Abstract
Although numerous studies have examined the relations between stress and memory in children, few studies have investigated physiological responses as predictors of children's memory for stressful events. In this study, 4- to 8-year-olds completed laboratory challenges and experienced a fire-alarm incident while their sympathetic and parasympathetic reactions were monitored. Shortly afterward, children's memory of the alarm incident was tested. As children's age and family income increased, memory performance improved. High sympathetic activation during the laboratory challenges was associated with enhanced memory. Also, a trend indicated that, among older children, greater general parasympathetic withdrawal was associated with poorer memory, but among younger children, parasympathetic withdrawal was unrelated to memory. Findings highlight the need to measure both sympathetic and parasympathetic responses when evaluating children's memory for mild stressors and to include a wide age range so that developmental changes in the relations between stress and memory in childhood can be identified.
Keywords: arousal, children, memory, psysiological reactivity, stress
Introduction
Although considerable attention has been directed toward understanding the connections between cognitive and emotional processes in childhood, much remains to be learned about their precise relations and influences on each other. The present study sought to advance knowledge in this area by investigating, in a controlled laboratory environment, the relations between children's physiological arousal and their memory for a mildly stressful fire-alarm incident. Before discussing the study, we first review previous research concerning the relations between stress and memory in childhood, including explanations that have been put forth to explain variable results across studies. Second, we discuss physiological stress response systems, including how arousal as driven by two of these systems—the sympathetic and parasympathetic systems—might differentially affect children's memory. Third, we present the study's hypotheses.
Stress and Memory
Despite a growing body of research examining children's memory for stressful events, results remain inconsistent. Some studies, for instance, have found positive associations between stress and memory (e.g., Alexander et al., 2002; Goodman, Hirschman, Helps, & Rudy, 1991; Quas & Lench, in press), whereas other studies have found negative associations (e.g., Bahrick, Parker, Fivush, & Levitt, 1998; Merritt, Ornstein, & Spicker, 1994; Peters, 1987). Still other studies have reported no significant or indirect associations (e.g., Fivush, Sales, Goldberg, Bahrick, & Parker, 2004; Howe, Courage, & Peterson, 1995; Vandermaas, Hess, & Baker-Ward, 1993).
Several reasons have been put forth to explain the variable results. One concerns the use of naturally occurring experiences as the to-be-remembered events. Although such events provide insight into how especially high levels of anxiety relate to children's memory, it is difficult to generalize results across studies, given the wide range of events experienced (e.g., inoculations, dentist visits, natural disasters). Also, the time frames associated with the measurement of stress during the events (e.g., distress when an inoculation occurs versus across an entire dental check-up) and the types of information assessed during the memory tests (e.g., face recognition vs. nurses' behavior) have varied. All of these factors could affect the patterns of results obtained. Moreover, in naturalistic settings, the events surrounding a stressor (e.g., parent or doctor behavior) are difficult to control but may exert an important influence on children's stress responses as well as memory for the stressor. Finally, naturalistic settings are often not conducive to obtaining assessments of children's distress as the events unfold. In some studies, stress ratings were made after the events (e.g., Peterson & Bell, 1996; Quas et al., 1999), relying on adults' memory of children's former distress. One alternative is to study children's memory for mildly stressful laboratory events. Although this approach necessarily reduces the absolute levels of arousal children may endure, the control it provides enables a clearer investigation of the mechanisms underlying associations between stress and memory and may result in more consistent findings. The mechanisms identified in the studies can then be tested using other to-be-remembered events to determine the generalizability of findings.
A second explanation for variable results in former studies concerns how distress has been measured. In some studies, observers documented the prevalence of behaviors typically associated with stress (e.g., crying, closing eyes, biting lip) or coping (e.g., talking, diverting attention) (e.g., Alexander et al., 2002; Burgwyn-Bailes, Baker-Ward, Gordon, & Ornstein, 2001; Chen, Zeltzer, Craske, & Katz, 2000; Vandermaas et al., 1993). In other studies, parents or other observers (e.g., nurses, researchers) rated children's stress levels (e.g., Chen et al., 2000; Goodman, Quas, Batterman-Faunce, & Riddlesberger, 1994; Merritt et al., 1994; Peters, 1987). A few studies have obtained children's own reports (e.g., Chen et al., 2000; Merritt et al., 1994; Vandermaas et al., 1993). Although the different ratings provide insight into children's overt or reported distress behaviors, the underlying meaning of the ratings can be difficult to interpret. For instance, measures are often uncorrelated statistically (e.g., Merritt et al., 1994; Quas, Hong, Alkon, & Boyce, 2000; Vandermaas et al., 1993), and, as might be expected due to nonsignificant relations among the measures, the different ratings' associations with memory vary (e.g., Chen et al., 2000; Merritt et al., 1994). Also, with development, children become increasingly able to mask their expression of emotions, including during negative events, and older children may not be as willing as younger children to articulate how they actually feel (Cole, 1986; Zeman & Garber, 1996). Related, children's overt displays of distress during negative experiences (e.g., kicking or crying) generally decrease with age, even though age at times is not related to self-reported distress (Rudolph, Dennig, & Weisz, 1995). Thus, behavioral and self-report indices may not fully capture children's actual experiences of distress. A few studies, described shortly, have included physiological measures (e.g., Bugental, Blue, Cortez, Fleck, & Rodriguez, 1992; Quas, Bauer, & Boyce, 2004). Because such measures do not rely on children's overt behavior or ability or willingness to talk about their feelings, the measures may provide unique insight into children's underlying stress experiences, insight not possible with observational or self-report methods alone.
A third factor that may explain some variable results across studies is that of child age. That is, the associations between stress and memory may vary developmentally, leading to different results in studies comprised of different-age samples. For instance, with age, children are better able to regulate their emotional responses, such as, by using self-initiated active coping strategies rather than by relying on others to help them cope (e.g. Compas & Boyer, 2001; Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001). These age-related changes in coping strategies may affect the magnitude of children's distress responses to their experiences as well as their attention during and later memory for those experiences. Insofar as older children are better than younger children at regulating their arousal during exposure to stress, older children who continue to have difficulty regulating their arousal may have more general problematic coping and regulation capabilities. Such problems could lead to poorer attention and memory for stressful experiences among older children, a possibility consistent with Vandermaas et al. (1993), who found negative relations between stress and memory for a dental visit in older children but no such relations in younger children. In contrast, insofar as young children rely on others to help them cope, young children who experience a novel stressor may become so aroused that they are not able to attend to the event. Lack of attention would lead to subsequently poorer memory, a pattern of results supported by other research (Bugental et al., 1992).
What is needed, therefore, is an investigation of children's memory for mildly stressful laboratory events that includes measures of physiological arousal as the events unfold and includes children across a wide age range, particularly ages across which children's stress experiences and coping behaviors vary substantially.
Stress, Autonomic Arousal, and Memory
When studying physiological stress responses as potential predictors of children's memory, it is important to distinguish between the sympathetic and parasympathetic branches of the autonomic nervous system, both of which are activated to various degrees in response to and following exposure to stress, threat, or challenge. Sympathetic activation is most commonly associated with the “fight or flight” response that results when a person is faced with a stressor external to the self (Cannon, 1914). During sympathetic arousal, pupils dilate, heart rate and blood flow to muscles increase, and metabolic output is produced to prepare an individual for physical activity that may be required (Cannon, 1939; Henry, 1992; Porges, 1995). In response to sympathetic activation, an individual needs to scan the environment, attend to important information, and decide an appropriate response (e.g., in classic terms, whether to remain and “fight” or depart and “flight”). Theoretically, attention should be focused on information related to the cause of the stress so that the situation and threat level can be evaluated and an action can be made (Christianson, 1992). Accordingly, sympathetic arousal during a to-be-remembered event should facilitate encoding and later memory for the event, at least for event-information directly related to the cause of the stress.
The parasympathetic system regulates internal and external demands on the body by either increasing parasympathetic activation (often called parasympathetic tone) or decreasing parasympathetic activation (often called parasympathetic withdrawal) (e.g., Berntson, Cacioppo, & Quigley, 1991; Porges, 1995; Salomon, Matthews, & Allen, 2000). When no external demands (e.g., stressors) are present, activation of the parasympathetic system helps promote growth in the body by maintaining a steady and decelerated heart rate. When an external demand presents itself, the parasympathetic system retains some internal regulation processes, but also withdraws its regulatory influence (e.g., on the cardiac cycle), increasing vigilance and arousal and allowing the individual to respond to the demand (Calkins & Dedmon, 2000). In fact, several studies indicate that parasympathetic withdrawal (increased arousal) compared to baseline is associated with increased vigilance during sustained attention tasks (Linnemeyer & Porges, 1986; Porges, 1992; Ruff & Rothbart, 1996; Suess, Porges, & Plude, 1994; Weber, Van der Molen, & Molenaar, 1994). Insofar as sustained attention tasks are similar to stressful events, parasympathetic withdrawal may enhance memory because of its positive association with sustained attention.
Other studies, however, suggest the opposite pattern, namely that parasympathetic withdrawal should be associated with poorer memory. Specifically, children who exhibit consistently high levels of parasympathetic withdrawal in laboratory contexts are at increased risk for internalizing behavior problems and extreme social and behavior inhibition (e.g., Boyce et al., 2001; Reznick et al., 1986). Behavioral problems are often related to difficulty with emotion regulation, especially during potentially arousing situations. Thus, parasympathetic withdrawal may lead to reduced attention to stressful environmental stimuli as a means of emotional self-regulation. Theoretically this reduced attention would also result in subsequently poor memory.
Despite reasons to expect that the activation of the sympathetic system and withdrawal of the parasympathetic system have different implications for memory, few studies have systematically examined these possibilities in children. Several studies have, however, examined relations between children's physiological arousal (primarily heart rate) and memory. Peters (1991), for example, investigated children's memory for events that took place when either a fire alarm or a radio sounded. Children in the fire-alarm, or “stressed,” group had significantly higher pulse rates than children in the radio, or “non-stressed,” group and later provided fewer correct and more incorrect statements about their experience. Bugental et al. (1992) found that 5-year-olds who exhibited increased heart rate while watching a video of a child receiving an inoculation made more mistakes when recalling the video than did 5-year-olds who did not exhibit an increase in heart rate. (No significant associations were found with older children, however). Chen et al. (2000) failed to uncover any significant associations between children's heart rate while enduring lumbar punctures and their later memory. Finally, both Stein and Boyce (1995) and Quas et al. (2004) compared children's general physiological response proclivities to their memory for a fire-alarm incident. Stein and Boyce found that children with consistently high heart rates during a series of laboratory tasks reported the least amount of information about the alarm. Quas et al. (2004) also found that children with consistently high levels of parasympathetic withdrawal during a similar set of laboratory tasks displayed poorer memory than children with lower levels of parasympathetic withdrawal. High parasympathetic withdrawal is often strongly related to high heart rate.
Despite these studies being suggestive of some associations between physiological arousal and memory, numerous questions remain. First, and perhaps most important, most prior studies only measured heart rate as an index of arousal. Heart rate is affected by both the sympathetic and parasympathetic systems (e.g., Cacioppo, Tassinary, & Berntson, 2000; Salomon et al., 2000), and as mentioned, the sympathetic and parasympathetic systems' stress responses may have different implications for memory. Thus, it is imperative to examine how each system uniquely predicts children's stress reaction and later memory. One study to date has included measures of both systems (Quas et al., 2004). Results revealed a significant relation between parasympathetic withdrawal and children's memory, but no relation between sympathetic activation and memory. However, children's stress responses were assessed across a series of laboratory tasks, and the age range was restricted, thus conclusions are tentative without further study.
Second, it is necessary to assess both children's general patterns of physiological reactivity, as conducted by Stein and Boyce (1995) and Quas et al. (2004), and their discrete physiological responses during the stressful event, as conducted by Bugental et al. (1992) and Chen et al. (2000). Consistently high levels of arousal to a range of stressors may be related to large responses to a discrete event (e.g., a fire alarm), but the two types of responses are not identical. Moreover, relatively brief increases in physiological arousal during a stressor, particularly that driven by the sympathetic nervous system, may be positively related to memory, orienting individuals to important environmental information. In contrast, consistently high levels of arousal, particularly that driven by parasympathetic withdrawal, may tax individuals' allostatic load, causing exposure to excessive stress hormones (McEwen, 1998, 2001) and demanding considerable resources, thereby limiting resources otherwise available to attend to and encode event-related information.
Third, as mentioned, age may affect the magnitude of children's stress responses, for instance, because of age-related improvements in children's ability to cope with challenge and stress (e.g., Brown, O'Keeffe, Sanders, & Baker, 1986; Compas, 1998; Compas et al., 2001; Rudolph, Dennig, & Weisz, 1995). Age may also affect the extent to which arousal is related to memory (Bugental et al., 1992), with such associations emerging in some age groups (e.g., older children) but not others (Vandermaas et al., 1993). For instance, younger children lack the ability to engage in sophisticated coping strategies without assistance from someone else. For them, arousal may constitute a normal developmental reaction to even mildly stressful experiences. However, with age, children increasingly engage in primary coping strategies to reduce their arousal (see Fields & Prinz, 1997). Accordingly, among older children, large stress responses may be indicative of problematic coping (e.g., the use of avoidant strategies) or poor emotion regulation capabilities, both of which have implications for children's ability to attend to and remember stressful information. By including a wide age range (e.g., children ranging from preschool to school age), it will be possible to determine how associations between children's physiological stress responses and memory change developmentally.
Present Study
In the present study, we examined the relations between 4- to 8-year-olds' physiological reactivity and memory for a fire-alarm incident. Children came to a laboratory and completed a series of mildly stressful laboratory tasks and experienced a fire alarm. Shortly afterward, a researcher asked children what happened. During the laboratory challenges and fire alarm, children's sympathetic and parasympathetic responses were monitored, allowing us to test how children's reactions both to a specific, discrete to-be-remembered event (i.e., the alarm) and across a range of other challenges (i.e., the laboratory tasks) related to their memory of the alarm.
For obvious ethical reasons, the laboratory tasks, including the fire alarm, were mildly (rather than extremely) arousing to children. Nonetheless, studies reveal substantial individual differences in children's reactions to these stressors. These differences have implications for a range of health and behavioral outcomes (e.g., Boyce et al., 2001; Monk et al., 2001; see Gunnar, Bruce, & Donzella, 2001) and, as tested here, possibly memory outcomes.
Based on the aforementioned literature, several hypotheses were advanced. First, children's physiological responses both during the alarm and other laboratory challenges were expected to decrease with age. Specifically, older children were hypothesized to be less likely to be aroused in response to the alarm due to prior exposure. Moreover, because older children have more sophisticated self-regulation coping strategies (Compas, 1998), they were expected to be better able to control their arousal or at least return to baseline levels afterward more quickly than younger children (Alkon et al., 2003). Second, increases in sympathetic “fight” or ‘“flight” responses should direct attention to information associated with the stressor (Christianson, 1992). Accordingly, sympathetic arousal should be positively related to children's memory. Third, although some studies suggest that parasympathetic withdrawal (greater arousal) is associated with sustained attention, high parasympathetic withdrawal during the laboratory challenges was expected to be associated with poorer memory, consistent with Quas et al. (2004) and with studies revealing positive relations between high parasympathetic withdrawal or reactivity and poor emotion regulation abilities (Beauchaine, 2001). When exposed to stress, chronically reactive children may need to attend to their own emotional reaction at the expense of attending to and encoding external information (Yuille & Tollestrup, 1992).
Fourth, two alternative hypotheses were examined regarding age-related changes in the relations between arousal and memory. On the one hand, consistent with Bugental et al.'s (1992) results, negative associations between arousal (particularly that driven by the parasympathetic system) and memory may be stronger in young than older children because of younger children's more limited coping abilities (e.g., see Band & Weisz, 1988; Compas, 1998). On the other hand, however, insofar as older children have more experience with fire alarms and are less aroused by them, older children who are particularly aroused may be those who are shy, inhibited, and have difficulty with self-regulation, leading to these children's greater difficulty attending to the stressor. As such, the negative relation between parasympathetically driven arousal and memory would be greater in the older than younger children (Vandermaas et al., 1993).
Method
Participants
One hundred and six children (58 boys and 48 girls), aged 4- to 8-years (M = 6.30), served as participants. This age range was selected to be comparable to the ages included in several prior studies of physiological stress reactivity and memory in children (Quas et al., 2004; Vandermaas et al., 1993) and for theoretical reasons concerning likely differences across this age range in children's physiological reactions to novel, laboratory challenges (Alkon et al., 2003; see Alpert & Wilson, 1992). Finally, the protocol employed in the study has been standardized for use with children within the study's age range (e.g., Alkon et al., 2003; Boyce et al., 2001).
Children were from diverse backgrounds: 52% were European American, 10% were African American, 10% were Asian American, 6% were Latino, and 20% were of mixed ethnicity. Three-fourths of the parents had at least a college education. Families were recruited from flyers in newspapers, at local childcare facilities, and by word of mouth. At the time of participation, none of the children had any chronic diseases, acute respiratory illnesses or mental health problems, or were on medication.
Due to equipment failure or noise in physiological data, or time constraints that precluded us from conducting the memory interview, the final sample ns ranged from 77 to 106 across the study measures. For the physiological data, children were most often missing pre-ejection period (PEP) data, ns ranged from 77 to 98, rather than respiratory sinus arrhythmia (RSA) data, ns ranged from 95 to 103 (RSA data collection requires fewer electrodes, so noise and movement artifact is less likely to interfere). Eleven children are did not complete the memory interview.
Questionnaires, Materials, and Procedures
Parents were contacted by phone, and their child was screened for eligibility (e.g., health status). The families were taking part in a larger study concerning physiological arousal and behavior in childhood (e.g., Alkon et al., 2003). When families arrived, the study was described in detail to parents and their consent was secured. Parents were given a demographic questionnaire, which concerned the child's family background (e.g., parents' income and education, and child's ethnicity), and other questionnaires regarding their child (e.g., health). None of the latter questionnaires is relevant to the present study or is discussed further.
While parents completed the questionnaires, the child took part in the laboratory challenges, fire-alarm incident, and memory interview. The entire session was conducted by one of four female research assistants (RAs), who were either hired staff or doctoral students with substantial experience working with children and administering cognitive and social tasks. They were trained to administer the protocol by one of the study's lead researchers. First, the RA explained the study in developmentally appropriate language to the child and obtained her/his assent to participate. The RA then brought the child into the laboratory room and showed the child the physiological monitoring equipment. Once the child was comfortable, the RA placed four spot electrodes on the child's right clavicle, right lower abdomen, left rib, and left lower abdomen (for the ground) to obtain electrocardiograph (ECG) data, used in the computation of children's parasympathetic reactions. She placed four additional electrodes on the child—two at the suprasternal notch and xiphoid process, and two slightly below the hairline and on the lumbar vertebra—to obtain impedance data and compute children's sympathetic reactions.
Once the equipment was in position, the RA administered a 15-min standardized protocol (termed “reactivity protocol;” Alkon et al., 2003; Boyce et al., 2001). The protocol was developed to assess physiological stress responses in children ages 4 to 8 years. The protocol has been used successfully in a number of studies and is effective at identifying individual differences in children's physiological response proclivities (Alkon et al., 2003; Boyce, Chesney, Alkon, & Tschann, 1995). The tasks include cognitive, social, and emotional challenges. Although not all children show exaggerated stress responses to all of the tasks, by including the range of tasks, the protocol allows for identification of children prone to high, prolonged arousal during novel, potential stressors and hence who may be at risk for physical and mental health problems (Boyce et al., 2001).
The protocol began with a female researcher reading the child a neutral story, included to allow time for the child to become familiar with the equipment and procedures. The child then completed four laboratory challenges, each of which lasted between 1 and 3 min. For the first task, the child answered questions from the social interview of the Gesell School Readiness Screening Test (Carlson, 1985). Questions concerned the child, and his/her family and school (e.g., “How many brothers or sisters do you have?” What do you like to play at school?”). Similar social interviews have been administered to identify individual differences in physiological reactivity in children and adolescents (e.g., Salomon et al., 2000). For the second task, the child completed a digit recall test adapted from the Kaufman Assessment Battery for Children (Kaufman & Kaufman, 1983), which requires the child repeat increasingly difficult sets of digits. Unlike the standardized battery administration, however, children were not given feedback, and the task ended when children made two consecutive errors. The child was then asked to sit quietly and rest for 1 min. The third task, a sensory/perceptual stressor, consisted of the child identifying an unknown substance (lemon juice) placed on the anterior of the tongue (Kagan & Snidman, 1991). For the fourth task, the child watched two brief emotionally evocative video clips (Eisenberg et al., 1988). The first clip was designed to elicit fear and depicted a boy frightened by a thunderstorm. The second clip was designed to elicit sadness and depicted a girl saddened by the death of her pet bird. Immediately following the videos, the researcher put the television monitor and headphones away, allowing a short amount of time to elapse before a second neutral story was read. After the story, a short break took place.
Next, the fire-alarm incident took place, based on a procedure developed by Stein and Boyce (1995). The researcher explained to the child that they were going to make hot chocolate. The researcher then followed a scripted protocol for preparing the hot chocolate. Once steam visibly appeared from a nearby kettle, a fire alarm sounded. The researcher acted surprised and stated that the fire alarm was sounding. The researcher explained that there was no fire; instead, steam from the kettle set off the alarm. The alarm sounded for approximately 20 s, during which the researcher engaged in various behaviors (e.g., waving hands to dissipate smoke) to stop the alarm. When the alarm stopped, the researcher finished making the hot chocolate.
As the hot chocolate cooled, the researcher told the child that, in order to make hot chocolate with other children, she needed the child to help her remember the events that took place. The researcher then administered the fire-alarm interview. It began with the researcher asking open-ended prompts to elicit narrative information from the child regarding what happened (e.g., “When I started making the hot chocolate, what was the first thing that I did?” “Then what happened?”). Next, she asked follow-up short-answer or yes/no questions that probed for details concerning the events that were involved in making hot chocolate; asked about what took place before, during, and after the alarm (e.g., “What made the fire alarm start making the noise?” “Was there a fire?” “What did I do after the alarm stopped?”); and asked for further clarification when a child was unclear (“What did I do again?”). The number of follow-up questions asked varied depending on the child's initial narrative responses (M = 12 questions, range = 5–21). A majority of the questions required was short answer (M = 9) as opposed to yes/no responses. As would be expected, with age, the number of follow-up questions asked decreased, r = −.42, p < .001. After the interview, the researcher read a final story, and the child was debriefed.
Scoring
Physiological Responses
Measures of PEP and RSA were used as indexes of sympathetic and parasympathetic activity, respectively. EP corresponds to the period of isovolumetric contraction during the cardiac cycle, and RSA reflects heart rate period controlling for respiration. To score PEP and RSA, the analog ECG and impedence data were edited for artifact (see Cacioppo, Uchino, & Berntson, 1994, for a description of the software) by raters trained and reliable on sample cases, r > .90.
For PEP, 1-min epochs were ensemble-averaged, with PEP quantified as the time (ms) between the ECG Q wave (onset of ventricular depolarization) and the B-point of the dZ/dt wave (onset of left ventricular ejection) (Cacioppo et al., 1994). Because sympathetic activation accelerates isovolumetric contractions, lower PEP scores correspond to greater activation or arousal, and higher PEP scores corresponds to lower arousal. RSA was estimated in 1-min epochs using the natural logarithm of the variance of high-frequency heart period within the frequency bandpass associated with respiration. Lower scores represent greater parasympathetic withdrawal (i.e., more arousal), and higher RSA scores reflect greater parasympathetic tone (i.e., less arousal).
Several summary scores were created from the PEP and RSA data (see Tab. 1 for a detailed description). First, baseline scores were computed, separately for PEP and RSA, as the average of children's responses while they listened to the second neutral story. Children's physiological responses during the second rather than first story were included to account for the possibility that children were still acclimating to the equipment during the first story. Paired t-tests revealed that children's PEP scores were significantly higher (indicating less sympathetic arousal) during the second than first story, although their RSA scores were significantly lower (indicating greater parasympathetic withdrawal), ts (96 or 102) ≥3.86, ps < .01. The first and second story scores were highly correlated, however, rs ≥ .86. Thus, there was a high degree of concordance across the stories. As such, the first story PEP scores were included for two children who were missing PEP scores during the second story.
Table 1. Definitions of Physiological and Memory Measures.
| Name of Measure | Definition | Larger Scores |
|---|---|---|
| Physiological scores | ||
| PEP baseline | Sympathetic response score while listening to story | Lower arousal |
| RSA baseline | Parasympathetic response score while listening to story | Lower arousal |
| PEP fire-alarm response | Sympathetic response score during the minute the fire alarm sounded | Lower arousal |
| RSA fire-alarm response | Parasympathetic response score during the minute the fire alarm sounded | Lower arousal |
| PEP fire-alarm difference | Sympathetic arousal during the fire alarm relative to baseline | Smaller stress response |
| RSA fire-alarm difference | Parasympathetic arousal during the fire alarm relative to baseline | Smaller stress response |
| PEP reactivity index | Sympathetic arousal across the laboratory challenges | Greater chronic arousal |
| RSA reactivity index | Parasympathetic arousal across the laboratory challenges | Greater chronic arousal |
| Memory scores | ||
| Free recall proportion | Proportion of features recalled during the initial narratives | Greater recall |
| Total proportion | Proportion of features recalled during the initial narratives and follow-up questions | Greater recall |
Second, PEP and RSA fire-alarm response scores correspond to children's overall arousal during the minute that the fire alarm sounded. Third, because these responses do not capture children's reactions per se to the sounding of the alarm, difference scores were computed by subtracting children's baseline PEP and RSA scores from their PEP and RSA fire-alarm response scores, respectively. The difference scores reflect the magnitude of change in children's arousal when the alarm sounded relative to their baseline arousal. As mentioned, for the baseline and response scores, lower PEP and RSA values indicate greater sympathetic arousal and parasympathetic withdrawal (i.e., arousal), respectively. For the difference scores, smaller values indicate greater arousal (as driven by either sympathetic activation, PEP, or parasympathetic withdrawal, RSA) specifically to the fire-alarm incident.
Finally, two reactivity index scores were created (Table 1). These scores reflect children's general arousal during the entire laboratory protocol (as opposed to their difference scores, which reflect their reactions specifically when the alarm sounded). Boyce et al. (2001) have relied on multiple dimensions to identify children who generally tend towards increased physiological arousal and poor physiological regulation. In the present study, two such indexes, one for PEP and one for RSA, were created according to procedures described by Boyce et al. (2001). Each index included four scores. First was the task mean score, computed by averaging children's minute-by-minute scores within each task and then averaging across the tasks. Smaller scores are associated with greater arousal. Second was a recovery score, computed by subtracting the rest minute (in which children were asked to sit quietly) from their arousal while engaged in the digit recall task, which immediately preceded the rest minute. The recovery scores reflect children's immediate ability to regulate their physiological arousal after a single stressor, and smaller scores indicate better regulation (Althaus et al., 2005). Third, slope scores were derived by regressing the four laboratory tasks on time; the coefficients reflect children's tendency to increase or decrease arousal across the four laboratory tasks. Smaller slope scores indicate greater arousal over time, that is, a lack of ability to self-regulate gradually. Fourth, variability scores were calculated as the within-subject standard deviation across the four laboratory tasks. Because high variability reflects individuals' ability to react to and recover from a challenge, lower scores are indicative of difficulty with self-regulation or greater arousal. The four scores were standardized so that they all were on comparable scales. Then, children's task mean, slope, and variability scores were inversed so that greater values were associated with higher arousal. Finally, these scores were averaged to create PEP and RSA index scores. Higher values indicate greater arousal throughout the laboratory protocol as driven by sympathetic activation (PEP) or parasympathetic withdrawal (RSA).
Memory Responses
The fire-alarm incident was separated into 15 unique features (e.g., stirring in hot chocolate, see Appendix). Two independent raters scored 20% of children's responses (randomly selected across age and gender) for the number of features reported in response to the free-recall and follow-up features. Proportion agreement was .98. Discrepancies were discussed, and one rater scored the remaining data. Proportion scores were computed using methods described in previous research employing similar interview protocols (e.g., Greenhoot, Ornstein, Gordon, & Baker-Ward, 1999; Gordon et al., 1993; Merritt et al., 1994). First, a “free-recall proportion score” was calculated by summing the number of components children described in their initial narratives and dividing by the total number of features possible (15). Second, a “total memory proportion” score was computed to reflect the total proportion of features recalled across the initial free-recall and follow-up questions. Because the number of follow-up questions varied per child, it was not appropriate to compute proportion scores for only children's responses to the follow-up questions.
Eleven children (10%) provided no substantive information about the alarm in free recall and hence received zeros for their free-recall proportion scores. The memory test took place shortly after the event. It was thus not the case that these children failed to encode or completely forgot the incident. Instead, they were unwilling or unable to provide narrative detail when initially asked what happened (they all provided information in response to the follow-up probes). Because of our interest in identifying physiological and developmental predictors of memory performance, these children were still included in the analyses. When analyses were reconducted with the 11 children removed, the direction and magnitude of the results were similar. Also, in part because of the short delay between the fire-alarm incident and the memory interview and because no false suggestions were included in the interview, children rarely provided incorrect information: 58% of the children never provided any incorrect information; 28% provided incorrect information relevant to 1 of the 15 fire-alarm features; 5% provided incorrect information relevant to two features, and one child provided incorrect information relevant to three features (mean proportion incorrect = .04). Incorrect responses are thus not considered further.
Results
First, we conducted preliminary analyses to examine patterns of missing data, investigate the relations among possible confounds, and assess the associations among children's physiological responses. Second, we conducted regression analyses to test our main hypotheses concerning predictors of children's memory. Descriptive data for variables of interest are presented in Table 2.
Table 2. Descriptive Statistics of Variables of Interest in the Study.
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Demographic characteristics | |||
| Children's age | 75.35 | 16.59 | 48–105 |
| Family income | 6.34 | 1.96 | 1–8 |
| Physiological scores | |||
| PEP baseline | 77.89 | 12.11 | 44.67–103.33 |
| RSA baseline | 6.09 | 1.16 | 3.72–9.61 |
| PEP fire-alarm response | 76.17 | 13.24 | 40–104 |
| RSA fire-alarm response | 6.32 | 1.30 | 4.06–9.34 |
| PEP fire-alarm difference | −.97 | 3.67 | −8.00–8.00 |
| RSA fire-alarm difference | .31 | .95 | −2.46–3.51 |
| Protocol PEP reactivity index | −.01 | 1.95 | −4.46–5.35 |
| Protocol RSA reactivity index | −.01 | 2.06 | −4.58–6.08 |
| Memory proportions | |||
| Free recall features recalled | .31 | .16 | .00–.73 |
| Total features recalled | .63 | .14 | .00–.93 |
Note: Family income was scored on an ordinal scale, with 1 = <$10000 and 8 = > $60000. PEP, pre-ejection period and RSA, respiratory sinus arrhythmia. For the PEP and RSA baseline and fire-alarm response scores, smaller values reflect greater absolute levels of responding. The PEP and RSA fire-alarm difference scores were computed by subtracting children's baseline scores from their fire-alarm response scores, with smaller values reflecting greater arousal in response to the fire alarm. For the reactivity indices, larger scores reflect greater reactivity or arousal during the laboratory tasks.
Preliminary Analyses
As mentioned, the final sample ranged from 77 to 106 participants across the different measures due to missing data. t-tests were conduced to determine whether the children who were missing either the physiological or memory data varied systematically from the children who were not missing data. With regard to the physiological measures, comparisons between children with and without PEP data revealed no significant differences in age, RSA data, family income, or memory performance, ts (93) ≤ 1.48. Too few children were missing RSA data to compare them to the remaining children across the other study variables. With regard to the memory measures, children who failed to complete the memory interview (due to time constraints) did not differ from children who completed the memory interview in their age, family income, PEP or RSA baseline, PEP or RSA difference and response scores, or PEP reactivity scores. However, children who did not complete the interview had significantly higher RSA general reactivity scores, M = 1.68, n = 8, than did children who did, M = −.16, n = 95; t (91) = 2.48, p < .05, a point to which we return in the Discussion.
Next, Spearman Rho correlations were computed to investigate whether children's gender or family income was related to their physiological responses and memory. Increases in family income were associated with better memory, as measured via both the free-recall and total proportion scores, rs (93) > .22, ps < .05. No other significant correlations emerged.
Bivariate correlations were computed among the hypothesized predictors of children's memory: children's age, and the sympathetic (PEP) and parasympathetic (RSA) physiological measures (Tab. 3). As can be seen, being older was associated with higher absolute levels of PEP and RSA responses during the minute the fire alarm sounded (i.e., lower arousal). Of note, because children's age was unrelated to their fire-alarm difference scores, the age-related changes in fire-alarm responses are indicative of decreases with age in children's general level of autonomic responses rather than in children's specific reactions to the fire-alarm event. (Age was similarly correlated with children's baseline PEP and RSA, rs > .28, which provides further evidence of age-related changes in general autonomic response levels). Finally, age was negatively associated with children's RSA reactivity index scores, which is suggestive of less parasympathetic withdrawal or lower arousal during the entire set of laboratory challenges.
Table 3. Bivariate Correlations Among the Predictors (dfs Ranged from 76 to 103).
| Child Age | Family Income | Fire-Alarm Response | Fire-Alarm Difference | Reactivity | ||||
|---|---|---|---|---|---|---|---|---|
| PEP | RSA | PEP | RSA | PEP | RSA | |||
| Child Age | — | |||||||
| Family income | .07 | — | ||||||
| Fire-alarm response | ||||||||
| PEP | .46*** | .09 | — | |||||
| RSA | .30** | .03 | .02 | — | ||||
| Fire-alarm difference | ||||||||
| PEP | .10 | .08 | .28** | .07 | — | |||
| RSA | .06 | .07 | .08 | .53*** | .09 | — | ||
| Reactivity index | ||||||||
| PEP | −.08 | .10 | −.47*** | .03 | .14 | .08 | — | |
| RSA | −.28** | −.09 | .08 | −.44*** | −.02 | .01 | −.15 | — |
p < .01.
p < .001.
Note: Family income was scored on an ordinal scale, with 1 = <$10000 and 8 = > $60000. PEP, pre-ejection period and RSA, respiratory sinus arrhythmia. For the PEP and RSA baseline and fire-alarm response scores, smaller values reflect greater absolute levels of responding. The PEP and RSA fire-alarm difference scores were computed by subtracting children's baseline scores from their fire-alarm response scores, with smaller values reflecting greater arousal in response to the fire alarm. For the reactivity indices, larger scores reflect greater reactivity or consistent arousal throughout the laboratory protocol.
Correlations among the physiological variables revealed several significant associations within the sympathetic and parasympathetic systems (Tab. 3). As would be expected, greater reactions to the fire alarm (i.e., fire-alarm difference scores) were associated with greater reactivity across the laboratory challenges. Of interest, no significant correlations emerged between children's sympathetic and parasympathetic responses. Thus, consistent with other studies (e.g., Boyce et al., 1995; Salomon et al., 2000), activation of the different branches of the autonomic nervous system appear to operate largely independently, supporting Cacioppo et al.'s (1994) point, “… that stress-induced changes in PEP and RSA can vary independently” (p. 120).
Finally, we tested whether the fire-alarm incident was arousing to children, as indexed via sympathetic activation or parasympathetic withdrawal. We conducted two paired-sample t-tests comparing children's PEP and RSA responses during the minute that the fire alarm sounded to their baseline PEP and RSA. Results revealed that children were indeed sympathetically aroused during the alarm. Specifically, children's PEP scores during the minute the fire alarm sounded (i.e., their fire alarm response scores) were significantly lower than their PEP scores while listening to the neutral story (i.e., their baseline scores), t (78) = 2.34, p < .05, d = 08 (see Tab. 2). At the same time, however, children exhibited greater parasympathetic tone. Specifically, children's RSA scores during the alarm minute were significantly higher (which is indicative of lower arousal) than their scores while listening to the story, t (94) = −3.15, p < .01, d = .25 (Tab. 2). Thus, at least during the brief exposure to the fire alarm, children exhibited greater sympathetic activation, but also greater parasympathetic tone.
Children's Physiological Reactivity and Memory for the Fire-Alarm Incident
The main goal of our study was to examine the relations between children's physiological arousal and memory for the fire-alarm incident, including the extent to which these relations varied with age. We were interested not only in the associations between children's arousal specifically during the fire-alarm event and their memory, but also the associations between children's more general profile of physiological reactivity across the laboratory challenges and their memory for the discrete alarm event. To address this goal, we conducted a series of linear regressions predicting children's free-recall and total memory proportions. Bivariate correlations between the predictors and children's memory performance are presented in Table 4.
Table 4. Bivariate Correlations Between the Predictor Variables (Age, Family Income, and Physiological Reactions) and Memory (dfs Range from 68 to 92).
| Memory Proportion Scores | ||
|---|---|---|
| Free-Recall | Total | |
| Age | .42** | .62** |
| Family income | .23* | .33** |
| Fire-alarm scores | ||
| PEP difference | .12 | .05 |
| RSA difference | −.04 | .01 |
| Reactivity protocol scores | ||
| PEP reactivity index | .21 | .09 |
| RSA reactivity index | −.11 | −.23* |
p < .05.
p < .01.
Note: Family income was scored on an ordinal scale, with 1 = <$10000 and 8 = > $60000. PEP, pre-ejection period and RSA, respiratory sinus arrhythmia. For the PEP and RSA baseline and fire-alarm response scores, smaller values reflect greater absolute levels of responding. The PEP and RSA fire-alarm difference scores were computed by subtracting children's baseline scores from their fire-alarm response scores, with smaller values reflecting greater arousal in response to the fire alarm. For the reactivity indices, larger scores reflect greater reactivity or consistent arousal throughout the laboratory protocol.
First, however, regressions were conducted to test for curvilinear relations between arousal and memory (Christianson, 1992; Yerkes & Dodson, 1908). Children's fire-alarm difference scores and reactivity index scores, along with the scores' squared terms (which capture curvilinear relations) were entered into regressions along with child age and family income. None of the squared terms emerged as significant predictors. Thus, consistent with findings concerning behavioral distress and memory in children (e.g., Alexander et al., 2002), no significant curvilinear relations emerged between children's sympathetic or parasympathetic arousal and their memory for the alarm, at least when tested shortly after it took place.
Two regressions were conducted to test relations between children's reactions specifically to the fire alarm and their memory. The dependent measures were children's free-recall proportion scores total memory proportion scores. On the first step, children's age, family income, and PEP and RSA fire-alarm difference scores were entered. Children's age and family income were included because of their significant associations with children's memory. On the second step, the age × PEP difference score and the age × RSA difference score interaction terms were entered. The inclusion of the interaction terms enabled us to investigate whether the relations between arousal and memory varied developmentally. All variables were centered on the mean prior to their inclusion according to procedures described by Cohen, Cohen, Aiken, and West (2003). Two children were missing data concerning family income. Mean values were substituted for these children's scores in the regressions.
Both models were significant, overall Fs (6, 62) ≥ 5.23, ps < .001, adjusted R2 = .27 and .49, for free-recall and total memory, respectively. However, neither of the R2Δ was significant at Step 2 when the interaction terms were entered. At Step 1, Fs ≥ 7.94, adjusted R2s = .29 and .49, with children's age and family income emerging as the only significant predictors (Tab. 5). With age, children reported more information about the fire alarm. Increases in family income were associated with better memory. Neither children's sympathetic nor parasympathetic arousal specifically to the fire alarm, directly or in conjunction with age, predicted their memory performance. Thus, stress, as measured via children's physiological reactions to the discrete mildly stressful alarm incident, was unrelated to their memory of that experience.
Table 5. Results of Regressions Predicting Children's Memory from Their Age, Income, and Sympathetic and Parasympathetic Reaction during the Fire Alarm.
| Memory Performance | ||||
|---|---|---|---|---|
| Free Recall | Total Memory | |||
| Model 1 β | Model 2 β | Model 1 β | Model 2 β | |
| Predictors | ||||
| Age | .47*** | .47*** | .61*** | .61*** |
| Family income | .26* | .28* | .34** | .32** |
| Fire-alarm PEP difference | .04 | .02 | −.06 | −.06 |
| Fire-alarm RSA difference | −.12 | −.13 | −.06 | −.07 |
| Age × fire-alarm PEP difference | — | .03 | — | .07 |
| Age × fire-alarm RSA difference | — | .06 | — | .06 |
p < .05.
p < .01.
p < .001.
Note: Family income was scored on an ordinal scale, with 1 = <$10000 and 8 = > $60000. PEP, pre-ejection period and RSA, respiratory sinus arrhythmia. The PEP and RSA fire-alarm difference scores were computed by subtracting children's baseline scores from their fire-alarm response scores, with smaller values reflecting greater arousal in response to the fire alarm.
Next, two regressions examined the relations between children's general reactivity (during the entire laboratory protocol) and their fire alarm memory. In the regressions, children's age, family income, the PEP and RSA reactivity indices (which reflect children's overall arousal, variability, and recovery across the reactivity protocol) were entered first, followed by the two interaction terms—between children's age and each the reactivity indices. Again, all variables were centered on the mean, and the free-recall and total memory scores served as separate dependent measures. Results are displayed in Table 6.
Table 6. Results of Regressions Predicting Children's Memory from Their Age, Income, and General Sympathetic and Parasympathetic Reactivity.
| Memory Performance | ||||
|---|---|---|---|---|
| Free Recall | Total Memory | |||
| Model 1 β | Model 2 β | Model 1 β | Model 2 β | |
| Predictors | ||||
| Age | .45*** | .43*** | .58*** | .56*** |
| Family income | .16 | .17 | .23** | .25** |
| PEP reactivity index | .24** | .25** | .13 | .14 |
| RSA reactivity index | −.03 | −.03 | −.11 | −.07 |
| Age × PEP Reactivity Index | — | −.04 | — | −.09 |
| Age × RSA reactivity index | — | .07 | — | .17* |
p < .05.
p < .01.
p < .001.
Note: Family income was scored on an ordinal scale, with 1 = <$10000 and 8 = >$60000. PEP, pre-ejection period and RSA, respiratory sinus arrhythmia. Higher PEP and RSA reactivity indices correspond to greater reactivity or consistent arousal throughout the laboratory protocol.
When children's free-recall memory was examined, the model was significant, overall F (6, 81) = 5.95, p < .001, adjusted R2 = .25. At Step 2, the R2Δ = .01, n.s. At Step 1, significant predictors included children's age and their PEP reactivity index score. Increasing age was associated with better memory. Also, as hypothesized, greater sympathetic arousal across the protocol was associated with enhanced memory, as reflected in the amount of narrative information children initially provided about the alarm. The model was also significant when total proportion features recalled was considered, F (6, 81) = 13.40, p < .001, adjusted R2 = .46. The R2Δ at Step 2 was .03, p < .10. Because we had a priori hypotheses concerning the interaction, results at Step 2 were examined further. Age, family income, and the age × RSA reactivity index interaction emerged as significant predictors (Tab. 6). Being older and having a family with a higher annual income predicted better overall memory. A plot of the significant interaction, in Figure 1, revealed that, among the older children, high parasympathetically driven arousal throughout the protocol (i.e., consistent parasympathetic withdrawal) was associated with poorer memory of the alarm. Among the younger children, however, heightened parasympathetically driven arousal was unrelated to memory. Thus, consistent with our hypothesis regarding the combined relation of age and arousal predicting memory, older but not younger children appeared to be adversely affected by chronic parasympathetic reactivity.
FIGURE 1.
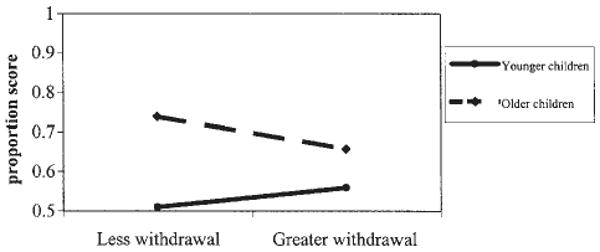
The significant interaction between children's age and parasympathetic reactivity during the laboratory challenges predicting the total amount of information recalled during the memory interview. The points on the lines denote 1 standard deviation above and below the mean for children's age and their RSA reactivity index scores, and the proportion scores reflect the number of features recalled out of the total number possible.
Discussion
The purpose of this study was to examine the relations between children's physiological reactivity and memory for a fire-alarm incident. Results reveal that arousal driven by the sympathetic and parasympathetic systems had different implications for memory, the latter possibly in conjunction with age. Further, although children's general proclivity to react mildly or strongly to multiple types of laboratory stressors was predictive of their memory, children's discrete reaction to the alarm was not. Next, we discuss the broader implications of our findings in terms of (a) methodology and measurement strategies in research concerning children's memory for stressful events, and (b) the relations between physiological reactivity and mnemonic processes in childhood.
Two noteworthy findings highlight the utility of studying children's memory for mild laboratory stressors. For one, our procedures were successful in eliciting stress responses in children. The use of a fire alarm as a discrete situationally specific stressor is not novel to our study (e.g., see Peters, 1991; Pillemer, Picariello, & Pruett, 1994; Quas et al., 2004; Stein & Boyce, 1995). Yet, our study is one of the first to investigate systematically both children's sympathetic and parasympathetic reactions to the potential stressor. Children indeed exhibited an increase in sympathetic arousal during the alarm, as expected. They also, however, displayed greater parasympathetic tone during the alarm, which theoretically lowers arousal. The different directions of responses between the two branches of the autonomic nervous system highlight the significance of collecting multiple measures of children's autonomic reactions to environmental stressors. Had only RSA, heart period, or heart rate (which is typically strongly correlated with parasympathetic withdrawal; Salomon et al., 2000) been measured, one might have concluded that the alarm was not arousing to children. Instead, children were aroused during the alarm, as indexed specifically by their sympathetic reactions, but children were, to some extent, engaging in self-regulation as well.
Second, no significant relations were found between the PEP and RSA difference or index scores. As such, the different measures were not tapping identical underlying physiological processes in children. Nor were the two branches of the autonomic nervous system uniformly acting in opposition to or conjunction with each other (Cacioppo et al., 1994). Instead, their reactions were largely independent, a pattern that could emerge even when the branches are well coordinated at a neurological level (Salomon et al., 2000; Stratakis & Chrousos, 1995). In previous studies of stress and memory in children, measurements of distress have varied considerably, ranging from assessments of children's discrete reactions to a single inoculation, to rating children's overall distress during an entire dental visit, to measuring children's reaction before, during, and after a medical procedure. Our findings indicate that the different measures may be tapping somewhat different types of reactions. A relatively short-lived, immediate reaction to a discrete event may or may not be related to children's more general reactivity predisposition and may vary across different physiological systems. As we discuss next, these different types of arousal had different implications for children's memory.
When we examined the relations between arousal and memory, children's age, family income, and physiological reactivity emerged as significant predictors. First, consistent with findings from several previous studies (e.g., Goodman et al., 1991; Vandermaas et al., 1993), age-related improvements in memory were evident. Older children are more knowledgeable about what is important to recount, are better able to conduct memory searches, can use strategies to help remember information, and are more detailed in their narrations about past events (Bjorklund & Douglas, 1997; Fivush & Hamond, 1990; see Ornstein & Haden, 2002), all of which enhance their memory for a range of events. Older children may also have more experience with fire alarms (e.g., in school or at home), leading possibly to a fire-alarm script (e.g., Farrar & Goodman, 1992; Hudson, Fivush, & Keubli, 1995) that facilitated their recall.
Second, family income predicted better memory. Although family income is only one component of socioeconomic status, a few studies have reported similar findings concerning associations between socioeconomic status and children's memory (e.g., Alexander et al., 2002; Howe, Cicchetti, Toth, & Cerrito, 2004). Higher socioeconomic status is related to higher scores on standardized IQ tests (Roberts, 1971). Also, in higher compared to lower income families, parents tend to read and talk more with their children, including about past events (Chaney, 1994), and children often have better vocabulary understanding and production. Such factors likely contribute to better narrative abilities in children from high socioeconomic families, irrespective of whether these children actually remember events better. Of note, relatively few children in our sample were from especially low-income families. It will be important, in subsequent research, to examine more directly the extent to which children from families with a broader range of incomes vary in their memory and reporting of different types of stressful experiences.
Third, and the main focus of our study, we uncovered important associations between children's autonomic arousal and memory, some direct and some in conjunction with age. Researchers have called for greater attention to be focused on assessing physiological arousal within the context of age-related differences (Beauchaine, 2001) and on evaluating complex patterns of physiological responses rather than only short-term discrete reactions within systems (Bauer, Quas, & Boyce, 2002; Salomon et al., 2000). Our findings support the researchers' calls and reveal that taking into account age and multiple physiological systems' reactions can advance knowledge concerning how children remember stressful events.
In our study, sympathetically versus parasympathetically driven arousal had different implications for children's memory, the latter albeit marginally. As predicted, children's sympathetic arousal during the entire reactivity protocol was positively associated with their fire-alarm memory, as reflected in the number of features the children reported in free recall. Quas et al. (2004) did not find a significant association between sympathetic arousal during a similar reactivity protocol and children's fire-alarm memory. However, Quas et al. measured sympathetic arousal according to a difference score, computed by subtracting children's PEP while listening to a neutral story from their mean PEP across the laboratory challenges. (The researchers also included a multidimensional index in their study, but this index combined the sympathetic and parasympathetic measures into a single score). In our study, with a larger sample and with a multidimensional index specifically of sympathetic arousal, we indeed uncovered a significant positive association between consistently high sympathetically driven arousal and children's fire-alarm memory. Sympathetically aroused individuals likely attend to the environment to evaluate the situation (Cannon, 1914; Henry, 1992), and increased attention should enhance memory, at least for information relevant to the cause of the stress and to deciding how best to respond. Our memory interview probed for details concerning what caused the alarm to start and stop. As such, benefits of sympathetic arousal on memory were anticipated. Had we probed for information unrelated to the alarm (e.g., features of the room), sympathetic arousal may not have helped memory or may even have inhibited memory (Peters, 1991; see Christianson, 1992), a possibility in need of empirical investigation.
Although a cautionary note is warranted in light of the small magnitude of the findings, children's parasympathetic responses interacted with age to predict their memory. With age, greater parasympathetic reactivity was associated with poorer overall memory. In other words, among older but not younger children, stress, as indicated by consistent parasympathetic withdrawal throughout the laboratory protocol, was related to providing less information about the alarm, a finding consistent with Quas et al. (2004), in a smaller sample of 4- to 6-year-old children (the researchers did not examine age differences). Of importance, in the present study, age was also negatively related to children's parasympathetic reactivity index scores, such that younger children were generally more parasympathetically withdrawn during the laboratory tasks than older children. As mentioned, high levels of parasympathetic withdrawal may be the “norm” or expected among younger, who are more likely to rely on others to help them cope with stressful experiences (see Fields & Prinz, 1997). In contrast, older children who exhibited high levels of parasympathetic withdrawal may have general difficulty regulating arousal during challenges. For instance, perhaps these older children are not efficient at enacting primary or active coping strategies (e.g., problem-solving) to reduce their arousal. Instead, they may have attempted to disengage from the fire-alarm situation or could not regulate their arousal, either of which may have led to lower encoding of the event or perhaps a reduced willingness to talk about it afterward.
Two other comments deserve mention regarding our findings concerning physiological reactivity and memory. For one, the different systems' associations with memory varied between the two memory measures: Greater sympathetic activation was associated with larger free-recall proportion scores, whereas greater parasympathetic withdrawal was associated with lower total memory proportions. It may be that sympathetic activation, by increasing attention to the to-be-remembered event, concurrently increased the event's salience, leading to children's greater willingness to describe the event when initially asked what happened. In contrast, parasympathetic withdrawal may be indicative of general inhibition tendencies or shyness more generally (Boyce et al., 2001; Kagan, Reznick, Snidman, & Gibbons, 1988) and hence an overall reduced willingness on the part of children to engage in the session, complete the tasks, and/or answer interview questions. This inhibition certainly could lead to lower performance overall on the memory test. As mentioned, children who failed to complete the memory interview due to time constraints had larger parasympathetic reactivity scores than did children who completed the memory interview. Although this difference could have emerged due to chance, such a finding is also consistent with the possibility that parasympathetically reactive children took longer to get accustomed to the protocol or needed more assistance during the tasks than less parasympathetically reactive children. In general, as research continues to examine relations between sympathetic and parasympathetic reactivity and memory, it will be important to consider how different memory prompts affect the associations obtained.
Also, children's general reactivity, as measured via sympathetic arousal and parasympathetic withdrawal during the reactivity protocol, the latter in conjunction with age, predicted their memory, whereas children's discrete arousal during the fire alarm did not. The laboratory event in which children took part was comprised of a series of tasks that are mildly challenging to children within the age range of the study (see Alkon et al., 2003). Studying physiological reactions across multiple (or prolonged) stressors rather to a discrete stressful event is common in investigations of physiological reactivity, health, and well being and is believed to provide information about individual differences in children's general physiologic regulatory abilities (e.g., Boyce et al., 1995, 2001; Buske-Kirschbaum, Jobst, Wustmans, & Kirschbaum, 1997; Dobkin, Tremblay, & Treiber, 1998; Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988). In our study, children's reactions to the range of laboratory tasks, as opposed to their reactions to the single, brief fire-alarm incident, may well have differentiated consistently aroused children who have a general limited ability to attend to and cope with stressful events from children who exhibit appropriate reactions to novelty but also recover quickly afterward. Of course, we only measured children's responses during a single laboratory visit. As such, our interpretation of our reactivity score reflecting a trait-like characteristic in children is speculative. However, even if the children's reactivity scores reflected only their state at the time of the laboratory experience, their responses across the entire session nonetheless were more predictive of their memory than were their responses during the discrete fire-alarm event. Thus, there remains considerable value in terms of predicting various outcomes when assessing children's physiological reactions over extended periods even within a single session.
Although this study represents an important step in the field of stress and memory in children, limitations must be acknowledged. First, despite our success at eliciting sympathetic reactions to the alarm, children's absolute levels of “distress” were minimal and are certainly not comparable to the levels children may experience during significant, prolonged, or unexpected stressors, that is, the types of events studied in former naturalistic research (e.g., Fivush et al., 2004; Goodman, Quas, Batterman-Faunce, Riddlesberger, & Kuhn, 1997; Merritt et al., 1994). The relatively small increase in arousal may have inhibited our ability to find curvilinear associations between stress and memory, although the lack of curvilinear relations we observed is consistent with prior studies that tested children's memory for arguably more distressing experiences (i.e., inoculations, dental visits; e.g., Alexander et al., 2002; Vandermaas et al., 1993). Nonetheless, it remains important to investigate children's autonomic reactions to different types of stressors to determine whether the associations between stress and memory (both direct and curvilinear) are consistent across events. The use of medical procedures as to-be-remembered events, for example, is particularly promising in light of research suggesting that it is possible to assess physiological responses in children as the procedures take place (e.g., Chen et al., 2001).
Second, the number of questions asked during the interview varied depending on children's initial free-recall responses. Although this procedure has been used by a number of researchers (e.g., Greenhoot et al., 1999; Merritt et al., 1994; Peterson & Rideout, 1998), the procedure does not allow children's responses only to follow-up probes to be compared directly. Standardized memory interviews would enable such comparisons to be made and to determine how age and stress are related to children's answers to a range of question types. Third, in the future it will be important to assess how other factors within children and their social environment, relate to children's memory. For instance, chronic stress, temperament, and attachment may differentially influence children's behavioral and/or physiological coping reactions (e.g., Compas, Connor-Smith, & Jaser, 2004; Goodman et al., 1997), and therefore have implications both for what children perceive as distressing and how children subsequently respond. Moreover, although there are no theoretical reasons to assume intelligence has implications for children's stress reactivity, both higher intelligence and more advanced verbal ability have been related to better memory (e.g., Quas, Wallin, Papini, Lench, & Scullin, 2005; Roebers & Schneider, 2001). Insofar as models concerning associations between stress and memory become increasingly complex, it will be important to take into account these other individual and social factors as independent or interactive contributors to children's memory.
Despite these limitations, our findings highlight the need to continue studying individual differences in children's physiological response patterns as predictors of their memory for stressful events. More specifically, our results suggest that children's general physiological reactivity to stress, rather than their discrete physiological responses to a single event, may have unique implications for children's ability to recount stressful experiences. The next step is to determine the extent to which our findings generalize to children's memory for a range of personal, significant experiences.
Acknowledgments
Contract grant sponsor: MacArthur Foundation Research Network on Psychopathology and Development
Contract grant sponsor: National Institute of Mental Health
Contract grant number: R01 MH44340
Appendix
Memory Checklist
Getting the kettle.
Getting the mugs.
Getting the hot chocolate mix.
Getting the marshmallows.
Getting the chocolate chips.
Explanation by the researcher that the water needs to boil before being poured into the mugs.
Fire alarm sounding.
Steam coming from kettle.
Researcher unplugging the kettle.
Explanation by the researcher that the steam is causing the alarm and the steam needs to be dissipated.
Researcher waving hands to dissipate steam.
Pouring water into the mugs.
Stirring the hot chocolate mix.
Adding whipped cream.
Letting the hot chocolate cool.
Footnotes
NOTES: Data examined in the present study were collected with support to W. Thomas Boyce from the MacArthur Foundation Research Network on Psychopathology and Development and from the National Institute of Mental Health (R01 MH44340). Portions of the study were presented at the 2003 Biennial Meeting of the Cognitive Development Society, Park City, Utah.
Contributor Information
Jodi A. Quas, Email: jquas@uci.edu, Department of Psychology and Social, Behavior, 3340 Social Ecology II, University of California, Irvine, Irvine, CA 92697-7085.
Nathalie Carrick, Department of Psychology and Social, Behavior, 3340 Social Ecology II, University of California, Irvine, Irvine, CA 92697-7085.
Abbey Alkon, University of California, San Francisco, CA.
Lauren Goldstein, University of California, Berkeley, CA.
W. Thomas Boyce, University of British Columbia, Vancouver, BC, Canada.
References
- Alexander K, Goodman G, Schaaf J, Edelstein R, Quas J, Shaver P. The role of attachment and cognitive inhibition in children's memory and suggestibility for a stressful event. Journal of Experimental Child Psychology. 2002;83:262–290. doi: 10.1016/s0022-0965(02)00149-2. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein L, Smider M, Essex N, Kupfer D, Boyce W. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Alpert BS, Wilson DK, editors. Stress Reactivity in Childhood and Adolescence. NY: Plenum Press; 1992. [Google Scholar]
- Althaus M, Gomarus HK, Wijers AA, Mulder LJM, van Velzen JL, Minderaa RB. Cortical and autonomic correlates of visual selective attention in introverted and extraverted children. Journal of Psychophysiology. 2005;19:35–49. [Google Scholar]
- Bahrick LE, Parker JF, Fivush R, Levitt M. The effects of stress on young children's memory for a natural disaster. Journal of Experimental Psychology: Applied. 1998;4:308–331. doi: 10.1037/1076-898X.12.3.142. [DOI] [PubMed] [Google Scholar]
- Band E, Weisz J. How to feel better when it feels bad: Children's perspectives on coping with everyday stress. Developmental Psychology. 1988;24:247–253. [Google Scholar]
- Bauer A, Quas J, Boyce W. Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development & Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Douglas RN. The development of memory strategies. In: Cowan N, editor. The development of memory in childhood. Hove, England: Psychology Press; 1997. pp. 201–246. [Google Scholar]
- Boyce W, Chesney M, Alkon A, Tschann J, Adams S, Chesterman B, Cohen F, Kaiser P, Folkman S, Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce W, Quas J, Alkon A, Smider N, Essex M, Kupfer D. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brown JM, O'Keeffe J, Sanders SH, Baker B. Developmental changes in children's cognition to stressful and painful situations. Journal of Pediatric Psychology. 1986;11:343–357. doi: 10.1093/jpepsy/11.3.343. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Blue J, Cortez V, Fleck K, Rodriguez A. The influence of witnessed affect on information processing in children. Child Development. 1992;63:774–786. [PubMed] [Google Scholar]
- Burgwyn-Bailes E, Baker-Ward L, Gordon B, Ornstein P. Children's memory for emergency medical treatment after one year: The impact of individual difference variables on recall and suggestibility. Applied Cognitive Psychology. 2001;15:25–48. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, Berntson GG. Handbook of psychophysiology. 2nd. New York, NY, US: Cambridge University Press; 2000. [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and pre-ejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins S, Dedmon S. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Cannon W. The interrelations of emotions as suggested by recent physiological researchers. American Journal of Psychology. 1914;25:256–282. [Google Scholar]
- Cannon WB. The Wisdom of the Body. New York, NY: W.W. Norton & Company.; 1939. [Google Scholar]
- Carlson R. Gesell School Readiness Test. In: Keyser D, Sweetland R, editors. Test critiques. Kansas City, KS: Test Corporation of America; 1985. [Google Scholar]
- Chaney C. Language development, metalinguistic awareness, and emergent literacy skills of 3-yr-old children in relation to social class. Applied Psycholinguistics. 1994;15:371–394. [Google Scholar]
- Chen E, Zeltzer K, Craske M, Katz E. Children's memories for painful cancer treatment procedures: Implications for distress. Child Development. 2000;71:933–947. doi: 10.1111/1467-8624.00200. [DOI] [PubMed] [Google Scholar]
- Christianson S. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, Aiken S, West L. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cole P. Children's spontaneous control of facial expression. Child Development. 1986;57:1309–1321. [Google Scholar]
- Compas B. An agenda for coping research and theory: Basic and applied developmental issues. International Journal of Behavioral Development. 1998;22:231–237. [Google Scholar]
- Compas B, Boyer MC. Coping and attention: Implications for child health and pediatric conditions. Journal of Developmental & Behavioral Pediatrics. 2001;22:323–333. doi: 10.1097/00004703-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical Child and Adolescent Psychology. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- Compas B, Connor-Smith J, Saltzman H, Thomsen A, Wadsworth M. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127:87–127. [PubMed] [Google Scholar]
- Dobkin P, Tremblay R, Treiber F. Cardiovascular reactivity and adolescent boys' physical health. Pediatrics. 1998;101:1–5. doi: 10.1542/peds.101.3.e11. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes R, Bustamante D, Mathy R, Miller P, Lindholm E. Differentiation of vicariously induced emotional reactions in children. Developmental Psychology. 1988;24:237–246. [Google Scholar]
- Farrar M, Goodman G. Developmental changes in event memory. Child Development. 1992;63:173–187. [PubMed] [Google Scholar]
- Fields L, Prinz RJ. Coping and adjustment during childhood and adolescence. Clinical psychology review. 1997;17:937–976. doi: 10.1016/s0272-7358(97)00033-0. [DOI] [PubMed] [Google Scholar]
- Fivush R, Hamond N. Autobiographical memory across the preschool years: Toward reconceptualizing childhood amnesia. In: Fivush R, Hudson J, editors. Knowing and remembering in young children Emory symposia in cognition. New York, NY: Cambridge University Press; 1990. pp. 223–248. [Google Scholar]
- Fivush R, Sales J, Goldberg A, Bahrick L, Parker J. Weathering the storm: Children's long-term recall of Hurricane Andrew. Memory. 2004;12:104–118. doi: 10.1080/09658210244000397. [DOI] [PubMed] [Google Scholar]
- Goodman G, Hirschman J, Helps D, Rudy L. Children's memory for stressful events. Merrill-Palmer Quarterly. 1991;37:109–158. [Google Scholar]
- Goodman G, Quas J, Batterman-Faunce J, Riddlesberger M. Predictors of accurate and inaccurate memories of traumatic events experienced in childhood. Consciousness & Cognition: An International Journal. 1994;3:269–294. [Google Scholar]
- Goodman GS, Quas JA, Batterman-Faunce JL, Riddlesberger M, Kuhn G. Children's reactions to and memory for a stressful event: Influences of age, anatomical dolls, knowledge, and parental attachment. Applied Developmental Science. 1997;1:54–75. [Google Scholar]
- Gordon B, Ornstein P, Nida R, Follmer A, Crenshaw MC, Albert G. Does the use of dolls facilitate children's memory of visits to the doctor? Applied Cognitive Psychology. 1993;7:459–474. [Google Scholar]
- Greenhoot AF, Ornstein PA, Gordon BN, Baker-Ward L. Acting out the details of a pediatric check-up: The impact of interview condition and behavioral style on children's memory reports. Child Development. 1999;70:363–3803. doi: 10.1111/1467-8624.00027. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Donzella B, editors. Stress physiology, health, and behavioral development. Ann Arbor, MI: The University of Michigan Press; 2001. [Google Scholar]
- Henry JP. Biological bases of the stress response. Integrated Physiological Behavioral Sciences. 1992;27:66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- Howe ML, Cicchetti D, Toth SL, Cerrito BM. True and false memories in maltreated children. Child development. 2004;75:1402–1417. doi: 10.1111/j.1467-8624.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Howe ML, Courage ML, Peterson C. Intrusions in preschoolers' recall of traumatic childhood events. Psychonomic Bulletin & Review. 1995;2:130–134. doi: 10.3758/BF03214419. [DOI] [PubMed] [Google Scholar]
- Hudson J, Fivush R, Keubli J. Scripts and episodes: The development of event memory. Applied Cognitive Psychology. Special Issue: Memory in Everyday Settings. 1995;6:483–505. [Google Scholar]
- Kagan J, Reznick J, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Assessment Battery for Children. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- Linnemeyer S, Porges S. Recognition memory and cardiac vagal tone in 6-month-old nfants. Infant Behavior & Development. 1986;9:43–56. [Google Scholar]
- McEwen BS. Seminars in Medicine of the Beth Israel Deaconess Medical Center: Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. From molecules to mind: Stress, individual differences, and the social environment. In: Damasio A, Harrington A, et al., editors. Unity of knowledge: The convergence of natural and human science. Annals of the New York Academy of Sciences. New York: New York Academy of Sciences; 2001. pp. 935pp. 42–49. [PubMed] [Google Scholar]
- Merritt KA, Ornstein PA, Spicker B. Children's memory for a salient medical procedure: Implications for testimony. Pediatrics. 1994;94:17–23. [PubMed] [Google Scholar]
- Monk C, Kovelenko P, Ellman LM, Sloan RP, Bagiella E, Gorman JM, Pine DS. Enhanced stress reactivity in paediatric anxiety disorders: Implications for future cardiovascular health. International Journal of Neuropsychopharmacology. 2001;4:199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- Ornstein P, Haden C. The development of memory: Toward an understanding of children's testimony. In: Eisen M, editor. Memory and suggestibility in the forensic interview. Personality and clinical psychology series. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 2002. pp. 29–61. [Google Scholar]
- Peters D. The impact of naturally occurring stress on children's' memory. In: Ceci SJ, Toglia MP, Ross DF, editors. Children's eyewitness memory. New York: Springer-Verlag; 1987. pp. 122–141. [Google Scholar]
- Peters D. The influence of stress and arousal on the child witness. In: John D, editor. The suggestibility of children's recollections. Washington, DC: American Psychological Association; 1991. pp. 60–76. [Google Scholar]
- Peterson C, Bell M. Children's memory for traumatic injury. Child Development. 1996;67:3045–3070. [PubMed] [Google Scholar]
- Peterson C, Rideout R. Memory for medical emergencies experienced by 1- and 2-year-olds. Developmental Psychology. 1998;34:1059–1072. doi: 10.1037//0012-1649.34.5.1059. [DOI] [PubMed] [Google Scholar]
- Pillemer DB, Picariello ML, Pruett JC. Very long-term memories of a salient preschool event. Applied Cognitive Psychology. 1994;8:95–106. [Google Scholar]
- Porges S. Autonomic regulation and attention. In: Campbell B, Hayne H, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1992. pp. 201–223. [Google Scholar]
- Porges S. Cardiac vagal tone: A physiological index of stress. Neuroscience & Biobehavioral Reviews. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Quas J, Bauer A, Boyce W. Physiological reactivity, social support, and memory in early childhood. Child Development. 2004;75:1–18. doi: 10.1111/j.1467-8624.2004.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas J, Goodman G, Bidrose S, Pipe M, Craw S, Ablin D. Emotion and memory: Children's long-term remembering, forgetting, and suggestibility. Journal of Experimental Child Psychology. 1999;72:235–270. doi: 10.1006/jecp.1999.2491. [DOI] [PubMed] [Google Scholar]
- Quas J, Hong M, Alkon A, Boyce WT. Dissociations between psychobiological reactivity and emotional expression in children. Developmental Psychobiology. 2000;37:153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Quas JA, Lench H. Effects of interviewer support and arousal on children's memory for emotion-eliciting events. Applied Cognitive Psychology in press. [Google Scholar]
- Quas JA, Wallin A, Papini S, Lench H, Scullin M. Suggestibility, social context, and memory for a novel experience in young children. Journal of Experimental Child Psychology. 2005;91:315–341. doi: 10.1016/j.jecp.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Kagan J, Snidman N, Gersten M, Baak K, Rodenberg A. Inhibited and uninhibted children: A follow-up study. In: Chess S, Thomas A, editors. Annual progress in child psychiatry and child development. Philadelphia, PA: Brunner/Mazel Inc.; 1986. pp. 256–290. [Google Scholar]
- Roberts J. Intellectual development of children as measured by the Wechsler Intelligence Scale. United States Vital & Health Statistics. 1971;41 [PubMed] [Google Scholar]
- Roebers CM, Schneider W. Individual differences in children's eyewitness recall: The influence of intelligence and shyness. Applied Developmental Science. 2001:59–20. [Google Scholar]
- Rudolph K, Dennig M, Weisz J. Determinants and consequences of children's coping in the medical setting: Conceptualization, review, and critique. Psychological Bulletin. 1995;118:328–357. doi: 10.1037/0033-2909.118.3.328. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development: Themes and variations. London: Oxford University Press; 1996. [Google Scholar]
- Salomon K, Matthews K, Allen M. Patterns of sympathetic and parasympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Stein N, Boyce T, Goodman G, Baker-Ward L. The role of physiological reactivity in attending to, remembering, and responding to an emotional event. Children' memory for emotional and traumatic events; Symposium conducted at the Society for Research in Child Development Meetings; Indianapolis, IN. 1995. Apri, [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals of New York Academy Science. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Suess P, Porges S, Plude D. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Vandermaas M, Hess T, Baker-Ward L. Does anxiety affect children's reports of memory for a stressful event? Applied Cognitive Psychology. 1993;7:109–127. [Google Scholar]
- Weber E, Van der Molen M, Molenaar P. Heart rate and sustained attention during childhood: Age changes in anticipatory heart rate, primary bradycardia, and respiratory sinus arrhythmia. Psychophysiology. 1994;31:164–174. doi: 10.1111/j.1469-8986.1994.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit formation. Journal of Comparative Neurology & Psychology. 1908;18:459–482. [Google Scholar]
- Yuille JC, Tollestrup PA. A model of the diverse effects of emotion on eyewitness memory. In: Christianson S, editor. The handbook of emotion and memory: Research and theory. England: Lawrence Erlbaum Associates, Inc.; 1992. pp. 201–215. [Google Scholar]
- Zeman J, Garber J. Display rules for anger, sadness, and pain: It depends on who is watching. Child Development. 1996;67:957–973. [PubMed] [Google Scholar]


