quickly than emmetropization, we argue that the choroidal thickness changes also are correlated with changes in the growth of the sclera, and hence of the eye. Because transient increases in choroidal thickness are followed by a prolonged decrease in synthesis of extracellular matrix molecules and a slowing of ocular elongation, and attempts to decouple the choroidal and scleral changes have largely failed, it seems that the thickening of the choroid may be mechanistically linked to the scleral synthesis of macromolecules, and thus may play an important role in the homeostatic control of eye growth, and, consequently, in the etiology of myopia and hyperopia.
1. Introduction
The choroid does not appear to be a mysterious tissue. It consists mostly of blood vessels, it supplies the outer retina, and choroidal defects cause degenerative changes and neovascularization. However, it is becoming increasingly evident that the choroid has at least three other functions: thermoregulation, adjustment of the position of the retina by changes in choroidal thickness, and secretion of growth factors. The last of these is likely to play an important role in emmetropization--the adjustment of eye shape during growth to correct myopia or hyperopia. What remains mysterious, at present, are the mechanisms behind the changes in choroidal thickness, the nature of its secretory functions and the relationship between these two processes.
In this review, we will summarize the anatomy, histology, innervation and functions of the choroid, discuss the control of choroidal thickness by visual signals, show evidence for a secretory role for the choroid and speculate on the relationship between changes in choroidal thickness and ocular elongation, and therefore, emmetropization.
2. Structure and “classical” functions of the choroid
The choroid is the posterior part of the uvea, the middle tunic of the eye (Fig. 1). The uvea develops from the mesenchyme surrounding the two vesicles that bud off the embryonic forebrain at the end of the first month in humans, eventually becoming the eyes. At about that time, melanocyte precursors migrate into the uvea from the neural crest; these do not differentiate into pigmented melanocytes until 7-8 months of gestation. The mesenchyme that forms the choriocapillaris at about 2 months must be in contact with the developing retinal pigment epithelium (RPE) in order to differentiate. Therefore, the choroid derives from different cell lines than do the retina and RPE, which both derive from the neural ectoderm. The choroid is comprised of blood vessels, melanocytes, fibroblasts, resident immunocompetent cells and supporting collagenous and elastic connective tissue. As one of the most highly vascularized tissues of the body, its main function has been traditionally viewed as supplying oxygen and nutrients to the outer retina, and, in species with avascular retinas, to the inner retina as well. Other likely functions include light absorption (in species with pigmented choroids), thermoregulation via heat dissipation, and modulation of intraocular pressure (IOP) via vasomotor control of blood flow. The choroid also plays an important role in the drainage of the aqueous humor from the anterior chamber, via the uveoscleral pathway. This pathway is responsible for approximately 35% of the drainage in humans, a higher percentage, between 40 and 60%, in non-human primates, and a much lower percentage in the cat (about 3%) and rabbit (3-8%) (Alm and Nilsson, 2009).
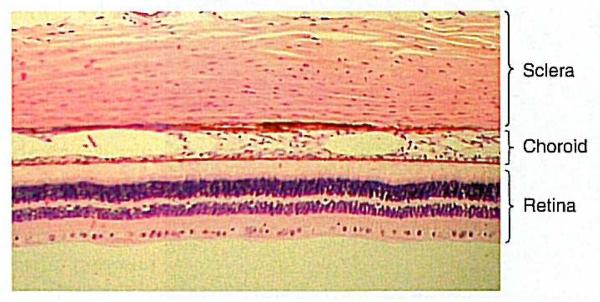
Figure 1.
Photomicrograph of the three tunics at the back of the primate eye. From: Remington, LA; Clinical Anatomy of the Visual System; 2nd Edition; 2005. Reproduced with permission © Elsevier.
2.1 Histology of the choroid
The choroid extends from the margins of the optic nerve to the pars plana, where it continues anteriorly, becoming the ciliary body. Its innermost layer is the complex 5-laminar structure of Bruch's membrane, and its outermost one is the suprachoroid outside of which is the suprachoroidal space between choroid and sclera.
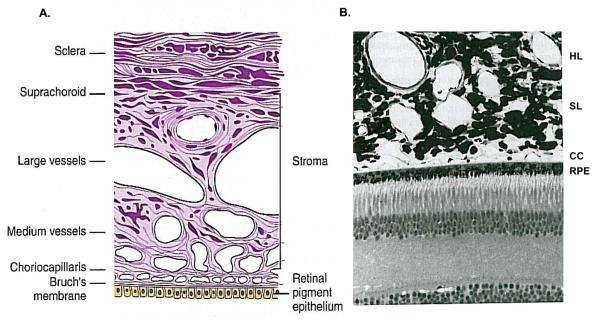
Histologically, the choroid has variously been divided into 4 to 6 layers, depending on whether the vascular region is considered as 1 or 2 layers (Sattler's and Haller's), and whether the lamina fusca is considered to be of scleral or choroidal origin. It is most commonly described as five layers: starting from the retinal (inner) side, these are Bruch's membrane, the choroiocapillaris, the two vascular layers (Haller's and Sattler's), and the suprachoroidea (Fig. 2A and B) (Hogan et al., 1971).
Figure 2.
Histology of the choroid. A. Schematic of the layers of the choroid. Reproduced with permission from Remington, LA. Clinical Anatomy of the Visual System. © Elsevier. B. Semithin resin section of the outer retina and choroid in the primate eye. RPE: retinal pigment epithelium ; CC, choriocapillaris; SL: Sattler's layer; HL: Haller's layer. Reproduced with permission from Forrester et al., 2002. The Eye: Basic Sciences in Practice © Elsevier.
In humans, the choroid is approximately 200 μm thick at birth and decreases to about 80 μm by age 90 (Ramrattan et al., 1994). In non-human primates, the choroid is thinner, approximately 95 μm at the fovea, thinning to 55 μm at the periphery (Krebs and Krebs, 1991). In chickens, it is about 250 μm thick in the center under normal visual conditions (Wallman et al., 1995; Nickla et al., 1998), thinning to about 100 μm in the periphery (Wallman et al., 1995). Large melanocytes are abundant in the primate choroid, imparting a dark pigmentation (Krebs and Krebs, 1991). In other species, such as birds, only sparse melanocytes are present in the outer choroid, resulting in lighter pigmentation (De Stefano and Mugnaini, 1997b) (although there is variability in pigmentation among avian groups).
2.1.1 Choriocapillaris
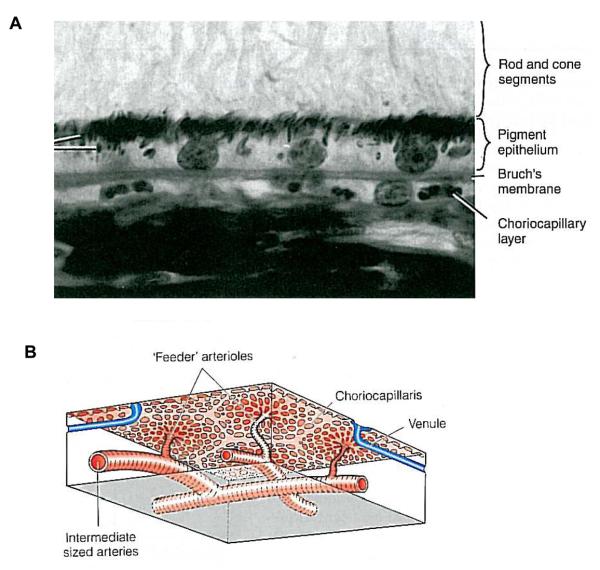
The choriocapillaris is a highly anastomosed network of capillaries, forming a thin sheet apposed to Bruch's membrane (Fig. 3A). The fibrous basement membrane of the capillary endothelial cells forms the outermost layer of Bruch's membrane in humans. The choriocapillaris is about 10 μm thick at the fovea, where there is the greatest density of capillaries, thinning to about 7 μm in the periphery. The capillaries arise from the arterioles in Sattler's layer, each of which gives rise to a hexagonal (or lobular)-shaped domain of a single layer of capillaries, giving a patch-like structure to the choriocapillaris (Fig. 3B). The capillaries are fenestrated and, in humans, are relatively large in diameter (20-40 μm), although possessing passages narrow enough to hinder the flow of 9 μm spheres (Bill et al., 1983). Because of the large area of the choriocapillaris, the velocity of red blood cells here is only about 77% that of the velocity of red blood cells in the retinal capillaries (Wajer et al., 2000). In birds, the capillaries are polarized, with the fenestrations almost exclusively facing the retina (De Stefano and Mugnaini, 1997b); in primates, this is mainly true, although there are a few fenestrations on the outer walls (McLeod et al., 2009). The fenestrated capillaries are highly permeable to proteins, contributing to the high oncotic pressure in the extravascular stroma, which fosters the movement of fluids from the retina to the choroid.
Figure 3.
Histology of the choriocapillaris. A. The choriocapillaris is located adjacent to Bruch's membrane. Reproduced with permission from Remington, LA (2005) Clinical Anatomy of the Visual System © Elsevier. B. Each feeder arteriole in Sattler's layer supplies a hexagonally-arrayed area of capillaries. From: Forrester et al., 2002 “The Eye: Basic Sciences in Practice. Reproduced with permissioin © Elsevier.
Adjacent to Sattler's layer at the outer choriocapillaris there is a fibrous layer which is attached to the outer fibrous layer of Bruch's membrane by columns of collagen fibers running between the capillaries; these columns (or “pillars”) may function to keep the capillary diameters constant (at least in primates) (Krebs and Krebs, 1991). Finally, the innermost choroidal layer is Bruch's membrane, a 5-layered structure consisting of (from outer to inner): basement membrane of the choroiocapillaris, outer collagenous zone, elastic layer, inner collagenous zone, and basement membrane of .the retinal pigment epithelium.
2.1.2 Choroidal vascular layers and suprachoroid
The vascular region of the choroid consists of the outer Haller's layer of large blood vessels and the inner Sattler's layer of medium and small arteries and arterioles that feed the capillary network, and veins (Figure 2). The stroma (extravascular tissue) contains collagen and elastic fibers, fibroblasts, non-vascular smooth muscle cells and numerous very large melanocytes that are closely apposed to the blood vessels. As in other types of connective tissue, there are numerous mast cells, macrophages, and lymphocytes.
The suprachoroid is a transitional zone between choroid and sclera containing elements of both -- collagen fibers, fibroblasts and melanocytes. In primates (Krebs and Krebs, 1988; Krebs and Krebs, 1991), birds (De Stefano and Mugnaini, 1997b; Junghans et al., 1998; Liang et al., 2004), and rabbits (Gomez et al., 1988), this layer contains large, endothelium-lined spaces (referred to as “lacunae” in the avian literature), which empty into veins (Fine and Yanoff, 1979; Krebs and Krebs, 1988; De Stefano and Mugnaini, 1997b). These presumably lymphatic structures will be discussed in more detail under “Special characteristic features of the choroid.”
The lamina fusca is the outermost layer of the suprachoroid (Fig. 4A), approximately 30 μm thick, consisting of several layers of closely-apposed, flattened fusiform melanocytes and fibroblast-like cells (Fig. 4B), with interspersed bundles of myelinated axons (De Stefano and Mugnaini, 1997b).1 The fibroblast-like cells form sheaths, or plates, that are connected to one another by adhering and occluding junctions (Krebs and Krebs, 1988). These cells synthesize and secrete the usual extracellular matrix components elastin, collagens and proteoglycans. The function of the melanocytes, apart from providing pigmentation, is unknown. However, their membranes contain endothelin-B receptors, which may mediate the endothelin 1- induced influx of calcium ions (Hirose et al., 2007), the functional implications of which are also not known.
Figure 4.
Light and electron micrographs of the lamina fusca. (a) Light micrograph (retina above, sclera below) showing the lamina fusca, unusually thick in this case, as a dark band at the bottom of the photograph. (b) Electron micrograph (same orientation) showing the stacked, tightly apposed cells making up the lamina fusca. Photographs courtesy of M. Egle De Stefano.
2.2 Innervation of the choroid
The smooth muscle of the vessel walls of the choroid, like those of skeletal and cardiac muscle blood vessels, are innervated by both divisions of the autonomic nervous system, which form dense plexuses of fibers around the vessels (“perivascular plexus”). Axon terminals are also found throughout the stroma, terminating on non-vascular smooth muscle, intrinsic choroidal neurons (ICNs), and possibly other cell types. There are also primary afferent sensory fibers that project to the trigeminal ganglion via the ophthalmic nerve; some of these give rise to peptide-positive collaterals (May et al., 2004) that terminate on and around the vessels and intrinsic choroidal neurons (Schrödl et al., 2001a; Schrödl et al., 2003).
2.2.1 Parasympathetic innervation
In mammals, the main parasympathetic input to the choroid originates from the pterygopalatine ganglion (Ruskell, 1971; Uddman et al., 1980; Bill, 1985; Stone, 1986; Stone et al., 1987; Bill, 1991), located within the pterygopalatine fossa or its equivalent. These fibers are predominantly cholinergic and are rich in the vasodilators vasoactive intestinal polypeptide (VIP) and nitric oxide (NO) (Uddman et al., 1980; Stone et al., 1987; Yamamoto et al., 1993; Alm et al., 1995) (Fig. 5A and B). Although there is some physiological evidence for innervation from the ciliary ganglion (Bill et al., 1976; Stjernschantz et al., 1976; Gherezghiher et al., 1990; Nakanome et al., 1995), this has not been documented anatomically.
Figure 5.
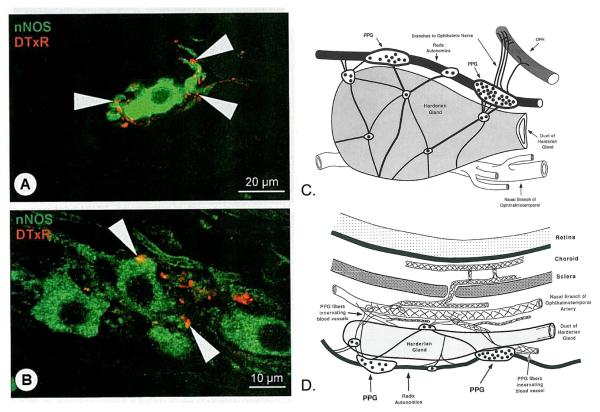
The pterygopalatine ganglion (PPG). A, B. Confocal micrograph of neurons in the PPG labeled for nNOS (green), and for Texas red (DtxR) anterogradely transported from the superior salivatory nucleus-PPG pathway (N VII). Postganglionic nitrergic neurons of the pterygopalatine ganglion were closely associated with anterogradely labeled preganglionic nerve fibers and boutons (yellow color at arrowheads represents sites of closest proximity). Reproduced with permission from Schrödl et al., 2006 © Association for Research in Vision and Ophthalmology. C. Schematic view of the left Harderian gland and associated pterygopalatine plexus from the nasal side. OPH: ophthalmic nerve; receives fibers from the PPG. Rostral is to the right and superior to the top. D. Schematic of the left Harderian gland, from the dorsal aspect. Nerves course to nearby artery; perivascular plexuses form on vessels in the choroid. Reproduced with permission from Cuthbertson et al., 1997 © Wiley.
In birds, on the other hand, the main parasympathetic innervation originates from a well-defined region of the ciliary ganglion, which receives input from the medial aspect of the Edinger-Westphal nucleus (Reiner et al., 1983; Fitzgerald et al., 1990; Reiner et al., 1991; Fitzgerald et al., 1996). These ciliary “choroid” neurons release somatostatin (Gray et al., 1989; Epstein et al., 1988; De Stefano et al., 1993; Schrödl et al., 2006) in addition to acetylcholine (Meriney and Pilar, 1987; Reiner et al., 1991; Cuthbertson et al., 1996) and nitric oxide (Sun et al., 1994). The other source of parasympathetic innervation is from the pterygopalatine ganglia, which in birds are an interconnected series of microganglia located adjacent to the Harderian gland on the nasal side of the orbit (Baumel, 1975; Nickel et al., 1977; Gienc and Kuder, 1985)(Fig. 5C). Two major microganglia are located along the superior aspect of the Harderian gland, the larger of these more rostrally (Cuthbertson et al., 1997). There are also numerous small microganglia, distributed along the upper, medial and lateral aspects of the gland. The preganglionic input to the pterygopalatine is called the radix autonomica (N VII). The pterygopalatine neurons are positive for VIP (Walcott et al., 1989; Cuthbertson et al., 1997), nitric oxide and choline acetyltransferase (ChAT) (Cuthbertson et al., 1997; Schrödl et al., 2006). In all species studied, parasympathetic fibers terminate on vessels in perivascular plexuses (Fig. 5D), and mediate increases in blood flow by vasodilation. These fibers also terminate on non-vascular smooth muscle cells and intrinsic choroidal neurons.
2.2.2 Sympathetic innervation
The sympathetic innervation of the choroid comes from the superior cervical ganglion (Kirby et al., 1978; Guglielmone and Cantino, 1982; Bill, 1985). These noradrenergic neurons terminate on the blood vessels and mediate vasoconstriction. In birds, the sympathetics also innervate non-vascular smooth muscle (Guglielmone and Cantino, 1982; Poukens et al., 1998) and intrinsic choroidal neurons (Schrödl et al., 2001b), the same targets as the parasympathetic system. Schrödl et al. (2001a,b) have speculated that the intrinsic choroidal neurons may act as intermediaries in the sympathetic system between the post-ganglionic neurons and the muscle, and increase smooth muscle tone, although the effects of noradrenaline on the ICNs and non-vascular smooth muscle are unknown.
2.2.3 Sensory innervation
Many organs, including the eye, have been shown to use peptides such as substance-P and calcitonin gene-related peptide in a pre-central reflex arc, or axon reflex, a non-synaptic response in which a local stimulus (chemical or mechanical) depolarizes a sensory terminal which travels to the nearest collateral (branch), releasing the peptide onto the effector tissue (reviews: Holzer, 1988; Bill, 1991). Evidence for this reflex has been found in the primary sensory afferents from the trigeminal ganglion in the uvea and choroid, which use both peptides; the reflex may mediate changes in blood flow or a variety of other functions. For instance, in both mammals and birds, sensory fibers projecting to the trigeminal ganglion from the choroid via the ophthalmic branch of the trigeminal nerve elicit vasodilation (Shih et al., 1999b). These terminals are positive for substance-P and calcitonin-gene-related peptide (Stone, 1985; Reiner, 1987; Stone and McGlinn, 1988); other vasoactive peptides such as cholecystokinin are also found, differing between species (Bill, 1991). Recent work has shown that these primary afferents also contact the intrinsic choroidal neurons, which are believed to be analogous to the intrinsic neurons in the enteric nervous system which function as local signal integrators responding to local mechanical, thermal or chemical stimuli (Schrödl et al., 2001a).
2.3 Special Characteristic Features of the Choroid
2.3.1 Intrinsic choroidal neurons
First described from choroids of higher primates including man (Mueller, 1859), intrinsic choroidal neurons are present in primates (humans and monkeys: Flugel et al., 1994; May et al., 1997; Bergua et al., 2003; Schrodl et al., 2003; May et al., 2004), tree shrews: (Flugel et al., 1994) and birds (Bergua et al., 1996; Bergua et al., 1998; Schrödl et al., 2004; review: Schrodl, 2009), but are scarce or absent in rats, rabbits and cats (Flugel et al., 1994). In humans, ICNs are especially numerous: choroids have approximately 2000 per eye, largely concentrated in the temporal and central regions, decreasing towards the periphery. In other primates, the numbers are lower, about 500 per eye, and are more evenly distributed than those of humans. In birds, the numbers vary, from fewer than 1,000 in quail and ducks, to about 6,000 in geese (Schrödl et al., 2004). These neurons are usually small (between 20 and 40 μm) and multipolar.
In all species, most ICNs are positive for NADPH-diaphorase and/or neuronal nitric oxide synthase (nNOS) (Flugel et al., 1994; Bergua et al., 1996; Cuthbertson et al., 1997; Bergua et al., 1998), indicating that they use nitric oxide as a transmitter (Fig. 6A). Many are also positive for VIP (Miller et al., 1983; Flugel et al., 1994; Cuthbertson et al., 1997) (Fig. 6B). In humans, about half the population are positive for calretinin (May et al., 2004). There is evidence that these neurons receive both sympathetic and parasympathetic innervation: They are contacted by boutons that are positive for both tyrosine hydroxylase and dopamine-B-hydroxylase, which in combination are a marker for postganglionic sympathetic neurons or collaterals; in addition they are contacted by boutons that are positive for nNOS and VIP, indicating inter-ICN connectivity and/or input from the pterygopalatine ganglia.
Figure 6.
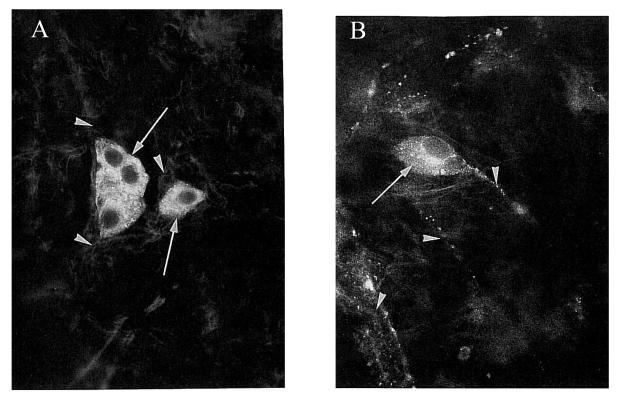
The intrinsic neurons of the human choroid immunolabeled for nNOS (left) and vasoactive intestinal polypeptide (VIP) (right). Left: Nitric oxide synthase labeling in the cytoplasm of ICNs (arrows) and weak staining in the axons of these neurons (arrowheads). Right: Staining for antibodies against VIP in cytoplasm (arrow) and axon (arrowhead). Approximately 200X magnification. Reproduced with permission from Flugel et al., 1994 © Association for Research in Vision and Ophthalmology.
Now, 150 years after their discovery, the functions of the ICNs remain unknown. They probably play a role in blood flow regulation because they terminate on the muscle walls of arteries (Meriney and Pilar, 1987; Flugel-Koch et al., 1996) and because they release NO, a potent vasodilator. Furthermore, because they are found adjacent to the non-vascular smooth muscle that span the stroma and suprachoroid around the large lymphatic lacunae and may innervate these muscles, it is possible that these cells are involved in changing the choroidal thickness in response to retinal defocus (Poukens et al., 1998), a topic which will be further discussed.
2.3.2 Non-vascular smooth muscle
Non-vascular smooth muscle cells were first described in human choroids by Mueller (1859). Subsequent work has found them in the choroids of birds (Kajikawa, 1923; Walls, 1942; Guglielmone and Cantino, 1982; Meriney and Pilar, 1987; Wallman et al., 1995), in which they are found in the stroma. These have been hypothesized to play a role in the changes in choroidal thickness in response to retinal defocus (Wallman et al., 1995).
In mammals, non-vascular smooth muscle cells have been found in primates (Krebs and Krebs, 1991; Flugel-Koch et al., 1996; Poukens et al., 1998) and rabbits (Haddad et al., 2001), but are absent in dog, cat, rat, tree shrew, pig and cow (May, 2003). Among primates, the number of smooth muscle cell are higher in species with well-defined foveas (May, 2003). These non-vascular smooth muscle are sometimes referred to as myofibroblasts, and are positive for α-actin. In primates, they are found in two populations: in the suprachoroid, forming a reticulum of flattened lamellae, and in a single layer immediately beneath and parallel to Bruch's membrane. In the suprachoroid, the lamellae run obliquely to the choroidal surface between “spaces”, presumably lacunae (Fig. 7A) (Poukens et al., 1998). Non-vascular smooth muscle cells are concentrated beneath the fovea and are sparcer anteriorly (although in a few cases connections with ciliary muscle fibers were seen). In the rabbit, they are arranged in bundles of up to 20 cell layers, running along the longitudinal axis of blood vessels (Haddad et al., 2001), possibly indicating a role in the choroidal blood flow autoregulation shown in this species (Kiel and Shepherd, 1992). Neighboring non-vascular smooth muscle cells are connected by zonula adherens, forming a syncytium, with their organelles clustered around the nucleus. These cells were identified as regular smooth muscle, as opposed to myofibroblasts (Haddad et al., 2001), which typically appear as isolated cells, do not have junctional complexes, nor are their organelles clustered near the nucleus. In birds, non-vascular smooth muscle cells are found in both the suprachoroid and the vascular layer (Wallman et al., 1995; Fig. 7B). The arrangement of the non-vascular smooth muscle oblique or tangential to the surface, between the lacunae, makes it plausible that they might play a role in the changes in choroidal thickness in response to retinal defocus, to be discussed later.
Figure 7.
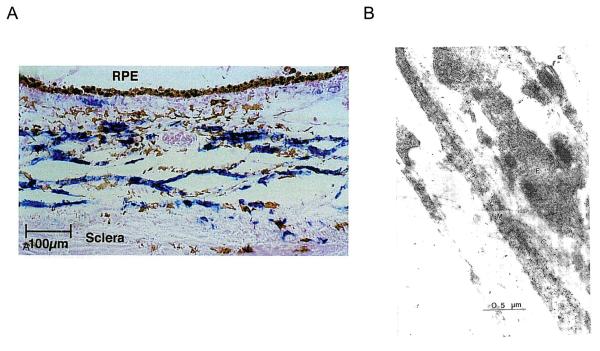
Non vascular smooth muscle of the primate (A) and chick (B) eyes. A. Suprachoroid and inner sclera contain cells positive for smooth muscle α-actin (blue chromogen counterstained with nuclear fast red). Reproduced with permission from Poukens et al., 1998 © Association for Research in Vision and Ophthalmology. B. Electron micrograph of choroidal cells labeled with antibodies to smooth muscle actin (black dots), showing long fibers not associated with blood vessels. M, muscle cell; F, fibroblast; C, collagen. Reproduced with permission from Wallman et al., 1995 © Association for Research in Vision and Ophthalmology.
The predominant sub-foveal localization has suggested to Flugel-Koch et al. (1996) that these cells may stabilize the position of the fovea against movement caused by the contracting ciliary muscle during accommodation. It is not certain whether these contractile cells are controlled by the autonomic nervous system, but the presence of nerve terminals positive for NADPH-diaphorase or tyrosine hydoxylase in close proximity suggests inputs from both parasympathetic nitrergic and sympathetic adrenergic systems (Poukens et al., 1998). 2
2.3.2.1 Are non-vascular smooth muscle cells myofibroblasts?
Non-vascular smooth muscle is prominent in the human uvea, including the iris constrictor and the ciliary muscles. The iris dilator, however, is not typical smooth muscle, but is classified as myofibroblast, a cell type intermediate between smooth muscle and fibroblasts, and not widely present in the body except around the lumena of glands. One distinguishing characteristic is the absence of the intermediate filament desmin found in “regular” smooth muscle (Flugel-Koch et al., 1996).
In the Poukens et al. (1998) study of primate choroids, the non-vascular smooth muscle cells were identified as predominantly myofibroblasts. These were arranged in flattened lamellae in the suprachoroid, oblique to the choroidal surface, between which were fluid-filled lacunae. In the inner part of the choroid they ran parallel to Bruch's membrane in the sub-foveal inner choriocapillaris. Similarly, Flugel-Koch et al. (1996) describe a network of α-actin-positive but desmin-negative, spindle-to star- shaped myofibroblasts in the suprachoroid, which were connected to an elastic fiber net of the stroma and to the adventitia of the vessels. Again, the arrangement was densest in the submacular region.
In contrast, an immunohistochemical study of 42 human eyes of a wide range of ages described all of the non-vascular smooth muscle cells as typical smooth muscle (May, 2005), based on the expression in these of the protein smoothelin, which is not expressed in myofibroblasts (van der Loop et al., 1996; Bar et al., 2002). The cells also stain for α-myosin, α-actin and caldesmon, markers for contractibility. The smooth muscles were located in three distinct subgroups: (1) in the suprachoroid and sclera forming a semi-circle around the entering posterior ciliary arteries, (2) in the outer vascular choroid (Haller's) between large blood vessels in the posterior segment, and (3) in the suprachoroid of the foveal region of the temporal quadrant, where they form dense plaques. Interestingly, this third group showed the greatest inter-individual variation, being extremely numerous in some people but absent in others. It was speculated that the location and variability of this population may indicate a visual function, as opposed to a vascular one, although without corroborating evidence (May, 2005).
2.3.3 Fluid-filled Lacunae: Lymphatics
Large, fluid-filled lacunae in the choroid were first observed over 50 years ago by Walls (1942). In 1987, Meriney & Pilar described fluid-filled lacunae in the stroma of chicken choroids that were connected to arterioles, and suggested that they functioned as a drainage reservoir involved in regulating intraocular pressure (Meriney and Pilar, 1987). Although the authors did not distinguish between the stroma and the suprachoroid, the photomicrographs show them in the suprachoroid as well. In 1997, a careful and thorough EM investigation of the chicken choroid showed that very large, endothelium-lined lacunae are present in the suprachoroid, continuing as smaller ones in the stroma of the vascular layers and ending in the choriocapillaris as blind-end structures (Fig. 8A and B; Liang et al., 2004; De Stefano and Mugnaini, 1997b). These structures were identified as true lymphatics based on (1) the endothelial lining being extremely thin, with elaborate junctional complexes, pinocytotic vesicles, and large fenestrations, all ultrastructural characteristics of lymphoendothelium (De Stefano and Mugnaini, 1997b) (Krebs and Krebs, 1988; Junghans et al., 1998), and (2) paracentesis (draining the anterior chamber) resulting in the backflow of blood cells into these spaces, indicating a connection with the venous system (De Stefano and Mugnaini, 1997b). These lymphatic spaces are surrounded by lamellae of elastin fibers and nerves, and very large melanocytes.
Figure 8.
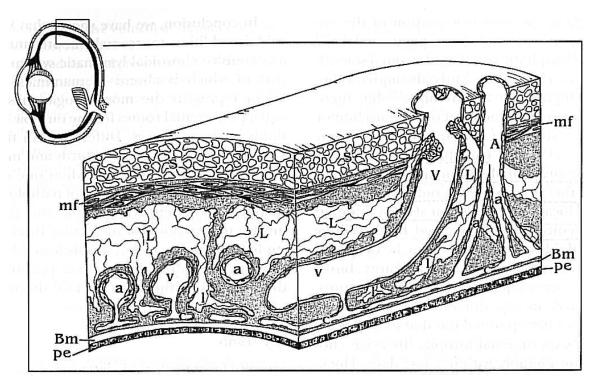
Diagram of the lymphatic system of the avian choroid. Veins (V) and arteries (A) traverse the eye wall from the sclera (S) through the suprachoroid and stroma, where they branch into arterioles (a) and venules (v), to the choriocapillaris where they form capillaries (c). Large lymphatic vessels (L) are in the suprachoroid and branch into lymphatic capillaries (l) that reach the choriocapillaris. Reproduced with permission from DeStefano & Mugnaini, 1997 © Association for Research in Vision and Ophthalmology.
It was presumed at the time of their discovery that these lacunae were a peculiarity of the bird eye. Indeed, the traditional view is that the primate eye does not contain a lymphatic drainage system (Bill, 1975), although the conjunctiva and eyelids drain into the submandibular and parotid lymph nodes (Wobig, 1981). However, some more recent work has described lymphatics in the baboon and macaque choroids (Krebs and Krebs, 1988; Sugita and Inokuchi, 1992; Poukens et al., 1998), which allegedly had been dismissed by earlier anatomists as artifacts. In these primates, the lymphatics are located both in the stroma, between arteries and veins, and in the suprachoroid (in the Japanese monkey (Macaca fuscata) they were found in the “outer choroid” (Sugita and Inokuchi, 1992)). In man, one study describes these lymphatics in the suprachoroid (Poukens et al., 1998). It should be emphasized, however, that the identification of these spaces as lymphatics in primate choroids, especially humans, is still controversial. For instance, Schrödl et al. (2008) found that LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1) and podoplanin, two markers for lymphatic endothelium, were absent in the vessel walls of the human choroid, although the same antibody labeled them in choroidal macrophages. On the other hand, positive labeling for both of these markers on vessel endothelium in the human ciliary body supports the existence of lymphatics in the primate uvea, perhaps affecting fluid dynamics via the uveoscleral outflow (Yucel et al., 2009). Regardless of their anatomical identity, these fluid reservoirs are the subject of interest because of evidence that they may contribute to the changes in choroidal thickness found in response to retinal defocus (Wallman et al., 1995; Junghans et al., 1998; Pendrak et al., 1998; Junghans et al., 1999; Liang et al., 2004), a topic to be discussed later.
2.4 Choroidal Blood flow: Nourishment of the retina
Despite the conspicuousness of the retinal blood vessels, the major blood supply to the retina is the choroid. Because the photoreceptors are extremely metabolically active, especially in darkness, when the light-gated ion channels are open, and active transport of ions is required to maintain ion homeostasis, over 90% of the oxygen delivered to the retina is consumed by the photoreceptors. In darkness, ninety percent of the oxygen comes from the choroidal circulation (Linsenmeier et al., 1981; Bill et al., 1983; Linsenmeier and Braun, 1992). To obtain this high transport of oxygen from the choroid, despite the barriers of Bruch's membrane and the retinal pigment epithelium, requires a steep gradient of oxygen tension, which is maintained by the high blood flow in the choroid, probably the highest of any tissue in the body per unit tissue weight, ten-fold higher than the brain (Alm and Bill, 1973; Alm, 1992). Consequently, the oxygen tension in the choroid stays high, with an arterial/venous difference of only 3% versus 38% for the retinal circulation.
In many species, the choroidal circulation supplies the inner retina, as well as the outer retina, because retinal blood vessels are absent (e.g., guinea pig) or sparse (e.g., rabbit) (Yu and Cringle, 2001). In guinea pigs, oxygen tension shows a steep decline, to almost 0 mm Hg, within about 70 μm of Bruch's membrane (Yu and Cringle, 2001); hence the inner retina functions in an anoxic environment, sustainable by anaerobic respiration. In contrast, in humans and other species with vascular retinas such as rats, the retinal vessels keep the inner retinal PO2 at about 20 mm Hg (Fig. 9; Yu and Cringle, 2001) (Wangsa-Wirawan and Linsenmeier, 2003). In all species, the oxygen tension of the inner retina is much lower than that at the photoreceptors (review: (Yu and Cringle, 2001).
Figure 9.
Oxygen tension profile through a vascular retina (rat). The measurements are from two sequential penetrations (circles) and withdrawals (triangles) of the electrode. The intraretinal oxygen distribution reflects the relative oxygen sources and sinks within the retina and choroid. Reproduced with permission from Yu and Cringle, 2001 © Elsevier.
In birds, a specialized vascular structure, the pecten, which projects into the vitreous from the optic nerve head, provides a supplemental oxygen supply to the inner retina (Wingstrand and Munk, 1965). Torsional oscillations of the eye during saccadic eye movements facilitate the diffusion of oxygen and nutrients across the retina by bulk flow (Pettigrew et al., 1990), a mechanism aided by the liquid phase of the vitreous being adjacent to the retina.
In the retina, the capillaries are the continuous type (the walls have no fenestrations), constituting the blood-ocular barrier, and are impermeable to even small molecular weight molecules such as glucose and amino acids, which require special transport systems to move them across the endothelium. The choroidal circulation is crucial in supplying nutrients as well as oxygen because the capillaries of the choroid are fenestrated, with especially large pores. These fenestrations have a high permeability not only to glucose but to low molecular weight substances such as albumen and myoglobulin. It was estimated that more than 50% of molecules the size of glucose or amino acids can pass through the fenestrations into the extracellular tissue, creating a very high glucose concentration there, thereby facilitating transport across the RPE to the retina (Bill et al., 1980). The high protein permeability of the choriocapillaris also allows the establishment of a high oncotic pressure, presumably contributing to movement of fluid out of the retina through the stroma and suprachoroid, and out the sclera (Bill, 1962; Marmor et al., 1980).
2.5 Does the choroidal blood flow exhibit autoregulation?
In most tissues of the body, blood flow is autoregulated, in that fluctuations in perfusion pressure (arterial minus venous pressure) do not cause proportional changes in blood flow because of compensatory dilation or constriction of the arterioles, metarterioles, and capillary sphincters, mediated locally. As a consequence, blood flow returns to normal in a short time after the pressure changes. Both the retinal circulation and the anterior uveal circulation exhibit autoregulation in response to fluctuations in systemic oxygen levels, IOP or blood pressure, maintaining oxygen tension at a constant level. Failure of the retinal circulation to autoregulate could lead to hypoxia and neovascularization, as occurs in diabetic retinopathy and in retinopathy of prematurity.
It has long been held that, in contrast to the retina and anterior uvea, the choroidal blood flow does not exhibit autoregulation (review: Delaey and Van De Voorde, 2000). The purported reason is that the high choroidal blood flow and low oxygen extraction precludes the need for it. However, this is still the subject of debate. In rabbits, for instance, when mean arterial pressure was decreased by partial occlusion of the thoracic vena cava, choroidal blood flow was maintained at control levels, possibly by a myogenic or vasomotor response (Kiel and Shepherd, 1992). A similar autoregulation was found in the pigeon, when arterial blood pressure was reduced by blood withdrawal (Reiner et al., 2003).
In human choroids as well, recent studies have found varying degrees of autoregulation. For example, changes in blood flow induced either by decreases in perfusion pressure elicited by step increases in intraocular pressure (IOP) (Riva et al., 1997b), or by increases in perfusion pressure induced by isometric exercises (Riva et al., 1997a; Lovasik et al., 2003; Polska et al., 2007) were not linearly related to the changes in perfusion pressure, indicating some degree of autoregulation. By the same token, increases in arterial carbon dioxide tension resulted in increases in choroidal blood flow of approximately 1.5% per mm Hg PCO2 (Geiser et al., 2000).
2.6 Choroidal Blood flow: Thermoregulation of the retina?
One proposed function of the extremely high blood flow of the choroid is that it protects the retina from damage in extreme hot or cold temperatures or from the heat generated during exposure to bright lights (Parver et al., 1980; Parver et al., 1982; Bill et al., 1983) by acting as a heat source in the cold (maintaining retinal temperature near core temperature) or as a heat sink for exogenous thermal radiation. In monkeys and rabbits, it was found that increasing the IOP to above the mean arterial pressure (thus preventing choroidal blood flow) under low ambient illumination resulted in a significant decrease in the temperature of the retina and choroid in the macula region (Bill, 1962). This was interpreted as showing that the choroid acts as a heat source for the retina under low illumination, when heat is lost through the cooler anterior chamber. Conversely, when flow was occluded under higher illumination, there was an increase in choroidal temperature, indicating the loss of the flow to the choroid acting as a heat sink (Parver et al., 1982a, b).
Although protection from these temperature changes could occur passively because of the high choroidal blood flow, as would be expected from the classical view that the choroidal circulation does not autoregulate, they could also be mediated by reflexive increases in choroidal blood flow in response to a stimulus (Parver, 1991). In addition to any tissue-level (local) autoregulation, there is evidence in humans and non-human primates for a centrally-controlled reflex arc regulating choroidal flow: In cynomolgus monkeys, increasing light intensity under constant IOP resulted in an increase in retinal/choroidal temperature and in choroidal blood flow (Parver et al., 1982). In humans, ocular surface temperature increases in response to increased light intensity, which may imply that choroidal blood flow is increased as well under these conditions (Parver et al., 1983). In birds, changes in light intensity mediate reflexive changes in choroidal blood flow, which may or may not be thermoregulatory (Fitzgerald et al., 1996). The nature of the thermal sensory receptors and the pathways mediating the reflex arc are both as yet unknown.
This view of temperature regulation by the choroid is not, however, universally accepted. An argument can be made that the light-evoked increase in choroidal/retinal temperature of 0.4 deg C with IOP held constant is evidence for a lack of thermoregulation and not the opposite. Furthermore, core body temperature shows fluctuations normally as large as those retinal ones measured under conditions of raised IOP and increased light intensity, hence it would be unlikely that core temperature would maintain a stable retinal temperature.
2.7 Choroid and Pathology: Age-related macular degeneration
Because water and ions, as well as nutrients and plasma-borne protein molecules, move in both directions across Bruch's membrane, impairment of this movement in some disease states and in the normal aging process can have serious consequences for visual function. In the normal process of aging, a thickening of Bruch's membrane and buildup of materials in the inner collagenous layer results in a decrease in water permeability; this is also seen in age-related macular degeneration (AMD), the cause of 70% of blindness (Zarbin, 1998; Zarbin, 2004). Impaired diffusion across Bruch's membrane may result in impaired diffusion of waste products from the RPE and impaired delivery of hormones and oxygen to the RPE, eventually leading to atrophy of the RPE and retina. The thickness of the choriocapillaris and the capillary lumen diameters also decrease with age and AMD (Ramrattan et al., 1994; however, see Spraul et al., 1999). If a decrease in choroidal blood flow results in decreased clearance of debris from the RPE cells, this might contribute to the pathological changes in Bruch's membrane that accompany AMD. It is also possible, however, that the RPE degeneration is the primary factor in the underlying deterioration of the choroid.
In the atrophic (dry) form of AMD the submacular choriocapillaris degenerates; it is unknown if this is a cause or consequence of the inflammatory response that causes the pathologic changes in the choroidal/RPE extracellular matrix (Zarbin, 2004). However, recent evidence showing a close association between degeneration of the RPE and that of the underlying choriocapillaris suggests that atrophy of the RPE occurs first (McLeod et al., 2009). In the exudative (wet) form of AMD there is choroidal neo-vascularization, which often leads to hemorrhage and retinal detachment. This neo-vascularization has been hypothesized to be the result of the RPE responding to the oxidative stress by synthesizing vascular growth factors such as vascular endothelial growth factor (VEGF).
In this form of AMD, choriocapillaris degeneration initially occurs in the presence of a viable RPE, suggesting that the neovascularization associated with it is a response to the ischemia induced by the primary capillary degeneration, with subsequent effects on the RPE (McLeod et al., 2009).
3. Modulation of Choroidal Thickness
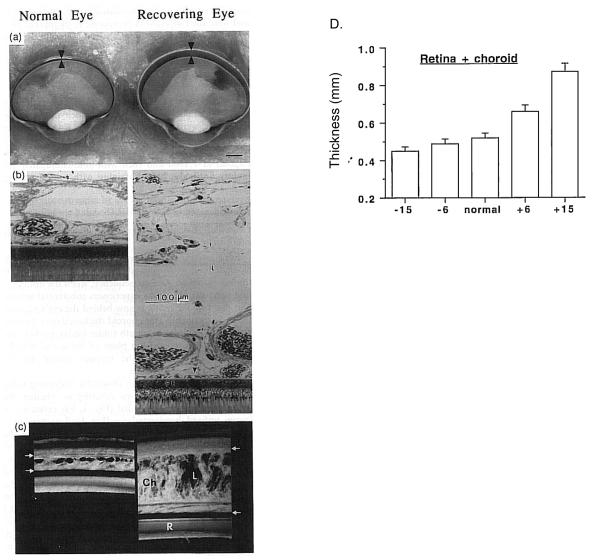
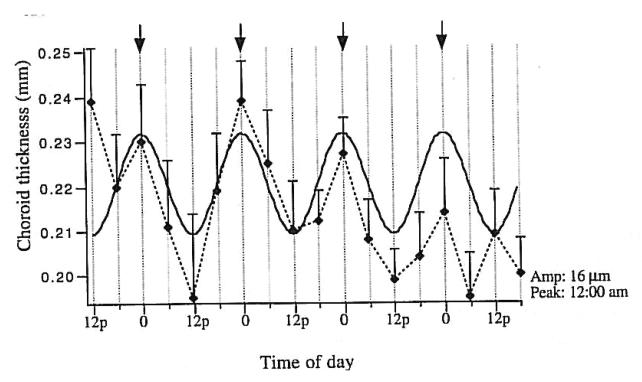
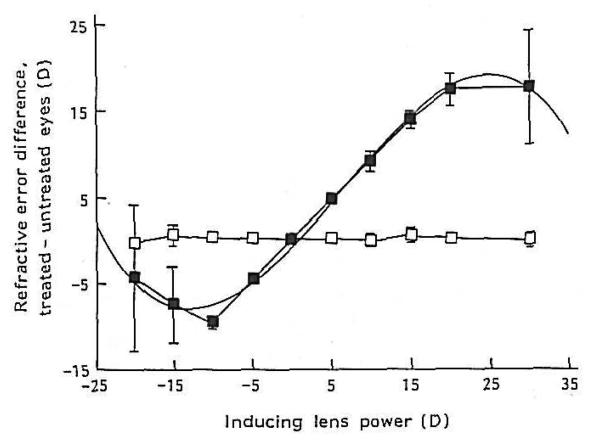
Long ago, it was suggested that the choroid might participate in refractive adjustment as a slow accommodative mechanism (Kajikawa, 1923; Walls, 1942). Over 50 years later, renewed interest in the choroid was sparked by the confirmation of this hypothesis by showing that, in chickens, the choroid can increase its thickness in response to myopic defocus (image focused in front of the retina) by as much as 1mm (> 17 D) pushing the retina towards the image plane and compensating for much of the imposed refractive error (Fig. 10 A-C) (Wallman et al., 1995). This occurs in situations that impose myopic defocus either by the wearing of positive spectacle lenses or by removing the diffuser from an eye that had developed form deprivation-induced myopia. The opposite happens in response to hyperopic defocus (image focused behind the retina by negative spectacle lenses): the choroid thins. Although the amount of thinning is more limited by mechanical constraints than that for thickening, there is a monotonic response between about −15 D to +15 D (Wildsoet and Wallman, 1995) (Fig. 10 D). The major anatomical correlate of the increase in thickness is a great expansion of the lacunae, which can change by approximately 3-fold over the course of 5 days (Fig. 10B and C) (Wallman et al., 1995; Junghans et al., 1999), reducing the myopia by about 7 diopters in eyes wearing +15 D lenses (Wallman et al., 1995). This response is very rapid: 100 μm changes are seen within several hours of the imposed defocus (Kee et al., 2001; Zhu et al., 2005; Nickla, 2007), and so it is intermediate in speed between accommodation and the growth-related changes in the length of the eye. Furthermore, the response is local, in that if only one half of the retina experiences myopic defocus, only the choroid underlying that part thickens (Wallman et al., 1995). Although large increases in choroidal thickness on the order of hundreds of microns have thus far only been documented in chickens, bidirectional but smaller changes are found in marmosets (Fig. 11A; Troilo et al., 2000) guinea pigs (Fig. 11B; Howlett and McFadden, 2009) and macaques (Fig. 11C; Hung et al., 2000). In addition to the modulation of choroidal thickness by refractive state, there is also a diurnal modulation of choroidal thickness, with the choroid being maximally thick at about midnight and thinnest at noon, with a peak-to-peak amplitude of 40 μm (Fig. 12; Nickla et al., 1998; Papastergiou et al., 1998; but also see Tian and Wildsoet, 2006). This rhythm free-runs in constant darkness, indicating that it is driven by a circadian oscillator (Nickla, 2006).
Figure 10.
Choroidal modulation of refractive state. A. Unfixed hemisected chick eyes. Arrowheads delimit choroidal boundaries. B. Plastic-embedded sections of the back of the eyes. L, lacuna; P, pigment cells; PE, retinal pigment epithelium; arrowhead, choriocapillaris. C, One-mm-thick section of the posterior eye wall. Ch, choroid, delimited by arrows. Reproduced with permission from Wallman et al., 1995 © Elsevier. D. Thickness of the retina+choroid as a function of retinal defocus induced by spectacle lenses. Reproduced with permission from Wildsoet & Wallman, 1995 © Elsevier.
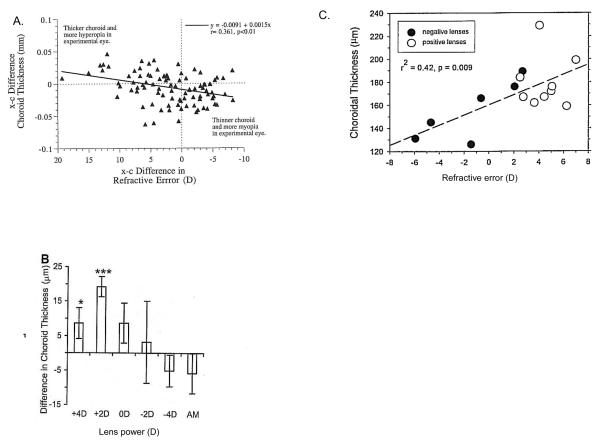
Figure 11.
Modulation of choroidal thickness in marmosets (A), guinea pigs (B) and macaques (C). A. Marmosets. Eyes were made hyperopic by lid suture and became myopic after lid opening. Interocular difference (experimental minus control eye) in refractive errors are plotted against interocular differences in choroidal thickness for all eyes. Eyes with more hyperopia had thicker choroids and vice versa. Reproduced with permission from Troilo et al., 2000 © Association for Research in Vision and Ophthalmology. B. Guinea pigs. Mean differences in choroidal thickness between eyes wearing lenses of different magnitude, or plano, and their respective fellow untreated eyes. The mean difference in untreated age-matched controls (AM) are also shown. * p<0.05; *** p<0.001. Reproduced with permission from Howlett & McFadden, 2009 © Elsevier. C. Macaques. Choroidal thickness as a function of refractive error for the right eyes of monkeys treated with binocular, equal-powered, negative (solid symbols) or positive (open symbols) lenses. Reproduced with permission from Hung et al., 2000 © Association for Research in Vision and Ophthalmology.
Figure 12.
The diurnal rhythm in the mean thickness of the chick choroid. Arrows denote midnight. Solid curve is the sine wave fit to the data (dotted line and standard error bars). Reproduced with permission from Nickla et al., 1998 © Elsevier.
3.1 Possible mechanisms underlying choroidal thickness changes
What could make the choroid increase its thickness by 50% in an hour, and quadruple its thickness in a few days? Because most of the expansion is of the lacunae (Wallman et al., 1995; Junghans et al., 1999), four hypotheses propose redistribution of fluid as the cause. First, it is possible that the increase in thickness is due to an increased synthesis of large, osmotically active proteoglycans, which would pull water into the choroid. Second, it could be the result of an increase in the size or number of the fenestrations in the choriocapillaris, which could similarly increase the amount of osmotically active molecules in the choroidal matrix. Third, fluid could enter the choroid as part of the drainage from the anterior chamber. Fourth, the fluid could be a result of altered transport of fluid from the retina across the RPE. Finally, in addition to other processes, changes in the tonus of the non-vascular smooth muscle that spans the width of the choroid in both birds and primates might play a role. It is likely that more than one of these is involved.
3.1.1- Changes in the synthesis of osmotically active molecules
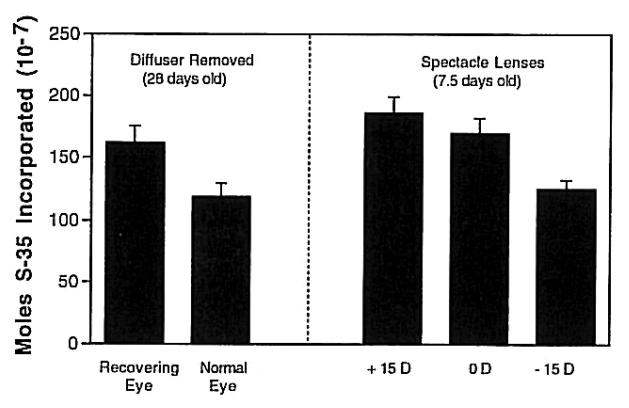
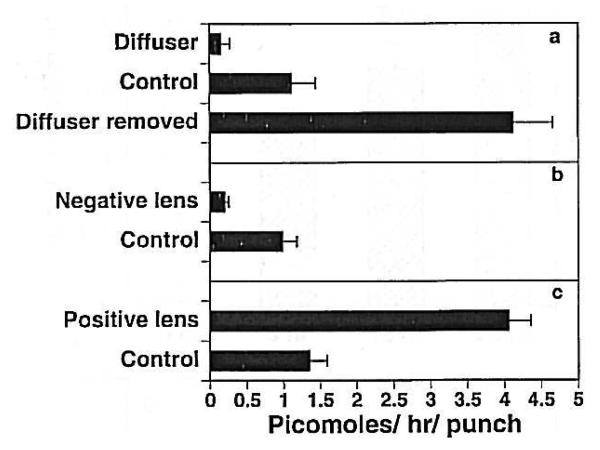
Because water is most commonly moved by moving osmotically active molecules, the most likely mechanism of choroidal expansion is by changing the tonicity of the choroid. This hypothesis is supported by the finding that choroids that are rapidly thickening because the eyes are recovering from form deprivation-induced myopia synthesize significantly higher amounts of proteoglycans than choroids from normal eyes (Fig. 13) (Wallman et al., 1995). Similarly, thickening choroids of eyes responding to myopic defocus induced by +15 D lenses synthesize greater amounts of proteoglycans than those wearing plano lenses, and thinning choroids of eyes responding to hyperopic defocus induced by −15 D lenses synthesize significantly less (Nickla et al., 1997) (Fig. 13). These large, highly sulfated, anionic molecules are extremely hydrophilic and function as “sponges” in extracellular tissues; increasing their synthesis could therefore cause changes in choroidal thickness. However, the relatively modest differences (less than two-fold) in proteoglycan synthesis between the choroids of eyes wearing positive versus negative lenses cast doubt on whether this mechanism is likely to be the primary one. It is also unknown whether the proteoglycans synthesized are located in the lacunae, the choroidal component that expands during choroidal thickening. In this regard it should be noted that Pendrak et al. (2000) found no difference in the amount of sulfated proteoglycans in suprachoroidal fluid from recovering versus form-deprived eyes.
Figure 13.
Incorporation of S-35 into proteoglycans in 6mm punches of posterior sclera in chicks. Left: Choroids from eyes recovering from deprivation myopia and normal eyes. Right: Choroids from eyes wearing positive, negative or plano lenses. Bars are standard errors of the mean. Reproduced with permission from Wallman et al., 1995 © Elsevier.
3.1.2- Changes in vascular permeability
Another way that the choroid could thicken is by an increased flux of fluid from the choroidal vasculature resulting from an increase in capillary permeability causing proteins to move into the extracellular matrix and/or lymphatics, followed by passive fluid flow (Junghans et al., 1999; Pendrak et al., 2000; Summers Rada and Palmer, 2007). Pendrak et al. (2000) found that the protein content in the suprachoroidal fluid (perhaps from the lacunae) decreased in choroids experimentally thinned by prior form deprivation, and increased by 30% in choroids thickened by the restoration of vision. In support of this hypothesis, chicks form-deprived for four weeks by lid-suture showed a decrease in the number of fenestrations facing Bruch's membrane in the choriocapillaris (Hirata and Negi, 1998). Furthermore, intravenous injections of fluorescein dextran resulted in a larger amount found in the choroids of recovering eyes compared to form-deprived or normal choroids, providing further evidence of increased vascular permeability to large molecules (Pendrak et al., 2000). Similarly, Summers Rada and Palmer (2007) found an increase in albumen in the suprachoroidal fluid of eyes recovering from form-deprivation myopia (and hence with thicker choroids), and a decrease in form-deprived eyes (thinner choroids). These studies conclude that changes in the vascular permeability to plasma proteins play a role in the changes in choroidal thickness.
3.1.3- Increases in fluid flux from the anterior chamber to the choroid
Because the choroid is part of the uveoscleral outflow pathway, changes in the amount of aqueous humor shunted through the ciliary muscle to the choroid could account for the increase in the size of the lacunae seen in eyes responding to myopic defocus. Evidence that the choroidal lacunae are connected to the anterior chamber comes from two studies. First, injections of horseradish peroxidase into the anterior chamber resulted in the tracer molecule being found in the lymphatic lacunae of the suprachoroid 4 hours later (Wallman et al., 1995). Similarly, Pendrak et al. (1998) found that a small amount of fluorescein-dextran injected into the anterior chamber appeared in the suprachoroid, although the authors speculate that this could have been due to artifactual disruption of the intraocular barriers. They also point out that one would expect that the increased aqueous flow in thickening choroids would dilute the proteins in the suprachoroidal fluid, whereas the opposite occurred. Because passage through the ciliary muscle is considered the rate-limiting step in uveoscleral outflow (Alm and Nilsson, 2009), an hypothesis that would couple the visual stimulus of retinal defocus to changes in the efficacy of the diffusional barrier of the ciliary muscle would be required.
3.1.4- Movement of fluid across the RPE
Because there is a continuous flow of ions and water across the retina and RPE into the choroid (review: Rymer and Wildsoet, 2005), modulation of this flow could modulate choroidal thickness, if the outflow from the choroid did not match the inflow across the RPE. Because there is also a flow of fluid and solutes from the blood vessels of the choriocapillaris into the tissues and back into the blood vessels, it is unclear whether the flow across the RPE would be large enough to upset this equilibrium.
One group of researchers has advanced the view that, at least in the case of the choroidal expansion that follows restoration of vision after form-deprivation, there is a region of hyperosmolarity that moves from the outer retina during form deprivation to the outer choroid after vision is restored (Junghans et al., 1999; Liang et al., 2004). Electron microscopy shows a sequential thickening of the retina, RPE and lastly the choroid, concurrent with changes in the concentrations of sodium and chloride in these tissues. The fact that these concentrations decrease over the first 72 hours of recovery, while choroidal thickness increases is presented as evidence that the choroidal thickening results from edema, and not from a visually driven refractive compensation.
Correlated with these changes in thickness, the basal membrane of the RPE cells has fewer infoldings in the deprived eye, which increase over the time of the restored vision, as do the number of membrane-bound fluid vesicles (Liang et al., 2004). The authors interpreted these changes as evidence of edema. Furthermore, the fenestrations of the endothelial cells of the lacunae increase in size in the thickened choroids of recovering eyes, consistent with changes in fluid flux (Junghans et al., 1999). Their interpretation is that the return to emmetropia from form- deprivation myopia represents the re-establishment of normal physiology and ultrastructure, as fluid moves from the vitreous to the choroidal lymphatics, and finally out of the eye, rather than being part of a homeostatic regulation of refractive state.
These findings, however, do not preclude the possibility that the choroidal changes are visually regulated; that is, that they are driven by changes in refractive error, rather than by passive edema. Furthermore, the fact that the choroidal thickness is closely related to lens-induced defocus in both directions, and that there is nearly precise refractive compensation for induced defocus, seriously weakens this hypothesis.
3.1.5- Changes in the tonus of non-vascular smooth muscle
Because the choroid can thin very rapidly, by about 100 μm in 3-4 hours in young chicks (Kee et al., 2001), one is inclined to look for muscular, rather than osmotic, mechanisms. Given that the choroid contains abundant non-vascular smooth muscle, probably controlled by both sympathetic and parasympathetic inputs, the contraction of these muscles might squeeze fluid out of the choroid, thereby thinning it. Thus, we can consider the possibility that the lacunae of the choroid are always somewhat hypertonic and tend to acquire fluid, and this tendency is opposed by the tonus of the non-vascular smooth muscle, so that if they contract, the choroid becomes thinner, whereas if they relax, the choroid becomes thicker. This hypothesis is supported by the finding that drastically lowering the IOP causes choroidal expansion (Abelsdoff and Wessely, 1909). However, because the non-vascular smooth muscle is not aligned perpendicular to the plane of the choroid, it is also possible that contraction of these muscles facilitate the filling of the lacunae.
While the innervation of the choroidal vasculature has been extensively studied, that of the non-vascular smooth muscle is still poorly understood. However, most evidence indicates that the non-vascular smooth muscle is under dual sympathetic and parasympathetic control. First, in human choroids, non-vascular smooth muscle cells are contacted by axon terminals that are positive for NADPH-diaphorase (a co-factor for NOS), as well as for tyrosine hydroxylase, the catecholamine rate-limiting enzyme (Schrödl et al., 2001b), suggesting innervation by the pterygopalatine or possibly the ciliary ganglion (although only 1% of these neurons are nitrergic), and the superior cervical ganglion (Poukens et al., 1998). Second, in birds, tyrosine hydroxylase is co-localized with dopamine-β-hydroxylase in terminals on the non-vascular smooth muscle, the double-localization of which is a marker for post-ganglionic sympathetic input (Schrödl et al., 2001b). Furthermore, lesions of the superior cervical ganglion result in loss of adrenergic fibers in the choroidal stroma, possibly reflecting an input onto non-vascular smooth muscle (Guglielmone and Cantino, 1982). In both humans and birds, the presence of terminals labeled for NADPH-diaphorase (Poukens et al., 1998) and nNOS and VIP (May et al., 2004), indicates innervation from the parasympathetic system, specifically the pterygopalatine ganglion, or the ICNs (Schrodl et al., 2003; May et al., 2004). The direction of action of the parasympathetic innervation is shown by the fact that electrical stimulation of the postganglionic axons from the chick ciliary ganglion cause contraction of explant choroids, and this contraction is blocked by atropine but not by curare, showing that acetylcholine causes contraction of at least some choroidal smooth muscle (Meriney and Pilar, 1987).
4. The choroid and emmetropization
As the eye develops from birth to maturity it undergoes adjustments of its optical components so that most eyes eventually become emmetropic (focused for objects at distance). It is generally accepted that this “emmetropization” is determined by a combination of environmental (i.e., visual) and genetic influences. When this process goes awry, the eyes develop refractive errors (hyperopia or myopia). In the United States approximately 25% of the population is myopic (Sperduto et al., 1983) while in other societies the vast majority of the educated population is myopic (Lin, 1996). Understanding the mechanisms underlying emmetropization has direct clinical relevance for the prevention of myopia.
Work with animal models has been crucial in establishing the importance of the visual environment in the regulation of ocular growth (reviews: Wallman, 1993; Norton, 1999; Wallman and Winawer, 2004). The original findings showed that depriving the eye of form vision by lid-suture or plastic diffusers resulted in excessive ocular elongation and consequent myopia in all species studied (Sherman et al., 1977; Wiesel and Raviola, 1977; Wallman et al., 1978). The strongest evidence for the visual regulation of eye growth however comes from studies using spectacle lenses to impose defocus on the retina. Chick eyes show nearly complete compensation to both the magnitude and the sign of the defocus while the lenses are worn (Schaeffel et al., 1990; Irving et al., 1992; Wildsoet and Wallman, 1995): Eyes made functionally myopic with positive lenses (image focused in front of the retina) compensate for the myopia by becaming hyperopic during the period of lens wear while those made functionally hyperopic with negative lenses (image focused behind the retina) became myopic (Fig. 14). After the lenses were removed, the eyes compensated in the opposite direction, returning to emmetropia (Wildsoet and Wallman, 1995). In chick eyes, the compensatory response has two components: (1) changes in the size of the globe, accompanied by changes in the synthesis of extracellular matrix macromolecules in the sclera (Rada et al., 1991; Rada et al., 1992), and (2) changes in the thickness of the choroid, moving the retina forward in the case of myopic defocus, or backward, in the case of hyperopic defocus (Wallman et al., 1995; Wildsoet and Wallman, 1995) (Fig. 10). We ask, first, in what ways does the choroid participate in the modulation of ocular growth; and second, what is the relationship between choroidal thickening and inhibition of ocular elongation? Are they independent homeostatic responses to defocus, or does the choroid influence ocular elongation?
Figure 14.
Interocular difference between lens-wearing eyes and fellow control eyes in chicks. The defocus-induced changes in refractive error are linear from −10 D to +15 D (solid symbols). Open symbols are from untreated birds. From: Irving et al., 1992.
4.1 Choroidal roles in controlling ocular elongation
In addition to the choroidal thickening and thinning moving the retina towards the plane of focus, the choroid almost certainly also plays a role in the modulation of ocular elongation in response to defocus. We assert this because (a) there are neurons in the retina that respond in opposite directions to the wearing of positive and negative lenses (the expression of ZENK immediate early genes by glucagonergic amacrine cells (Fischer et al., 1999a) and the synthesis of retinoic acid by unidentified cells (Mertz and Wallman, 2000; McFadden et al., 2004)); (b) the sclera is not innervated; (c) the signal cascade starting at the retina ends in molecular signals that modulate scleral growth; these must either originate in the choroid or pass through it (review: Wallman and Winawer, 2004).
We can envision three ways in which the choroid can control the growth of the sclera and hence the length of the eye. First, in response to signals from the retina and RPE, the choroid may secrete growth factors that stimulate or inhibit scleral growth, independent of the choroidal thickness. Second, the thickness of the choroid might intrinsically affect the molecular signals reaching the sclera. This could occur either because the choroid's synthetic activities are related to its thickness, or because a thicker choroid might constitute a greater barrier to signals from the RPE or retina, or because a thicker choroid might act as a sponge, facilitating access of molecules to the sclera. Third, the area of the choroid (that is, its lateral extent) might (mechanically) influence the area of the sclera, and hence the size of the globe. Any or all of these processes may be operative.
4.1.1 Does the size of the choroid determine the size of the eye or vice versa?
To consider the third, and most speculative, hypothesis first, because the sclera defines the size of the globe, and because it is more rigid than the layers within it, it seems reasonable to suppose that the size of the eye is controlled by the biosynthetic and tissue-remodeling activities of the sclera together with the hydrostatic force exerted by the intraocular pressure, and that the growth of the other layers (retina, RPE, choroid) responds accordingly. However, this may not be correct. It might be the case that the area of the choroid determines the area of the sclera, and hence the size of the globe.
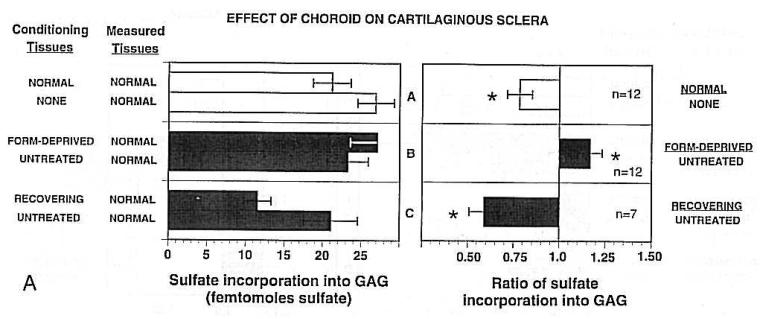
The plausibility of this explanation rests largely on the work of van Alphen (1986), who showed that when the entire posterior sclera is removed from post-mortem eyes and normal intraocular pressure is applied, the choroid retains the size and shape of the eye. When the intraocular pressure was increased, the choroid did not expand like a balloon, increasing its radius of curvature, but rather maintained its normal curvature, like a rigid shell, and expanded backwards, presumably by stretching the ciliary muscle, which attaches the choroid to the anterior part of the eye (Fig. 15). Furthermore, van Alphen reported that there was normally a small pressure differential across the choroid, presumably as a result of the choroid being under tension from the ciliary muscle. One can envision that if the choroid grew in extent relative to the sclera, this pressure differential would be abolished and the choroid would press against the sclera. This pressure might stimulate biosynthetic changes either in the sclera or in the choroid, perhaps in the lamina fusca, which lies in between the sclera and the vascular choroid. The end-result of the increased pressure might be that the fibroblasts of the sclera reduce their synthesis of proteins and proteoglycans, as occurs when the rate of ocular elongation is increased, both in mammals (tree shrews: Norton and Rada, 1995; McBrien et al., 2000; Moring et al., 2007; monkeys: Rada et al., 2000; Troilo et al., 2006) and birds (Marzani and Wallman, 1997), thereby increasing ocular elongation.
Figure 15.
Human eye with the sclera removed from the equator to the posterior pole; the cornea is facing down. Yellow PbO powder was dusted onto the choroid and then the IOP was increased by saline injections. Note that the eye does not balloon, but expands in the antero-posterior direction assuming an ellipsoid shape. Reproduced with permission from Van Alphen, 1985 © Elsevier.
In birds, the situation is slightly more complicated; the sclera as a whole increases its rate of synthesis of proteoglycans and proteins when the eye accelerates its rate of elongation (Christensen and Wallman, 1991; Rada et al., 1991; Rada et al., 1992; Rada and Matthews, 1994; Nickla et al., 1997), because the sclera also contains a cartilaginous layer, the chondrocytes of which respond in the opposite direction as the fibroblasts (Gottlieb et al., 1990; Marzani and Wallman, 1997; Kusakari et al., 2001; Zhu and Wallman., 2009). However, because the chondrocytes appear to be controlled by the activity of the fibroblasts (Marzani and Wallman, 1997), the perichondrial fibroblasts adjacent to the choroid might be the cells that respond to the pressure. A difficulty raised by this conjecture is that it would seem that a thickened choroid would also exert more force on the sclera, and yet choroidal thickening is associated with decreased ocular elongation.
One could also envision the opposite relationship: The choroid could grow to fit the sclera. In this scenario, the same mechanosensory stimulation just mentioned of the choroid pushing up against the sclera could operate to inhibit choroidal expansion, rather than to stimulate scleral expansion. In either case, it seems plausible that the coordination of the growth of the tissues at the back of the eye may be guided by mechanical forces, which modulate the release of growth factors. Other examples of such mechanosensory homeostasis are changes in fibroblast gene expression resulting from mechanical forces on the extracellular matrix (Chiquet et al., 2009) and the expression of growth factor receptors being modulated by mechanical factors during angiogenesis (Mammoto et al., 2009).
4.1.2 Are the choroidal and scleral responses independent?
The general association of choroidal thinning with acceleration of ocular elongation and of choroidal thickening with slowed ocular elongation raises the question of whether these two processes are causally linked. That is, does the choroid have a single phenotype or physiological state that determines both whether the choroid thickens or thins and whether ocular elongation (and therefore, scleral growth) is stimulated or inhibited, or are the two responses independent?
4.1.2.1.Evidence for independent responses
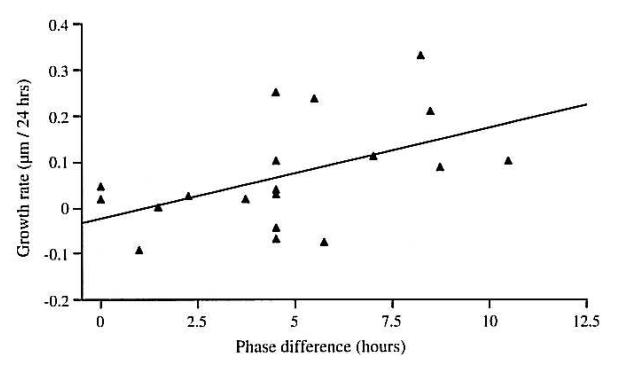
In support of the independence of the choroidal thickening and scleral modulating responses there are several studies that show differential effects of particular visual conditions on the two responses. For example, when the eye receives myopic defocus from positive lenses for brief but infrequent periods (darkness for the rest of the time), there is inhibition of axial growth, without choroidal thickening. Conversely, when the eye receives hyperopic defocus from negative lenses for brief but infrequent periods, there is choroidal thinning without axial elongation (Winawer and Wallman, 2002). Furthermore, if a light diffuser is put over a positive lens, this eliminates the choroidal thickening that the positive lens would normally provoke, but with no effect on the lens-induced inhibition of ocular elongation (Park et al., 2003). Most provocatively, if chicks wear lenses in dim blue or red light, such that only the short-wavelength-sensitive cones or long-wavelength-sensitive cones are stimulated, the eyes compensate in both cases in terms of refractive status, but only the choroid thickness changes in the case of the red light, and only the ocular length changes in the case of the blue light (Rucker and Wallman, 2008).
Although these examples are certainly compatible with separate visual signals guiding the choroidal and scleral responses, they could also indicate that the same visual signals are integrated differently, so that visual signal are more effective in thinning than thickening the choroid, and in decelerating than accelerating ocular elongation. When tested explicitly by giving episodes of lens-wear at a range of different durations and intervals (with darkness in between), the major result was that episodes of positive lens-wear had a four times more enduring effect on inhibiting ocular elongation than on thickening the choroid, whereas episodes of negative lens-wear had an eight times more enduring effect on thinning the choroid than on accelerating ocular elongation (Zhu and Wallman, 2009).
While the integration of these episodes of lens-wear could be occurring in either the retina, the RPE or the choroid, the implication of the differential action on choroid and sclera might be that the same signals act differently on different tissues, depending on their intensity and time course. The implication of different temporal requirements for the effects of negative versus positive lenses might be that the signals controlling the thickening versus thinning of the choroid are different from those controlling the acceleration versus deceleration of the ocular elongation, or it might simply imply that some signals are easier to turn up than to turn down, or vice versa. For example, one can imagine a brief episode of lens-wear causing an increase in transcription of a gene, resulting in a long-lasting increase in a peptide, whereas a decrease in the same peptide might require a more continuous expression of a molecule that degrades the peptide or its mRNA.
4.1.2.2. Evidence for choroidal thickness modulating ocular elongation
The preceding section has shown that the usual linking of changes in choroidal thickness and rate of ocular elongation can be disrupted by experimental manipulations. However, more recent work has shown that brief exposure to myopic defocus causes transient thickening of the choroid, lasting only hours (Nickla, 2007), which therefore would not be detected in the usual experiments, such as those in the preceding section in which eyes are measured at the start and end of the experiment. Because brief exposure to myopic defocus has an effect on the rate of ocular elongation that is over 10 times more enduring than the effect of hyperopic defocus (Zhu and Wallman, 2009), it may be that the transient episodes of choroidal thickening are responsible for the enduring effect of brief periods of myopic defocus.
4.1.2.2.1 The enduring effects of brief episodes of myopic defocus and transient choroidal thickening
It is known that long periods of form deprivation can be largely overridden by brief daily periods of either unrestricted vision (Napper et al., 1995), or myopic defocus (Winawer and Wallman, 2002; Nickla, 2007), suggesting a long time-constant for myopiagenic stimuli. Similarly, negative lenses need to be worn nearly continuously to produce myopia in chicks (Schmid and Wildsoet, 1996; Winawer and Wallman, 2002), tree shrews (Shaikh et al., 1999) and macaques (Kee et al., 2007). On the other hand, if a chick living in a normal visual environment wears a positive lens for only 2 minutes four times a day, hyperopia and a slowing of ocular elongation results (Zhu et al., 2005). Consistent with this asymmetry between signs of defocus, when negative and positive lenses are alternated, brief periods of positive lens-wear balance out much longer periods of negative lens wear (Winawer and Wallman, 2002; Zhu and Wallman, 2009). The developmental utility of it being easier to halt ocular elongation than to accelerate it might be to prevent the development of myopia from transient visual disturbances (such as periods of near vision with hyperopic defocus because of accommodative lag), given that the eye can correct its refractive error more easily by growing than by shrinking.
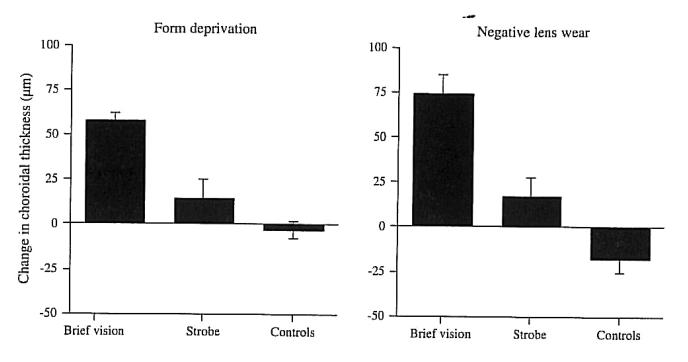
Because even 10 minutes of myopic defocus induces transient choroidal thickening within an hour (Zhu et al., 2005), perhaps this transient thickening causes the ensuing longer-lasting ocular growth inhibition. This hypothesis is supported by the finding that several daily visual manipulations that block myopia also cause transient choroidal thickening. Specifically, whether the myopiagenic stimulus was form deprivation or negative lens-wear, myopia was inhibited by 2 daily hours of unrestricted vision or by one daily hour of stroboscopic illumination, both of which produced transient choroidal thickening within 3 hours (Fig. 16) (Nickla, 2007) that tapered off over the ensuing 24 hours. Similarly, if the myopia caused by constant darkness was inhibited by either a half hour per day of myopic defocus, 2 minutes per hour per day of myopic defocus, or one daily hour of unrestricted vision, again, all three stimuli resulted in transient choroidal thickening (Nickla, 2007). Interestingly, in the one case in which the stimulus was ineffective in myopia prevention (dark-reared birds with infrequent wearing of positive lenses) was there no transient choroidal thickening.
Figure 16.
Transient increases in choroidal thickness measured 3 hours after daily brief vision or stroboscopic stimulation, both of which inhibit the development of myopia in response to form deprivation or negative lens wear. Reproduced with permission from Nickla, 2007 © Elsevier.
4.1.2.2.2 Pharmacological treatments affect both choroidal thickening and ocular elongation
In the following sections, we will discuss pharmacological manipulations that affect ocular elongation and myopia. It is striking to what extent the same molecules affect both the choroidal thickness and the rate of ocular elongation. In the case of molecules that exert their effect on the retina, this is not surprising because it may affect the production of molecular signals that direct both the choroidal and scleral effects. However, drugs affecting nitric oxide, which is known to act on the choroid, also have linked effects on transient choroidal thickening and on ocular elongation. The overall pattern is that molecules that affect ocular elongation also affect choroidal thickening in a consistent manner, suggesting that the two response mechanisms of the choroid—thickening and secretory—are linked.
4.1.3 The choroid as a secretory tissue: Evidence for a direct role in ocular growth
Because of the vascular nature of the choroid, it is not surprising to find choroidal synthesis of growth factors involved in angiogenesis, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF) (Hu et al., 2009), as well as signal molecules involved in functions such as extravasation and vaso-motor changes. In addition, several of the matrix metalloproteases (MMP1, MMP2, MMP3, MMP9) and their tissue inhibitors (TIMP3) are synthesized in the choroid (Janssen et al., 2008) and mediate changes in neovascularization, which may be involved in the etiology of diseases such as AMD. Apart from these vascular-related signal molecules, however, the choroid has been largely ignored as a potential source of growth factors/signal molecules related to other ocular functions. We put forth the hypothesis that the choroid contains secretory cells which function in the visual regulation of ocular growth, by influencing the biosynthetic activity of the sclera.
One of the first investigations providing evidence that choroidal cells secrete growth factors acting on other tissues demonstrated that trophic factors from the choroid were necessary for the development and survival of the ciliary ganglion neurons (Wentzek et al., 1993). That neuronal survival was also promoted by the smooth muscle from avian amnion suggested that the choroidal smooth muscle was the source of the trophic molecules. Furthermore, when dissociated ciliary neurons were grown in culture, their axons contacted smooth muscle cells, strengthening the hypothesis that these were the source; cililary neurons were not rescued in medium conditions which promoted a de-differentiation of smooth muscle into a non-contractile phenotype. The results of using molecular weight cut-off filters determined that the trophic molecule was over 10K; possible candidates were ciliary neurotrophic factor (CNTF), growth-promoting activity (GPA), or platelet-derived growth factor (PDGF). Subsequent work has supported a primary role for GPA in this rescue effect (Nishi, 1994).
The 15 intervening years have yielded other potential signal molecules, involved in a number of ocular functions. For example, a study of rat eyecups in superfusion culture which used successive tissue layer ablations demonstrated that the choroid secretes large amounts of tissue plasminogen activator (t-PA) (Wang et al., 1995). Moreover, the source of the t-PA must be extravascular, because the plasma contains much lower amounts than that released into the superfusate. t-PA cleaves plasminogens to plasmin, a protease which degrades extracellular matrices, and which activates other mediators of matrix degradation such as the metalloproteinases and collagenases (review: Smalley et al., 1994). It was hypothesized that the choroid is the major source of t-PA in aqueous and vitreous humors, and may influence outflow resistance and regulate fluid flux between the anterior chamber and the outflow pathways. We postulate that t-PA might play a role in the scleral matrix remodeling that occurs during the development of myopia.
Another signal molecule synthesized in the choroid is transforming growth factor-ß (TGF-ß), a cytokine found in the endothelial cells of the vessels (TGF-ß1) and in the extravascular stroma (TGF-ß2 and TGF-ß3) of the human eye (Lutty et al., 1993), that has a myriad of functions, ranging from cell proliferation and differentiation, to extracellular matrix remodeling, to immune regulation. All three isoforms are found in the choroid of the tree shrew eye (Jobling et al., 2009) and TGF-ß2 is found in the choroid of the chicken eye (Simon et al., 2004). There is evidence that this molecule plays a role in the visual regulation of ocular growth because it counters the growth inhibitory effects of bFGF (Rohrer and Stell, 1994) and is found in greater amounts in the ocular tissues of form-deprived chicken eyes (Seko et al., 1995), supporting the hypothesis that it is a growth stimulator. However, another study found that choroidal levels of TGF-ß2 mRNA are rapidly and significantly decreased in eyes wearing negative lenses (Simon et al., 2004). It is possible that the tissue-specific isoforms of TGF-ß have different effects within the different tissues and do not act as part of the signal cascade between retina, choroid and sclera (Jobling et al., 2009). This is supported by the finding that the levels of the three isoforms did not change in the choroid or the retina of tree shrews during the development of form-deprivation myopia (Jobling et al., 2009).
Three studies have shown that choroidal state influences scleral growth. The first involved co-culturing scleral tissue with choroids from eyes that were either recovering from myopia and so had decreased their rate of elongation, or were responding to form deprivation and so had increased their rate of elongation. Scleras co-cultured with the choroids from slow-growing eyes showed significantly decreased scleral glycosaminoglyscan (GAG) synthesis (a reliable indicator of the rate of growth of the eye (Rada et al., 1991; Rada et al., 1992; Rada and Matthews, 1994)) compared to normal eyes, whereas those co-cultured with choroids from fast-growing eyes showed an increase in GAG synthesis (Fig. 17), suggesting that the state of the choroid influences scleral growth (Marzani and Wallman, 1997).
Figure 17.
The effect of choroidal conditioned medium from normal eyes, form-deprived eyes, and recovering eyes, compared to the fellow controls, on glycosaminoglycan synthesis in cartilaginous sclera from normal eyes. Left: sulfate incorporation into scleral glycosaminoglycans. Right: Data converted to ratios between the experimental and control eye conditions. Reproduced with permission from Marzani & Wallman, 1997 © Association for Research in Vision and Ophthalmology.
Second, choroids isolated from eyes whose elongation had been inhibited by either positive lenses or the removal of diffusers synthesized three times as much retinoic acid in culture as did normal choroids, and choroids from eyes elongating rapidly because they were wearing either negative lenses or diffusers synthesized one-fifth as much retinoic acid as normal choroids (Fig. 18) (Mertz and Wallman, 2000). In fact, the rate of retinoic acid synthesis in normal choroids (Mertz and Wallman, 2000) is higher than in the retina or liver, major sources of retinoic acid (Blaner and Olson, 1994). Furthermore, treating scleral tissue in culture with retinoic acid caused a dose-dependent inhibition of GAG synthesis (Mertz and Wallman, 2000). Finally, the choroid synthesizes the enzymes involved in retinoic acid synthesis and contains the proteins involved in transporting it (Fischer et al., 1999b). All the above is strong evidence for a role for retinoic acid in ocular growth regulation and for the choroid being the source of the retinoic acid. The questionable part of this nice story is that although scleral proteoglycan synthesis decreases within the first day of positive lens-wear and retinoic acid synthesis increases (Mertz and Wallman, 2000), the choroids from these eyes do not inhibit scleral GAG synthesis in culture until two days later (Summers Rada and Folger, 2009).
Figure 18.
The effects of visual manipulations on choroidal retinoic acid synthesis. Conditions that increase ocular elongation (form deprivation and negative lens wear) result in significant decreases in choroidal retinoic acid synthesis, whereas conditions that inhibit ocular growth (removing the diffuser, and positive lens wear) result in significant increases in retinoic acid synthesis. Reproduced with permission from Mertz & Wallman, 2000 © Elsevier.
Third, significantly more ovotransferrin is released into the culture medium from choroids of eyes with their axial elongation inhibited by the removal of diffusers than from choroids of normal eyes, and ovotransferrin also inhibits GAG synthesis by scleral punches in culture (Rada et al., 2001). Finally, another molecule associated with ocular growth inhibition is avian thymic hormone, the mRNA of which was upregulated in the choroids of eyes one day after removal of the diffuser (myopic defocus), and the protein of which was upregulated three days later (Rada and Wiechmann, 2009). This upregulation approximately temporally corresponds to the increase in choroidal thickness and vascular permeability associated with the decrease in growth rate on day four.
4.2 Pharmacological effects on choroidal thickness and ocular elongation
To clarify whether the choroidal thickness and ocular elongation components of compensation for defocus are intrinsically linked, we will consider the effect of three molecular signals known to be important in ocular physiology. One of these—dopamine—is likely to have its action on retinal neurons, although it may have choroidal actions as well. Another—acetylcholine—may act on a variety of ocular tissues, including the choroid. The third—nitric oxide—is most likely to have its principal actions on the choroid. In each case, agonists and antagonists of the signaling molecules affect both choroidal thickness and the rate of ocular elongation in a consistent manner.
4.2.1 Dopamine
4.2.1.1 Evidence for a role in emmetropization
The potential role of dopamine, a neuromodulator synthesized by amacrine and interplexiform cells, in the signal cascade mediating emmetropization has been the subject of interest and debate for about 20 years (Stone et al., 1989; Iuvone et al., 1991; Rohrer et al., 1993). The initial interest was sparked by the finding that dopamine was decreased in the retinas of form-deprived eyes (Stone et al., 1989) and negative lens-wearing eyes (Guo et al., 1995) and increased in retinas of eyes recovering from form deprivation myopia (Pendrak et al., 1997). Interestingly, only daytime levels of dopamine were affected, suggesting the involvement of a circadian component. Furthermore, the development of both form-deprivation myopia (Stone et al., 1989; Iuvone et al., 1991; Rohrer et al., 1993) and lens-induced myopia (Schmid and Wildsoet, 2004) was inhibited by the administration of the non-specific dopamine agonist apomorphine, further supporting a role for dopamine in ocular growth inhibition. The D2 receptor agonist quinpirole, but not the D1 receptor agonist SKF-38393, inhibited the development of myopia in eyes wearing diffusers or negative lenses, suggesting that the site of action was on the D2 receptors in the retina rather than the D1 receptors on RPE cells (Schaeffel et al., 1995). Accordingly, the D2 receptor antagonist sulpiride enhanced deprivation myopia (Schaeffel et al., 1995) and prevented brief periods of daily vision from blocking the development of deprivation myopia (McCarthy et al., 2007), consistent with the idea that dopamine is required for this inhibition of excessive growth.
However, other studies using dopaminergic agents have yielded conflicting results that make this interpretation problematic (Schaeffel et al., 1995). For instance, intravitreal injections of haloperidol, a dopamine antagonist, suppressed the development of deprivation myopia (Stone et al., 1989). Similarly, 6-hydroxy-dopamine, a neurotoxin that depletes catecholamine levels, reduced the development of deprivation myopia (Li et al., 1992) and did not alter the response to lens-wear (Schaeffel et al., 1994). The same was true of reserpine, a depletor of both dopamine and serotonin (Schaeffel et al., 1995).
Despite these problematic issues, dopamine is still a potential candidate in ocular growth regulation (McCarthy et al., 2007). Furthermore, it has been shown that dopamine stimulates the production of nitric oxide (Melis et al., 1996), another potential growth candidate molecule synthesized in the choroid, making it possible that dopamine is part of the signal cascade upstream of the choroidal changes. It is also possible, however, that there is an as yet unknown source of dopamine in the chicken choroid: the co-localization of dopamine-B-hydroxylase and tyrosine hydroxylase in nerve terminals in the duck choroid indicates that dopamine is synthesized as an intermediate product in the biosynthesis of noradrenaline, but in the chicken choroidal terminals, dopamine-β -hydroxylase appears to be absent, suggesting the possibility that dopamine rather than noradrenaline might be the transmitter for these neurons (Falk Schrödl, personal communication).
4.2.1.2 Effects on choroidal thickness and ocular elongation
If dopamine were part of the signal cascade mediating ocular growth inhibition, and if it acted upstream of the choroid, then injections of the effective agonists should cause transient increases in choroidal thickness, similar to the effects induced by 2 hours of myopic defocus or removing the diffuser or negative lens. Intravitreal injections of both apomorphine (a non-specific agonist) and quinpirole (a D2 agonist), both effective inhibitors of myopia, into eyes wearing negative lenses produced significant transient increases in choroidal thickness (Fig. 19) that were associated both with the inhibition of axial elongation and the resultant myopia (Dhillon and Nickla, 2008). The D1 agonist SKF 38393, which did not prevent the development of myopia, also did not elicit choroidal thickening (Dhillon and Nickla, 2008). Together these results support a role for dopaminergic D2 receptor-mediated mechanisms, and choroidal thickening, in ocular growth inhibition.
Figure 19.
Effects of dopamine agonists on changes in choroidal thickness over 3 hours from the injection into eyes wearing −10 D lenses. Both effective growth inhibitors result in transient increases in choroidal thickness. Unpublished data.
4.2.2 Acetylcholine
4.2.2.1 Evidence for a role in emmetropization
Atropine, a non-specific muscarinic acetylcholine antagonist, has long been known to inhibit the development of myopia in humans (Bedrossian, 1971; Shih et al., 1999a; Kennedy, 1995; Kennedy et al., 2000), and to prevent form-deprivation myopia in several species (chicks: Stone et al., 1991; McBrien et al., 1993b; Schmid and Wildsoet, 2004; Schwann et al., 2000; Luft et al., 2003; tree shrews: McKanna and Casagrande, 1981; monkeys: Young, 1965; Raviola and Wiesel, 1985; Tigges et al., 1999). The effect is believed to be mediated by M1 or M4 receptors, because pirenzepine (M1 and M4 antagonist) is also effective (Stone et al., 1991; McBrien et al., 1993; Leech et al., 1995; Cottriall and McBrien, 1996; Schwahn et al., 2000; Luft et al., 2003). Of a large number of muscarinic antagonists examined, only pirenzepine and oxyphenonium (non-selective), but not scopolamine (M1) were effective in inhibiting axial elongation in response to form deprivation in chicks (Luft et al., 2003). Because M1 receptors are reportedly absent in chicks, it is likely that pirenzepine acts on M4, rather than M1 receptors (McBrien et al., 2009).
It is unclear on what tissue of the eye atropine acts to exert its anti-myopiagenic effects. Contrary to the early views, the suppression of myopia by atropine does not depend on its cycloplegic action, as atropine is also effective in preventing myopia in birds (Stone et al., 1991; McBrien et al., 1993b), in which the ciliary muscle, being striated, is nicotinic, and in a non-accommodating mammal (McBrien et al., 1993a). The view that it acts at the retinal level is not supported, as lesions of cholinergic retinal neurons do not alter the effects of form deprivation, nor do they affect the inhibitory influence of atropine (Fischer et al., 1998). It is possible that atropine has an indirect retinal action, by causing the release of dopamine or other neurotransmitters, or by inducing spreading depression in the retina (Schwahn et al., 2000). Finally, evidence in tree shrews (Cottriall et al., 2000), guinea pigs (Liu et al., 2007) and chicks (Lind et al., 1998) indicate that the site of action may be scleral: form deprivation causes an increase in the expression of M1 and M4 receptor mRNA in the guinea pig sclera (Liu et al., 2007).
4.2.2.2 Effects on choroidal thickness and ocular elongation
The connection between muscarinic receptors and both transient choroidal thickening and inhibition of the excessive ocular elongation is shown by the finding that, when injected into the vitreous of birds wearing –10D lenses, atropine, pirenzepine and oxyphenonium, drugs that are effective in eliciting ocular growth inhibition (Luft et al., 2003) also caused a significant and transient choroidal thickening of approximately 75 μm (Nickla and Cheng, 2009), whereas dicyclomine, which did not prevent the excessive growth (Luft et al., 2003), had no effect on choroidal thickness (Nickla and Cheng, 2009). If the muscarinic system is involved in both the choroidal and the growth responses, then agonists should have the effect of thinning the choroid and increasing ocular growth. In a pilot experiment, the non-specific agonist carbachol was injected into normal eyes, and produced choroidal thinning by 3 hours; this effect was dose-dependent (unpublished data).
4.2.3 Nitric Oxide
4.2.3.1 Evidence for a role in emmetropization
Nitric oxide is a gaseous neurotransmitter that relaxes smooth muscle by stimulating guanylyl cyclase to make cGMP, and is a potent vasodilator. In vertebrate eyes, it is present in retinal neurons (Fischer and Stell, 1999), RPE (Goldstein et al., 1996), intrinsic choroidal neurons (Flugel et al., 1994; Bergua et al., 1996; Schrödl et al., 2000; Schrödl et al., 2004), and in terminals from the parasympathetic ciliary (Sun et al., 1994; Nilsson, 1996) and pterygopalatine ganglia (Yamamoto et al., 1993; Cuthbertson et al., 1997). Therefore, if the nonvascular smooth muscle cells in the choroid oppose the tendency of the lacunae to gain fluid via osmotic forces, it seems likely that nitric oxide is involved because NOS-positive axon terminals are found on these cells. Perhaps nitric oxide determines the degree of contraction.
Unlike other neurotransmitters, nitric oxide is synthesized immediately before release. There are three isoforms of the enzyme nitric oxide synthase. The neuronal form, nNOS, and the endothelial form, eNOS (formerly known as endothelium-derived relaxing factor) are activated by calcium binding to calmodulin, and the activation is short-lived, appropriate for involvement in short-term physiological phenomena. The inducible form, iNOS, is controlled at the level of transcription and is much longer-lasting, being involved in immune responses and in pathologies such as retinal ischemias (reviews: Snyder and Bredt, 1992; Dawson and Snyder, 1994; Stewart et al., 1994; Goldstein et al., 1996).
4.2.3.2 Effects on choroidal thickness and ocular elongation
Inhibiting the formation of nitric oxide using NOS inhibitors prevents the increase in choroidal thickness normally induced by myopic defocus. When the non-specific NOS inhibitor L-NAME (Nickla and Wildsoet, 2004) or L-NMMA (Nickla et al., 2009) was injected into eyes receiving myopic defocus by either positive spectacle lenses or by having the diffuser removed from a form-deprived eye, the choroid did not increase in thickness (Fig. 20). This effect is transient, lasting about 24 hours, and dose-dependent (Nickla and Wildsoet, 2004). Interestingly, blocking the synthesis of nitric oxide also blocks the defocus-induced inhibition of axial growth in these eyes, supporting the hypothesis that choroidal thickening is causally linked to the ocular growth inhibition (Nickla and Wildsoet, 2004; Nickla et al., 2006). Furthermore, if the NOS inhibitor was injected several hours after the defocus-induced thickening of the choroid had taken place, there was no effect on the rate of ocular elongation, linking the choroidal and axial responses (Nickla et al., 2006). Finally, if L-NAME was injected prior to giving form-deprived eyes brief daily periods of unrestricted vision over the course of 4 days (which normally inhibits the development of deprivation myopia), thus preventing the transient daily increases in choroid thickness (Fig. 21A), the eyes did develop axial myopia (compare L-NAME to saline, Fig. 21B) (Nickla et al., 2006). L-NAME also prevented the increase in choroidal synthesis of retinoic acid normally produced by myopic defocus (Nickla and Mertz, 2003), linking these molecules in the signal cascade. The tight temporal association between the effect of L-NAME on the choroid and its subsequent effect on ocular growth implies either that nitric oxide participates in the choroidal thickening, which in turn inhibits ocular elongation, or that nitric oxide participates in the retinal processing of the myopic defocus. There is no evidence for nitric oxide acting directly on the sclera.
Figure 20.
The effects of L-NAME on the choroidal response to myopic defocus measured after 7 hours. The non-specific NOS inhibitior L-NAME inhibits the choroidal thickening in response to myopic defocus caused by prior form deprivation (Recovery) and positive lens wear (Lens). Redrawn from: Nickla & Wildsoet, 2004.
Figure 21.
The effects of L-NAME on choroidal thickness (A) and refractive error (B) when given prior to brief daily periods of vision over 4 days, during the development of form deprivation myopia. A. The change in choroidal thickness measured after 2 hours of vision. L-NAME prevented the thickening in response to vision (L-NAME vs saline). B. L-NAME prevented the inhibitory effects of vision on the development of myopia: eyes become significantly more myopic than saline controls. Reproduced with permission from Nickla et al., 2006 © Elsevier.
The localization of NOS to retinal neurons, intrinsic choroidal neurons and axon terminals from the autonomic nervous system makes it possible that any one of the three isozymes might be involved. If relaxation of the non-vascular smooth muscle contributes to choroidal thickening, nNOS might be the relevant one, as both the intrinsic choroidal neurons and the pterygopalatine terminals are positive for nNOS, and both contact non-vascular smooth muscle cells (Poukens et al., 1998; Schrödl et al., 1998). If the response is mediated by changes in vascular permeability, and/or changes in blood flow, then eNOS would be the likely candidate. The transient nature of the choroidal response (i.e. less than 24 hours (Nickla and Wildsoet, 2004)) makes it unlikely that iNOS is involved. A test of several more specific inhibitors showed that only the nNOS inhibitor N-ω -propyl-L-arginine, but not the inhibitors more specific for eNOS or iNOS, behaved similar to L-NAME in blocking the defocus-induced choroidal thickening (Fig. 22A), the defocus- induced refractive hyperopia (Fig. 22B) and the defocus-induced growth inhibition (Fig. 22C) (Nickla et al., 2009). To summarize, the results of these studies suggest that (1) NO from a neuronal source may underlie the thickening of the choroid, possibly by changing the tonus of the non-vasuclar smooth muscle, and (2) NO may be part of the signal cascade mediating growth inhibition in response to myopic defocus.
Figure 22.
The effects of several NOS inhibitors on the responses to positive lens-induced myopic defocus on (A) choroidal thickness, (B) refractive error and (C) ocular elongation. A. Change in choroidal thickness measured 7 hours after the injection. Only the inhibitor most specific for nNOS (Nw-PLA) prevented the choroidal thickening response in a dose-dependent manner (bottom panel). B. Refractive error measured 48 hours after the injection. Only Nw-PLA prevented the refractive response to the myopic defocus; eyes became significantly less hyperopic than saline controls. C. Change in axial length measured 24 hours after the injection. Nw-PLA prevented the growth inhibition in response to the myopic defocus; eyes grew significantly longer than saline controls. Fellow: untreated fellow eyes. Nw-PLA, n-omega propyl-L-arginine (nNOS inhibitor); L-NIO, N-(1-iminoethyl)-L-ornithine dihydrochloride (less specific eNOS inhibitor); L-NIL, N-(1-iminoethyl)-L-Lysine hydrochloride (less specific iNOS inhibitor). Reproduced with permission from Nickla et al., 2009 © Elsevier.
4.3 Implications of these pharmacological effects
It is striking that these three very different signaling molecules all affect both choroidal thickness and the rate of ocular elongation. Because the three molecules are unlikely to act with the same time course and because it is unlikely that all three molecules act on the same cells, these results are further evidence that there is a causal link between the choroidal thickness response system and the control of ocular elongation.
5. The Diurnal Rhythm in Choroidal Thickness and Ocular Growth
As mentioned above, the thickness of the chicken choroid shows diurnal oscillations, thickening during the night and thinning during the day (Nickla et al., 1998; Papastergiou et al., 1998), in approximate antiphase to the diurnal rhythm in axial elongation. Both rhythms free-run in darkness and so are driven by an endogenous circadian oscillator, rather than being a response to darkness and light (Nickla et al., 2001). Furthermore, isolated scleral tissue exhibits a circadian rhythm of proteoglycan synthesis that continues for days, indicating the existence of an oscillator within the sclera (Nickla et al., 1999).
The most provocative aspect of these studies is that the phase difference between the rhythms of choroidal thickness and ocular elongation predicts the rate at which the eye elongates: the phase difference shifts in one direction (towards anti-phase) in eyes becoming myopic and elongated, and it shifts in the opposite direction (towards being in phase) in eyes becoming hyperopic with elongation inhibited (Nickla et al., 1998). Indeed, when rhythms were measured in eyes subjected to myopic and hyperopic defocus by the wearing of spectacle lenses, the phase differences between the rhythms of choroidal thickness and axial length in individual animals predicted the growth rate on the following day (Fig. 23) (Nickla, 2006). Also, the young, faster-growing eyes of juvenile marmosets have axial and choroidal rhythms in approximate anti-phase while the slower growing eyes of adolescents have phases that are closer together (Nickla et al., 2002), similar to the pattern in fast versus slower-growing eyes of chicks. The recent description of diurnal rhythms in axial length (Stone et al., 2004) and choroidal thickness (Brown et al., 2009) in humans lends credence to the notion that these rhythms are evolutionarily conserved, and possibly important in the control of eye growth.
Figure 23.
Phase differences and growth rates in chicks exposed to myopic and hyperopic defocus induced by spectacle lenses. Mean ocular growth rate (μm/24 hrs) as a function of the mean difference in phase on the preceding day, between the rhythms in axial length and choroidal thickness. The correlation is significant at p<0.05. Reproduced with permission from Nickla, 2006 © Springer.
We hypothesize that the mechanism underlying this phase-dependent growth modulation may be that the thickness of the choroid is directly associated with the production of a growth signal, either inhibitory or excitatory (Marzani and Wallman, 1997; Mertz et al., 2000; Rada et al., 2001; Rada and Wiechmann, 2009), which acts most strongly at a particular phase of the scleral cell's growth cycle. Alternatively, choroidal thickness may influence the ability of a signal molecule from the retina or RPE to reach the sclera, again acting most strongly at a particular phase of the growth cycle.
6. Significance to Human Refractive Disorders
The reasons behind the high incidence of myopia, particularly among educated people, remain obscure. The finding that animals can be made myopic by presenting them with hyperopic defocus by spectacle lenses argues that refractive error is under homeostatic control. Because the eye can alter its growth even if the optic nerve is severed, it follows that the retina is able to detect the sign of the defocus and control the eye's growth. Because chemical signals from the retina must pass through the choroid to reach the sclera, and because the choroid produces growth-modulating molecules in response to visual conditions, it seems clear that the choroid plays an important role in the homeostatic control of refractive error, and therefore is likely important to understanding the etiology of myopia.
Despite decades of work, there is no effective prophylaxis against childhood myopia other than atropine, which has undesirable side effects, and perhaps contact lenses that reshape the cornea. The possibility that ocular elongation is directly modulated by the choroid opens up another avenue of treatment, which is potentially less dangerous than interfering with the retina. In particular, if nitric oxide in required for choroidal expansion and, consequently, for inhibition of ocular elongation, rather benign treatments might be possible that would increase the nitric oxide synthase activity and, perhaps, reduce ocular elongation and therefore myopia.
7. Future Directions
In this review, we have tried to bring together the literature bearing on the unusual aspects of the anatomy and function of the choroid. The knowledge concerning most of these aspects remains incomplete and hypotheses about choroidal functions remain tentative. In these final paragraphs, we will point to some research directions that might prove useful.
The most puzzling component of the choroid is the lamina fusca, about which nearly nothing is known. It would be interesting to assess what it secretes, either by in situ hybridization or by dissecting it out and assessing the messenger RNAs or proteins produced. Alternatively, the possibility of its being a mechanosensory tissue could be assessed by electrophysiological recording. Similarly, electrophysiological recording from the nearly equally unknown intrinsic choroidal neurons might reveal whether they respond to osmotic or mechanical forces.
The non-vascular smooth muscle might act either to thin or thicken the choroid or to alter blood flow. Directly stimulating the motor nerves innervating it, either in vivo or in vitro might clarify its function.
The dramatic ability of the choroid to thin and thicken demands an explanation of the underlying mechanism. We considered three possibilities: If synthesis of osmotically active molecules in the choroid modulates its thickness, clarifying the contents of the lacunae by histology or by microanalysis under thickened and thinned conditions might ascertain the plausibility of this potential mechanism. The possibility that choroidal thickness is modulated by changes in vascular or lymphatic permeability might be addressed by manipulating water and ion channels. Although modulation of uveoscleral outflow of aqueous humor seems unlikely to be the only mechanism of changing choroidal thickness given that the choroid can be made to thicken or thin in vitro, and that local regions of the choroid thicken if the adjacent retina is made myopic, it would be informative to measure uveoscleral outflow during visual circumstances that cause the choroid to thicken or thin.
Much of this review has been devoted to the relationship of the choroid to emmetropization. We have considered the possibility that the area of the choroid may determine the size of the globe more than the sclera does. If this is so, one should be able to measure evidence of vascular growth preceding scleral enlargement. We have discussed the evidence for the choroid being a secretory tissue thereby directing the growth of the sclera. A more complete analysis of what the choroid secretes under different eye-growth conditions awaits experimentation. Finally, we discussed the possibility that the choroidal thickening may intrinsically determine the growth-altering molecules presented to the sclera. Perhaps this could be explored by altering the choroidal thickness pharmacologically in vitro and either assessing the effect of the tissue culture medium conditioned by thick or thin choroids on scleral tissue or measuring alterations in the secretion of candidate molecules such as retinoic acid, transforming growth factor beta or transferrin under thick and thin conditions.
Last of all, we discussed the fact that choroidal thickness changes over the course of the day in both birds and humans. We discussed evidence that the phase of this cycle may predict the rate of subsequent ocular elongation, conceivably by linking the secretion of some growth factor to a period of scleral responsiveness to that growth factor. This might be explored by treating scleral tissue with choroid-conditioned medium collected at different times of day.
The question in the background throughout this review is how evolutionarily conserved are these various choroidal functions. Although the choroidal thickness changes are not as dramatic in mammals as in birds, they seem to occur in the same circumstances, and three of the unusual features of choroid —intrinsic choroidal neurons, endothelium-lined lacunae, and non-vascular smooth muscle cells, occur in both birds and humans. How deep these similarities extend remains a question for the future.
8. Acknowledgements
Supported by: NIH-EY-013636 (DLN) and EY-02727 and RR03060 (JW).
The authors are grateful to the following people for their important contributions: Jeffrey Kiel for his insightful discussions on the choroid and thermoregulation, and Falk Schrödl, Maria Egle De Stefano and Gerald Lutty for their excellent critical readings of the manuscript.
Footnotes
Some anatomists identify the lamina fusca as the innermost layer of the sclera (Forrester et al., 2002; Watson and Young, 2004).
In fact, these authors noted that the arrangement of the reticulum of the non-vascular smooth muscle and the adjacent open spaces is reminiscent of the lacunae in the suprachoroid of the bird, and hence might mediate similar functions, notably, that of regulating fluid flux between the vasculature and the extracellular matrix.
9. REFERENCES
- Abelsdoff G, Wessely K. Vergleichendphysiologische Untersuchungen fiber den Flussigkeit-swechsel des auges in der Wirbeltierreihe. Arch. Augenheilk. 1909;64:65–124. [Google Scholar]
- Alm A. Ocular CIrculation. In: Hart WM, editor. Adler's Physiology of the Eye. Mosby-Year Book Inc.; St. Louis, MO: 1992. pp. 198–227. [Google Scholar]
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressure in monkeys. Exp. Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Alm A, Nilsson FE. Uveoscleral outflow: A review. Exp. Eye Res. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Alm P, Uvelius B, Ekstrom J, Holmqvist B, Larsson B, Andersson KE. Nitric oxide synthase-containing neurons in rat parasympathetic, sympathetic and sensory ganglia: a comparative study. Histochem. J. 1995;27:819–831. [PubMed] [Google Scholar]
- Bar H, Wende P, Watson L. Smoothelin is an indicator or reversible phenotype modulation of smooth muscle cells in baloon-injured rat carotid arteries. Basic Res. Cardio. 2002;97:9–16. doi: 10.1007/s395-002-8382-z. [DOI] [PubMed] [Google Scholar]
- Baumel JJ. Sisson and Grossman's The Anatomy of Domestic Animals. W. B. Saunders; Philadelphia: 1975. Aves Nervous System; pp. 2019–2066. [Google Scholar]
- Bedrossian RH. The effect of atropine on myopia. Ann. Ophthalmol. 1971;3:891–897. [PubMed] [Google Scholar]
- Bergua A, Junnemann A, De Laet A, Timmermans JP, Brehmer A, Neuhuber W, Schrodl F. Topography of nitrergic intrinsic choroidal neurons (ICN) in the human eye. Invest. Ophthalmol. Vis. Sci. 2003:44. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- Bergua A, Mayer B, Neuhuber W. Nitrergic and VIP-ergic neurons in the choroid and ciliary ganglion of the duck Anis carina. Anat. Embryol. 1996;193:239–248. doi: 10.1007/BF00198327. [DOI] [PubMed] [Google Scholar]
- Bergua A, Schrodl F, Brehmer A, Neuhuber WL. Ultrastructural characteristic of different neuron types in the choroid of the duck. Exp. Eye Res. 1998;67:78. [Google Scholar]
- Bill A. Intraocular pressure and blood flow through the uvea. Arch Ophthal. 1962;67:336–348. doi: 10.1001/archopht.1962.00960020338010. [DOI] [PubMed] [Google Scholar]
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- Bill A. Some aspects of the ocular circulation. (Friedenwald Lecture) Invest. Ophthalmol. Vis. Sci. 1985;26:410–424. [PubMed] [Google Scholar]
- Bill A. Effects of some neuropeptides on the uvea. Exp. Eye Res. 1991;53:3–11. doi: 10.1016/0014-4835(91)90138-5. [DOI] [PubMed] [Google Scholar]
- Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int. Ophthalmol. 1983;6:101–107. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- Bill A, Stjernschantz J, Alm A. Effects of hexamethonium, biperiden and phentolamine on the vasoconstrictive effects of oculomotor nerve stimulation in rabbits. Exp. Eye Res. 1976;23:615–622. doi: 10.1016/0014-4835(76)90220-7. [DOI] [PubMed] [Google Scholar]
- Bill A, Tornqvist P, Alm A. The permeability of the intraocular blood vessels. Trans. Ophthal. Soc. UK. 1980;100:332–336. [PubMed] [Google Scholar]
- Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. Raven Press; 1994. pp. 229–255. [Google Scholar]
- Brown JS, Flitcroft DI, Ying G, Francis EL, Schmid GF, Quinn GE, Stone RA. In vivo human choroidal thickness measurements: Evidence for diurnal fluctuations. Invest. Ophthalmol. Vis. Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest. Ophthalmol. Vis. Sci. 1991;32:2143–2150. [PubMed] [Google Scholar]
- Cottriall CL, McBrien N. The m1 muscarinic antagonist pirenzepine reduces myopia and eye enlargment in the tree shrew. Invest. Ophthalmol. Vis. Sci. 1996;37:1368–1379. [PubMed] [Google Scholar]
- Cottriall CL, Truong HT, Gentle A, McBrien NA. Changes in scleral metabolism following in vivo application of pirenzepine to prevent axial myopia. Invest. Ophthalmol. Vis. Sci. 2000;41(Suppl):S133. [Google Scholar]
- Cuthbertson S, Jackson B, Toledo C, Fitzgerald MEC, Shih YF, Zagvazdin Y, Reiner A. Innervation of orbital and choroidal blood vessels by the pterygopalatine ganglion in pigeons. J. Comp. Neurol. 1997;386:422–442. [PubMed] [Google Scholar]
- Cuthbertson SL, White J, Fitzgerald M, Shih YF, Reiner A. Distribution within the choroid of cholinergic nerve fibers from the ciliary ganglion in pigeons. Vision Res. 1996;36:775–786. doi: 10.1016/0042-6989(95)00179-4. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: Nitric oxide and carbon monoxide in the brain. J. Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano M, Ciofi Luzzatto A, Mugnaini E. Neuronal ultrastructure and somatostatin immunolocalization in the ciliary ganglion of chicken and quail. J. Neurocytol. 1993;22:868–892. doi: 10.1007/BF01186358. [DOI] [PubMed] [Google Scholar]
- De Stefano M, Mugnaini E. Fine structure of the choroidal coat of the avian eye: lymphatic vessels. Invest. Ophthalmol. Vis. Sci. 1997b;38:1241–1260. [PubMed] [Google Scholar]
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- Dhillon B, Nickla D. The ocular growth inhibition effected by dopamine agonists and atropine is associated with transient increases in choroidal thickness in chicks. Invest. Ophthalmol. Vis. Sci. 2008 E-Abstract 1732. [Google Scholar]
- Epstein ML, Davis JP, Gellman LE, Lamb JR, Dahl JL. Cholingergic neurons of the chicken ciliary ganglion contain somatostatis. Neuroscience. 1988;25:1053–1060. doi: 10.1016/0306-4522(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Fine BS, Yanoff M. Ocular Histology. Harper & Row; Hagerstown, MD: 1979. p. 195. [Google Scholar]
- Fischer A, McGuire J, Schaeffel F, Stell W. Light and defocus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 1999;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer A, Miethke P, Morgan I, Stell W. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form deprivation myopia. Brain Res. 1998;794:48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Fischer A, Stell W. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J. Comp. Neurol. 1999;405:1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fischer A, Wallman J, Mertz J, Stell W. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J. Neurocytol. 1999;28:597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Gamlin P, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye. Visual Neurosci. 1996;13:655–669. doi: 10.1017/s0952523800008555. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Vana BA, Reiner A. Control of choroidal blood flow by the nucleus of Edinger-Westphal in pigeons: A laser doppler study. Invest. Ophthalmol. Vis. Sci. 1990;31:2483–2492. [PubMed] [Google Scholar]
- Flugel C, Tamm ER, Mayer B, Lutjen-Drecoll E. Species differences in choroid vasodilative innervation: evidence for specific intrinsic nitrergic and VIP-positive neurons in the human eye. Invest. Ophthalmol. Vis. Sci. 1994;35:592–599. [PubMed] [Google Scholar]
- Flugel-Koch C, May A, Lutjen-Drecoll E. Presence of a contractile cell network in the human choroid. Ophthalmologica. 1996;210:296–302. doi: 10.1159/000310728. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Dick AD, McMenamin PG, Lee WR. The Eye. Basic Sciences in Practice. Saunders; Edinburgh: 2002. [Google Scholar]
- Geiser MH, Riva C, Dorner GT, Diermann U, Luksch A, Schmetterer L. Response of choroidal blood flow in the foveal region to hyperoxia and hyperoxia-hypercapnia. Curr. Eye Res. 2000;21:669–676. [PubMed] [Google Scholar]
- Gherezghiher T, Hey J, Koss M. Parasympathetic nervous control of intraocular pressure. Exp. Eye Res. 1990;50:457–462. doi: 10.1016/0014-4835(90)90032-p. [DOI] [PubMed] [Google Scholar]
- Gienc J, Kuder T. Morphology and topology of the pterygopalatine ganglion in pigeons. Zool. Pol. 1985;32:229–235. [Google Scholar]
- Goldstein IM, Ostwald P, Roth S. Nitric oxide: A review of its role in retinal function and disease. Vision Res. 1996;36:2979–2994. doi: 10.1016/0042-6989(96)00017-x. [DOI] [PubMed] [Google Scholar]
- Gomez DG, Manzo RP, Fenstermacher JD, Potts DG. Cerebrospinal fluid absorbtion in the rabbit. Graefe's Arch Clin Exp Ophthalmol. 1988;266:17. doi: 10.1007/BF02172707. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form deprivation myopia. Curr Eye Res. 1990;9:1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- Gray DB, Pilar GR, Ford MJ. Opiate and peptide inhibition of transmitter release in p- arasy.m- pathetic nerve terminals. J. Neurosci. 1989;9:1683–1692. doi: 10.1523/JNEUROSCI.09-05-01683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone R, Cantino D. Autonomic innervation of the ocular choroid membrane in the chicken. Cell Tissue Res. 1982;222:417–431. doi: 10.1007/BF00213222. [DOI] [PubMed] [Google Scholar]
- Guo SS, Sivak JG, Callender MG, Diehljones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr. Eye Res. 1995;14:385–389. doi: 10.3109/02713689508999936. [DOI] [PubMed] [Google Scholar]
- Haddad A, Laicine EM, Tripathi BJ, Tripathi RC. An extensive system of extravascular smooth muscle cells exists in the choroid of the rabbit eye. Exp. Eye Res. 2001;73:345–353. doi: 10.1006/exer.2001.1042. [DOI] [PubMed] [Google Scholar]
- Hirata A, Negi A. Morphological changes of choriocapillaris in experimentally induced chick myopia. Graefe's Arch Clin Exp Ophthalmol. 1998;236:132–137. doi: 10.1007/s004170050053. [DOI] [PubMed] [Google Scholar]
- Hirose A, Azuma H, Tokoro T, Hirai K. Endothelin-B receptors on suprachoroidal melanocytes mediate and endothelin-1-induced increase in the intracellular calcium concentration of rabbit ocular suprachoroidal tissue. Curr. Eye Res. 2007;32:585–591. doi: 10.1080/02713680701391529. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye. Saunders Company; Philadelphia: 1971. [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Howlett M, McFadden S. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hu W, Criswell MH, Fong SL, Temm CJ, Rajashekhar G, Cornell TL, Clauss MA. Differences in the temporal expression of regulatory growth factors during choroidal neovascular development. Exp. Eye Res. 2009;88:79–91. doi: 10.1016/j.exer.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Hung L-F, Wallman J, Smith E. Vision-dependent changes in the choroidal thickness of Macaque monkeys. Invest. Ophthalmol. Vis. Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthal. Physiol. Optics. 1992;12:448–456. [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest. Ophthalmol. Vis. Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- Janssen A, Hoellenriegel J, Fogarasi M, Schrewe H, Seeliger M, Tamm E, Ohlmann A, May CA, Weber BH, Stohr H. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest. Ophthalmol. Vis. Sci. 2008;49:2812–2822. doi: 10.1167/iovs.07-1444. [DOI] [PubMed] [Google Scholar]
- Jobling AI, Wan R, Gentle A, Bui BV, McBrien N. Retinal and choroidal TGF-B in the tree shrew model of myopia: Isoform expression, activation and effects on function. Exp. Eye Res. 2009;88:458–466. doi: 10.1016/j.exer.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Junghans B, Crewther S, Liang H, Crewther D. A role for choroidal lymphatics during recovery from form deprivation myopia? Optom. Vis. Sci. 1999;76:796–803. doi: 10.1097/00006324-199911000-00028. [DOI] [PubMed] [Google Scholar]
- Junghans BM, Crewther SG, Crewther DP. Choroidal lympthatics: An active storage reservoir? Invest. Ophthalmol. Vis. Sci. 1998;39:S504. [Google Scholar]
- Kajikawa J. Beiträge zur Anatomie und Physiologie des Vogelauges. Albrecht von Graefes Arch Ophthalmol. 1923;112:260–346. [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest. Ophthalmol. Vis. Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL. Temporal constraints on experimental emmetropization in infant monkeys. Invest. Ophthalmol. Vis. Sci. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RH. Progression of myopia. Trans. Am. Ophthalmol. Soc. 1995;93:755–800. [PMC free article] [PubMed] [Google Scholar]
- Kennedy RH, Dyer JA, Kennedy MA, Parulkar S, Kurland LT, Herman DC, McIntire D, Jacobs D, Luepker RV. Reducing the progression of myopia with atropine: a long term cohort study of Olmsted County students. Binocul. Vis. Strabismus Q. 2000;15:281–304. [PubMed] [Google Scholar]
- Kiel JW, Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest. Ophthalmol. Vis. Sci. 1992;33:2399–2410. [PubMed] [Google Scholar]
- Kirby ML, Diab IM, Mattio TG. Development of adrenergic innervation of the iris and fluorescent ganglion cells in the choroid of the chick eye. Anat. Rec. 1978;191:311–320. doi: 10.1002/ar.1091910304. [DOI] [PubMed] [Google Scholar]
- Krause WJ, Cutts JH. Concise Text of Histology. Williams and Wilkens; Baltimore: 1981. [Google Scholar]
- Krebs W, Krebs I. Primate Retina and Choroid: Atlas of Fine Structure in Man and Monkey. Springer-Verlag; New York: 1991. [Google Scholar]
- Krebs W, Krebs IP. Ultrastructural evidence for lymphatic capillaries in the primate retina. Arch Ophthal. 1988;106:1615–1616. doi: 10.1001/archopht.1988.01060140783055. [DOI] [PubMed] [Google Scholar]
- Kusakari T, Sato T, Tokoro T. Visual deprivation stimulates the exchange of the fibrous sclera into the cartilaginous sclera in chicks. Exp. Eye Res. 2001;73:533–546. doi: 10.1006/exer.2001.1064. [DOI] [PubMed] [Google Scholar]
- Leech EM, Cottriall CL, McBrien N. Pirenzapine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol. Opt. 1995;15:351–356. [PubMed] [Google Scholar]
- Li XX, Schaeffel F, Kohler K, Zrenner E. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis. Neurosci. 1992;9:483–492. doi: 10.1017/s0952523800011287. [DOI] [PubMed] [Google Scholar]
- Liang H, Crewther S, Crewther D, Junghans B. Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest. Ophthalmol. Vis. Sci. 2004;45:2463–2474. doi: 10.1167/iovs.03-1009. [DOI] [PubMed] [Google Scholar]
- Lin LL. Epidemiological study of ocular refractions among school children (aged 6 through 18) in Taiwan. Invest. Ophthalmol. Vis. Sci. 1996;37:S1002. [Google Scholar]
- Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest. Ophthalmol. Vis. Sci. 1998;39:2217–2231. [PubMed] [Google Scholar]
- Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxia. J. Gen. Physiol. 1992;99:177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Goldstick TK, Blum RS, Enroth-Cugell C. Estimation of retinal oxygen transients from measurments made in the vitreous humor. Exp. Eye Res. 1981;32:369–379. doi: 10.1016/s0014-4835(81)80016-4. [DOI] [PubMed] [Google Scholar]
- Liu O, Wu J, Wang X, Zeng J. Changes in the muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs. Mol. Vis. 2007;13:1234–1244. [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Riva C, Petrig BL, Geiser M. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest. Ophthalmol. Vis. Sci. 2003;44:2126–2132. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- Luft W, Ming Y, Stell W. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest. Ophthalmol. Vis. Sci. 2003;44:1330–1338. doi: 10.1167/iovs.02-0796. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest. Ophthalmol. Vis. Sci. 1993;34:477–487. [PubMed] [Google Scholar]
- Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Abdul-Rahim AS, Cohen SD. the effect of metabolic inhibitors on retinal adhesion and subretinal fluid reabsorbtion. Invest. Ophthalmol. Vis. Sci. 1980;19:893–903. [PubMed] [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- May CA. Nonvascular smooth muscle alpha-actin positive cells in the choroid of higher primates. Curr. Eye Res. 2003;1:1–6. doi: 10.1076/ceyr.27.2.1.15459. [DOI] [PubMed] [Google Scholar]
- May CA. Non-vascular smooth muscle cells in the human choroid: distribution, development and further characterization. J. Anat. 2005;207:381–390. doi: 10.1111/j.1469-7580.2005.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CA, Hayreh S, Furuyoshi N, Ossoinig K, Kaufman PL, Lutjen-Drecoll E. Choroidal ganglion cell plexus and retinal vasculature in monkeys with laser-induced glaucoma. Ophthalmologica. 1997;211:161–171. doi: 10.1159/000310784. [DOI] [PubMed] [Google Scholar]
- May CA, Neuhuber W, Lutjen-Drecoll E. Immunohistochemical classification and functional morphology of human choroidal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2004;45:361–367. doi: 10.1167/iovs.03-0624. [DOI] [PubMed] [Google Scholar]
- McBrien N, Lawlor P, Gentle A. Scleral remodeling druing the development of and recovery from axial myopia in the tree shrew. Invest. Ophthalmol. Vis. Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- McBrien N, Sahebjada S, Arumugam B, Chow A, Jobling AI, Gentle A. Role of M4 receptor antagonist in control of myopic eye growth. Invest. Ophthalmol. Vis. Sci. 2009 E-Abstract #3850. [Google Scholar]
- McBrien NA, Moghaddam HO, New R, Williams LR. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J. Physiol. 1993a;469:427–41. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest. Ophthal. Vis. Sci. 1993b;34:205–215. [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan I. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp. Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- McFadden S, Howlett M, Mertz J. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development: Experimental studies of chronic atropinization in tree shrews. Doc. Ophthalmol. 1981;28:187–192. [Google Scholar]
- McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Succu S, Argiolas A. Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus: correlation with penile erection and yawning. Eur. J. Neurosci. 1996;8:2056–2063. doi: 10.1111/j.1460-9568.1996.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Meriney S, Pilar G. Cholinergic innervation of the smooth muscle cells in the choroid coat of the chick eye and its development. J. Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JR, Wallman J. Choroidal retinoic acid synthesis: A possible mediator between refractive error and compensatory eye growth. Exp. Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- Miller AS, Coster DJ, Costa M, Furness JB. Vasoactive intestinal polypeptide immunoreactive nerve fibers in the human eye. Aust. J. Ophthalmol. 1983;11:185–193. doi: 10.1111/j.1442-9071.1983.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton T. Modulation of glycosaminoglycan levels in the tree shrew sclera during lens-induced myopia development and recovery. Invest. Ophthalmol. Vis. Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. Ueber glatte Muskeln und Nervengeflechte der chorioidea im menschlichen Auge. Vehr physik-med Ges Wurzburg. 1859;10:179–192. [Google Scholar]
- Nakanome Y, Karita K, Izumi H, Tamai M. Two types of vasodilation in cat choroid elicited by electrical stimulation of the short ciliary nerve. Exp. Eye Res. 1995;60:37–42. doi: 10.1016/s0014-4835(05)80081-8. [DOI] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vis. Res. 1995;35:1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E. Anatomy of the domestic birds. Springer-Verlag; New York: 1977. [Google Scholar]
- Nickla D. The phase relationships between the diurnal rhythm in axial length and choroidal thickness and the association with ocular growth rate in chicks. J. Comp. Physiol. A. 2006;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- Nickla D. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp. Eye Res. 2007;84:951–959. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Nickla D, Damyanova P, Lytle G. Inhibiting the neuronal form of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp. Eye Res. 2009;88:1092–1099. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla D, Mertz J. Inhibiting the recovery from form deprivation myopia pharmacologically results in decreased choroidal all-trans-retinoic acid synthesis. Invest. Ophthalmol. Vis. Sci. 2003 E-Abstract, 2806. [Google Scholar]
- Nickla D, Wildsoet C. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom. Vis. Sci. 2004;81:111–118. doi: 10.1097/00006324-200402000-00009. [DOI] [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: Implications for ocular growth regulation. Invest. Ophthalmol. Vis. Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest. Ophthalmol. Vis. Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- Nickla D, Wilken E, Lytle G, Yom S, Mertz J. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor L-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp. Eye Res. 2006;83:456–464. doi: 10.1016/j.exer.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Cheng AT. Atropine and Apomorphine in Emmetropization: Evidence for Inhibitory Interactions on a Common Growth Regulatory Pathway. Invest. Ophthalmol. Vis. Sci. 2009 E-Abstract 3848. [Google Scholar]
- Nickla DL, Rada JA, Wallman J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J. Comp. Physiol. [A] 1999;185:81–90. doi: 10.1007/s003590050368. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr. Eye Res. 1997;16:320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp. Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest. Ophthalmol. Vis. Sci. 1996;37:2110–2119. [PubMed] [Google Scholar]
- Nishi R. Target-derived molecules that influence the development of neurons in the avian ciliary ganglion. J. Neurobiol. 1994;25:612–619. doi: 10.1002/neu.480250604. [DOI] [PubMed] [Google Scholar]
- Norton T. Animal models of myopia: Learning how vision controls the size of the eye. Instit. Lab. Animal Res. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton T, Rada J. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp. Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- Park T, Winawer J, Wallman J. Further evidence that chicks use the sign of blur in spectacle lens compensation. Vision Res. 2003;43:1519–1531. doi: 10.1016/s0042-6989(03)00180-9. [DOI] [PubMed] [Google Scholar]
- Parver LM. Temperature modulating action of choroidal blood flow. Eye. 1991;5:181–185. doi: 10.1038/eye.1991.32. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J. Ophthalmol. 1980;89:641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO. The stabilizing effect of the choroidal circulation on the temperature environment of the macula. Retina. 1982;2:117–120. doi: 10.1097/00006982-198200220-00008. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO. Choroidal blood flow III. Reflexive control in human eyes. Arch Ophthal. 1983;101:1604–1606. doi: 10.1001/archopht.1983.01040020606021. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO, Doyle T. Choroidal blood flow II. Reflexive control in the monkey. Arch Ophthal. 1982;100:1327–1330. doi: 10.1001/archopht.1982.01030040305021. [DOI] [PubMed] [Google Scholar]
- Pendrak K, Nguyen T, Lin T, Capehart C, Zhu XS, Stone RA. Retinal dopamine in the recovery from experimental myopia. Curr. Eye Res. 1997;16:152–157. doi: 10.1076/ceyr.16.2.152.5090. [DOI] [PubMed] [Google Scholar]
- Pendrak K, Papastergiou GI, Lin T, Laties AM, Grimes PA, Stone RA. Origin of fluid in chick choroidal lacunae. Invest. Ophthalmol. Vis. Sci. 1998;39:S504. [Google Scholar]
- Pendrak K, Papastergiou GI, Lin T, Laties AM, Stone RA. Choroidal vascular permeability in visually regulated eye growth. Exp. Eye Res. 2000;70:629–637. doi: 10.1006/exer.2000.0825. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD, Wallman J, Wildsoet C. Saccadic oscillations facilitate ocular perfusion from the avian pecten. Nature. 1990;343:362–363. doi: 10.1038/343362a0. [DOI] [PubMed] [Google Scholar]
- Polska E, Simader C, Weigert G, Doelemeyer A, Kolodiaschna J, Scharmann O, Schmetterer L. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest. Ophthalmol. Vis. Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest. Ophthalmol. Vis. Sci. 1998;39:1765–1774. [PubMed] [Google Scholar]
- Rada J, Huang Y, Rada K. Identification of choroidal ovotransferrin as a potential ocular growth regulator. Curr. Eye Res. 2001;22:121–132. doi: 10.1076/ceyr.22.2.121.5525. [DOI] [PubMed] [Google Scholar]
- Rada J, Wiechmann A. Ocular expression of avian thymic hormone: changes during the recovery from induced myopia. Mol. Vis. 2009;15:778–792. [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Matthews AL. Visual deprivation upregulates extracellular matrix synthesis by chick scleral chondrocytes. Invest. Ophthalmol. Vis. Sci. 1994;35:2436–2447. [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr. Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest. Ophthalmol. Vis. Sci. 2000;41:2050–2058. [PubMed] [Google Scholar]
- Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Developmental Biology. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PGH, de Jong P. Morphometric analysis of Bruch's membrane, the choriocapillaris and the choroid in aging. Invest. Ophthalmol. Vis. Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. An animal model of myopia. New England Journal of Medicine. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- Reiner A. The presence of substance P/CGRP-containing nerve fibers, VIP-containing fibers and numerous cholinergic fibers on blood vessels of the avian choroid. Invest. Ophthalmol. Vis. Sci. 1987;28:S81. [Google Scholar]
- Reiner A, Erichsen J, Cabot JB, Evinger C, Fitzgerald M, Karten HJ. Neurotransmitter organization of Edinger-Westphal and its projection to the avian ciliary ganglion. Visual Neurosci. 1991;6:451–472. doi: 10.1017/s0952523800001310. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ, Gamlin PDR, Erichsen JT. Parasympathetic ocular control: Functional subdivisions and circuitry of the avian nucleus of Edinger-Westphal. Trends Neurosci. 1983;6:140–145. [Google Scholar]
- Reiner A, Zagvazdin Y, Fitzgerald M. Choroidal blood flow in pigeons compensates for decreases in arterial blood pressure. Exp. Eye Res. 2003;76:273–282. doi: 10.1016/s0014-4835(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Remington LA. Clinical Anatomy of the Visual System. 2nd Ed. Elsevier; 2005. [Google Scholar]
- Riva C, Titze T, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest. Ophthalmol. Vis. Sci. 1997;38:2338–2343. [PubMed] [Google Scholar]
- Riva C, Titze T, Hero M, Petrig BL. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Invest. Ophthalmol. Vis. Sci. 1997;38:1752–1760. [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Visual Neuroscience. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Stell WK. Basic Fibroblast Growth-Factor (Bfgf) and Transforming Growth-Factor-Beta (TGF-Beta) Act as Stop and Go Signals to Modulate Postnatal Ocular Growth in the Chick. Exp. Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Cone signals for spectacle lens compensation: differential responses to short and long wavelengths. Vision Res. 2008;48:1980–1991. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp. Eye Res. 1971;12:166–172. doi: 10.1016/0014-4835(71)90085-6. [DOI] [PubMed] [Google Scholar]
- Rymer J, WIldsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: A review. Visual Neurosci. 2005;22:251–261. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35:1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K. 6-hydoxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis. Neurosci. 1990;4:177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- Schmid K, Wildsoet CF. Effects of the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Invest. Ophthalmol. Vis. Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Schrödl F. Intrinsic choroidal neurons. In: Troger J, Kieselbach G, Gechrakis N, editors. Neuropeptides in the Eye. Research Signpost; Kerala: 2009. pp. 169–197. [Google Scholar]
- Schrödl F, De Laet A, Tassignon MJ, Van Bogaert P, Brehmer A, Neuhuber W, Timmermans JP. Intrinsic choroidal neurons in the human eye: Projections, targets, and basic electrophysiological data. Invest. Ophthalmol. Vis. Sci. 2003;44:3705–3712. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- Schrödl F, Brehmer A, Neuhuber W, Kruse FE, May CA, Cursiefen C. The normal human choroid is endowed with a significant number of lymphatic vessel endoethelial hyaluronate receptor 1(LYVE-1)-positive macrophages. Invest. Ophthalmol. Vis. Sci. 2008;49:5222–5229. doi: 10.1167/iovs.08-1721. [DOI] [PubMed] [Google Scholar]
- Schrödl F, Bergua A, Brehmer A, Neuhuber WL. Nonvascular smooth muscle as a main target for intrinsic choroid neurons in duck. Exp. Eye Res. 1998;67:78. [Google Scholar]
- Schrödl F, Brehmer A, Neuhuber W. Intrinsic choroidal neurons in the duck eye express galanin. J. Comp. Neurol. 2000;425:24–33. [PubMed] [Google Scholar]
- Schrödl F, Schweigert M, Brehmer A, Neuhuber WL. Intrinsic neurons in the duck choroid are contacted by CGRP-immunoreactive nerve fibers: Evidence for a local pre-central reflex arc in the eye. Exp. Eye Res. 2001a;72:137–146. doi: 10.1006/exer.2000.0940. [DOI] [PubMed] [Google Scholar]
- Schrödl F, Tines R, Brehmer A, Neuhuber W. Intrinsic choroidal neurons in the duck eye receive sympathetic input: anatomical evidence for adrenergic modulation of nitrergic functions in the choroid. Cell Tissue Res. 2001b;304:175–184. doi: 10.1007/s004410100362. [DOI] [PubMed] [Google Scholar]
- Schrödl F, De Stefano M, Reese S, Brehmer A, Neuhuber W. Comparative anatomy of nitrergic intrinsic choroidal neurons (ICN) in various avian species. Exp. Eye Res. 2004;78:187–196. doi: 10.1016/j.exer.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Schrödl F, Brehmer A, Neuhuber W, Nickla D. The autonomic facial nerve pathway in birds: a tracing study. Invest. Ophthalmol. Vis. Sci. 2006;47:3225–3233. doi: 10.1167/iovs.05-1279. [DOI] [PubMed] [Google Scholar]
- Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis. Neurosci. 2000;17:165–176. doi: 10.1017/s0952523800171184. [DOI] [PubMed] [Google Scholar]
- Seko Y, Shimokawa H, Tokoro T. Expression of bFGF and TGF-B2 in experimental myopia in chicks. Invest. Ophthalmol. Vis. Sci. 1995;36:1183–1187. [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton T. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom. Vis. Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J. Ocular Pharm. Therapeut. 1999;15:85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- Shih YF, Fitzgerald M, Cuthbertson SL, Reiner A. Influence of opthalmic nerve fibers on choroidal blood flow and myopic eye growth in chicks. Exp. Eye Res. 1999;69:9–20. doi: 10.1006/exer.1999.0692. [DOI] [PubMed] [Google Scholar]
- Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFB-2, RALD-2, and ZENK following imposed positive and negative defocus in chickens. Mol. Vis. 2004;10:588–597. [PubMed] [Google Scholar]
- Smalley DM, Fitzgerald JE, Taylor DM, O'Rourke J. Tissue plasminogen activator in human aqueous humor. Invest. Ophthalmol. Vis. Sci. 1994;35:48–53. [PubMed] [Google Scholar]
- Snyder SH, Bredt D. Biological roles of nitric oxide. Scient. Amer. 1992;266:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch. Ophthalmol. 1983;101:405–407. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Survey of Ophthalmol. 1999;44:10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Stewart AG, Phan L, Grigoriadis G. Physiological and pathophysiological roles of nitric oxide. Microsurgery. 1994;15:693–702. doi: 10.1002/micr.1920151006. [DOI] [PubMed] [Google Scholar]
- Stjernschantz J, Alm A, Bill A. Effects of intracranial oculomotor nerve stimulation on ocular blood flow in rabbits: modification by indomethacin. Invest. Ophthalmol. Vis. Sci. 1976;18:99–103. doi: 10.1016/0014-4835(76)90175-5. [DOI] [PubMed] [Google Scholar]
- Stone RA. Substance P-like immunoreactive nerves in the human eye. Arch Ophthalmol. 1985;103:1207–1211. doi: 10.1001/archopht.1985.01050080119031. [DOI] [PubMed] [Google Scholar]
- Stone RA. Vasoactive intestinal polypeptide and the ocular innervation. Invest. Ophthalmol. Vis. Sci. 1986;27:951–957. [PubMed] [Google Scholar]
- Stone RA, Kuwayama Y, Laties AM. Regulatory peptides in the eye. Experentia. 1987;43:791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp. Eye Res. 1991;52:755–7588. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc. Nat. Acad. Sci. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, McGlinn AM. Calcitonin gene-related peptide immunoreactive nerves in human and rhesus monkey eyes. Invest. Ophthalmol. Vis. Sci. 1988;29:305–310. [PubMed] [Google Scholar]
- Stone RA, Quinn GE, Francis EL, Ying G, Flitcroft DI, Parekh P, Brown J, Orlow J, Schmid G. Diurnal axial length fluctuations in human eyes. Invest. Ophthalmol. Vis. Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- Sugita A, Inokuchi T. Lymphatic sinus-like structures in choroid. Jpn. J. Ophthalmol. 1992;36:436–442. [PubMed] [Google Scholar]
- Summers Rada J, Folger RH. Biphasic scleral response during the recovery form induced myopia: Is the choroid involved? Invest. Ophthalmol. Vis. Sci. 2009 E-Abstact 3930. [Google Scholar]
- Summers Rada J, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest. Ophthalmol. Vis. Sci. 2007;48:2957–2966. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]
- Sun W, Erichsen JT, May P. NADPH-diaphorase reactivity in ciliary ganglion neurons: a comparison of distributions in the pigeon, cat and monkey. Vis. Neurosci. 1994;11:1–5. doi: 10.1017/s0952523800003965. [DOI] [PubMed] [Google Scholar]
- Tian Y, Wildsoet CF. Diurnal fluctuations and developmental changes in ocular dimensions and optical aberrations in young chicks. Invest. Ophthalmol. Vis. Sci. 2006;47:4168–4178. doi: 10.1167/iovs.05-1211. [DOI] [PubMed] [Google Scholar]
- Tigges M, Iuvone PM, Fernandes A, Sugrue M, Mallorga P, Laties AM, Stone R. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis. Sci. 1999;76:397–407. doi: 10.1097/00006324-199906000-00020. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Mertz J, Summers-Rada J. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest. Ophthalmol. VIs. Sci. 2006;47:1767–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla D, Wildsoet C. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest. Ophthalmol. Vis. Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- Uddman RJ, Alumets B, Ehinger R, Hakanson I, Loren I, Sundler F. Vasoactive intestinal peptide nerves in ocular and orbital structures of the cat. Invest. Ophthalmol. Vis. Sci. 1980;19:878–885. [PubMed] [Google Scholar]
- van Alphen GWHM. Choroidal stress and emmetropization. Vision Res. 1986;26:723–734. doi: 10.1016/0042-6989(86)90086-6. [DOI] [PubMed] [Google Scholar]
- van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J. Cell Biol. 1996;134:401–411. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajer SD, Taomoto M, McLeod DS, McCally RL, Nishiwaki H, Fabry RL, Lutty GA. Velocity measurements of normal and sickle cell red blood cells in the rat retinal and choroidal vasculatures. Microvasc. Res. 2000;60:281–293. doi: 10.1006/mvre.2000.2270. [DOI] [PubMed] [Google Scholar]
- Walcott B, Sibony PA, Keyser KT. Neuropeptides and the innervation of the avian lacrimal gland. Invest. Ophthalmol. Vis. Sci. 1989;30:1666–1674. [PubMed] [Google Scholar]
- Wallman J. Retinal Control of Eye Growth and Refraction. In: Osbourne N, Chader G, editors. Progress in Retinal Res.earch. Vol. 12. Pergamon Press; Oxford: 1993. pp. 133–153. [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest changes in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb M, Nickla D, Marran L, Krebs W, Christensen A. Moving the retina: Choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Walls GL. The Vertebrate Eye and its Adaptive Radiations. Bloomfield Hills, Mich.: 1942. [Google Scholar]
- Wang Y, Gilliles C, Cone RE, O'Rourke J. Extravascular secretion of t-PA by the intact superfused choroid. Invest. Ophthalmol. Vis. Sci. 1995;36:1625–1632. [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Watson PG, Young RD. Scleral structure, organization and disease. A review. Exp. Eye Res. 2004;78:609–623. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Wentzek LAF, Bowers CW, Khairallah L, Pilar G. Choroid tissue supports the survival of ciliary ganglion neurons in vitro. J. Neurosci. 1993;13:3143–3154. doi: 10.1523/JNEUROSCI.13-07-03143.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Wingstrand K, Munk O. The pecten oculi of the pigeon with particular regard to its function. Biol. Skr Dan Vid Selsk. 1965;14:1–89. [Google Scholar]
- Wobig JL. The blood vessels and lymphatics of the orbit and lid. In: Wobig JL, Reeh MJ, Wirtschafter JD, editors. Ophthalmic Anatomy. American Academy of Ophthalmology; San Francisco: 1981. p. 77. [Google Scholar]
- Yamamoto R, Bredt DS, Dawson TM, Snyder SH, Stone RA. Enhanced expression of nitric oxide synthase by rat retina following pterygopalatine parasympathetic denervation. Brain Res. 1993;631:83–88. doi: 10.1016/0006-8993(93)91190-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Bredt DS, Snyder SH, Stone RA. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993;54:189–200. doi: 10.1016/0306-4522(93)90393-t. [DOI] [PubMed] [Google Scholar]
- Young FA. The effect of atropine on the developement of myopia in monkeys. Am J Optom and Arch Am Acad Optom. 1965;42:439–449. doi: 10.1097/00006324-196508000-00001. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle S. Oxygen distribution and consumption within the retina in vascularized and avascular retinas and in animal models of retinal disease. Prog. Retinal Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yucel YH, Johnston MG, Ly T, Patel M, Drake B, Gumus E, Fraenkl SA, Moore S, Tobbia D, Armstrong D, Horvath E, Gupta N. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89:810–819. doi: 10.1016/j.exer.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Age-related macular degeneration: review of pathogenesis. Eur. J. Ophthalmol. 1998;8:199–206. doi: 10.1177/112067219800800401. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- Zhu X, Park T, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest. Ophthalmol. Vis. Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest. Ophthalmol. Vis. Sci. 2009;50:37–46. doi: 10.1167/iovs.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]