Abstract
The activation of N-methyl-D-aspartate (NMDA) receptors and subsequent release of nitric oxide (NO) are likely contributors to the delayed neuronal damage that accompanies ischemia and other neurodegenerative conditions. NMDA receptor antagonists and inhibitors of NO synthesis, however, are of limited benefit when administered following excitotoxic events, suggesting the importance of determining downstream events that result in neuronal degeneration. Inhibition of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), a key glycolytic enzyme, which may result in glycolytic impairment, is one of the biological targets of NO. This suggests that alternative energy substrates may prevent neuronal damage. Using rat hippocampal slices from juvenile rats, we examined the role of glycolytic impairment in NMDA mediated excitotoxicity and whether pyruvate, an end product of glycolysis, prevents the excitotoxic neuronal injury. We observed that administration of NMDA acutely depresses ATP levels and result in a slowly developing inhibition of GAPDH. Unlike NMDA receptor antagonists or NO inhibitors, exogenously applied pyruvate is effective in restoring ATP levels and preventing delayed neuronal degeneration and synaptic deterioration when administered in the period following NMDA receptor activation. This raises the possibility that treatment with agents that maintain cellular energy function can prevent delayed excitotoxicity.
Keywords: sodium nitroprusside, monocarboxylate, glycolysis, nitric oxide, energy metabolism
Introduction
N-methyl-D-aspartate receptors (NMDARs) participate in delayed neuronal death in a variety of neurodegenerative conditions, including hypoxia and stroke21. Nitric oxide (NO) release following NMDAR activation may contribute to the toxic cascade, and NMDAR antagonists and NO synthase (NOS) inhibitors attenuate neuronal degeneration caused by NMDAR activation3,11,21. The effectiveness of these agents, however, is markedly diminished when they are administered following initial excitotoxic events5. To identify regimens for neuronal protection after excitotoxic injury, it is important to determine downstream targets that lead to neuronal degeneration.
The adverse effects of NO include alterations in cellular energy metabolism2. These effects lead to inhibition of oxidative metabolism10 and glycolysis7, and activation of poly-ADP- ribose synthetase26 resulting in energy depletion and neurodegeneration1. A slow but substantial inhibition of the glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), is observed after NO release during brain ischemia8,15,16. This GAPDH inhibition may result from NO-mediated ADP-ribosylation and S-nitrosylation6,28 or formation of peroxynitrite anions24. Because alternative glycolytic pathways around GAPDH do not exist, GAPDH inhibition causes severe neurodegeneration12. We hypothesize that the inhibition of glycolysis caused by NO is responsible, at least in part, for neuronal deterioration following excitotoxic insults. If glycolytic suspension participates in NMDAR-mediated neuronal degeneration then administration of glycolytic end products may provide effective ways to protect neurons and preserve neuronal function following acute insults. Although glucose is a primary energy source for neurons, it has been shown that during glucose deprivation lactate and pyruvate can preserve neuronal integrity12,23 and adenosine triphosphate (ATP) levels14.
In this study we used rat hippocampal slices to examine the role of glycolytic inhibition on NMDA-mediated excitotoxicity and also examined the ability of pyruvate to preserve neuronal integrity following NMDAR activation.
Materials and Methods
All experiments were performed in accordance with the guidelines of the Washington University Animal Study Committee. Every effort was made to minimize the number of animals used and their suffering in all experimental procedures. Transverse slices were prepared from the septal half of the hippocampus using standard techniques29. Albino rats (PND 30 ± 2) were anaesthetized with halothane and decapitated. The hippocampi were rapidly dissected at 4 to 6 °C and cut into 500 μm slices using a Campden vibrotome (Campden Instruments, Sileby, Loughborough, U.K.). Slices were then kept in artificial cerebrospinal fluid (ACSF) containing (in millimolar): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2-5% CO2 in an incubation chamber for at least 60 min at 30°C.
ATP levels were determined by luminometry (Zylux, Maryville, TN) using a firefly luciferase-based spectrofluorometric assay (Turner Systems) with a Calbiochem-Novabiochem ATP Assay Kit (Kit 119108) 13. Protein levels in all biochemical assays were determined using a standard BioRad procedure (BioRad, Hercules, CA) by reading the absorbency at 595 nm in a recording spectrometer. Four hippocampal slices were used for each GAPDH assay and at least five assays were repeated for each experimental condition. After an experiment, slices were homogenized in 250 mM sucrose, 10 mM imidazole and 10 mM KCl on ice. GAPDH activity was measured in 100 mM triethanolamine buffer (pH 7.6), 500 mM sodium arsenate (pH 8.8), 24 mM reduced glutathione, 5 mM NAD+ and 10 mg/ml glyceraldehyde-3-phosphate by reading the absorbency at 340 nm in a recording spectrophotometer. LDH activity was determined with an LDH Assay Kit (Sigma, St. Louis, MO) by reading the absorbency at 340 nm with NADH and pyruvate. ATP concentrations, LDH and GAPDH activities from each whole slice were compared to matched controls incubated and measured simultaneously during each experiment from the same hippocampus incubated and measured simultaneously during each experiment.
For histological assays, hippocampal slices were fixed in a solution containing 1% paraformaldehyde and 1.5% glutaraldehyde overnight at 4°C. The fixed slices were rinsed in 0.1 M pyrophosphate buffer, placed in 1% buffered osmium tetroxide for 60 min, dehydrated with alcohol and toluene, embedded in araldite, cut into sections 1 μm thick, stained with methylene blue and azure II and evaluated by light microscopy. Damage in the CA1 region was rated on a 0 (completely intact) to 4 (severe morphological changes in pyramidal neurons) scale by a rater who was unaware of the experimental condition11. Using this system, control slices incubated for 120 min in the standard solution were rated as 0.2 ± 0.3 (N=34). Slices treated with 100 μM NMDA for 20 min followed by 90 min post incubation in standard solution exhibited damage scores of 3.7 ± 0.4 (N=34).
For electrophysiological experiments, slices were transferred to a submersion-recording chamber where they were continuously perfused with ACSF (2 ml/min) at 30°C. Extracellular recordings were obtained from the pyramidal cell layer and dendritic region of CA1 using 5 to 10 MΩ electrodes filled with 2 M NaCl. During an experiment, the Schaffer collateral-commissural fibers were stimulated in stratum radiatum with bipolar electrodes and 0.1 to 0.2 ms constant current pulses at an intensity sufficient to evoke a 50% maximal EPSP slope based on a baseline stimulus-response curve. The use of half-maximal stimulus intensities allows reliable detection of changes in synaptic transmission. The initial slope of the EPSP was used for data analysis. Results are shown as a percentage of the average baseline EPSP slope. Only a single slice from each hippocampus was used for each group of experiments.
All chemicals, except for the ATP and protein assay kits were purchased from Sigma Chemical Co. (St. Louis, MO). Data in the text and figures are expressed as means ± SEM. Differences between groups were analyzed using Student's t-test and Mann Whitney's U-test and paired Student's t-test if data are paired (SigmaStat, Jandel Scientific Software, San Rafael, CA).
Results
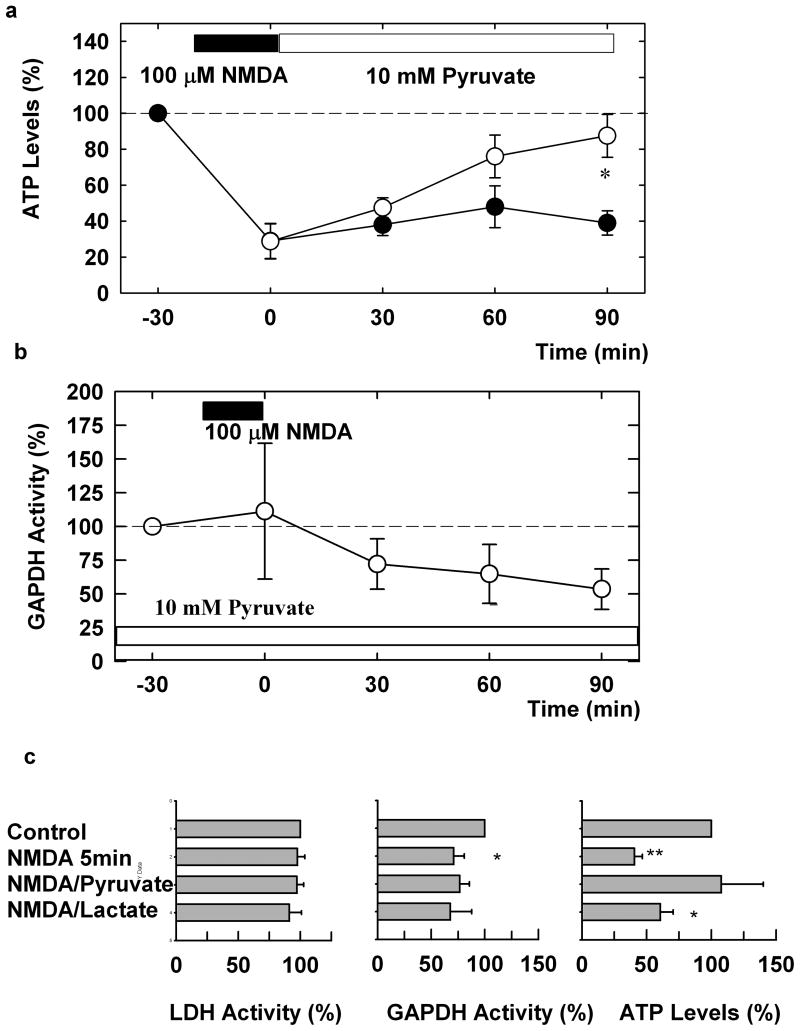
In whole hippocampal slices, we examined the effects of NMDA administration on ATP levels. When hippocampal slices were exposed to 100 μM NMDA for twenty minutes, we observed a rapid depression of ATP levels that was apparent immediately following NMDA treatment. The depression of ATP levels persisted for at least 90 minutes following washout of NMDA (Figure 1a, N=5). Administration of 10 mM pyruvate after NMDA washout gradually restored ATP levels to near baseline as shown in Fig. 1 (P<0.05, N=5). In contrast, administration of pyruvate only during the 20 min NMDA exposure failed to alter either the immediate or persisting depression of ATP levels (see Table). Similar to the effects of 20 min administration of NMDA, ATP levels were depressed 90 min after 5 min administration of NMDA. The depression was prevented by the presence of pyruvate applied both during and after NMDA exposure. However, the presence of 10 mM L-lactate failed to preserve ATP levels (Fig. 1c).
Figure 1.
Pyruvate overcomes the depression of ATP and GAPDH by NMDA. a) NMDA (100 μM, filled bar) promptly and persistently depresses ATP levels following a 20 min exposure (filled circles). Administration of 10 mM pyruvate (open bar) immediately after NMDA exposure restores ATP levels (open circles). b) NMDA causes a slow inhibition of GAPDH activity in hippocampal slices. Twenty min exposure to 100 μM NMDA (filled bar) slowly depresses GAPDH activity in the presence of 10 mM pyruvate. Values are ratios relative to pyruvate alone in each experiment. Three or more slices were done at each time point. c) LDH and GAPDH activities and ATP levels were determined 90 min after 5 min exposure to NMDA in 8 hippocampi. ATP level were restored by the presence of 10 mM pyruvate but not by 10 mM L-lactate. Results are normalized with respect to untreated controls. **<0.01, *<0.05 vs. control by paired t-test.
Table.
Effects of 100 μM NMDA (20 min + 90 min washout) and effects of continous administration of sodium nitroprusside (SNP) on ATP levels and GAPDH activity in the absence and presence of 10 mM pyruvate.
| Conditions | ATP levels (%) |
N | GAPDH activity (%) |
N |
|---|---|---|---|---|
| 20 min NMDA | 39 ± 7 | 5 | ||
| Pyruvate during NMDA | 43 ± 11 | 3 | ||
| Pyruvate after NMDA | 88 ± 2 | 5 | ||
| Pyruvate during/after NMDA | 91 ± 13 | 4 | 46 ± 7 | 4 |
| 2 hour SNP + Vit.C | N.D. | 48 ± 7 | 5 | |
| 3 hour SNP + Vit.C | 45 ± 10 | 5 | 45 ± 12 | 5 |
| 3 hour SNP + Vit.C + pyruvate | 83+7 | 5 | N.D. |
One way that NMDA can deplete ATP is via release of NO and subsequent inhibition of GAPDH2. To determine whether this is a relevant mechanism, we examined the effects of NMDA on GAPDH activity in hippocampal slices. Because 20 min administration of NMDA results in substantial neuronal degeneration over the period of interest, it is difficult to determine whether loss of activity results from enzyme inhibition or cellular injury. To overcome this limitation, we took advantage of the fact that pyruvate preserves ATP levels in slices exposed to NMDA. Similar to pyruvate administered after NMDA exposure (Figure 1a), continuous administration of 10 mM pyruvate during 20 min exposure to 100 μM NMDA and for another 90 min after NMDA exposure sustained ATP levels (see Table). Administration of 10 mM pyruvate alone for 2 hours did not alter basal GAPDH activity (100 ± 8% of control, N=10). However, GAPDH activity in the presence of 10 mM pyruvate was reduced within 30-90 min following 20 min administration of 100 μM NMDA (Figure 1b and Table). Similarly, only 5 min administration of NMDA significantly depressed GAPDH activity in the absence of pyruvate (Fig. 1c), though the depression was not statistically significant in the presence of pyruvate or L-lactate. In this set of experiments, LDH activity was not altered by NMDA exposure.
We also found that NMDA does not alter GAPDH activity in the presence of 10 μM MK-801 (94 ± 10% of control, N=4). Similarly, GAPDH activity was not depressed following NMDA administration if NMDA was administered with 100 μM L-monomethylarginine, a NOS inhibitor (115 ± 28 % of control, N=3). We also examined more directly whether NO release alters GAPDH activity in hippocampal slices. We found that release of NO by administration of 3 mM sodium nitroprusside (SNP) in the presence of 1mM ascorbate for two- or three-hours reduced GAPDH levels (Table). This reduction in GAPDH activity results in an inhibition of glycolytic metabolism and a depression of ATP levels in the slices (Table). Administration of SNP plus ascorbate in the presence of 10 mM pyruvate reduced ATP levels only partially (P<0.05 compared to SNP and ascorbate alone by paired t-test).
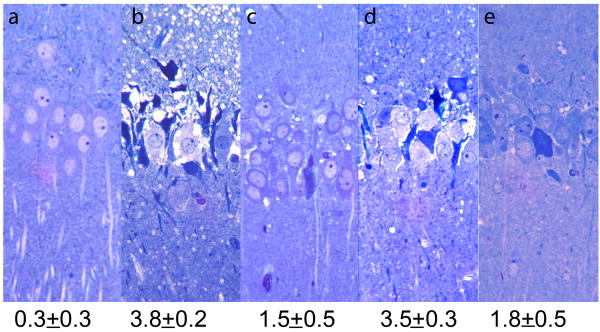
In the CA1 region of hippocampal slices, severe histological damage was observed 90 min following 20 min administration of 100 μM NMDA (N=6, Figure 2b) as previously reported11. This neuronal damage was blocked by administration of 10 μM MK-801, a non-competitive NMDAR antagonist, or 100 μM 7-nitroindazole (7-NIA), an inhibitor of neuron specific NOS, when these agents were present during the NMDA exposure (damage scores: 1.3 ± 0.3, N=3 for MK-801 and 2.0 ± 0.2, N=4 for 7-NIA; see also ref.10). However, consistent with prior studies5,20, MK-801 and 7-NIA were not effective when applied immediately after NMDA administration (damage scores: 3.6 ± 0.2, N=5; 3.5 ± 0.3, N=4, respectively). If defects in energy metabolism and/or energy depletion participate in NMDA-mediated excitotoxicity, then administration of glycolytic end products might preserve neuronal integrity following NMDA exposure. We observed that damage in the CA1 region induced by NMDA (Figure 2b) was attenuated by continuous administration of 10 mM pyruvate during and after NMDA exposure (Figure 2c, N=6, p<0.05 in damage score). Although pyruvate failed to protect neurons when administered only during NMDA exposure (Figure 2d), pyruvate administered immediately following washout of NMDA also attenuated the damage (Figure 2e, N=6, p<0.05 in damage score). Lactate, another glycolytic end product, provided similar but somewhat less effective protection compared to pyruvate (damage scores: 2.2 ± 0.5, N=5 when applied continuously throughout the experiment; 2.5 ± 1.0, N=5 when applied only after wash out of NMDA, data not shown).
Figure 2.
Pyruvate attenuates NMDA-mediated damage in the CA1 region. The photomicrographs depict a) the control appearance of the CA1 region after incubation in standard solution for 110 min; b) the pattern of damage induced by 20 min exposure to 100 μM NMDA followed by 90 min post-incubation in drug free solution; c) preservation of morphological integrity by 10 mM pyruvate administered during NMDA exposure and continuously during the post-incubation period; d) administration of pyruvate only during NMDA exposure; and e) pyruvate administered only in the period after NMDA exposure. NMDA mediated damage in the CA1 region is typically characterized by marked changes in the pyramidal cell layer with severely swollen (pale) neurons interspersed with shrunken (dark) neurons and an overall torn appearance. Numbers below panels depict each damage score. Magnification 275×.
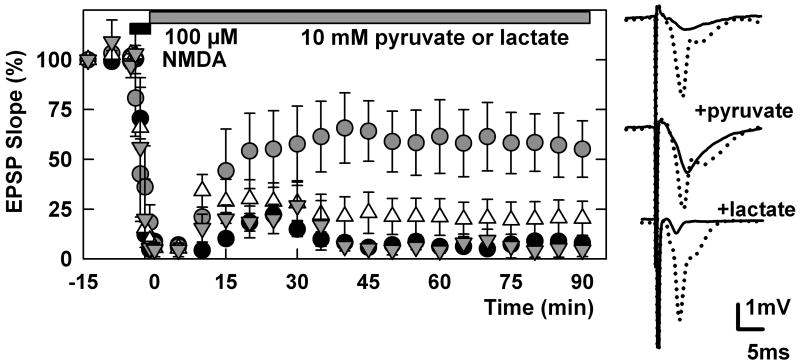
We also sought to determine whether pyruvate restores neuronal synaptic function after NMDA administration in the CA1 region. Administration of 100 μM NMDA for 5 min quickly depressed glutamate-mediated EPSPs in the CA1 region (Figure 3). Although we observed a small transient recovery after NMDA washout, synaptic responses were almost totally suppressed 90 min after NMDA exposure (5.4 ± 3.2 % of control, N=5). Administration of 10 mM pyruvate immediately following NMDA exposure restored EPSPs, though the recovery was only partial (53.4 ± 17.4% of control baseline EPSPs, N=5, P<0.01 vs. no pyruvate 90 min after NMDA washout). In contrast to pyruvate, administration of 10 mM L-lactate after NMDA exposure failed to restore EPSPs (20.4 ± 8.6%, N=5). Administration of pyruvate only during the period of NMDA exposure did not restore synaptic transmission (4.5 ± 3.3% of control, N=3, data not shown).
Figure 3.
Pyruvate overcomes the depression of EPSPs by NMDA. The graph shows the effects of a 5 min perfusion of 100 μM NMDA (filled bar) on dendritic EPSP slopes in control slices (filled circles, N=5) and in slices treated with 10 mM pyruvate in the period following NMDA (open circles, N=5), or just during the period of NMDA administration (triangles, N=3). Traces were sampled before and 90 min after NMDA administration in the presence of pyruvate or L-lactate.
Discussion
When NMDAR antagonists are applied following acute excitotoxic events, neuroprotective effects are partial at best5,20. This suggests that delayed NMDAR-mediated neuronal death does not result from sustained NMDAR activation but rather from cellular events downstream of these receptors. Several lines of evidence suggest that NOS activation and NO release are involved in the neurodegeneration triggered by NMDARs3,11. However, the short half life of NO following its release, coupled with the ineffectiveness of NOS inhibitors administered following the initial excitotoxic insult19, suggests the importance of identifying targets of NO involved in neurodegeneration in order to develop treatments that can be administered in the post-insult period.
Depression of cellular energy status, resulting in part from inhibition of GAPDH, is likely to represent an effect of NO that contributes to neural degeneration. In this scenario, excessive activation of NMDARs ultimately results in neuronal starvation even in the presence of glucose because GAPDH inhibition precludes effective use of glucose. The neurodegeneration induced by NMDA shares features with the damage produced by the GAPDH inhibitor, iodoacetate (IA), and the effects of IA can be overcome by administration of pyruvate12. The inhibition of GAPDH by NMDA, however, occurs relatively slowly. This slow inhibition is consistent with the delayed onset of neurodegeneration after NMDA exposure and provides a potential window during which specific treatments that enhance metabolism might be effective when applied following NMDAR activation. Alternative energy sources that circumvent points of disrupted metabolism represent one such approach18,27. In mouse cultured striatal neurons, pyruvate and lactate preserve ATP levels after NMDA exposure17. Moreover, in organotypic hippocampal slice cultures β-hydroxybutyrate appears to prevent NMDA-mediated excitotoxicity22. We observed that pyruvate is effective against NMDA toxicity when applied following NMDA exposure but not when applied only during the period of NMDAR activation. This is in striking contrast to the effects of the NMDAR antagonists and NOS inhibitors, which are effective only when present during NMDA exposure. Thus, alternative energy substrates are potential candidates for inclusion in neuroprotective and neurorestorative treatment regimens. Based on our observations, it appears that alternative energy substrates require continuous administration until GAPDH activity recovers under conditions in which GAPDH inactivation is involved in excitotoxicity.
To the extent that GAPDH inactivation following NMDA exposure determines the decline in cellular energy metabolism, we expected that the depression of ATP levels would follow a similar time course. Rather, NMDA administration in hippocampal slices resulted in an immediate and persistent depression of ATP levels. Even in the presence of normal energy metabolism, however, ATP levels will be depressed when ATP consumption exceeds production. Thus, excessive neuronal activity and energy demands may acutely suppress ATP levels despite preserved GAPDH activity during NMDA exposure. Similarly, the acute depression of EPSPs during NMDA exposure appears to be independent of the impairment in glycolytic metabolism. A small but transient recovery of synaptic responses after toxic NMDA exposure suggests that multiple mechanisms are involved in suppressing synaptic function including delayed effects on neuronal survival. Although the recovery of EPSPs in the presence of pyruvate suggests that impaired glycolytic energy metabolism contributes to the longer-term effects, pyruvate-mediated synaptic recovery and histological preservation are only partial, indicating that mechanisms other than glycolytic inhibition are involved in the ultimate synaptic effects of excitotoxic events. In addition, the partial prevention of histological damage by pyruvate suggests that specific cells or limited functions of neurons are preserved by supplemental alternative energy substrates.
It is also important to consider that the actions of pyruvate may not be limited to its role as an energy source because pyruvate can also alter calcium homeostasis and other biochemical pathways9,25. Furthermore, free radical scavenging may also contribute to pyruvate's neuroprotective effects27. The superiority of pyruvate to lactate in preserving neuronal integrity in this study may suggest additional properties of pyruvate4.
Our results indicate two important features about the potential utility of monocarboxylates and other alternative energy substrates in disorders involving acute excitotoxic neural injury. First, our synaptic data indicate that alternative energy substrates can preserve and/or restore neural function following adverse events. Thus, treatments like the monocarboxylates that restore this function could have a significant impact on functional recovery and quality of life. Second, these energy substrates can prevent damage when administered in the post-event period. This is important in clinical settings because in most cases the excitotoxic cascade will already be underway before patients come to clinical attention. At such a time, NMDAR antagonists and NOS inhibitors are unlikely to be of benefit. The significance of our study is limited, however, because we used only juvenile rat slices. Further studies using older animals will be needed in the future.
Acknowledgments
This work was supported in part by National Institute of Health grants MH077791, AA017413, Neuroscience Blueprint Grant NS57105 and the Bantly Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ Depletion Is Necessary and Sufficient for Poly(ADP-Ribose) Polymerase-1-Mediated Neuronal Death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brorson JR, Schumacker PT, Zhang H. Nitric oxide acutely inhibits neuronal energy production. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desagher S, Glowinski J, Prémont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dezsi L, Greenberg JH, Sladky J, Araki N, Hamar J, Reivich M. Prolonged effects of MK-801 in the cat during focal cerebral ischemia and recovery: survival, EEG activity and histopathology. J Neurol Sci. 1994;121:110–120. doi: 10.1016/0022-510x(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 6.Dimmeler S, Brüne B. L-arginine stimulates an endogenous ADP- ribosyltransferase. Biochem Biophys Res Commun. 1991;178:848–855. doi: 10.1016/0006-291x(91)90968-d. [DOI] [PubMed] [Google Scholar]
- 7.Dimmeler S, Brüne B. Nitric oxide preferentially stimulates auto-ADP- ribosylation of glyceraldehydes-3-phospate dehydrogenase compared to alcohol or lactate dehydrogenase. FEBS Lett. 1993;315:21–24. doi: 10.1016/0014-5793(93)81124-i. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S, Lottspeich F, Brüne B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992;267:16771–16774. [PubMed] [Google Scholar]
- 9.Frenzel J, Richter J, Eschrich K. Pyruvate protects glucose-deprived Muller cells from nitric oxide-induced oxidative stress by radical scavenging. Glia. 2005;52:276–288. doi: 10.1002/glia.20244. [DOI] [PubMed] [Google Scholar]
- 10.Heales SJ, Bolanos JP, Stewart VC, Brookes PS, Land JM, Clark JB. Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 11.Izumi Y, Benz AM, Clifford DB, Zorumski CF. Nitric oxide inhibitors attenuate N-methyl-D-aspartate excitotoxicity in rat hippocampal slices. Neurosci Lett. 1992;135:227–230. doi: 10.1016/0304-3940(92)90442-a. [DOI] [PubMed] [Google Scholar]
- 12.Izumi Y, Benz AM, Zorumski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. Neuroreport. 1994;5:617–620. doi: 10.1097/00001756-199401000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Izumi Y, Izumi M, Matasukawa M, Funatasu M, Zorumski CF. Ammonia-mediated LTP inhibition: Effects of NMDA receptor antagonists and l-carnitine. Neurobiol Dis. 2005;20:615–624. doi: 10.1016/j.nbd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Kanatani T, Mizuno K, Okada Y. Effects of glycolytic metabolites on preservation of high energy phosphate level and synaptic transmission in the granule cells of guinea pig hippocampal slices. 1995 doi: 10.1007/BF01931098. [DOI] [PubMed] [Google Scholar]
- 15.McDonald LJ, Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA. 1993;90:6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin P, Maus M, Bockaert J, Glowinski J, Premont J. Oxygen free radicals enhance the nitric oxide-induced covalent NAD(+)-linkage to neuronal glyceraldehyde-3-phosphate dehydrogenase. Biochem J. 1995;309:891–898. doi: 10.1042/bj3090891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maus M, Marin P, Israël M, Glowinski J, Prémont J. Pyruvate and lactate protect striatal neurons against N-methyl-D-aspartate-induced neurotoxicity. Eur J Neurosci. 1999;11:3215–3224. doi: 10.1046/j.1460-9568.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamichi N, Kambe Y, Oikawa H, Ogura M, Takano K, Tamaki K, Inoue M, Hinoi E, Yoneda Y. Protection by exogenous pyruvate through a mechanism related to monocarboxylate transporters against cell death induced by hydrogen peroxide in cultured rat cortical neurons. J Neurochem. 2005;93:84–93. doi: 10.1111/j.1471-4159.2005.02999.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima MN, Yamashita K, Kataoka Y, Yamashita YS, Niwa M. Time course of nitric oxide synthase activity in neuronal, glial, and endothelial cells of rat striatum following focal cerebral ischemia. Cell Mol Neurobiol. 1995;15:341–349. doi: 10.1007/BF02089944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nellgard B, Wieloch T. Postischemic blockade of AMPA but not NMDA receptors mitigates neuronal damage in the rat brain following transient severe cerebral ischemia. J Cereb Blood Flow Metab. 1992;12:2–11. doi: 10.1038/jcbfm.1992.2. [DOI] [PubMed] [Google Scholar]
- 21.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 22.Samoilova M, Weisspapir M, Abdelmalik P, Velumian AA, Carlen PL. Chronic in vitro ketosis is neuroprotective but not anti-convulsant. J Neurochem. 2010;113:826–835. doi: 10.1111/j.1471-4159.2010.06645.x. [DOI] [PubMed] [Google Scholar]
- 23.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 24.Souza JM, Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch Biochem Biophys. 1998;360:187–194. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 25.Villalba M, Martinez-Serrano A, Gomez-Puertas P, Blanco P, Borner C, Villa A, Casado M, Gimenez C, Pereira RE, Bogoez E, Pozzan T, Satdstegui J. The role of pyruvate in neuronal calcium homeostasis. Effects on intracellular calcium pools. J Biol Chem. 1994;269:2468–2476. [PubMed] [Google Scholar]
- 26.Wallis RA, Panizzon KL, Henry D, Wasterlain CG. Neuroprotection against nitric oxide injury with inhibitors of ADP-ribosylation. Neuroreport. 1993;5:245–248. [PubMed] [Google Scholar]
- 27.Zeng J, Yang GY, Ying W, Kelly M, Hirai K, James TL, Swanson RA, Litt L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J Cereb Blood Flow Metab. 2007;27:304–315. doi: 10.1038/sj.jcbfm.9600335. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Snyder SH. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA. 1992;89:9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorumski CF, Mennerick S, Izumi Y. Assessment of synaptic effects of nitric oxide in hippocampal neurons. Methods Neurosci. 1996;31:282–299. [Google Scholar]