Abstract
Background
Adults with severe mental illness (SMI) display an increased prevalence of HIV compared with the general population. Recommendations for provider-initiated testing in South Africa lack robust evidence because the studies – mainly from low-prevalence regions – overestimate the risk of HIV among adults with SMI.
Objective
We aimed to assess whether the mentally ill are a vulnerable population in South Africa.
Methods
All new admissions to an acute psychiatric ward from July to December 2000 were tested for HIV anonymously and the results linked with socio-demographic and clinical data. We did a restricted analysis of black females from a population-based survey of black females with SMI.
Results
There were 216 admissions, of whom 206 were included in the analysis. The seroprevalence of HIV-1 was 29.1% (confidence interval 27.8 – 32.4); seroprevalence of HIV among the women was 40%. The following predicted HIV positivity: female (odds ratio (OR) 3.5 (1.7 – 6.9)), infections of the chest or central nervous system (OR 3.2 (1.4 – 7.5)), age group 30 – 39 years (OR 2.3 (1.1 – 4.8)) and aggression on admission. The age and sex ratios among the SMI group were similar to the general population. Adults with SMI have an almost three times greater prevalence of HIV than the general population, after controlling for age and gender.
Conclusions
Adults with SMI are a vulnerable population; therefore, provider-initiated HIV testing and other prevention and treatment programmes must be tailored to their needs.
HIV is associated with major psychiatric disorders (also termed severe mental illness (SMI)), including major depression and bipolar and psychotic disorders.1,2 Adults with SMI have a 2 – 36 times higher seroprevalence of HIV than the general population,3–6 and HIV-infected people have a higher prevalence of SMI.7 A systematic review concluded: ‘… adults with SMI are at high risk for HIV/STD’.8 In their review, Joska et al. recommended provider-initiated testing and counselling (PITC) for adults with SMI.9 The World Health Organization (WHO) recommends PITC in generalised epidemics and prioritises implementation in vulnerable groups (e.g. medical inpatients10), but adults with SMI are not explicitly identified. PITC can be implemented subject to the following: informed consent, the right to refuse, the availability of counselling, follow-up, and antiretroviral (ARV) treatment.10 PITC has drawn much criticism;11 its prerequisites cannot be assured in patients with SMI. The Mental Health Care Act (MHCA) allows medical superintendents to approve HIV testing. There is limited evidence from South Africa to recommend policy changes that have serious ethical and public health implications. We studied whether adults with SMI can be considered as a vulnerable population.
The recommendation for PITC is based on evidence mainly from cross-sectional studies in the developed world on the prevalence of SMI among HIV-infected people or seroprevalence among those with SMI.9 The strength of association between HIV and mental illness varies according to the presence of other risk factors in these different populations. There are risk factors (e.g. substance abuse) common to HIV, and psychiatric disorders that may act as mediators, moderators, proxies, confounders or overlapping risk factors. There are also conceptual reservations about how these risk factors work together and whether statistical tests can fully adjust for these effects.12 The increased HIV prevalence in patients with SMI may be overestimated because studies included high-risk groups such as the homeless and intravenous drug users, and the treatment setting varied, with inpatient, outpatient and forensic units. Himelhoch et al. found that the elevated prevalence of HIV among those with SMI is due to the HIV-related risk factors that underlie the association between HIV and SMI,13 viz. age <39 years, male, African-American, Latino, schizophrenia, dual diagnosis, and high-risk behaviour (prostitution, sex with parenteral drug-taker, male-male sex, drug injection).7 Mentally ill patients with HIV in the developed world have multiple HIV risk factors that account for the increased prevalence of HIV.
Despite more than 68% of the world’s HIV burden being in sub-Saharan Africa,14 few studies from the region describe the seroprevalence of HIV among the mentally ill.9,15–17 Our study describes the seroprevalence of HIV in an acute psychiatric ward in South Africa, which has a generalised epidemic. In a region with a generalised epidemic, HIV is firmly established and can be sustained by sexual networking alone. Therefore, people with HIV may not have multiple risk factors for HIV. This state contrasts with the multiple risk factors for HIV among those with SMI, as described in low-prevalence regions. We may obtain a more valid assessment of the association between HIV and SMI because there are fewer statistical adjustments and the unknown effect of multiple overlapping risk factors is not applicable. We compared HIV seroprevalence in the SMI group with the general population; if higher, this group should be considered to be a vulnerable population. If adults with SMI are a vulnerable population, it would partly counter the arguments against PITC11 and support special HIV prevention and treatment programmes targeted at this group.
Methods
We undertook a descriptive study from 1 July to 31 December 2000 in the in-patient psychiatric ward at King Edward VIII Hospital (KEH), a public sector hospital affiliated with the Nelson R Mandela School of Medicine in Durban, KwaZulu-Natal (KZN). KZN has a population of over 9.5 million people of whom only about 9% have private health insurance. The HIV seroprevalence among 15 – 49-year-olds in KZN was 15.7% in 2002.18 This figure has since increased; it is estimated that, in 2008, 5.7 million South Africans were HIV infected.
Patients are referred to the KEH psychiatric department from outlying community clinics and other hospital departments. The psychiatric ward is a 20-bed acute psychiatric unit for males and females; only those with SMI are admitted, and only medically stable patients are admitted to the psychiatric ward as those with serious medical conditions are managed in the medical wards. All patients admitted to this ward during this period were tested for HIV using the enzyme-linked immunosorbent assay (ELISA) and Western blot tests. The attending clinician completed a questionnaire to collect data on age, sex, medical history, substance abuse in the last 3 months, physical findings, duration of hospital stay, and final diagnosis, which were linked with HIV test results for the analysis.
There were 216 admissions, and no patient was counted twice; 8 with incomplete clinical data or missing sero-status, and 2 with equivocal test results, were excluded. We present the data from more than 95% of all admissions from the study period.
The Nelson R Mandela School of Medicine Bioethics Research Ethics Committee and the Columbia University Institutional Review Board (IRB) approved this study.
HIV infection status
Discarded blood taken for routine chemistry as part of baseline investigations was tested for HIV by the ELISA test. All identifiers, except a unique 6-digit number assigned for the purpose of the study, were removed. Reactive samples were confirmed with the Western blot test and classified as recommended by the Centers for Disease Control. A specimen was considered positive if antibodies to two of the following were detected: p24, gp41 and gp120/160. The presence of a single band on the Western blot was considered an indeterminate result. Retesting when results were inconclusive was impossible as testing was anonymous.
Data analysis
Psychosis was defined as the presence of two or more of the following: hallucinations, delusions, abnormal behaviour (e.g. removing clothes, aggression) or formal thought disorder. Aggression was assessed from historical data and clinical assessment.
Substance abuse was defined as a pattern of use that caused clinically significant distress or impairment. The type of substance was specified.
Examples of medical co-morbidity are pulmonary tuberculosis and meningitis, and chronic disorders included hypertension and asthma. Patients with delirium and unstable medical co-morbidities were managed in the medical wards, or their conditions were stabilised before they were transferred to the psychiatric ward.
Age and duration of hospital stay were measured continuously. The distributions were checked for normality and log transformed. Age was divided into categories commonly presented in other HIV epidemiological surveys.18
Methods of analysis
Descriptive statistics were used to describe the study population. To determine significant differences, t-tests, chisquared and Fisher’s exact tests were used. Odds ratios (ORs) were used to evaluate the association between each covariate and the outcome. Results were considered statistically significant where p<0.05. We compared our data with a valid estimate of HIV seroprevalence in the general population of South Africa. The Nelson Mandela/Human Sciences Research Council (HSRC) study of HIV/AIDS was a national, population-based survey that included a representative sample of more than 9 900 people from all provinces, localities and races.18,19 Data for this study were collected a year after our study was completed. Therefore, we have a valid estimate of the seroprevalence of HIV in the general population in the year 2000. We obtained the sample size in specific age, race and gender categories, and did a restricted analysis of the HIV-positive women in our study. To adjust for the confounding effects caused by the differing age structure of our study population, we standardised age-specific and overall prevalence.
Differences between HIV-positive and negative admissions on various characteristics were assessed using Pearson chisquared tests (categorical data) and t-tests (continuous data). Variables that were statistically significant in the univariate analysis were entered into a stepwise multivariate logistic regression model to predict HIV status. To distinguish acute diseases from chronic medical disorders, infections of the central nervous system (e.g. meningitis) and chest (e.g. pulmonary tuberculosis) were collapsed into one category. ORs and 95% confidence intervals (CIs) are provided. Data were analysed using the Statistical Package for the Social Sciences (SPSS), version 16.0.
Results
Overall seroprevalence of HIV and socio-demographic characteristics of HIV-positive and HIV-negative patients
A complete set of confirmed HIV results and clinical records were available for 206 patients. The overall prevalence of HIV was 29.1% (CI 27.8 – 32.4% – Table I). Women had an approximately twofold increase in seroprevalence compared with men (female:male ratio = 2.1:1). The majority of patients were <30 years old, and no HIV-positive patient was >50 years. All patients had SMI and most were psychotic. The spectrum of substance abuse was limited and did not include intravenous drugs. Cannabis was the most common drug. A higher proportion of HIV-negative than HIV-positive patients took cannabis (p=0.007).
Table I.
Socio-demographic, clinical and psychiatric characteristics of patients in a ward, 2000
| HIV positive (N=60) |
HIV negative (N=146) |
p-value | |
|---|---|---|---|
| Female (%) | 60 | 36 | 0.001 |
| Age (yrs) (%) | 30.2 | 30.6 | |
| 15 – 29 | 50 | 58 | |
| 30 – 39 | 37 | 20 | 0.02 |
| 40 – 49 | 13 | 12 | |
| ≥50 | 0 | 10 | |
| Black (%) | 100 | 100 | |
| Substance abuse (%) | |||
| Cannabis | 18 | 38 | 0.007 |
| Alcohol | 12 | 15 | |
| Mandrax | 1.5 | 0.5 | |
| Glue | 1.5 | 0.5 | |
| Physical findings (%) | |||
| Normal | 60 | 77 | |
| Chest infections | 13 | 2 | 0.001 |
| (pulmonary TB, pneumonia) | |||
| CNS infections | 13 | 8 | 0.04 |
| (e.g. cryptococcal meningitis) | |||
| Chronic disorders | 8 | 8 | |
| (e.g. hypertension, asthma) | |||
| Symptoms | |||
| Hallucinations – | 65 | 63 | |
| any type (%) | |||
| Auditory only | 37 | 41 | |
| Visual only | 2 | 14 | 0.009 |
| Auditory and visual | 25 | 8 | |
| Auditory and tactile | 2 | 1 | |
| Mood symptoms: any (%) | 40 | 29 | |
| Depressive symptoms | 18 | 12 | |
| Manic symptoms | 22 | 17 | |
| Formal thought | 10 | 15 | |
| disorder (%) | |||
| Abnormal behaviour (%) | 67 | 74 | |
| Aggression (%) | 52 | 43 | |
| Delusions (any type) (%) | 48 | 54 | |
| Psychosis (%) | 90 | 90 | |
| Total stay in hospital (days) | 10.8 | 14 | 0.05 |
CNS = central nervous system.
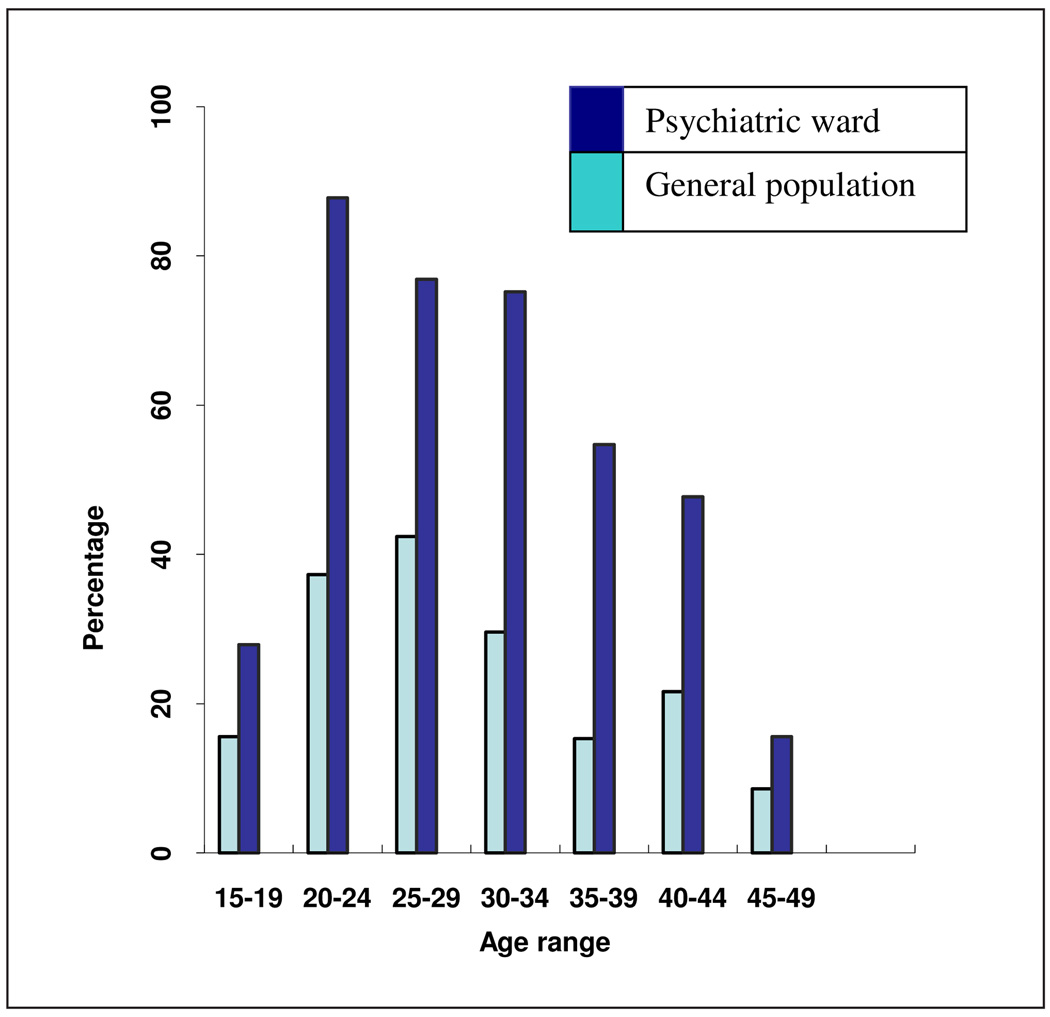
The prevalence of HIV among black women aged 15 – 49 years in the general population in South Africa was 20.7%.18 The prevalence of HIV among the women in this study was 40.1% (36/88). To control for the confounding effects of gender and age, we standardised the age and did a restricted analysis of the women in our study (all black) and the black women in the HSRC study (Fig. 1). Black women with SMI had a consistently higher prevalence of HIV in each age category. The difference was largest (3.6 times) in the 35 – 39-year age group.
Fig. 1.
Comparison of the prevalence of HIV among black women in the general population and black women with severe mental illness after standardising for age.
Multiple logistic regression analysis confirmed that socio-demographic characteristics (female and age group 30 – 39 years) and certain clinical characteristics (aggression and meningitis or tuberculosis) were independently associated with HIV (Table II). The adjusted OR showed that women have a 3.5 times greater probability of being HIV positive than men.
Table II.
Multiple logistic regression to determine predictors of HIV seropositivity
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Male | 1.0 | ||
| Female | 3.45 | 1.73 – 6.91 | 0.000 |
| Normal | 1.0 | ||
| Infections in the | 3.22 | 1.38 – 7.51 | 0.007 |
| CNS or chest | |||
| Age 15 – 29 yrs | 1.0 | ||
| Age 30 – 39 yrs | 2.31 | 1.13 – 4.75 | 0.022 |
| No aggression | 1.0 | ||
| Aggression | 2.11 | 1.05 – 4.21 | 0.035 |
Discussion
The overall prevalence of HIV among adults with SMI was 29.1% (CI 27.8 – 32.4). This was almost three times higher than that of the general population. The seroprevalence among black women in our study was 40%. HIV seroprevalence was higher among mentally ill women than women in the general population across all age categories. Socio-demographic characteristics (age and sex) and known clinical characteristics (past or current infections, e.g. meningitis and pulmonary TB) were more predictive of HIV status than psychiatric symptoms (aggression). There were no cases of intravenous drug use.
The seroprevalence of HIV in our study was higher than in all other published studies of adults with SMI, notwithstanding the differences of study populations and HIV testing methods.3–6,15–17,20–22 To assess if adults with SMI have a higher HIV seroprevalence than the general population, a valid estimate of the HIV seroprevalence in the general population must be used. There is marked heterogeneity of HIV, even within a country;18 HIV varies by race, sex and age.18 Antenatal surveys only include sexually active, fertile, pregnant females from a limited age category, and who are therefore unsuitable for estimating the prevalence of HIV in the general population. Population-based surveys, of which the HSRC study is the latest,18,19 provide a more valid estimate of the prevalence of HIV in the general population. In 2002, the overall prevalence of HIV in South Africa was 11.4%.18
HIV is a female-predominant epidemic in sub-Saharan Africa. In South Africa, women between the ages of 15 and 24 years were four times more likely to acquire HIV than men (16.9% v. 4.4%).18 In our study, the overall and gender-specific HIV seroprevalence was higher than that of the general population. Adults with SMI have a higher prevalence of HIV than the general population, even after considering racial, gender and age differences.
Compared with the general population, there was an almost tripled increase of HIV in adults with SMI (11.4% v. 29.1%). The prevalence of HIV in anonymous linked studies that were conducted in low-prevalence regions vary from 1% to 7%,22,23 which is 2 – 12 times higher than the prevalence of HIV in the general population. This difference is greater in studies that included patients with common risk factors for HIV and mental illness.6–8 The joint risk of acquiring HIV from other high-risk behaviours that accompany mental and co-morbid disorders (e.g. substance abuse) may account for the increased seroprevalence among the mentally ill compared with the general population. An HIV seroprevalence of 19.4% was reported in adults with SMI who were homeless and had high levels of alcohol abuse.6 Schizophrenics without substance abuse were found to have a lower risk for HIV.13 In this study, the prevalence of substance abuse (mainly cannabis) among the HIV-positive patients was low.
HIV-positive patients with SMI in low-prevalence regions have multiple risk factors for HIV.7,8 The age and sex ratios of the HIV-positive patients in our study were similar to those of the HIV-positive patients in the general population.18 The multivariate analysis confirms that age and sex are associated with HIV seropositivity, which suggests that the same factors driving the epidemic regionally are responsible for the high HIV prevalence among the mentally ill. However, the remaining excess seroprevalence of HIV suggests an increased risk to the mentally ill for contracting HIV. This claim is made cautiously as cross-sectional studies cannot establish causality, and our study was not designed to examine the nature of the association. Others have reported that patients with schizophrenia and SMI have poor HIV/AIDS knowledge, cognitive deficits, affective instability, behavioural impulsivity, and high rates of HIV risk-behaviour, which all contribute to HIV acquisition. In summary, the seroprevalence of HIV among those with SMI appears to be a function of their risk networks. In low-prevalence regions, those with SMI have multiple risk factors for HIV, including sexual and drug-related behaviours. However, in a generalised epidemic, sexual contact is the main mode of transmission.
After considering possible biases that might have been introduced through methodological and statistical differences, we conclude that the severely mentally ill is a vulnerable population, and we recommend provider-initiated testing and counselling. Public health benefits must not undermine the human rights of patients with SMI; PITC may increase stigma, the right to autonomy must be respected, and those with SMI may often not be able to decline testing or give informed consent. PITC must not be implemented in a vacuum; confidentiality, post-test counselling when patients can assimilate information, and access to ARVs and ongoing HIV care must be assured.
Our study has the following strengths: We have a valid estimate of the seroprevalence of HIV. As a result of anonymous testing, we included more than 95% of all admissions for the study period, which increased the power and reduced selection bias. The seroprevalence of HIV ranged from 1% to 10% in studies that tested patients anonymously for HIV,20,21 and from 0% to 28% in studies with voluntary testing.4,5,21 Anonymous testing is more accurate because it includes patients who may otherwise not consent to HIV testing, or patients incapable of giving informed consent. Studies with informed consent may self-select patients who suspect they may be HIV-positive. Almost all our patients were psychotic on admission and might have been unable to consent to HIV testing. Ethical problems with anonymous testing include the consideration that it would be considered unethical to test an individual and not disclose the result if effective treatment is available. At the time of this study (2000), no ARV therapy was available in the public sector in South Africa. It is unlikely that another, similar study would be approved by any IRB, and future studies will have selection bias. We acknowledge that the data were collected in 2000 but, given the ethical considerations of anonymous testing and easier access to ARVs, it is unlikely this study design will be replicated. We had a valid estimate of HIV among the general population at the time of our study.
Our findings are limited mainly to black females with SMI. Almost all patients were psychotic, and this study was conducted in a tertiary hospital in an urban centre. HIV-negative patients with less severe or less common mental disorders might have been treated in the community. Psychosis per se might have been a bias, as the patients with positive features (e.g. aggression and hallucinations) might have accessed care, and people with negative symptoms might have been managed in the community. Patients presenting with aggression were more likely to be HIV positive. However, these factors may explain why the total length of stay for the HIV-positive patients was shorter (Table I), as aggressive or unmanageable patients were transferred to another, longer-term ward. We did not do in-depth interviews and did not use validated scales. Therefore, we came to no firm conclusions regarding risk behaviour and clinical findings. We did not measure other potential confounders, e.g. income, sexual behaviour, etc. The socio-demographic characteristics were reliably determined. We have confidence in the data, as the socio-demographic findings were replicated in the univariate and multivariate analysis. Access to health care and criteria for admission to hospital are other factors that will affect the prevalence of HIV in a hospital setting. An equal proportion of HIV-positive and negative patients were psychotic on admission. Only patients with SMI were admitted to hospital, and access to care was not differential.
Conclusion
Notwithstanding its limitations, this study adds to studies of the mentally ill in a generalised epidemic. Our HIV seroprevalence finding among those with SMI was larger than that of the general population, even after considering variation by sex, age and race, which supports our conclusion that those with SMI are a vulnerable population and that HIV prevention and treatment programmes must be scaled up to address their needs. Our study provides empirical data to support the recommendations for provider-initiated testing for SMI patients.9 More public debate is encouraged on the ethics and human rights aspects of PITC in the mentally ill. In South Africa, provisions within the Mental Health Care Act allow for implementation of PITC.
Acknowledgments
Dinesh Singh thanks Margaret G Nair, MD, for her contribution to earlier drafts of this paper. Dinesh Singh was supported by Fogarty International Centre, NIH, grant 5-D43-TW00231 (AIDS International Training and Research Program, Quarraisha Abdool Karim, PhD, Principal Investigator).
Contributor Information
Dinesh Singh, Department of Psychiatry, University of KwaZulu-Natal and HIV Prevention Research Unit, Medical Research Council, Durban.
Alan Berkman, Mailman School of Public Health, Columbia University, New York, USA.
Michaeline Bresnahan, Mailman School of Public Health, Columbia University, New York, USA.
References
- 1.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 2.Harris MJ, Jeste DV, Gleghorn A, Sewell DD. New-onset psychosis in HIV-infected patients. J Clin Psychiatry. 1991;52:369–376. [PubMed] [Google Scholar]
- 3.Ayuso-Mateos JL, Montanes F, Lastra I, Picazo de la Garza J, Ayuso-Gutierrez JL. HIV infection in psychiatric patients: an unlinked anonymous study. Br J Psychiatry. 1997;170:181–185. doi: 10.1192/bjp.170.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Meyer I, McKinnon K, Cournos F, et al. HIV seroprevalence among long-stay patients in a state psychiatric hospital. Hosp Community Psychiatry. 1993;44:282–284. doi: 10.1176/ps.44.3.282. [DOI] [PubMed] [Google Scholar]
- 5.Naber D, Pajonk FG, Perro C, Lohmer B. Human immunodeficiency virus antibody test and seroprevalence in psychiatric patients. Acta Psychiatr Scand. 1994;89:358–361. doi: 10.1111/j.1600-0447.1994.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 6.Susser E, Valencia E, Conover S. Prevalence of HIV infection among psychiatric patients in a New York City men’s shelter. Am J Public Health. 1993;83:568–570. doi: 10.2105/ajph.83.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cournos F, McKinnon K. HIV seroprevalence among people with severe mental illness in the United States: a critical review. Clin Psychol Rev. 1997;17:259–269. doi: 10.1016/s0272-7358(97)00018-4. [DOI] [PubMed] [Google Scholar]
- 8.Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25:433–457. doi: 10.1016/j.cpr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Joska JA, Kaliski SZ, Benatar SR. Patients with severe mental illness: A new approach to testing for HIV. S Afr Med J. 2008;98:213–217. [PubMed] [Google Scholar]
- 10.World Health Organization. Geneva: World Health Organization; [accessed 20 December 2008];Guidance on Provider-initiated HIV Testing and Counseling in Health Facilities. 2007 http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf.
- 11.Human Rights Watch. [accessed 20 December 2008];Comments on WHO/UNAIDS draft ‘Guidance on Provider Initiated HIV Testing and Counseling in Health Facilities’ Submission to WHO by Human Rights Watch. http://hrw.org/pub/2007/hivaids/hrwWhoGuidance.pdf.
- 12.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 13.Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, Blow FC. Understanding associations between serious mental illness and HIV among patients in the VA Health System. Psychiatr Serv. 2007;58:1165–1172. doi: 10.1176/ps.2007.58.9.1165. [DOI] [PubMed] [Google Scholar]
- 14.UNAIDS. Geneva: World Health Organization; 2008 Report on the Global AIDS Epidemic: Executive Summary. 2008
- 15.Acuda SW, Sebit MB. Serostatus surveillance testing of HIV-1 infection among Zimbabwean psychiatric inpatients, in Zimbabwe. Cent Afr J Med. 1996;42:254–257. [PubMed] [Google Scholar]
- 16.Sebit MB. Neuropsychiatric HIV-1 infection study: in Kenya and Zaire cross-sectional phase I and II. Cent Afr J Med. 1995;41:315–322. [PubMed] [Google Scholar]
- 17.Sebit MB, Tombe M, Siziya S, Balus S, Nkomo SD, Maramba P. Prevalence of HIV/AIDS and psychiatric disorders and their related risk factors among adults in Epworth, Zimbabwe. East Afr Med J. 2003;80:503–512. doi: 10.4314/eamj.v80i10.8752. [DOI] [PubMed] [Google Scholar]
- 18.Shisana O, Simbayi LC. Nelson Mandela HSRC Study of HIV/AIDS: South African National HIV Prevalence, Behavioural Risks and Mass Media. Household Survey 2002. Cape Town: Human Science Research Council of South Africa Publishers; 2002. [Google Scholar]
- 19.Shisana O, Stoker D, Simbayi LC, et al. South African national household survey of HIV/AIDS prevalence, behavioural risks and mass media impact – detailed methodology and response rate results. S Afr Med J. 2004;94:283–288. [PubMed] [Google Scholar]
- 20.Dasananjali T. The prevalence of HIV infection among mentally ill offenders in Thailand. J Med Assoc Thai. 1994;77:257–260. [PubMed] [Google Scholar]
- 21.Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91:31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tharyan P, Ramalingam S, Kannangai R, Sridharan G, Muliyil J, Tharyan A. Prevalence of HIV infection in psychiatric patients attending a general hospital in Tamil Nadu, south India. AIDS Care. 2003;15:197–205. doi: 10.1080/0954012031000068344. [DOI] [PubMed] [Google Scholar]