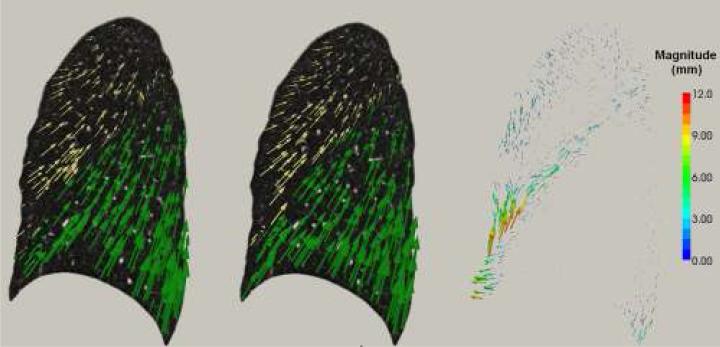Abstract
The human lungs are divided into five independent compartments called lobes. The lobar fissures separate the lung lobes. It is hypothesized that the lobar surfaces slide against each other during respiration. We propose a method to evaluate the sliding motion of the lobar surfaces during respiration using lobe-by-lobe mass-preserving non-rigid image registration. We measure lobar sliding by evaluating the relative displacement on both sides of the fissure. The results show a superior-inferior gradient in the magnitude of lobar sliding. We compare whole-lung-based registration accuracy to lobe-by-lobe registration accuracy using vessel bifurcation landmarks.
1 Introduction
The human lungs are divided into five independent compartments called lobes. A lobar fissure is a thin space (approximately 0.5mm depending on volume of pleural fluid) separating the lung lobes. The left lung is divided into the left upper (LUL) and left lower (LLL) lobes, separated by the oblique fissure. The right lung is partitioned into the right upper lobe, middle lobe, and the lower lobe, separated by the oblique and horizontal fissures. The branching patterns of the bronchial and vascular trees also follow the lobar structure of the lung.
Regional function and biomechanics depend on the material properties of the lung parenchyma and the complex interaction between the lobes, diaphragm, and chest wall. Hubmayr et al. [1] have used embedded metal markers and X-ray projection images to study regional lung mechanics. Recently, image registration has been used to assess regional lung function and tissue biomechanics using multiple 3D images at different lung volumes by CT [2–4] and MRI [5]. Although those results show regional changes in lung function and mechanics, they do not explicitly account for the interaction between the lung lobes. It is believed that during respiration the lobes move relative to each other (sliding and rotation), and this motion may provide a means to reduce the lung parenchymal distortion and avoid regions of high local stress [1]. In addition, understanding of how lobes slip relative to one another is of importance to the understanding of how the lung accommodates chest wall shape changes while minimizing effects on regional distribution of ventilation.
In this paper, we investigate lung biomechanics using a lobe-by-lobe registration technique. Our approach explicitly accounts for the registration displacement field discontinuity at the fissure (due to lobar sliding), and should provide more accurate image registration near the fissure and, as a result, better biomechanical measurements. We measure lobar sliding by evaluating the relative displacement on both sides of the fissure. We compare whole-lung-based registration accuracy to lobe-by-lobe registration accuracy using vessel landmarks.
2 Materials and Methods
2.1 Data Acquisition
All data were gathered under a protocol approved by our institutional review board. Three pairs of volumetric CT data sets from three normal human subjects were used in this study. Each image pair was acquired with a Siemens Sensation 64 multi-detector row CT scanner (Forchheim, Germany) during breath-holds near functional residual capacity (FRC) and total lung capacity (TLC) in the same scanning session. Each volumetric data set was acquired at a section spacing of 0.5 ~ 0.6 mm and a reconstruction matrix of 512 × 512. In-plane pixel spacing is approximately 0.6 mm × 0.6 mm.
2.2 Automatic Lobe Segmentation
To perform the lobe-by-lobe registration, the lobes are first automatically segmented using the method from [6]. The lobar segmentation begins with automatic lung, airway tree, and vessel tree segmentation. A watershed transform, applied to a distance map derived from the original CT image and the vessel segmentation, provides an initial lobar segmentation. The lobar surfaces are refined using a 3D optimal surface detection that divides the lungs at the fissure surfaces. For complete details on the lobar segmentation method, see [6].
2.3 Image Registration
The CT scans at FRC and TLC are registered for each subject. The FRC–TLC image pairs show large lung volume change, large tissue deformation, and large voxel intensity changes. To account for these differences between the images during registration, we used a lung mass preserving registration method [7]. The method uses a similarity metric that estimates the local tissue and air fraction within the lung and minimizes the local tissue mass difference. This method has been shown to be effective at registering across large lung volume changes (such as FRC–TLC pairs) [7].
From the CT value of a given voxel, the tissue volume can be estimated as
| (1) |
where v(x) denotes the volume and I(x) is the intensity of a voxel at position x. HUair and HUtissue refer to the intensity of air and tissue, respectively [8]. In this work, we assume that air is -1000 HU and tissue is 55 HU. γ(I(x)) is introduced for notational simplicity.
Given (1), we can then define the similarity measure as the sum of squared local tissue volume difference:
| (2) |
where Ω denotes the overlapping lung regions in the two images, and T (x) is the warping function. In this work, T (x) is a cubic B-splines transform:
| (3) |
where ϕ describes the displacements of the control nodes and β(x) is a three-dimensional tensor product of basis functions of cubic B-Spline.
Given a warping function T (x), If (T (x)) can be interpolated from the moving image. vf (T (x)) can be calculated from the Jacobian J(x) of the deformation as vf (T (x) = J(x)vr(x). Note that the Jacobian value must be positive here, which can be achieved by using displacement constraints on the control nodes.
2.4 Computational Setup
In this study, the lobe-by-lobe registration is used to investigate lobar sliding. Our current analysis is limited to the upper and lower lobes of the left lung, since the three lobes in the right lung will likely have more complicated interaction.
We start with the lobar segmentations of the TLC and FRC images as described in 2.2. After segmentation we match the TLC left upper lobe to the FRC left upper lobe, and the TLC left lower lobe to the FRC left lower lobe. After registration, the displacement fields are recombined into one left lung displacement field. We also perform conventional lung-by-lung registration to match the TLC left lung to the FRC left lung, using the same registration algorithm.
Discontinuities of the displacement field along the fissure surface are indications of lobar sliding. Figure 1 shows the displacement fields generated by lobe-by-lobe and lung-by-lung registration methods for one subject. The figure shows a considerable difference between these methods along the fissure surface.
Fig. 1.
Comparison of displacement field between the lobe-by-lobe registration (left column) and the lung-by-lung registration (middle column) for the LUL (yellow) and LLL (green). The right column is the difference of the two displacement fields with the magnitude indicated by the color bar.
2.5 Assessment of Image Registration Accuracy
Vascular bifurcation points are used as landmarks to evaluate registration accuracy. An observer uses a landmark annotating system [9] to find corresponding landmarks in the FRC and TLC images. For each landmark, the actual landmark position is compared to the registration-derived estimate of landmark position from the two registration methods and the landmark error is calculated.
2.6 Evaluation of Local Lobar Sliding
Once the lobe segmentations are obtained, the oblique fissure surface between LUL and LLL is extracted as a triangular mesh. The normal direction is then calculated at each vertex of the mesh. The sliding motion is quantified for each point along the fissure surface by looking at the discontinuity in the line profile perpendicular to the fissure surface as shown in Figure 2. On each side, the profile of tangent component of the displacement is fitted as a 3rd order polynomial function d of the distance to the fissure surface. The sliding distance s(x) at fissure surface position x is then defined as
| (4) |
where is the predicted value on the fissure surface from the polynomial function along the positive normal direction (we define the normal direction pointing the LUL as positive.) while is the predicted value from the other side.
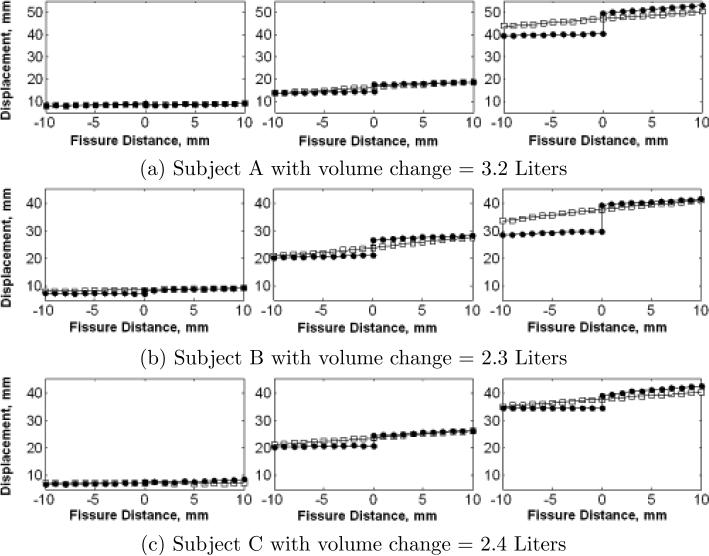
Fig. 2.
Displacement profile of tangent components along a line perpendicular to the fissure surface at three different locations (left: near apex; middle: near lingula; and right: near base) for both the whole-lung-based (square) and the lobe-based (solid circle) methods.
3 Results
3.1 Registration Accuracy
For each lobe, 20 to 40 landmarks are identified. Table 1 shows the results of the landmark distance before and after registration for the lobe-based and whole-lung based registrations. The average landmark errors are 0.83 mm and 0.73 mm for whole-lung-based registration and lobe-based registration.
Table 1.
Comparison of registration accuracy between lobe-based and whole-lung-based registrations. Distances in mm.
| Subject | Lobe | Before Registration | Whole-lung-based Registration | Lobe-based registration |
|---|---|---|---|---|
| A | LUL | 19.08 ± 8.25 | 0.99 ± 0.99 | 0.95 ± 0.81 |
| LLL | 35.79 ± 12.69 | 0.94 ± 1.12 | 0.71 ± 0.41 | |
| B | LUL | 15.09 ± 4.03 | 0.72 ± 0.81 | 0.57 ± 0.30 |
| LLL | 38.33 ± 6.55 | 0.87 ± 0.48 | 0.75 ± 0.43 | |
| C | LUL | 13.45 ± 6.59 | 0.78 ± 0.72 | 0.78 ± 0.83 |
| LLL | 35.45 ± 10.76 | 0.68 ± 0.24 | 0.67 ± 0.30 |
3.2 Local Lobar Sliding
The sliding distance at each fissure surface point was calculated to evaluate the local lobar sliding. A step length of 1 mm and 10 sample points were used along the normal direction on each side of the surface. A surface point was not taken into consideration if any of its 20 sample points were outside the lobes. Figure 2 shows the displacement profile of the tangent component along a line perpendicular to the fissure surface at three different locations (near the apex, near the lingula and near the base) for the whole-lung-based (square) and the lobe-based (solid circle) methods. The results show increased sliding (larger discontinuity) in the more basal positions using the lobe-by-lobe analysis. However, these discontinuities are not apparent using the lung-by-lung analysis.
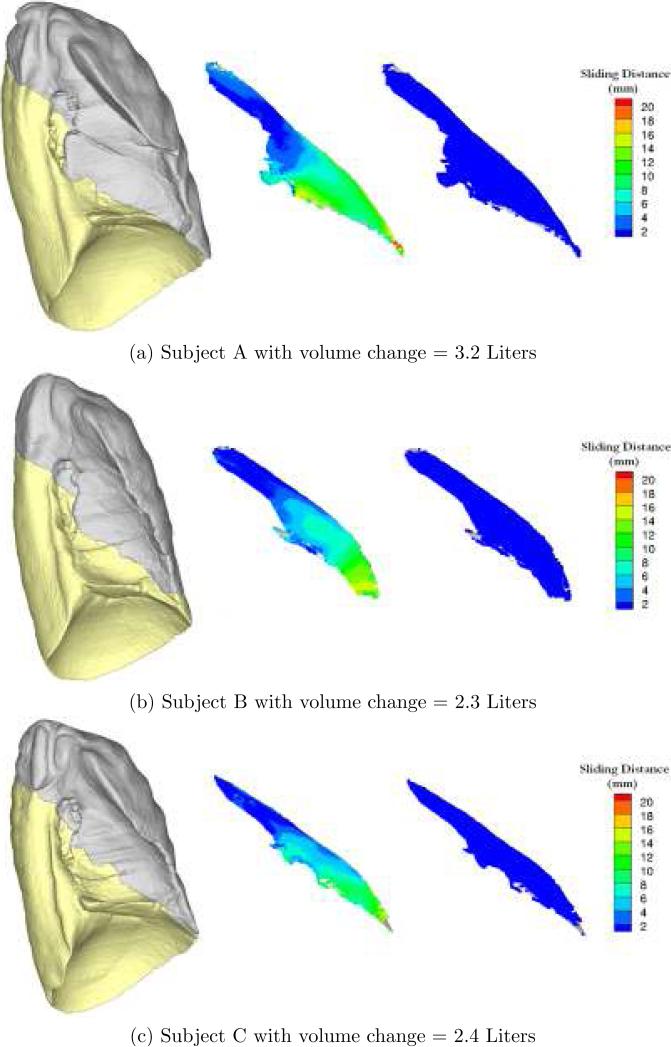
Figure 3 compares the estimated lobar sliding distances between two different registration methods. The whole-lung-based registration shows small sliding distance (≤ 1 mm) because the transformation model enforces displacement field smoothness across the fissure, while the lobe-by-lobe registration method recovers the displacement field discontinuity along the fissure.
Fig. 3.
The color-coded sliding distance map overlays on the fissure surface. Left most column is the surface rendering of LUL (gray) and the LLL (gold); second column shows the sliding distance from the lobe-based registration; and right most column shows the sliding distance from the whole-lung registration.
4 Discussion and Summary
We proposed a method to estimate local lobe sliding using lobe-by-lobe lung-mass-preserving registration. In addition, we compared the displacement field, the landmark error, and the sliding distance between the lobe-by-lobe registration and the lung-by-lung registration for the left lungs of three normal human subjects. We used major vascular bifurcation as landmarks. Thus, there are not large numbers of landmarks near fissures. As seen in Figure 1, both methods yield similar matching results in the center regions of the lobes while a considerable difference is observed in the vicinity of fissure. Thus, as seen in Table 1 there is not a very significant decrease in the overall landmark error while there are significant regional differences. Moreover, the lung-by-lung registration is not able to capture the sliding between the lobes while the lobe-by-lobe registration shows the same superior-inferior gradient of sliding distance in all three cases. One possible explanation for this pattern is that the lungs contract and expand more at the diaphragm than at the apex and the LUL is more firmly anchored to the chest wall than LLL.
In addition to evaluating lobar sliding, the lobe-by-lobe registration may yield more physiologically meaningful assessments of regional lung function and mechanics. Registration transformation functions that do not explicitly model the lobar fissure are not able to capture lobar sliding and thus experience more registration errors near the fissure. These findings may have implications in using registration to estimate lung function (specific volume change and lung expansion) and for tracking lung tissue and lung nodules, across the respiratory cycle. These methods can be directly extended to respiratory-gated CT of the lung, where CT data is reconstructed at multiple points across the respiratory cycle.
In conclusion, we have described a method to evaluate the local lobar sliding using a lobe-by-lobe lung-mass-preserving registration. Application of these methods may be useful for increasing our understanding of function and biomechanical behavior of the respiratory system.
5 Acknowledgments
The authors thank Ms. Keelin Murphy and Dr. Bram van Ginneken for providing the software iX for annotating landmarks, and Mr. Matthew Moehlmann and Mr. Luke Gabe for the data analysis. This work was supported in part by NIH grants HL079406, HL064368, EB005823, RR022421, EB004126, HL080285 and a University of Iowa CTSA NIH/NCRR grant 1UL1RR024979.
References
- 1.Hubmayr RD, Rodarte JR, Walters BJ, Tonelli FM. Regional ventilation during spontaneous breathing and mechanical ventilation in dogs. J Appl Physiol. 1987;63(6):2467–2475. doi: 10.1152/jappl.1987.63.6.2467. [DOI] [PubMed] [Google Scholar]
- 2.Christensen GE, Song JH, Lu W, Naqa IE, Low DA. Tracking lung tissue motion and expansion/compression with inverse consistent image registration and spirometry. Med Physics. 2007 June;34(6):2155–2165. doi: 10.1118/1.2731029. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero T, Sanders K, Noyola-Martinez J, Castillo E, Zhang Y, Tapia R, Guerra R, Borghero Y, Komaki R. Quantification of regional ventilation from treatment planning CT. Int. J. Radiation Oncology Biol. Phys. 2005 Jul 1;62(3):630–634. doi: 10.1016/j.ijrobp.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, Bodas SV. Registration-based estimates of local lung tissue expansion compared to xenon-CT measures of specific ventilation. Medical Image Analysis. 2008 December;12(6):752–763. doi: 10.1016/j.media.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gee J, Sundaram T, Hasegawa I, Uematsu H, Hatabu H. Characterization of regional pulmonary mechanics from serial magnetic resonance imaging data. Acad. Radiol. 2003;10:1147–1152. doi: 10.1016/s1076-6332(03)00329-5. [DOI] [PubMed] [Google Scholar]
- 6.Ukil S, Reinhardt JM. Anatomy-guided lung lobar surface detection in X-ray CT images. IEEE Trans. Medical Imaging. 2009 Feb.28(2):202. doi: 10.1109/TMI.2008.929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y, Hoffman EA, Lin CL. Local tissue-weight-based nonrigid registration of lung images with application to regional ventilation. SPIE. 2009;7262:72620C. [Google Scholar]
- 8.Hoffman EA, Ritman EL. Effect of body orientation on regional lung expansion in dog and sloth. J Appl Physiol. 1985;59(2):481–491. doi: 10.1152/jappl.1985.59.2.481. [DOI] [PubMed] [Google Scholar]
- 9.Murphy K, van Ginneken B, Pluim J, Klein S, Staring M. Semi-automatic reference standard construction for quantitative evaluatoin of lung CT registration.. Proc. of International Conference on Medical Image Computing and Computer-Assisted Intervention 2008.; 2008. pp. 1006–1013. [DOI] [PubMed] [Google Scholar]