Summary
Synaptic Vesicle 2 (SV2) proteins, critical for proper nervous system function, are implicated in human epilepsy, yet, little is known about their function. We demonstrate for the first time, using direct approaches, that loss of the major SV2 isoform in a central nervous system nerve terminal is associated with an elevation in both resting and evoked presynaptic Ca2+ signals. This increase is essential for the expression of the SV2B−/−secretory phenotype, characterized by changes in synaptic vesicle dynamics, synaptic plasticity, and synaptic strength. Short-term reproduction of the Ca2+ phenotype in wild-type nerve terminals reproduces almost all aspects of the SV2B−/− phenotype, while rescue of the Ca2+ phenotype in SV2B−/− neurons relieves every facet of the SV2B−/− secretory phenotype. Thus, SV2 controls key aspects of synaptic functionality via its ability to regulate presynaptic Ca2+, suggesting a potential new target for therapeutic intervention in the treatment of epilepsy.
Keywords: synapse, vesicle, bipolar cell, epilepsy, homeostatic plasticity
INTRODUCTION
SV2 proteins are a group of homologous integral membrane proteins found on secretory vesicles that undergo regulated release (Bajjalieh et al., 1992; Buckley and Kelly, 1985; Feany et al., 1992). They have 12 transmembrane domains and are members of the major facilitator superfamily of membrane transporter proteins (Janz et al., 1998; Saier et al., 1999). In mammals, the three isoforms, SV2A, SV2B and SV2C, are each encoded by a separate gene (Bajjalieh et al., 1994; Janz and Südhof, 1999). Inactivation of the gene coding for SV2A, by far the most widely-distributed isoform in the mouse brain, results in early postnatal-lethal epileptic-like seizures (Crowder et al., 1999; Janz et al., 1999). In humans, SV2A has been identified as a target of levetiracetam, a highly effective drug used in the treatment of epilepsy (De Smedt et al., 2007; Lynch et al., 2004), and a reduction in SV2A has been recently reported in patients with temporal lobe epilepsy (Feng et al., 2009). SV2 proteins can also interact with botulinum neurotoxins (Dong et al., 2008; Dong et al., 2006; Mahrhold et al., 2006), allowing their entry into nerve terminals during exocytosis, where they exert their toxic effects.
Despite their critical role in nervous system health, the function of SV2 proteins is far from clear. While some researchers have suggested that SV2 proteins govern synaptic plasticity by regulating residual calcium (Chang and Südhof, 2009; Janz et al., 1999), others have argued that SV2 proteins act independently of changes in presynaptic calcium (Custer et al., 2006; Xu and Bajjalieh, 2001); evidence for both viewpoints is largely indirect. In addition, SV2 proteins have been suggested to act either before or after a priming step in the secretory pathway (Chang and Südhof, 2009; Xu and Bajjalieh, 2001). Unfortunately, precise elucidation of the function of SV2 proteins has been hindered by several factors, including the severe seizures and early lethality associated with the absence of the major brain isoform of SV2, SV2A. In addition, biochemical approaches have been hampered by the fact that SV2 proteins are heavily glycosylated, polytopic membrane proteins that are difficult to express functionally in recombinant systems. Physiological approaches have been hampered by the lack of a neuronal preparation that permits quantitative and simultaneous measurement of presynaptic calcium and synaptic vesicle dynamics in neurons with SV2 deficiency.
In this study, we took advantage of a neuronal preparation recently developed for the direct, biophysical study of exocytosis and presynaptic calcium in the mammalian central nervous system: the mouse rod bipolar cell (Wan et al., 2008; Zhou et al., 2006). In addition, we took advantage of the fact that SV2B is the major SV2 isoform expressed in these neurons (Wang et al., 2003) and that SV2B knockout mice are viable and do not suffer from seizures (Janz et al., 1999). This allowed us to investigate SV2 function in neurons isolated from mature, healthy animals. The only known deficit in SV2B knockout animals resides in the retina, consistent with a major role for SV2B in ribbon synapses of the rod visual pathway (Lazzell et al., 2004; Morgans et al., 2009).
We compared the presynaptic Ca2+ and secretory responses of rod bipolar cells acutely isolated from the retinae of adult SV2B−/− and wild-type mice using quantitative fluorescence measurements of presynaptic Ca2+ in combination with time-resolved membrane capacitance measurements (Wan et al., 2008; Zhou et al., 2006). Our data provide direct evidence that SV2B is important for the regulation of presynaptic Ca2+ levels and consequently, Ca2+-dependent synaptic vesicle dynamics. In addition, we identified a role for SV2B that is independent of short-term changes in presynaptic Ca2+ and is reminiscent of the previously described peri-priming defect (Chang and Südhof, 2009; Custer et al., 2006). Strikingly, every aspect of the SV2B knockout secretory phenotype, including the functional reduction in the rapidly-releasing pool, was relieved by the restoration of presynaptic Ca2+ signaling to wild-type levels. Thus, not only does this SV2 protein play a key role in regulating presynaptic Ca2+, but presynaptic Ca2+ signaling plays a key role in the expression of the SV2-deficiency phenotype.
RESULTS
SV2B regulates presynaptic Ca2+ signaling
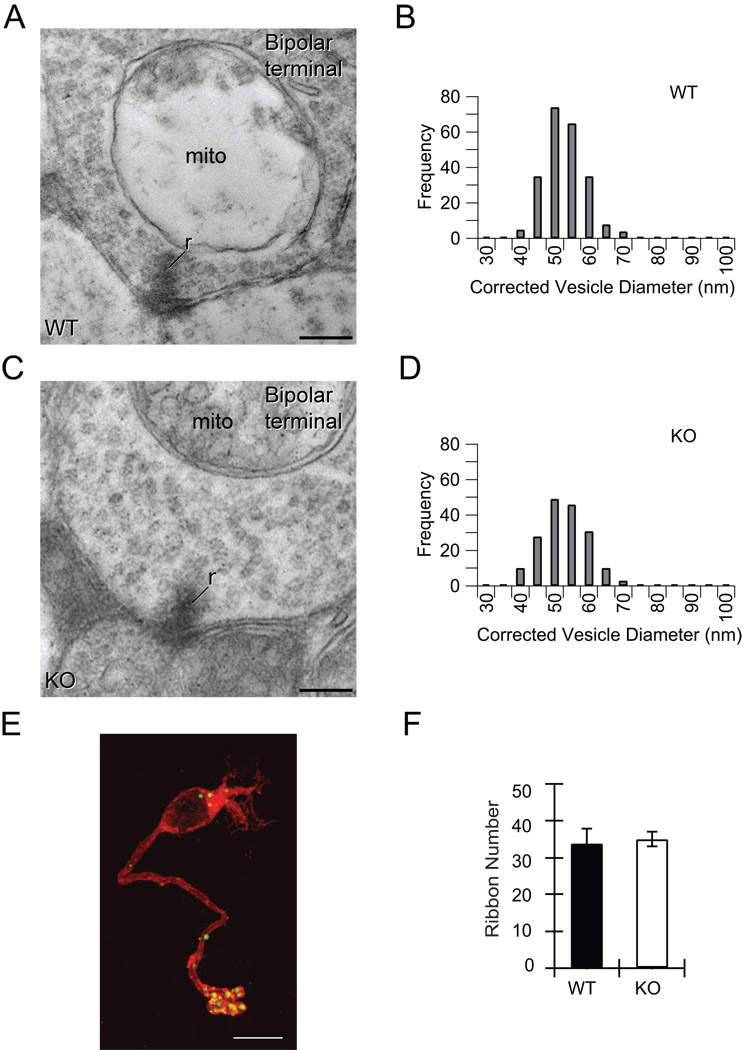
Previous ultrastructural analysis of conventional synapses from SV2A/B double-knockout mice have shown that the lack of SV2 proteins does not affect the formation of morphologically-normal synapses or synaptic vesicles (Crowder et al., 1999; Janz and Südhof, 1999). Similarly, we found that the absence of SV2B did not alter the structure and size of synaptic vesicles or the number or length of synaptic ribbons at the ribbon-style synapses of the mouse rod bipolar cell (Figure 1 and Supplementary Table S1). Neither did the absence of SV2B disrupt formation of normal synaptic connections by bipolar cells (Figure 1).
Figure 1. Absence of SV2B does not affect synaptic architecture.
(A and C) The absence of SV2B does not disrupt bipolar cell ribbon synapse ultrastructure or organization. Ribbon synapses in bipolar cell terminals of WT and SV2B−/− (KO) retina show similar ultrastructure, with short synaptic ribbons (r) attached to the presynaptic membrane and surrounded by numerous synaptic vesicles. Postsynaptic processes from amacrine or ganglion cells flank the synaptic ribbon in the normal diad organization (Dowling, 1968). Mito, mitochondrion. Scale bars = 0.2 µm. (B and D) The distribution of synaptic vesicle diameters in bipolar cell terminals in WT and KO retina is comparable. Feret’s diameter was measured from digitized electron micrographs and adjusted using Abercrombie’s correction factor to adjust for systematic underestimation of the size of spherical objects in thin sections (Abercrombie, 1946). (E–F) Loss of SV2B did not alter the number of synaptic ribbons in bipolar cell terminals. (E) Confocal fluorescence image of a freshly isolated rod bipolar cell from an SV2B−/− mouse double immunostained for the synaptic ribbon marker CtBP2 (green) and the rod bipolar cell marker Protein Kinase C-α (PKC-α, red). Scale bar = 10 µm. (F) The number of ribbons in terminals did not differ between WT and KO mice. The number of synaptic ribbons in WT and KO rod bipolar terminals was determined by counting the number of CtBP2-positive puncta in the terminals of rod bipolar cells positively identified as rod bipolar cells by PKC-α labeling. WT ribbon counts from Wan et. al., (2008).
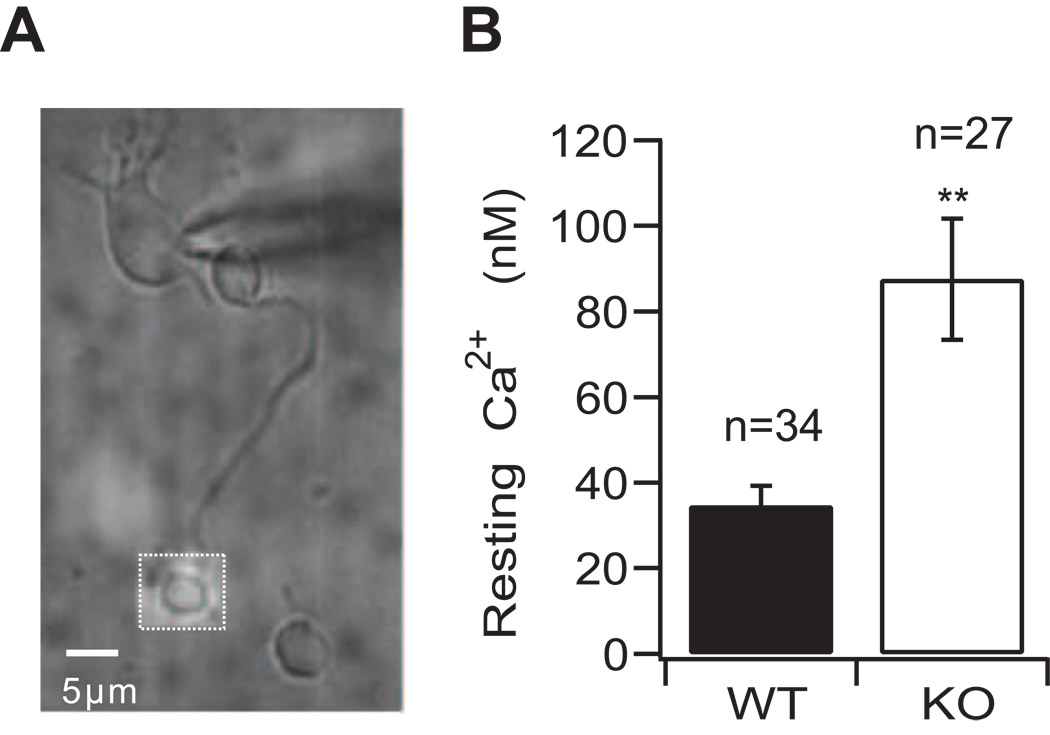
To ascertain whether SV2 proteins regulate the resting presynaptic Ca2+ concentration, we performed quantitative measurements of spatially-averaged presynaptic Ca2+ in bis-fura-2-loaded terminal boutons of isolated, mature rod bipolar neurons from SV2B knockout mice (SV2B−/−) and wild-type littermates (Figure 2). To control for changes in membrane potential that could alter calcium signaling, neurons were held under voltage-clamp control at −70 mV. Under these conditions, the mean spatially-averaged intraterminal Ca2+ concentration of neurons obtained from SV2B−/− mice was 88 ± 14 nM (n=27), compared with 35 ± 5 nM (n=34) in wild-type (WT) littermates (p=0.00028) (Figure 2b).
Figure 2. Absence of SV2B is associated with elevated resting presynaptic Ca2+.
(A) An acutely isolated rod bipolar neuron is shown with a patch pipette on its soma. The dotted square over the terminal denotes the region from which the emitted bis-fura-2 fluorescence signal was collected. (B) The average resting Ca2+ concentration in synaptic terminals held at −70 mV is significantly higher in neurons from SV2B−/− mice (KO) than WT littermates. Error bars represent s.e.m. **p<0.01.
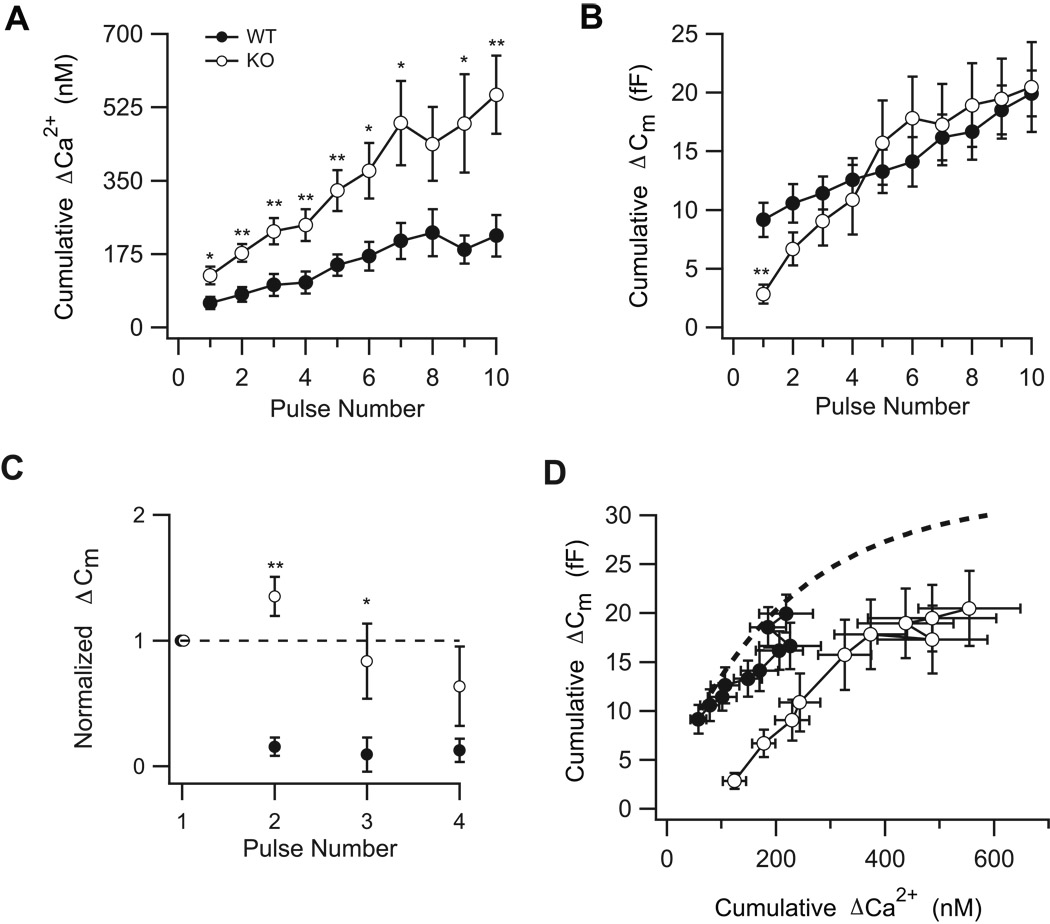
To establish whether or not SV2 proteins influence evoked Ca2+ signals, we applied a brief 4 Hz stimulus train to bipolar neurons from SV2B−/− and WT mice. Each pulse in the train was of a magnitude and duration sufficient to deplete the rapidly-releasing pool of vesicles (Wan et al., 2008). We simultaneously monitored the spatially-averaged presynaptic Ca2+ concentration and changes in membrane capacitance as an index of synaptic vesicle fusion (Figure 3; see also Supplementary Figure S1). The average cumulative increase in presynaptic Ca2+ concentration was greater in SV2B−/− terminals than in WT terminals for each pulse in the train (Figure 3A). In general, the rise in presynaptic free Ca2+ evoked by each pulse was approximately double in SV2B−/− terminals relative to WT (Pulse 1: WT: 58 ± 16 nM, n=13; SV2B−/−: 124 ± 21 nM, n=14; p=0.018; Pulse 10: WT: 219 ± 50; SV2B−/−: 555 ± 94 nM, p=0.0046; Figure 3A). Taken with results from the previous section, our results provide strong evidence for a role for SV2 proteins in the regulation of both resting and evoked Ca2+ signaling in nerve terminals.
Figure 3. Absence of SV2B is associated with a larger increase in cumulative Ca2+ and a change in the pattern of exocytosis.
(A) The mean cumulative ΔCa2+ concentration for each pulse in the train was higher in terminals from SV2B−/− mice (KO) relative to WT littermates. *p<0.05, **p<0.01. (B) Despite greater increases in intraterminal Ca2+ concentration in SV2B−/− terminals, the ΔCm increase evoked by the first pulse of the stimulus train was smaller in SV2B−/− terminals than in WT terminals, while the cumulative ΔCm increase after the 10th pulse was similar. Furthermore, the slope of the cumulative ΔCm during the initial 3 pulses of the train was steeper in SV2B−/− neurons. (C) Facilitation precedes depression in SV2B−/−neurons but not WT neurons. The normalized ΔCm responses evoked by the stimulus for WT rod bipolar cells (filled circles) shows a pronounced synaptic depression. By contrast, SV2B−/− (KO) neurons (empty circles) exhibit facilitation that is followed by a slower rate of depression. The average ΔCm evoked by each pulse is normalized to that of the first; only the first four responses to the train are shown. (D) The cumulative ΔCm with respect to the cumulative ΔCa2+ concentration was significantly shifted to the right in SV2B−/− compared to WT terminals. The estimated size of the shift was 7 fF. Dotted line shows the relationship predicted from Zhou et al. (2006). See also Figure S1.
SV2B regulates synaptic vesicle dynamics
We next examined the extent of exocytosis evoked by the stimulus train in neurons with and without SV2B. Figure 3B presents our analysis of the cumulative capacitance change as a function of pulse number (see also Figure S1). In SV2B−/− neurons, there was a dramatic decrease in the magnitude of the first exocytotic response relative to WT neurons (SV2B−/−: 2.8 ± 0.8 fF, n=12; WT: 9.2 ± 1.5 fF, n=13; p=0.0015), suggesting that there is a decrease in the fusion of rapidly-releasing vesicles in the absence of SV2B. This decrease was accompanied by an increase in the slope of the cumulative capacitance increase early in the pulse train (Slopepulses1–3 SV2B−/−: 3.1 ± 0.4; Slopepulses1–3 WT: 1.2 ± 0.2; PROC Mixed analysis, p=0.0393). This enhancement briefly converts depression in the WT to facilitation in the SV2B−/− neurons (Figures 3B and 3C). However, the maximum cumulative release was essentially identical (SV2B−/−: 20.5 ± 3.8 fF, n=12; WT: 19.9 ± 1.9 fF, n=13; p=0.9), indicating that the total number of vesicles released by the whole train was similar between SV2B−/− and WT neurons.
Comparison of Figure 3A and 3B raised the possibility that the extent of exocytosis in SV2B−/− neurons began to approximate that of WT neurons as the relative change in cumulative Ca2+ concentration exceeded that of WT neurons. To evaluate this possibility, for each pulse in the train, we graphed the cumulative change in membrane capacitance as a function of the cumulative increase in presynaptic Ca2+ concentration. Figure 3D demonstrates that relative to WT neurons, the curve for SV2B−/− neurons was shifted to the right by approximately 7 fF (PROC Mixed analysis, p<0.0001, estimated shift 7 fF). This finding could indicate that the absence of SV2B is associated with a decrease in the apparent Ca2+ sensitivity of release, assuming that the spatially-averaged presynaptic Ca2+ concentration and Ca2+ concentration at the release site are correlated (Naraghi and Neher, 1997).
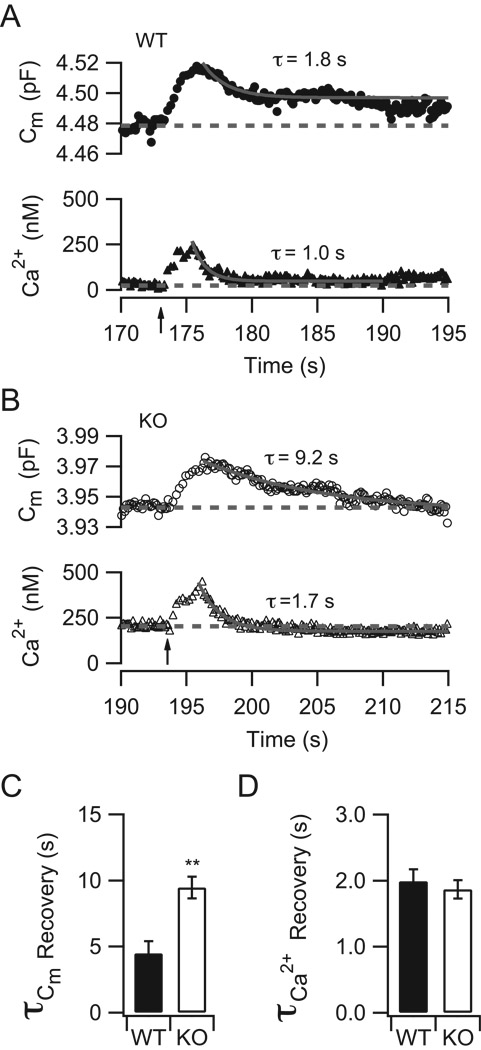
The time course for the restoration of membrane capacitance following a stimulus train also differed between SV2B−/− and WT neurons (Figure 4). SV2B−/− terminals displayed a slower average time constant for membrane recovery than WT terminals (SV2B−/−: 9.5 ± 0.8 s, n=8; WT: 4.5 ± 0.9 s, n=12; p=0.0015). This may reflect either a decrease in the rate of endocytosis, an increase in asynchronous release, or a combination of both. By contrast, Ca2+ concentration recovered to the prestimulus baseline with a time constant of a few seconds in both neurons (Figure 4: SV2B−/−: 1.9 ± 0.1 s, n=17; WT: 2.0 ± 0.2 s; n=16; p=0.59), indicating that other presynaptic Ca2+ handling mechanisms remained intact (Zenisek and Matthews, 2000).
Figure 4. The rate of membrane recovery following a stimulus train is prolonged in SV2B−/− terminals.
(A–B) Typical capacitance (circles) and Ca2+ (triangles) responses following a pulse train in WT (filled circles) and SV2B−/− terminals (KO, empty circles). Superimposed curves in (A) and (B) represent the single exponential fit for each trace. Time constants are as indicated. (C–D) Following the pulse train, membrane recovery was significantly slower in SV2B−/− terminals than in WT terminals (panel C; **p<0.01). However, there was no difference between SV2B−/− and WT terminals in the time course of recovery of Ca2+ concentration to baseline following the stimulus train (D).
Taken together, our data demonstrate that SV2-deficiency gives rise to both a Ca2+ phenotype and a secretory phenotype. The former is characterized by elevated spatially-averaged resting Ca2+ concentration and an enhanced evoked Ca2+ response in nerve terminals. The latter is characterized by a decrease in the number of rapidly-releasing vesicles, a decrease in the apparent Ca2+ sensitivity of exocytosis, an early enhancement of the secretory response evoked by a stimulus train, and a prolongation of membrane recovery.
Restoration of presynaptic Ca2+ signaling rescues the SV2B−/− secretory phenotype
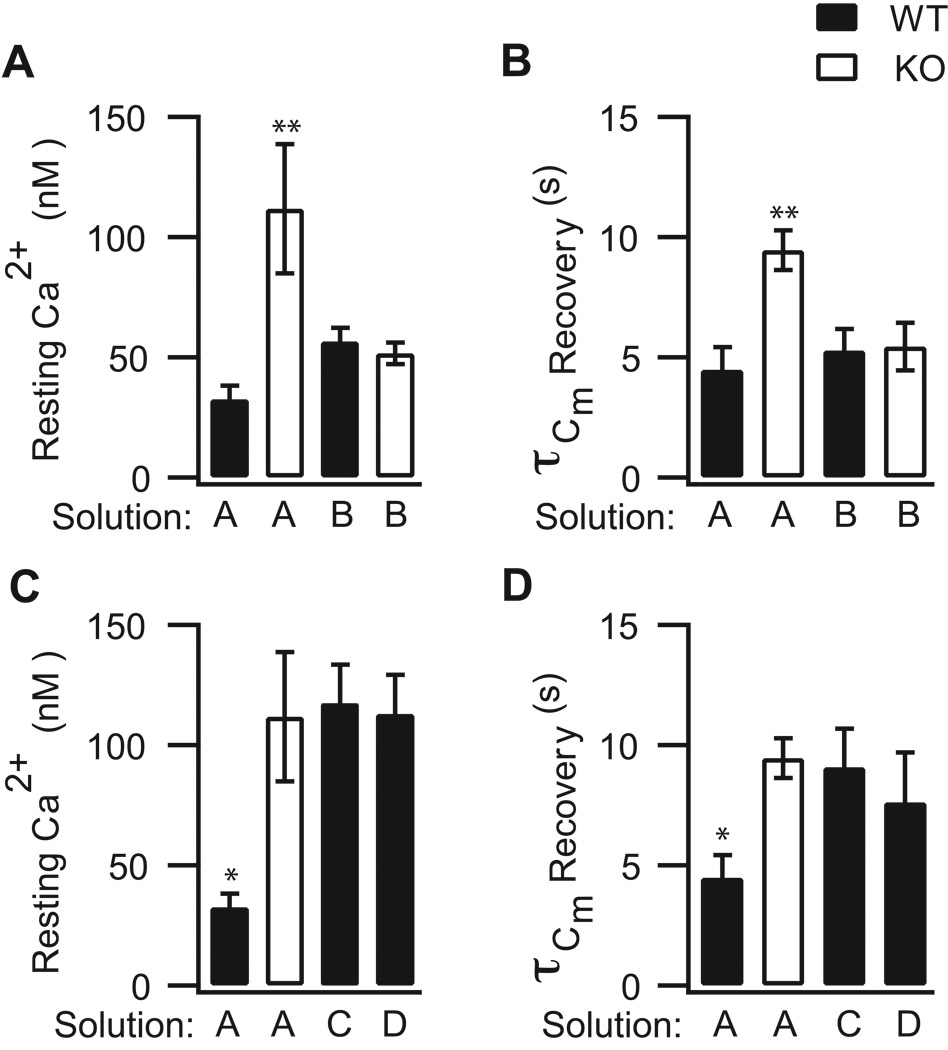
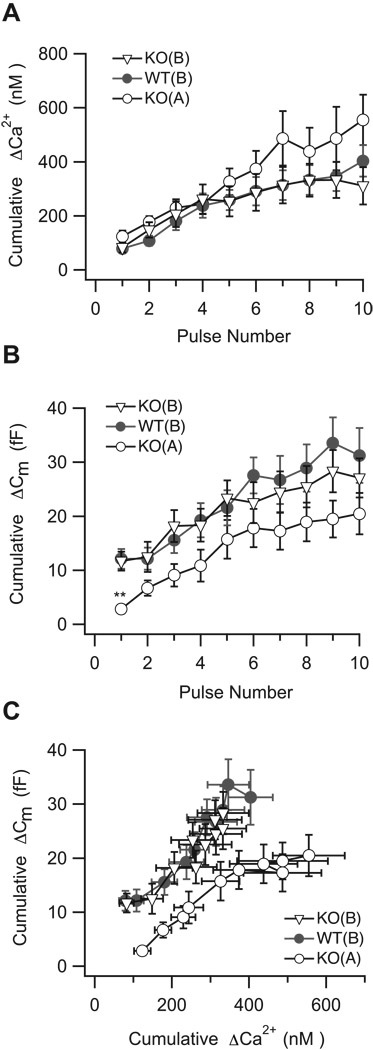
Several aspects of secretory vesicle dynamics are regulated by intraterminal Ca2+ (Heidelberger, 2001; Neher and Sakaba, 2008). We therefore asked whether we could rescue the SV2B−/− secretory phenotype by restoring resting intraterminal Ca2+ levels to near WT values. To this end, we clamped the resting intraterminal Ca2+ concentration in SVB−/− and WT terminals via the use of a Ca2+-buffered internal recording solution (solution B; Table 1). Following two minutes of dialysis with solution B, the mean intraterminal free Ca2+ concentration in SV2B−/− terminals held at −70 mV was 52 ± 4 nM (n=16), representing a ≈ 40% decrease from what was measured for SV2B−/− terminals using our standard recording solution (solution A; Figure 5A; 112 ± 27nM, n=14; p=0.037). The mean intraterminal free Ca2+ concentration in SV2B−/− neurons dialyzed with solution B was indistinguishable from that of WT terminals dialyzed with solution B (Figure 5A; WTsolutionB: 57 ± 6 nM, n=17). With solution B, the rise in cumulative intraterminal Ca2+ concentration in SV2B−/− neurons evoked by the pulse train was essentially identical to that of WT neurons (Figure 6A), and the total cumulative Ca2+ concentration rise was reduced to 312 ± 70 nM (n=16), representing an ≈ 40% decrease from what was measured using our standard solution (solution A; Figure 3 A). The time constant of Ca2+ recovery was similar between SV2B−/− and WT terminals (not shown), as expected from our earlier findings (i.e., Figure 4D).
Table 1.
Table of intracellular solutions
| Solution (mM) | A | B | C | D |
|---|---|---|---|---|
| CaCl2 | 0 | 0.05 | 0.3 | 0.15 |
| EGTA | 0.5 | 0.55 | 0.5 | 0.2 |
| Calculated Free EGTA | 0.5 | 0.48 | 0.25 | 0.1 |
| Calculated Free Ca2+ (nM) | 0 | 14 | 173 | 163 |
| Measured free Ca2+ (nM) | 0 | 12 | 241 | 225 |
Figure 5. The SV2B−/− membrane retrieval phenotype is sensitive to manipulation of presynaptic Ca2+.
(A–B) Lowering presynaptic Ca2+ concentration with solution B restores the mean rate of membrane retrieval in SV2B−/− (KO) neurons (empty bars) to that of WT neurons (filled bars) dialyzed with standard recording solution A. (C–D) In WT neurons, solutions C or D mimicked the KO phenotype of higher resting Ca2+ concentration and slower rate of membrane retrieval. Data were analyzed by one-way ANOVA followed by Dunnett’s Multiple Comparisons Test. **p<0.01, *p<0.05.
Figure 6. Restoration of presynaptic Ca2+ concentration to WT levels rescues the secretory phenotype of SV2B−/− neurons.
(A) Restoration of presynaptic Ca2+ concentration to WT levels for ≈ two minutes in SV2B−/− neurons with solution B (KO(B), open triangles) was sufficient to rescue the cumulative Ca2+ concentration response evoked by the pulse train. (B) When resting Ca2+ concentration was lowered in SV2B−/− neurons with solution B (KO(B), open triangles), the magnitude of the 1st Cm response became identical to that of WT neurons (WT(B), filled circles; one-way ANOVA, followed by Dunnett’s Multiple Comparisons Test. **p<0.01) (C) Upon restoration of presynaptic Ca2+ concentration in SV2B−/− neurons to WT levels (KO(B), open triangles), the mean cumulative ΔCa2+ concentration as a function of the mean cumulative ΔCm became superimposable on that of WT neurons.
What happens to exocytosis in SV2B−/− terminals with a WT Ca2+ phenotype? We addressed this question in SV2B−/− and WT neurons dialyzed with the Ca2+-defined internal recording solution described above (solution B). In contrast to what was observed previously in response to the pulse train, the average magnitude of the 1st capacitance jump in SV2B−/− neurons dialyzed with solution B was no longer suppressed (Figure 6B). Rather, it was indistinguishable from that of WT terminals (Figure 6B; SV2B−/−solutionB: 11.7 ± 1.8 fF, n=16; WTsolutionB: 12.1 ± 1.8 fF, n=17) and significantly different from SV2B−/− neurons dialyzed with solution A. In addition, there was no longer a difference in the slope of the cumulative capacitance increase as a function of pulse number for the first three points in the train (Figure 6B; Slope SV2B−/−solutionB: 3.2 ± 1.4; Slope WTsolutionB: 1.8 ± 1.0, PROC Mixed analysis, p=0.1574), indicating that the early enhancement seen in Figure 3B may represent a Ca2+-dependent process. Furthermore, with solution B, the apparent Ca2+ sensitivity of release for SV2B−/− neurons was no longer shifted to the right, but rather was superimposable upon that of WT neurons (Figure 6C, PROC Mixed analysis, WTsolutionB vs KOsolutionB, p=0.4). Thus, restoration of presynaptic Ca2+ signaling to wild-type patterns relieves the SV2B−/− secretory phenotype.
If the loss of SV2 proteins indirectly alters the time course of membrane recovery following exocytosis via an increase in presynaptic Ca2+ concentration, then mimicking the WT Ca2+ phenotype in an SV2B−/− neuron should restore the time constant of membrane recovery of SV2B−/− terminals to control values. This is precisely what we observed (Figure 5B). For example, following the pulse train, the average time constant of membrane recovery for SV2B−/− neurons was 5.4 ± 1.0 s (solution B; n=9) compared with 5.3 ± 0.9 s for WT neurons (solution B; n=10). Given previous results indicating that larger rises in spatially-averaged Ca2+ concentration are associated with longer time constants of membrane recovery in bipolar neurons (von Gersdorff and Matthews, 1994; Wan et al., 2008), these results suggest that the prolonged time course of membrane recovery in SV2B−/− neurons dialyzed with our standard recording solution (solution A) may be directly attributed to the SV2B−/− Ca2+ phenotype.
Mimicking the SV2B−/− Ca2+ phenotype in WT neurons does not reproduce the full SV2B−/− secretory phenotype
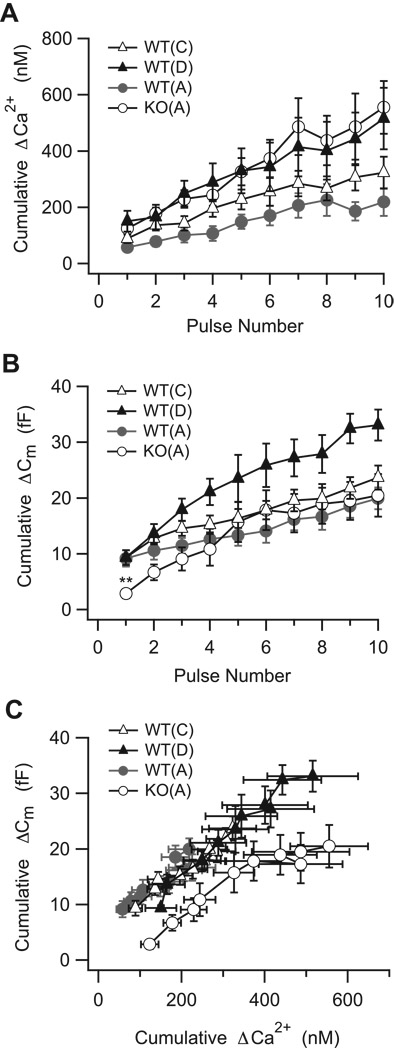
If the secretory phenotype of SV2B−/− neurons is a direct consequence of the SV2B−/− Ca2+ phenotype, then WT neurons with an SV2B−/− Ca2+ phenotype should display the SV2B−/− secretory phenotype. To test this hypothesis, we manipulated the Ca2+ profile in WT and SV2B−/− neurons by internal dialysis with either high Ca2+ solution C or D; these solutions differ in the amount of free EGTA (Table 1). With either solution, approximately two minutes after achieving the whole-cell recording configuration, the resting Ca2+ in WT terminals was increased to a level similar to SV2B−/− terminals (Figure 5C; SV2B−/−solutionA: 112 ± 27 nM, n=14; WTsolutionC: 117 ± 16nM, n=20; WTsolution D: 113 ± 16 nM, n=5) and significantly higher than WT control (32 ± 6 nM, n=12, one-way ANOVA with Dunnett Multiple Comparisons Test, p=0.011). Furthermore, as shown in Figure 7A, dialysis with solution D fully mimicked the elevated train-evoked Ca2+ response of SV2B−/− neurons, while dialysis with solution C, which had a higher concentration of free EGTA (Table 1), had a Ca2+ profile that was intermediary between the WT and SV2B−/− Ca2+ phenotype.
Figure 7. Replication of the SV2B−/− Ca2+ phenotype in WT neurons does not reproduce the SV2B−/− secretory phenotype.
(A) Elevation of the resting Ca2+ concentration in WT neurons to SV2B−/− (KO) levels with solution C for ≈ two minutes did not mimic the cumulative Ca2+ concentration phenotype (WT(C), gray triangles). However, when free EGTA in the high Ca2+ recording solution was reduced with solution D (WT(D), black triangles; see Table 1), the cumulative Ca2+ concentration record resembled that of SV2B−/− neurons (KO(A), open circles). (B) Elevation of resting Ca2+ concentration in WT neurons (WT(C), gray triangles) did not suppress the secretory response to the first pulse in the train relative to WT controls (WT(A), filled circles; one-way ANOVA, followed by Dunnett’s Multiple Comparisons Test. **p<0.01). (C) Elevation of presynaptic Ca2+ concentration, with or without a decrease in free EGTA (WT(C) and WT(D), respectively, failed to reproduce the rightward shift in the mean cumulative ΔCm with respect to the mean cumulative ΔCa2+ concentration that was observed in SV2B−/− neurons.
What happens to exocytosis in WT terminals with an SV2B−/− Ca2+ phenotype? Figure 7B demonstrates that there was no difference in the amplitude of the 1st capacitance jump between WT neurons with a SV2B−/− Ca2+ phenotype (WTsolutionC: 9.3 ± 1.3fF, n=21; WTsolutionD: 9.4 ± 0.5 fF, n=5) and our standard solution A (WTsolutionA: 9.2 ± 1.5 fF, n=13). All three differed from the amplitude of the 1st Cm jump in SV2B−/− neurons dialyzed with standard solution A (Figure 7B; Figure 2.8 ± 0.8 fF, n=12; one way ANOVA with Dunnett Multiple Comparisons Test, p=0.0035). In addition, WT neurons with an SV2B−/−Ca2+ phenotype did not exhibit an apparent decrease in the calcium sensitivity of release (Figure 7C, PROC Mixed analysis, WTsolutionC-WTsolutionA: p=0.21; WTsolutionD-WTsolutionA: p=0.34; WTsolutionC-KOsolutionA: p<0.0001; WTsolutionD-KOsolutionA: p<0.0001). Thus, while elevated resting and evoked presynaptic Ca2+ reveals both the early secretory defect and the decrease in the apparent Ca2+-sensitivity of release, short-term elevation of presynaptic Ca2+ of the type tested here is insufficient to trigger these changes.
Two aspects of the SV2B−/− secretory phenotype, however, were reproduced in WT neurons with an SV2B−/− Ca2+ phenotype. First, similar to what was observed in SV2B−/−neurons (Figure 7B), WT neurons with an SV2B−/− Ca2+ phenotype showed a significant early enhancement in the cumulative capacitance response that was reminiscent of SV2B−/− neurons (Slope WTsolutionD: 4.3 ± 0.02) and different from control WT neurons (Figure 3B; PROC Mixed analysis, slope WTsolutionA vs slope WTsolutionD, p=0.05). Secondly, following the stimulus train, the time constants of membrane recovery in WT neurons with an SV2B−/− Ca2+ phenotype were prolonged relative to WT controls (Figure 5D; WTsolutionA 4.5 ± 0.9 s, n=12; WTsolutionC: 9.1 ± 1.6 s, n=11; WTsolutionD: 7.6 ± 2.1 s, n=5), and approached values observed in SV2B−/− neurons (SV2B−/−solutionA: 9.5 ± 0.8 s, n=8). These results, together with those from the previous section, demonstrate that enhancement and membrane recovery are sensitive to the manipulation of presynaptic Ca2+ signaling in both directions. Thus, these two features of the SV2B−/− secretory phenotype are likely to be a direct consequence of the changes in presynaptic Ca2+ signaling associated with SV2-deficiency.
DISCUSSION
SV2B regulates presynaptic Ca2+
We took advantage of the relatively large and accessible nerve terminal of the mouse rod bipolar neuron to make time-resolved quantitative measurements of presynaptic Ca2+ concentration from SV2-deficient and WT neurons. Our data provide the first direct demonstration that an SV2 protein affects presynaptic Ca2+ signaling. The loss of SV2B, the major SV2 isoform expressed by rod bipolar cells, was associated with an increase in the basal Ca2+ concentration in rod bipolar cell nerve terminals and an increased accumulation of presynaptic Ca2+ during a stimulus train. That these changes in Ca2+ concentration are consequential was evidenced by altered synaptic vesicle dynamics and dramatic changes in the pattern of neurotransmitter release. Our data extend previous studies that have hinted at a role for SV2 in the regulation of presynaptic Ca2+ based upon the action of an exogenous Ca2+ chelator (Chang and Südhof, 2009; Janz et al., 1999). While our results appear to contrast with a recent study in endocrine cells (Iezzi et al., 2005), it is important to note that in that particular study, Ca2+ measurements were made with low temporal resolution and in response to strong stimulation. Thus, small differences in Ca2+ signaling that may have occurred on a fast timescale or in advance of the saturation of other Ca2+ handling mechanisms were unlikely to be detected.
How might SV2 proteins regulate presynaptic Ca2+? SV2 proteins belong to a large superfamily of transporter molecules and contain two conserved negatively charged amino acids in the first transmembrane spanning domain (Janz and Südhof, 1999). This has led to the hypothesis that SV2 proteins are Ca2+ transporters (Janz et al., 1999), moving Ca2+ ions from the cytosol into secretory vesicles, a compartment suggested to contain Ca2+ ions (Kendrick et al., 1977; Schmidt et al., 1980; Xin and Wightman, 1998). We estimate that SV2 could potentially provide at least 10,000 Ca2+ binding sites per bouton, assuming a conservative vesicle density per synaptic bouton in mouse rod bipolar cells approximately 1/5th that of the goldfish Mb1 bipolar terminal (von Gersdorff et al., 1996) and an average of 2 copies of SV2 per synaptic vesicle (Takamori et al., 2006). By contrast, the cumulative train-evoked ΔCa2+ in the SV2B−/− terminals was 337 nM higher than that of wildtype. For a single 3 µm bouton, this corresponds to ≈ 2,869 additional free Ca2+ ions. Thus, the number of Ca2+ binding sites afforded by SV2 molecules would be more than sufficient to account for the difference in stimulus-evoked free Ca2+. In our experiments, loss of SV2 proteins had the most pronounced effect on Ca2+ within the interstimulus interval (250 ms). SV2 proteins might therefore serve as fast, high-affinity Ca2+ transporters or binding proteins.
SV2 proteins also could conceivably interact with voltage-gated Ca2+ channels or Ca2+ pumps to affect changes in Ca2+ signaling. However, the localization of SV2 in the synaptic vesicle membrane makes an interaction with plasma membrane Ca2+ channels or Ca2+ pumps, located on the plasma membrane, mitochondria and endoplasmic reticulum (Krizaj et al., 2002), unlikely. Furthermore, Ca2+ clearance following stimulation, dominated by plasma membrane Ca2+ pumps in rod-dominant bipolar cells (Zenisek and Matthews, 2000), was not affected by the loss of SV2B (Figure 4).
SV2 deficiency alters Ca2+-regulated synaptic vesicle dynamics
There are multiple Ca2+-regulated steps in the secretory pathway (Heidelberger, 2001; Neher and Sakaba, 2008). These include vesicle mobilization and the refilling of vesicle pools (Gomis et al., 1999; Mennerick and Matthews, 1996; Sakaba and Neher, 2001; von Ruden and Neher, 1993; Wang and Kaczmarek, 1998). In response to a stimulus train, rod bipolar cells lacking SV2B exhibited a secretory response that was initially suppressed but was followed by an enhancement that briefly converted depression to facilitation (Figure 3). These findings are congruent with the loss of enhancement and marked synaptic depression observed in cultured SV2A/B−/− neurons following treatment with an exogenous Ca2+-buffer (Chang and Südhof, 2009; Janz et al., 1999). Enhancement was also observed in wild-type neurons with an SV2 Ca2+-phenotype (Figure 7). We therefore propose that altered presynaptic Ca2+ signaling underlies the pronounced changes in synaptic strength observed in SV2-deficient neurons.
Endocytosis is also Ca2+-regulated (Balaji et al., 2008; Ceccarelli and Hurlbut, 1980; Gad et al., 1998; Marks and McMahon, 1998; Sankaranarayanan and Ryan, 2001; Wu et al., 2009). In retinal bipolar cells, elevation of bulk presynaptic Ca2+ levels inhibits compensatory endocytosis (Rouze and Schwartz, 1998; von Gersdorff and Matthews, 1994; Wan et al., 2008). Here, we show that SV2B loss slows membrane recovery following a pulse train and that this is a direct consequence of the change in Ca2+ signaling attributable to SV2B loss (Figures 4 and 5). The mechanism underlying the Ca2+-dependent slowing of endocytosis is not well understood, however it does not appear to be caused by the saturation of the rod bipolar cell endocytotic machinery (Wan et al., 2008). Rather, it may relate to on-going release following Ca2+ channel closure, inhibition of endocytosis by elevated Ca2+ (Rouze and Schwartz, 1998; von Gersdorff and Matthews, 1994), or changes in internal Cl− resulting from activation of a Ca2+-dependent Cl− current (Hull and von Gersdorff, 2004). In this context it is important to note that the slowing of membrane recovery is unlikely to have an immediate impact on pool refilling, as multiple rounds of release can occur in bipolar cells in the absence of endocytosis (Heidelberger et al., 2002; von Gersdorff and Matthews, 1997), presumably because these neurons refill the releasable pool from their unusually large reserve pool (von Gersdorff et al., 1996).
SV2 is required for maintenance of the rapidly-releasing pool
We observed a pronounced exocytotic defect characterized by a reduction in vesicle fusion evoked by a single, non-saturating depolarization and a decrease in the apparent Ca2+ sensitivity of release in SV2B−/− neurons (Figure 3). Both effects are in the range of 6–7 fF, a magnitude similar to that of the rapidly-releasing pool of vesicles in rodent rod bipolar cells (Singer and Diamond, 2006; Wan et al., 2008). Given that there was no change in vesicle diameter or in the number of synaptic ribbons (Figure 1 and Supplementary Table 1) and that the changes in presynaptic Ca2+ concentration were sufficient to evoke release, SV2 deficiency is most likely associated with a defect in the rapidly-releasing pool. Similarly, a decrease in the number of secretory granules available for rapid release has been described in endocrine cells lacking SV2A (Xu and Bajjalieh, 2001), and in SV2A/B−/− cultured neurons, the amplitude of the first postsynaptic current evoked by a train was decreased (Chang and Südhof, 2009), potentially indicating a decrease in the number of vesicles available for release. Thus, SV2A and SV2B modulate the number or function of vesicles available for rapid release. Further, this role is conserved across large, dense-core secretory granules and small, clear-core synaptic vesicles. Interestingly, the total extent of exocytosis evoked by a stimulus train was comparable between WT controls and SV2B−/− neurons (Figure 3B), indicating that the build-up of presynaptic Ca2+ during the pulse train in SV2-deficient neurons and the consequent enhancement overcomes the defect.
SV2 proteins interact with synaptotagmin 1 (Bennett et al., 1992; Lazzell et al., 2004; Schivell et al., 1996; Schivell et al., 2005), a Ca2+ sensor for fast exocytosis that is present in rod bipolar cell terminals (Heidelberger et al., 2003). Synaptotagmin is also important for the positional priming of synaptic vesicles and maintenance of vesicle pools (Young and Neher, 2009). This raises the possibility that the SV2-deficiency secretory phenotype results from altered synaptotagmin function. However, there is almost tenfold more synaptotagmin on synaptic vesicles isolated from the brain than SV2 (Takamori et al., 2006). Thus, if the same ratio holds for synaptic vesicles of retinal ribbon synapses, the relevant synaptotagmin molecules must specifically be those that interact with SV2. However, this scenario does not readily account for the rescue of the SV2B−/− secretory phenotype upon restoration of presynaptic Ca2+ signaling to WT levels. One would need to postulate an additional action such as the presence of SV2 makes the release machinery less sensitive to Ca2+-adaptation, an interesting hypothesis that warrants future investigation.
In some studies, a reduction in synaptotagmin levels has been described in SV2 deficient neurons (Morgans et al., 2009; Yao et al., 2010, but see Crowder et al., 1999; Janz et al., 1999) . In SV2B−/− retinae, photoreceptor terminals, but not bipolar cells, show a decrease in synaptotagmin immunolabeling (Lazzell et al., 2004; Morgans et al., 2009). If the functional reduction in the rapidly-releasing pool and the decrease in the apparent Ca2+ sensitivity of release were due to a loss of synaptotagmin in rod bipolar cells, lowering the SV2B−/− Ca2+ phenotype to wild-type levels should worsen the secretory defect. However, we observed the opposite effect (Figure 6). Furthermore, the rapidity with which the secretory defect was rescued upon lowering presynaptic Ca2+ to WT levels indicates that the synaptotagmin levels in the terminal could support normal secretion and is difficult to reconcile with a reduction in synaptotagmin concentration.
We can similarly rule out changes in the concentrations of other synaptic proteins as mediators of the SV2-deficiency secretory phenotype. In agreement with quantitative immunoblots performed on brains from SV2A/B−/− mice (Janz and Südhof, 1999), analysis of immunolabeling in SV2B−/− retinae did not reveal changes in the levels of other major synaptic vesicle proteins (Lazzell et al., 2004; Morgans et al., 2009). Morgans et al. (2009) did report reductions in synaptobrevin/VAMP, VGluT 1 and synaptophysin in SV2B−/− mouse eye extracts, but these reductions were not of a magnitude known to disrupt synaptic transmission (Fremeau et al., 2004; McMahon et al., 1996; Schoch et al., 2001; Wojcik et al., 2004). As with synaptotagmin, the rapid rescue of the secretory phenotype upon restoration of presynaptic Ca2+ signaling is difficult to reconcile with the reduction of a major vesicular protein.
The hypothesis most consistent with our data is that the role of SV2 in exocytosis is mediated via its ability to regulate presynaptic Ca2+. Potentially, the increase in basal presynaptic Ca2+ concentration could trigger vesicle fusion (Lagnado et al., 1996), decreasing the number of vesicles available for stimulus-driven release (Wasser and Kavalali, 2009; Xu et al., 2009). However, although we observed an increase in capacitance fluctuations in SV2B−/− bipolar cells that might reflect an increase in spontaneous fusion and retrieval events (not shown), our high Ca2+ recording solutions increased the spontaneous capacitance fluctuations of WT neurons to comparable levels without reproducing the secretory defect (Figure 7). In addition, cultured neurons from SV2-deficient mice exhibit a similar secretory defect in the absence of an increase in spontaneous release (Chang and Südhof, 2009; Crowder et al., 1999; Custer et al., 2006). Thus, simple, use-dependent exhaustion of the rapidly-releasing pool is probably insufficient to account for the observed secretory defect.
A more likely explanation for the decrease in the rapidly-releasing pool of vesicles and the decrease in the apparent Ca2+-sensitivity of release is adaptation induced by the chronic elevation of presynaptic Ca2+. Elevated Ca2+ is a well-known stimulus for adaptation in neurons, including at the level of the release machinery (Eatock et al., 1987; Kline et al., 2007; Kurahashi and Shibuya, 1990; Wu et al., 2008). Chronic, mild stimulation has also been suggested to decrease vesicle priming and the number of vesicles available for release (Belair et al., 2005; Millar et al., 2005; Moulder et al., 2006). Synaptic adaptation in the face of mild stimulation requires days, rather than minutes (Moulder et al., 2006), consistent with our inability to reproduce all aspects of the SV2B−/− secretory phenotype upon short-term doubling of the resting presynaptic Ca2+. Our anatomical analyses of SV2-deficient neurons revealed no alteration in the number of synaptic ribbons and no gross deficiencies in the synaptic architecture. Presumably therefore, any adaptive modification associated with SV2-deficiency occurs at the molecular level, consistent with its rapid reversibility. This type of Ca2+-dependent adaptation may play a critical role in the maintenance of synaptic homeostasis in the face of chronically-enhanced Ca2+ associated with SV2-deficiency. Given that all aspects of the SV2-deficiency secretory phenotype are rapidly reversed upon restoration of the Ca2+-phenotype to that of WT controls, our results suggest that therapeutic regulation of presynaptic Ca2+ signaling and Ca2+-accelerated vesicle supply are areas worthy of further exploration as potential interventions for some forms of epilepsy.
METHODS
Animals
All animal procedures conformed to NIH guidelines and were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston. The generation of the SV2B−/− mice has been previously described (Janz et al., 1999). The SV2B−/− mice were bred against C57Bl6 mice for at least 6 generations and were maintained as a heterozygous line. Matching SV2B−/− and WT animals were generated by heterozygous interbreeding. Animals were genotyped using PCR as described (Janz et al., 1999). The person performing the electrophysiology had no knowledge of the genotype of the animals used during the experiment.
Cell isolation
Rod bipolar neurons were isolated from the retinas of 2–6 month old C57Bl6 or SV2B−/− mice by enzymatic digestion followed by mechanical trituration as described previously (Wan et al., 2008; Zhou et al., 2006). Isolated rod bipolar neurons were identified and selected for recording based on morphological criteria as previously described (Zhou et al., 2006).
Electron microscopy and measurement of vesicle size
Following euthanasia of wildtype or SV2B−/− mice, eyeballs were rapidly enucleated, and the anterior segment was removed. The resulting eyecups were fixed in 2% paraformaldehyde + 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) overnight at 4°C. Eyecups were postfixed in 1% OsO4 for 1 hr at 4°C, dehydrated through 50%-100% ethanol followed by propylene oxide and embedded in LX112-araldite epoxy resin (Ladd Research Industries, Williston, VT). Thin sections of gold interference color (approximately 150 nm thickness) were collected onto copper grids, post-stained with lead citrate and uranyl acetate, and examined using a transmission electron microscope (Technai G2 Biotwin, FEI, Hillsboro, OR; or H-7600, Hitachi, Tokyo, Japan). To analyze synaptic structure and vesicle size, images of bipolar cell terminals in wildtype and SV2B knockout retinas were imaged digitally at 20,000x-50,000x. Images were imported into NIH ImageJ and image scale was calibrated. Individual vesicles were traced manually along their outer circumference and the following variables were measured: vesicle area (nm2), vesicle perimeter (nm), major axis and minor axis (determined by the best-fit ellipse), and the maximum diameter of the vesicle (Feret’s diameter). The synaptic vesicle diameters measured in our studies are similar to the synaptic vesicle diameter reported previously for mouse rod bipolar cells (Spiwoks-Becker et al., 2001) and slightly larger than the diameter reported for synaptic vesicles in goldfish Mb bipolar cell terminals (von Gersdorff et al., 1996). The small differences in vesicle sizes observed across these studies likely arise from differences in fixation, tissue processing and embedding media. We also calculated an adjusted Feret’s diameter using Abercrombie’s correction factor to compensate for systematic underestimation of objects that can occur in thin sections (Abercrombie, 1946). Statistical comparisons were performed using Student’s two-tailed t-test for unpaired samples. The critierion for statistical significance was set at p< 0.05.
Immunocytochemistry and Imaging
Freshly isolated SV2B−/− mouse retinal cells were deposited onto glass coverslips coated with 0.1 mg/ml poly-D-lysine and allowed to settle for 15 min. The cells were washed in 0.1M phosphate buffer (PB), fixed in 4% paraformaldehyde for 15 min at RT, and then rinsed. Cells were incubated in primary antibodies diluted in 1% normal goat serum in PB for 24 hours at 4°C. To positively identify synaptic ribbons in rod bipolar cells, isolated cells were labeled simultaneously using a mouse monoclonal antibody against the synaptic ribbon marker, CtBP2/Ribeye (1:200; BD Transduction Laboratories, San Jose, CA) and a rabbit polyclonal antiserum against the rod bipolar cell marker PKC-α (1:1000; Calbiochem, San Diego, CA). Secondary antisera specific for rabbit or mouse immunolglobulins were raised in goat and were conjugated to either AlexaFluor 568 or AlexaFluor 488 (1:1000 dilution; Molecular Probes, Eugene, OR). Immunolabeling was imaged with a Zeiss Laser Scanning Microscope 510 META (Zeiss, Thornwood, NY) using either Plan Apochromat 63x (1.4 NA) or Plan Neofluar 40X (1.3 NA) oil immersion objective lenses. Images in the two fluorescent channels were collected sequentially and laser power and detector sensitivity were adjusted to prevent spectral bleed-through during image acquisition. To determine the number of synaptic ribbons in rod bipolar cell terminals of cells isolated from WT and SV2B−/− retina, short stacks of optical sections through the terminals of PKC-α positive rod bipolar cells were acquired (typically 6 optical sections at a step size of 0.2 µm) to ensure that individual ribbons were only counted once. Image stacks were compressed into a maximum intensity projection and the number of synaptic ribbons in rod bipolar cell terminals was determined by counting the number of CtBP2-positive fluorescent puncta within the PKCα-positive terminals.
Electrophysiological and intraterminal Ca2+ measurements
All experiments were performed at room temperature (~25°C). Whole-cell recordings from the soma of intact rod bipolar neurons were performed with 5–6 MΩ pipette pulled from borosilicate glass using a Sutter Instruments P-97 puller (Novato, CA, USA). Electrophysiological and capacitance measurements were made using an EPC-9 patch-clamp amplifier controlled via PULSE LOCK-IN software (HEKA Electroniks, Lambrecht, Germany). To monitor membrane capacitance, a 30 mV peak-to-peak, 800 Hz sinusoidal voltage command was applied about the holding potential of −70 mV, and the resultant signal was processed by the Lindau-Neher technique to calculate Cm, Gm and Gs (Wan et al., 2008; Zhou et al., 2006). For time-resolved capacitance measurements, one capacitance point was generated per each 100 ms sweep. For high-resolution capacitance measurements during the train, one capacitance data point was generated per sine-wave cycle. Briefly, a 4 Hz train of ten 100 ms depolarizing pulses (−70 to 0 mV) was given (Figure S1). Five ms after each depolarizing pulse, a 50 ms sinusoidal voltage command was superimposed over the holding potential of −70 mV. The first 25 ms interval following the 100 ms depolarization was excluded from analysis due to potential complications associated with gating currents and/or the decay of depolarization evoked conductance changes (Gillis, 1995; Horrigan and Bookman, 1994; Wan et al., 2008). The mean capacitance value for each depolarization in the train was determined from the average capacitance value of the remaining 30 ms period.
The standard external recording solution contained (in mM): 127 NaCl, 5 CsCl, 20 TEA-Cl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH = 7.4, 315 – 320 mOsm. Unless noted, recordings were made using the standard intracellular solution (Table 1, solution A) which contained (in mM): 125 Cs-gluconate, 10 TEA-Cl, 3 MgCl2, 2 Na2ATP, 0.5 GTP, 0.5 EGTA, 0.2 bis-fura-2, 35 HEPES, pH = 7.2, 310 – 315 mOsm. Additional intracellular solutions used to clamp presynaptic Ca2+ and free EGTA to specific concentrations are defined in Table 1. To ensure that terminals were fully dialyzed with the intracellular solution, we allowed at least 2 minutes for the bis-fura-2 fluorescence signal to reach a plateau before presenting the first stimulus. Intraterminal Ca2+ measurements were performed using a computer-controlled monochrometer-based system (ASI/TILL Photonics, Eugene, OR (Messler et al., 1996). Intraterminal Ca2+ concentration was determined from the ratio of the fluorescence signals excited at 340 and 380 nm, using calibration constants obtained as described previously (Heidelberger and Matthews, 1992). An adjustable aperture was used to position the collection field for emitted fluorescence selectively over the terminal (Wan et al., 2008; Zhou et al., 2006).
Data Analysis
Cells with leak currents larger than 30 pA or access resistances > 35 MΩ were excluded from analysis. Cells were also excluded if changes in Cm were correlated with changes in membrane conductance (Gm) and series conductance (Gs). Data analysis was performed using IGOR Pro software (Wavemetrics, Lake Oswego, OR, USA). Results are presented as mean ± SEM. To calculate the cumulative ΔCm, the membrane capacitance after each depolarization pulse was subtracted from the resting Cm, which was defined as the average Cm value over the 5 s period immediately before the first depolarization. To calculate cumulative ΔCa2+, the Ca2+ concentration immediately after each depolarization pulse was subtracted from the resting Ca2+ concentration, which was defined as the average Ca2+ concentration over the 5 s period immediately before the first depolarizing stimulus pulse. To measure the rate of membrane recovery, for each record, the falling phase of the capacitance response was fit with a single exponential function (Wan et al., 2008).
Statistical comparisons were performed using the two-tailed, unpaired Student's t-test or one-way ANOVA with Dunnett’s Multiple Comparisons Test (Sigmaplot 11.0 software, Access Softek, San Jose, CA, USA), unless indicated otherwise. To estimate the effects of mean cumulative ΔCa2+ on mean cumulative ΔCm among groups, an analysis of covariance with repeated measurements (PROC Mixed) was performed using SAS for Windows 9.1 software (SAS Institute, Cary, NC). The dependent variable was the mean cumulative ΔCm and independent variables were the mean cumulative ΔCa2+, group, and intergroup interaction. If the effect of the interaction term was not significant, it indicated that the slopes of linear trend did not differ among the groups and a model with different intercepts and same slope was further studied. PROC Mixed analysis with a repeated measurement model was used to estimate the slope of the mean cumulative ΔCm during the initial 3 pulses of the train among groups. Statistical significance was set at p ≤ 0.05.
Highlights
Loss of SV2 results in elevated resting and evoked Ca2+ signals in nerve terminals.
Loss of SV2 alters synaptic vesicle dynamics, synaptic strength and plasticity.
Most of the secretory phenotype is reproduced in WT terminals upon elevating Ca2+.
Restoration of Ca2+ signaling completely rescues the SV2 secretory phenotype.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Eye Institute Grant EY-12128 to R. Heidelberger, EY-16452 to R. Janz and Core Grant EY-10608. We thank Dr. Alice Z. Chuang for her assistance with the statistical analysis and Margaret Gondo and Joe Wilkerson for technical assistance with electron microscopy. Electron microscopy at the University of Houston was supported by an NIH CORE grant to the University of Houston College of Optometry (P30 EY07751). Electron microscopy at the University of Oklahoma Health Sciences Center was performed at the Oklahoma Medical Research Foundation Imaging Core Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Q.-F.W, Z.-Y.Z, P.T., D.S., R.J. and R.H. contributed to the design and interpretation of the experiments. Q.-F.W, Z.-Y.Z, and P.T. conducted the electrophysiological experiments. A.V. and P.T. performed the immunocytochemistry. D.S. performed the electron microscopy. R.H., Q.-F.W., and R.J. wrote the manuscript. R.H. and R.J. supervised the project. All authors have approved the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
REFERENCES
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec (Hoboken) 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belair EL, Vallee J, Robitaille R. Long-term in vivo modulation of synaptic efficacy at the neuromuscular junction of Rana pipiens frogs. J Physiol. 2005;569:163–178. doi: 10.1113/jphysiol.2005.094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Südhof TC. SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J Neurosci. 2009;29:883–897. doi: 10.1523/JNEUROSCI.4521-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci U S A. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci. 2006;26:1303–1313. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell. 2008;19:5226–5237. doi: 10.1091/mbc.E08-07-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc R Soc Lond B Biol Sci. 1968;170:205–228. doi: 10.1098/rspb.1968.0034. [DOI] [PubMed] [Google Scholar]
- Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987;7:2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, Xi Z, Wang X. Down-regulation Synaptic Vesicle Protein 2A in the Anterior Temporal Neocortex of Patients with Intractable Epilepsy. J Mol Neurosci. 2009 doi: 10.1007/s12031-009-9288-2. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Neher BSE, editor. In Single-Channel Recording. New York and London: Plenum Press; 1995. pp. 155–198. [Google Scholar]
- Gomis A, Burrone J, Lagnado L. Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J Neurosci. 1999;19:6309–6317. doi: 10.1523/JNEUROSCI.19-15-06309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R. Electrophysiological approaches to the study of neuronal exocytosis and synaptic vesicle dynamics. Rev Physiol Biochem Pharmacol. 2001;143:1–80. doi: 10.1007/BFb0115592. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Wang MM, Sherry DM. Differential distribution of synaptotagmin immunoreactivity among synapses in the goldfish, salamander, and mouse retina. Vis Neurosci. 2003;20:37–49. doi: 10.1017/s095252380320105x. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Zhou ZY, Matthews G. Multiple components of membrane retrieval in synaptic terminals revealed by changes in hydrostatic pressure. J Neurophysiol. 2002;88:2509–2517. doi: 10.1152/jn.00267.2002. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci. 2005;118:5647–5660. doi: 10.1242/jcs.02658. [DOI] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Janz R, Hofmann K, Südhof TC. SVOP, an evolutionarily conserved synaptic vesicle protein, suggests novel transport functions of synaptic vesicles. J Neurosci. 1998;18:9269–9281. doi: 10.1523/JNEUROSCI.18-22-09269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R, Südhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience. 1999;94:1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- Kendrick NC, Blaustein MP, Fried RC, Ratzlaff RW. ATP-dependent calcium storage in presynaptic nerve terminals. Nature. 1977;265:246–248. doi: 10.1038/265246a0. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Demarco SJ, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol. 2002;451:1–21. doi: 10.1002/cne.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P. Levetiracetam: the profile of a novel anticonvulsant drug-part I: preclinical data. CNS Drug Rev. 2007;13:43–56. doi: 10.1111/j.1527-3458.2007.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Gomis A, Job C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron. 1996;17:957–967. doi: 10.1016/s0896-6273(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem. 2004;279:52124–52131. doi: 10.1074/jbc.M407502200. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS letters. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Bolshakov VY, Janz R, Hammer RE, Siegelbaum SA, Südhof TC. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc Natl Acad Sci U S A. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Messler P, Harz H, Uhl R. Instrumentation for multiwavelengths excitation imaging. Journal of neuroscience methods. 1996;69:137–147. doi: 10.1016/S0165-0270(96)00032-5. [DOI] [PubMed] [Google Scholar]
- Millar AG, Zucker RS, Ellis-Davies GC, Charlton MP, Atwood HL. Calcium sensitivity of neurotransmitter release differs at phasic and tonic synapses. J Neurosci. 2005;25:3113–3125. doi: 10.1523/JNEUROSCI.4717-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Kensel-Hammes P, Hurley JB, Burton K, Idzerda R, McKnight GS, Bajjalieh SM. Loss of the Synaptic Vesicle Protein SV2B results in reduced neurotransmission and altered synaptic vesicle protein expression in the retina. PLoS ONE 4, e5230. 2009 doi: 10.1371/journal.pone.0005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Taylor AA, Olney JW, Mennerick S. Physiological activity depresses synaptic function through an effect on vesicle priming. J Neurosci. 2006;26:6618–6626. doi: 10.1523/JNEUROSCI.5498-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of Ca2+ at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Rouze NC, Schwartz EA. Continuous and transient vesicle cycling at a ribbon synapse. J Neurosci. 1998;18:8614–8624. doi: 10.1523/JNEUROSCI.18-21-08614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem. 1996;271:27770–27775. doi: 10.1074/jbc.271.44.27770. [DOI] [PubMed] [Google Scholar]
- Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci. 2005;29:56–64. doi: 10.1016/j.mcn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Zimmermann H, Whittaker VP. Metal ion content of cholinergic synaptic vesicles isolated from the electric organ of Torpedo: effect of stimulation-induced transmitter release. Neuroscience. 1980;5:625–638. doi: 10.1016/0306-4522(80)90060-3. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Südhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular Anatomy of a Trafficking Organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Wan QF, Vila A, Zhou ZY, Heidelberger R. Synaptic vesicle dynamics in mouse rod bipolar cells. Vis Neurosci. 2008;25:523–533. doi: 10.1017/S0952523808080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wang MM, Janz R, Belizaire R, Frishman LJ, Sherry DM. Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. J Comp Neurol. 2003;460:106–122. doi: 10.1002/cne.10636. [DOI] [PubMed] [Google Scholar]
- Wasser CR, Kavalali ET. Leaky synapses: regulation of spontaneous neurotransmission in central synapses. Neuroscience. 2009;158:177–188. doi: 10.1016/j.neuroscience.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Surmeier DJ, Disterhoft JF. Coupling of L-type Ca2+ channels to KV7/KCNQ channels creates a novel, activity-dependent, homeostatic intrinsic plasticity. J Neurophysiol. 2008;100:1897–1908. doi: 10.1152/jn.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Q, Wightman RM. Simultaneous detection of catecholamine exocytosis and Ca2+ release from single bovine chromaffin cells using a dual microsensor. Anal Chem. 1998;70:1677–1681. doi: 10.1021/ac970746o. [DOI] [PubMed] [Google Scholar]
- Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol. 2001;3:691–698. doi: 10.1038/35087000. [DOI] [PubMed] [Google Scholar]
- Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci. 2010;30:5569–5578. doi: 10.1523/JNEUROSCI.4781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SM, Jr, Neher E. Synaptotagmin has an essential function in synaptic vesicle positioning for synchronous release in addition to its role as a calcium sensor. Neuron. 2009;63:482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Zhou ZY, Wan QF, Thakur P, Heidelberger R. Capacitance measurements in the mouse rod bipolar cell identify a pool of releasable synaptic vesicles. J Neurophysiol. 2006;96:2539–2548. doi: 10.1152/jn.00688.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.