Abstract
Salmonella enterica serovar Typhimurium is an animal and zoonotic pathogen of worldwide importance. In pigs, transport and social stress are associated with reactivation and spread of Salmonella Typhimurium infection. The stress-related catecholamine norepinephrine (NE) has been reported to activate growth and virulence factor expression in Salmonella; however the extent to which NE contributes to stress-associated salmonellosis is unclear. We studied the impact of releasing NE from endogenous stores during Salmonella Typhimurium infection of pigs by administration of 6-hydroxydopamine (6-OHDA), which selectively destroys noradrenergic nerve terminals. Treatment of pigs with 6-OHDA 7 or 16 days post-oral inoculation with Salmonella Typhimurium produced elevated plasma NE levels and transiently, but significantly, increased faecal excretion of the challenge strain. Oral administration of NE to Salmonella Typhimurium-infected pigs also transiently and significantly increased shedding; however pre-culture of the bacteria with NE did not alter the outcome of infection. Salmonella has been proposed to sense and respond to NE via a homologue of the adrenergic sensor kinase QseC. A ΔqseC mutant of Salmonella Typhimurium was consistently excreted in lower numbers than the parent strain post-oral inoculation of pigs, though not significantly so. 6-OHDA treatment of pigs infected with the ΔqseC mutant also increased faecal excretion of the mutant strain, albeit to a lesser extent than observed upon 6-OHDA treatment of pigs infected with the parent strain. Our data support the notion that stress-related catecholamines modulate the interaction of enteric bacterial pathogens with their hosts.
Keywords: Salmonella, stress, norepinephrine, virulence, colonisation
1. INTRODUCTION
Salmonella enterica serovar Typhimurium is responsible for over 60% of acute porcine salmonellosis in the United Kingdom [2]. In a UK-wide survey of pigs at slaughter, Salmonella Typhimurium was detected in 11.1% of caeca and 2.1% of carcases [10], indicating significant potential for entry of the pathogen into the human food chain and environment. It is evident that stress influences susceptibility of pigs to Salmonella and the risk of zoonosis. Transportation has been correlated with reactivation of subacute Salmonella Typhimurium infections in pigs and intestinal and carcass contamination increases with time spent in lairage [7, 20, 21, 29]. Moreover, social stress caused by mixing increases faecal excretion and translocation of Salmonella Typhimurium to intestinal lymph nodes in early-weaned pigs [8]. Such phenomena do not appear restricted to Salmonella, as mixing of litters, isolation from the sow or short-lived cold stress has been reported to increase faecal excretion of enterotoxigenic Escherichia coli relative to control piglets [22]. Even mild physical handling of pigs involving a daily weight measurement increased the faecal excretion of E. coli and total coliforms relative to control animals [12]. A plausible explanation for such links is that activation of adrenal axes under stress leads to the release of stress-related catecholamines that may impair innate and adaptive immunity. In recent years however, it has become clear that many bacteria are able to sense mediators of the host stress response and respond by activating growth and the expression of virulence factors (reviewed in [17, 41]).
A key response of the enteric nervous system to stress is the release of norepinephrine (NE) from sympathetic nerve fibres that originate in the prevertebral ganglia and innervate the gut mucosa. Elevated NE levels could be found in the intestines of mice subject to partial hepatectomy compared to control animals and were associated with increased susceptibility to gut-derived sepsis and virulence gene expression by Pseudomonas aeruginosa [1, 24]. In relation to Salmonella, NE has been reported to resuscitate Salmonella Typhimurium from a viable non-culturable state [35] and to enhance its growth from low inocula in serum-rich nutrient-limited medium [15]. The ability of NE to activate growth in such media has been associated with binding of NE to ferric iron complexed with lactoferrin and transferrin and its reduction to Fe(II), for which the proteins have a lower affinity [36]. NE has also been reported to activate the expression of virulence-associated factors in Salmonella Typhimurium, including flagella-mediated motility [5, 28] and Type III protein secretion [28, 32], though others have not reproduced such effects [31]. Exogenous iron can activate both motility and Type III secretion system (T3SS)-1 [6, 13], indicating that the effect could be a consequence of NE-mediated iron supply. An alternative explanation is that Salmonella senses and responds to stress-related catecholamines via homologues of adrenergic receptors identified in E. coli O157:H7 called QseC [9] and QseE [34]. In E. coli O157:H7 these proteins autophosphorylate on binding of epinephrine (Epi) and transfer the phosphate moiety to cognate transcriptional regulators, modulating the expression of genes under their control [9, 34]. QseC has been reported to be required for full virulence of Salmonella Typhimurium in pigs [5, 6] and mice [28]. Moreover, an inhibitor of E. coli O157:H7 QseC signalling (LED209) controls systemic salmonellosis in a murine model [32]. However, two-component sensory systems are known to integrate multiple signals and the extent to which such sensors influence virulence by sensing stress-related catecholamines is unclear.
The ability of NE to alter the outcome of Salmonella infection has been suggested in several reports. NE augments Salmonella-induced enteritis in a bovine ligated ileal loop model of infection in a manner independent of QseC and QseE [31]. Analysis of plasmid partitioning in NE-stimulated and unstimulated bacteria in this model indicated that the effect was associated with enhanced net replication of Salmonella Typhimurium [31]. Though useful for evaluating multiple strains, neurochemicals and doses for their effects on local secretory and inflammatory responses, ligated intestinal loop models may be considered artificial in the context of stressed animals in that high inocula and hormone concentrations are held statically over surgically-manipulated mucosa for protracted periods. Oral dosing of mice or chickens with NE enhances intestinal and systemic Salmonella infection [27, 46], albeit that high hormone doses were again used. In calves, intramuscular dosing with NE enhances translocation of various S. enterica serovars from the intestines and promotes encephalopathy [26]. Pre-culture of Salmonella Typhimurium with NE has been reported to result in higher levels of colonisation of the porcine intestinal tract [43]. However, NE-treated and untreated bacteria were cultured in distinct media and it is likely that the physiological status of the bacteria differed substantially, making it difficult to separate effects of NE from growth or media-related effects [16]. In transgenic mice unable to produce Epi or NE owing to mutation of dopamine beta-hydroxylase (dbh −/−), Salmonella Typhimurium transcribed T3SS-1 genes at a lower level than in wild-type mice, consistent with a role for stress-related catecholamines in control of virulence gene expression in vivo [28]. However, dbh −/− mice lacking NE/Epi were more sensitive to Salmonella Typhimurium infection than dbh +/− mice owing to immune deficiency and it is therefore difficult to separate direct effects of NE/Epi on Salmonella from the immune status of the animals [28].
Here, we sought to mimic the effect of acute stress during recovery from experimental oral Salmonella infection in pigs. The selective neurotoxin 6-hydroxydopamine (6-OHDA) was used to invoke the release of NE by destruction of noradrenergic nerve terminals in the periphery without crossing the blood-brain barrier or affecting cholinergic neurons [11, 25]. The effect is transient and damaged noradrenergic neurons heal and recover, enabling the course of Salmonella infection to be monitored in the days post-treatment. Intraperitoneal administration of 6-OHDA to mice invokes the outgrowth of coliforms relative to controls, though coliform numbers returned to normal as damage to noradrenergic neurons was repaired over a two week period [25]. Having established such a model in pigs, we evaluated the role of the QseC sensor kinase in both intestinal colonisation and 6-OHDA-induced responses. Moreover, we examined the impact of treating the inoculum, or infected animals, directly with NE. Our data provide valuable insights into the role of stress-related catecholamines in the outcome of Salmonella infection in reservoir hosts.
2. MATERIALS AND METHODS
2.1. Bacterial strains and media
A spontaneous nalidixic acid resistant mutant of Salmonella Typhimurium strain ST4/74 of well-defined virulence in pigs was used [30, 45]. ST4/74 is the parent of strain SL1344, a hisG auxotroph derived by transduction [19], that has previously been reported to respond to NE [15, 28, 35]. A ST4/74 nalR deletion mutant lacking qseC (also known as preB or STM3178) was created by lambda Red recombinase-mediated integration of a linear PCR amplicon comprising a kanamycin resistance gene flanked by the sequences 5′ and 3′ of qseC, followed by P22int transduction of the mutated allele into the archived strain and flippase-mediated excision of the kanamycin resistance cassette as described [31]. Bacteria were cultured in Luria-Bertani medium containing 20 μg/mL nalidixic acid.
2.2. Experimental animals
Animal experiments were conducted according to the Animal (Scientific Procedures) Act 1986 (license 30/2485) with the approval of the local Ethical Review Committee. Large-White × Landrace pigs of mixed sex and aged ca. 6 weeks old were obtained from a commercial supplier with no history of acute salmonellosis and housed in containment level 2 accommodation with access to antibiotic-free irradiated weaner pellets and water ad libitum. Pigs were confirmed to be culture-negative for Salmonella prior to infection by overnight enrichment of rectal swabs in Rappaport broth (at 37 °C) and selenite brilliant green broth (at 42 °C) followed by plating to modified brilliant green agar (Oxoid, Basingstoke, UK).
2.3. Salmonella infection
ST4/74 nalR or its isogenic ΔqseC mutant were grown to stationary phase in Luria-Bertani broth at 37 °C with shaking. In studies to evaluate the impact of pre-culture with NE, ST4/74 nalR was cultured as above, then subcultured 1:10 into fresh LB broth containing L-norepinephrine bitartrate (Sigma, Poole, UK, reference A9512) at a final concentration of 5 mM or diluent for 2 h at 37 °C with shaking. For inoculation, approximately 1 × 108 colony-forming units (CFU) were suspended in 10 mL antacid (5% (w/v) Mg(SiO3)3, 5% (w/v) NaHCO3 and 5% (w/v) MgCO3 in H2O) and administered by oral gavage using a 10FG catheter. The number of bacteria delivered was determined by retrospective plating of serial ten-fold dilutions of inocula. Rectal temperatures were recorded and animals monitored for clinical signs of disease twice daily. The magnitude and duration of faecal excretion of the challenge strain was followed by plating of serial ten-fold dilutions of fresh faeces collected by rectal palpation to MacConkey agar supplemented with 20 μg/mL nalidixic acid. Data presented are the least square mean viable count (expressed as log10 CFU/g) and the limit of detection by direct plating was 2.0 log10 CFU/g.
2.4. 6-hydroxydopamine and L-norepinephrine treatment
Seven or 16 days post-inoculation with Salmonella Typhimurium groups of pigs were dosed by the intravenous route with 6-hydroxydopamine or placebo. A 240 mg/mL (0.96 M) solution of 6-OHDA (Fluka Chemicals, Gillingham, UK, reference 55238) was freshly prepared in 0.9% (w/v) saline containing 1 mM ascorbic acid and sterilised by passing through a 0.22 μm filter. In the first study, three pigs were given 6-OHDA 7 days post-inoculation with ST4/74 nalR in a series of rising doses of 1 mg/kg, 4 mg/kg then 35 mg/kg at intervals of 20 min via an ear vein. The total dose of 6-OHDA was similar to that used to produce chemical sympathectomy in adult miniature swine (50 mg/kg intravenously) [42]. At the same time, 3 age-matched control pigs infected with ST4/74 nalR were given three injections of an equal volume of diluent at the same intervals and site. All reasonable precautions were taken to minimise handling effects between treated and control groups. In the second experiment, 4 pigs were given a single dose of 40 mg/kg and 2 pigs were given diluent via the vena cava at 16 days post-inoculation with ST4/74 nalR. The latter regime was also used to dose groups of 3 pigs with 6-OHDA or diluent at 16 days post-inoculation with ST4/74 nalR ΔqseC. In all cases, a sample of venous blood was withdrawn from the vena cava using a needle into heparin-coated tubes under vacuum immediately before and 1 h after 6-OHDA treatment, and plasma stored at −80 °C for quantification of circulating NE. Animals were observed continuously for 4 h post-treatment and at 8 h intervals thereafter. Faecal samples were collected immediately before and twice daily after dosing with 6-OHDA or diluent and viable nalidixic acid resistant bacteria were enumerated as above.
To determine the effect of oral dosing of Salmonella Typhimurium-infected pigs with NE, 15 mL of a 167 mg/mL freshly prepared solution of L-norepinephrine or diluent (PBS) was given to duplicate pigs using a 10FG catheter 17 days after oral inoculation with ST4/74 nalR. The final estimated dose was ca. 200 mg/kg.
2.5. NE enzyme-linked immunosorbent assay (ELISA)
Plasma NE levels in pigs pre- and post-treatment with 6-OHDA or diluent were quantified using a sandwich ELISA designed for human plasma and urine according to the manufacturer’s instructions (IBL International GMBH, Hamburg, Germany). NE standards of known concentration were used to convert absorbance readings to NE concentrations in ng/mL.
2.6. Statistical analysis
Measured traits were analysed for the effect of strain or treatment by two-way analysis of variance (Statistical Analysis System 1995, SAS Institute, Cary, USA). The magnitude and duration of faecal excretion of strains was analysed after a log10 transformation of the number of excreted bacteria, with daily values taken as repeated measurements (Proc Mixed). Where strain or treatment was identified as a significant variable, pair-wise comparisons of least square means were performed. p values ≤ 0.05 were considered significant.
3. RESULTS
3.1. 6-OHDA-mediated release of NE increases faecal excretion of Salmonella Typhimurium in pigs
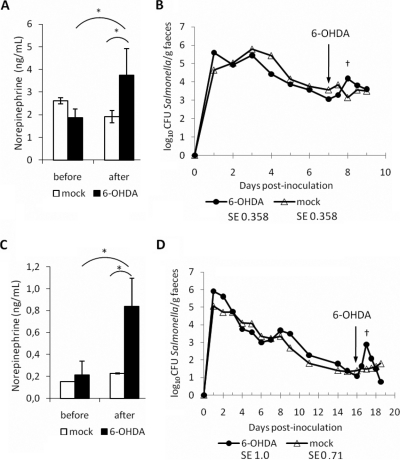
To mimic the impact of acute stress during Salmonella Typhimurium infection, two groups of six 6-week-old pigs were inoculated via the oral route with ST4/74 nalR then treated at 7 or 16 days post-inoculation with 6-OHDA or diluent. In both sets of pigs, ST4/74 nalR induced rapid but short-lived pyrexia and diarrhoea, and recoveries of viable bacteria in the faeces over time were comparable to those recorded previously [30, 45]. By 3–4 days post-inoculation rectal temperatures and clinical signs returned to normal levels. In the first study, 3 pigs were dosed at 7 days post-inoculation with 3 doses of 6-OHDA 20 min apart (1, 4 and 35 mg/kg) via an ear vein. Three control pigs were given an equivalent volume of diluent via an ear vein at the same intervals. Only upon administration of 35 mg/kg did pigs exhibit an overt response to 6-OHDA, characterised by pyrexia, rapid breathing, increased salivation and slight ataxia. Control pigs appeared normal throughout. 6-OHDA treatment was confirmed to elicit a significant increase in plasma NE levels 1 h post-exposure compared to control pigs (p = 0.0027) and pre-treatment levels (p = 0.003; Fig. 1A). Analysis of the course of faecal excretion revealed that 6-OHDA treatment produced a transient increase in faecal excretion of ST4/74 nalR of ca. 1 log10 CFU/g 1 day post-treatment relative to control pigs (Fig. 1B). The difference in the number of Salmonella Typhimurium excreted 1 day after 6-OHDA treatment was not significantly different to the diluent-treated group by a narrow margin (p = 0.057). However, there was a highly significant rise in the mean log10 CFU/g Salmonella Typhimurium excreted 1 day after 6-OHDA treatment compared to faecal samples from the same animals immediately prior to treatment (p = 0.0003; mean log10 CFU/g 1.43 fold higher, equivalent to a rise from 1.14 × 103 CFU/g to 1.53 × 104 CFU/g). By 2 days post-treatment, a comparable number of viable ST4/74 nalR were excreted by 6-OHDA- and diluent-treated pigs.
Figure 1.
Intravenous administration of 6-OHDA to pigs increases plasma NE levels and faecal excretion of Salmonella Typhimurium. Plasma NE (ng/mL) was quantified by ELISA immediately before and 1 h after treatment with a total of 40 mg/kg 6-OHDA or diluent 7 days post-inoculation with ST4/74 nalR (panel A) or 16 days post-inoculation (panel C). The impact of treatment on the course of faecal excretion is shown for pigs treated at 7 and 16 days post-inoculation in panels B and D, respectively. Values shown are the least square means (LSM) ± standard error (SE) of the LSM. p values ≤ 0.05 are marked with an asterisk. † denotes significant differences in the 6-OHDA group relative to pre-treatment samples.
In the second trial, a single dose of 40 mg/kg 6-OHDA was given via the vena cava to 4 pigs 16 days post-oral inoculation with ST4/74 nalR. The 6-OHDA-treated pigs showed significantly elevated plasma NE levels 1 h post-treatment compared to 2 control pigs given an equivalent volume of diluent via the same route (Fig. 1C; p = 0.0041). Plasma NE levels after 6-OHDA treatment were also significantly higher than the corresponding pre-treatment samples (p = 0.0004). In 6-OHDA-treated animals, the number of ST4/74 nalR excreted in the faeces was again elevated after 6-OHDA treatment relative to the control group, this time by ca. 2 log10 CFU/g 1 day post-inoculation, but the difference between the two groups was not significant (Fig. 1D; p = 0.1). The mean log10 CFU/g Salmonella Typhimurium excreted was significantly higher after 6-OHDA treatment than in faeces samples from the same animals immediately prior to treatment (p = 0.0047; mean log10 CFU/g 2.68 fold higher, equivalent to a rise from 1.19 × 101 CFU/g to 7.60 × 102 CFU/g). The effect was sustained for longer than observed upon 6-OHDA treatment 7 days after ST4/74 nalR challenge, albeit not significantly so relative to control pigs at 2 days post-treatment.
3.2. Oral administration of NE, but not pre-culture with NE, alters the course of Salmonella Typhimurium infection in pigs
Consistent with the effect of releasing NE from endogenous stores, we observed that oral dosing of Salmonella Typhimurium-infected pigs with NE transiently increases faecal excretion of Salmonella. Groups of two pigs inoculated with ST4/74 nalR as above were allowed to recover then given a single oral bolus of ca. 200 mg/kg NE or diluent 17 days after Salmonella Typhimurium infection by oral gavage with a catheter. Owing to inter-animal variation in shedding levels, data are presented as the fold-change in mean log10 CFU of the challenge strain excreted per gram of faeces relative to the pre-treatment sample. In the NE-treated animals, the mean log10 CFU/g ST4/74 nalR excreted rose by c. 1.4 fold 1 and 2 days after NE treatment, with significant differences to the pre-treatment levels in the same animals being detected (Fig. 2A; p ≤ 0.007). No significant change in the course of excretion of ST4/74 nalR was seen in the group given diluent (Fig. 2A). Clinical signs were similar in both groups.
Figure 2.
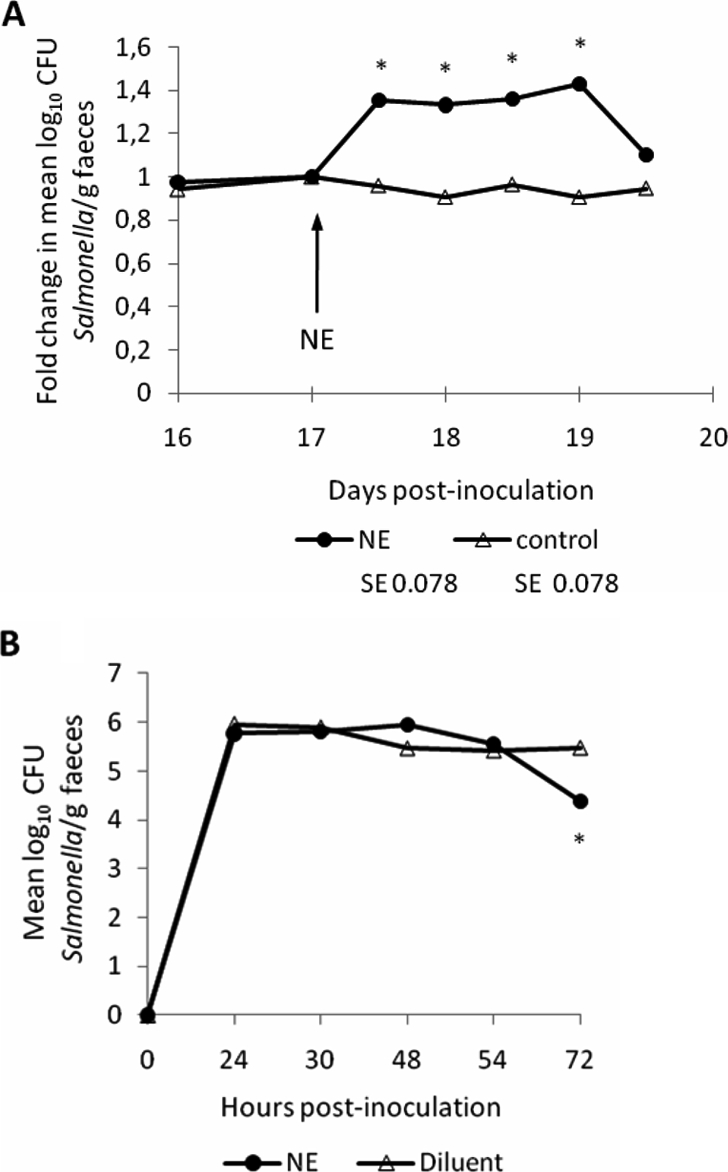
Oral administration of NE, but not pre-culture with NE, alters the course of Salmonella Typhimurium infection in pigs. Panel A shows the impact of oral administration of c. 200 mg/kg NE or diluent to groups of 2 pigs 17 days post-inoculation with ST4/74 nalR. Values are the fold change in mean log10 CFU Salmonella/g relative to samples collected immediately prior to treatment. Panel B shows the magnitude of faecal excretion of Salmonella Typhimurium after oral dosing of groups of 3 pigs with c. 1 × 108 CFU ST4/74 nalR amplified in LB medium for 2 h in the presence of 5 mM NE or diluent. Values shown are the least square means (LSM) ± standard error (SE) of the LSM. p values ≤ 0.05 are marked with an asterisk.
It has been reported that cultivation of Salmonella Typhimurium in serum-rich nutrient-limited medium containing 2 mM NE enhances the number and tissue distribution of bacteria in the porcine intestines after oral dosing relative to bacteria grown in LB medium [43]. However, the use of different media raises the possibility that the effect is due to distinct physiological states of the bacteria rather than direct effects of NE, as discussed elsewhere [16]. Given the assertion that 50 μM NE activates motility and Type III secretion [5, 28] and evidence that the transcriptome of Salmonella Typhimurium during growth in LB broth alters in response to Epi [23] and NE [38], we examined if pre-culture of ST4/74 nalR in LB medium with or without 5 mM L-norepinephrine for 2 h altered the outcome of infection of pigs. This NE concentration was selected as it augments enteritis induced by LB-grown Salmonella Typhimurium in calf ileal loops [31]. Groups of 3 pigs were inoculated with c. 1 × 108 CFU NE-treated or untreated LB culture and faecal excretion monitored twice daily for 3 days. No statistically significant differences were detected in the magnitude of faecal excretion (Fig. 2B), except at 72 h when the number of NE-pretreated bacteria excreted was lower than in the pigs given untreated bacteria. Similarly, there were no statistical differences in pyrexial responses or colonisation of intestinal sites at post mortem examination between the two groups (data not shown).
3.3. Role of the putative adrenergic sensor kinase QseC in intestinal colonisation of pigs by Salmonella Typhimurium and the response to 6-OHDA
A qseC mutant of Salmonella Typhimurium has been reported to be significantly impaired in intestinal colonisation at 1–7 days after co-infection of 13-week-old pigs with the parent strain [5] or 2–7 days post-inoculation of 7-week-old pigs when given separately [6]. However, only modest attenuation of a Salmonella Typhimurium qseC mutant was detected by co-infection of mice [28] and no significant defect in colonisation of most intestinal sites was observed in calves [31]. As QseC has been proposed as a novel drug target for Salmonella [32, 33], we evaluated its role in colonisation of pigs by separately dosing 6-week-old pigs by the oral route with ST4/74 nalR (n = 5) or its isogenic ΔqseC mutant (n = 6). The magnitude and duration of faecal excretion of the challenge strains was followed daily for 16 days. ST4/74 nalR ΔqseC was consistently shed in lower numbers than the parent strain, though not statistically significantly so (Fig. 3). Values at days 14 and 15 post-inoculation showed a tendency toward significance (p ≤ 0.1). No significant differences in rectal temperature or diarrhoea severity were recorded between the wild-type and ΔqseC mutant groups.
Figure 3.
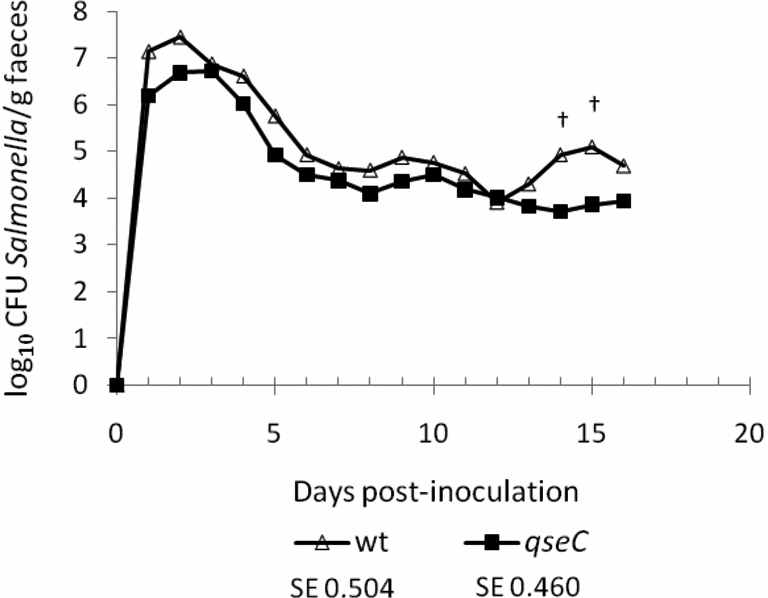
The putative adrenergic sensor kinase QseC does not significantly influence intestinal colonisation of pigs. 6-week-old pigs were inoculated with 1.50 × 109 CFU ST4/74 nalR (n = 5) or 1.53 × 109 CFU ST4/74 nalR ΔqseC (n = 6) and the course of faecal excretion of the bacteria followed daily for 16 days. Values shown are the least square means (LSM) ± standard error (SE) of the LSM. † denotes p values ≤ 0.1.
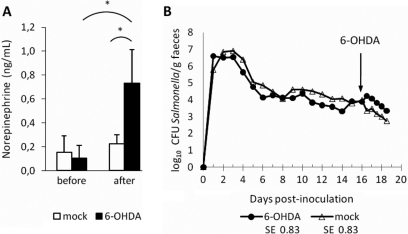
To determine if QseC is required for the response of Salmonella Typhimurium to 6-OHDA treatment, 16 days after inoculation of pigs with ST4/74 nalR ΔqseC 3 pigs were given 40 mg/kg 6-OHDA into the vena cava and 3 control pigs were given an equivalent volume of diluent. 6-OHDA elicited a significant increase in plasma NE levels relative to the control group (p = 0.008) and pre-treatment samples (p = 0.01; Fig. 4A). The mean log10 CFU/g of the ST4/74 nalR ΔqseC mutant excreted in the faeces rose in response to 6-OHDA treatment compared to animals given diluent, but not significantly so (Fig. 4B). The peak fold change in mean log10 CFU/g ST4/74 nalR ΔqseC excreted after 6-OHDA treatment relative to samples collected from the same animals immediately prior to treatment was 1.09 (equivalent to a rise from 7.67 × 103 CFU/g to 1.75 × 104 CFU/g) and was not statistically significant. The data suggest that QseC contributes to the 6-OHDA-mediated increase in faecal excretion of Salmonella Typhimurium.
Figure 4.
QseC influences, but is not essential for, increased faecal excretion of Salmonella Typhimurium in response to 6-OHDA treatment. Plasma NE (ng/mL) was quantified by ELISA immediately before and 1 h after treatment with 40 mg/kg 6-OHDA or diluent 16 days post-inoculation with CFU ST4/74 nalR ΔqseC (panel A). The impact of 6-OHDA treatment on the course of faecal excretion of ST4/74 nalR ΔqseC is shown in panel B. Values shown are the least square means (LSM) ± standard error (SE) of the LSM. p values ≤ 0.05 are marked with an asterisk.
4. DISCUSSION
Epidemiological and experimental studies support a role for stress in the development of porcine salmonellosis. Stress modulates multiple processes, and the contribution of stress-related catecholamines to observed phenomena is unclear. Our studies show that release of NE from endogenous stores by administration of the selective neurotoxin 6-hydroxydopamine can enhance faecal excretion of S. enterica serovar Typhimurium during recovery from acute infection. Intravenous delivery of 6-OHDA markedly elevated plasma NE levels, and though NE levels in the intestines are difficult to quantify for reasons we have discussed [44], the enteric nervous system is densely innervated by noradrenergic neurons and it is likely that significant spill over of NE into the intestinal lumen occurred. The ability of 6-OHDA to invoke responses that are specifically due to NE has previously been demonstrated using the catecholamine uptake blocker desipramine hydrochloride [25], and we propose that the model may be suitable for studies with other pig pathogens. Previous studies using a glucose analogue, 2-deoxy-D-glucose, indicated that it could induce many of the hallmark parameters of physiological stress in pigs [39], yet it failed to reactivate Salmonella Choleraesuis at enteric or systemic sites when given to carrier animals [40]. However, catecholamine levels in treated and control animals were not measured and further studies are required to determine how intestinal levels of stress-related catecholamines in experimental animal models compare to those of stressed animals in the field.
Though Salmonella Typhimurium was excreted in the faeces in significantly greater numbers in 6-OHDA-treated pigs relative to pre-treatment samples, it is noteworthy that the effect was relatively modest and transient even though there was clear evidence of physiological shock. Furthermore, the reactivation of Salmonella Typhimurium infection was less pronounced when 6-OHDA was given at day 7 post-inoculation (Fig. 1B) than when 6-OHDA was given at day 16 post-inoculation (Fig. 1D). In both cases, an explanation for the short-lived effect may be that priming of innate immunity during acute infection allows pigs to more effectively control rising Salmonella levels. It is known for example that prior infection of gnotobiotic piglets with avirulent S. enterica serovar Infantis can protect the animals from subsequent challenge with virulent Salmonella Typhimurium in a manner associated with neutrophil recruitment to the intestines [14]. The elevated faecal excretion of Salmonella Typhimurium upon 6-OHDA treatment at day 16 compared to day 7 post-challenge may therefore be associated with a waning protective innate response.
The ability of NE to alter the magnitude of Salmonella Typhimurium excretion by pigs was confirmed by oral administration of NE to recovering animals. This invoked a substantial rise in the number of excreted bacteria compared to controls, consistent with the ability of orally-administered NE to promote intestinal colonisation and systemic translocation by Salmonella Enteritidis in chicks [27] and Salmonella Typhimurium in mice [27, 46]. The concentrations required to promote this effect were very high in a physiological context, however the survival and transit times of orally administered NE through anatomical compartments are unknown. It is conceivable that elevated excretion of Salmonella in response to 6-OHDA or NE treatment may reflect changes in intestinal motility.
To investigate the mechanism(s) by which exogenous or endogenous NE alter the outcome of Salmonella infection in pigs, we examined the role of the putative adrenergic sensor kinase QseC. A Salmonella Typhimurium ΔqseC null mutant was consistently shed in lower numbers than the parent strain, though not significantly so. The data are consistent with the absence of significant attenuation of the same mutant in calves [31] and the modest attenuation of an independent ΔqseC mutant in mice [28], but differ markedly from other studies with a Salmonella Typhimurium qseC mutant in pigs [5, 6]. The disparities between pig studies may be due to use of distinct mutants (a qseC::cat insertion mutant was used by Bearson et al.), inoculation routes (intranasal vs. intragastric), or other parameters. Given the assertion that QseC signalling represents a novel drug target [32, 33], further studies are required to evaluate the role of QseC and inhibitors thereof in reservoir hosts.
Faecal excretion of the Salmonella Typhiumurium ΔqseC mutant could be increased by 6-OHDA treatment, indicating that QseC is not absolutely essential for the effect. However, the magnitude of the increase in faecal excretion of ST4/74 nalR ΔqseC was lower than observed with the parent strain when pigs were treated with 6-OHDA at the same time post-inoculation and when Salmonella levels in the faeces were similar (1.09 fold increase in mean log10 CFU/g ST4/74 nalR ΔqseC compared to a 2.68 fold increase for the parent strain upon 6-OHDA treatment 16 days post-infection), and was not statistically significant. This suggests that QseC plays a role in the response to 6-OHDA, but that other factors may be involved. We caution that the effect of 6-OHDA on faecal excretion of the ∆qseC mutant and parent strains was tested in separate experiments and direct comparisons within a single cohort of treated pigs are required. The potential for QseC-independent phenomena is consistent with the existence of at least one other adrenergic sensor kinase in E. coli O157:H7 (QseE: [34]), and with the ability of NE to modulate other processes such as growth, iron supply and virulence in a manner that is independent of QseCE [31]. Another possible explanation for the reduced response of the ∆qseC mutant to 6-OHDA treatment (Fig. 4B) when compared to wild-type bacteria tested separately but in the same way (Fig. 1D), may be that the mutant is subtly attenuated and unable to replicate as efficiently upon release of NE. In this respect, the lower response of the ∆qseC mutant to 6-OHDA treatment could be due to impaired fitness rather than absence of adrenergic sensing, and studies with other attenuated mutants may support this.
It is also plausible that release of NE mediated by 6-OHDA or exogenous NE may promote Salmonella infection via changes in the microflora. It is known that 6-OHDA profoundly enhances the number of coliforms, particularly E. coli, in the murine intestinal tract [25]. Furthermore, deep-sequencing of intestinal microflora in a restraint-based mouse model of chronic stress was recently reported to alter the composition of the microflora in a manner associated with elevated susceptibility to the murine enteric pathogen Citrobacter rodentium [3]. It is established that the microflora plays an important role in determining the outcome of Salmonella infection, since streptomycin-mediated depletion of the microflora allows Salmonella Typhimurium to produce colitis in inbred mice, whereas mice with intact gut flora exhibit minimal enteric pathology [4]. The bacterial families and genera perturbed by antibiotics that alter susceptibility to salmonellosis have been defined at low resolution [18, 37], and it will be of interest to determine if the same groups are affected by stressors. Further studies are also required to examine the relative importance of growth activation, putative adrenergic sensor kinases, and the modulation of host processes in the context of stress and susceptibility to microbial infection.
In conclusion, we report that the selective neurotoxin 6-OHDA can be used to elicit the release of NE in pigs and enhances the faecal excretion of Salmonella Typhimurium. Our findings are consistent with the ability of NE to augment Salmonella pathogenesis in other animal models [26, 27, 31, 46] and may provide a partial explanation for the elevated excretion of zoonotic pathogens by livestock subject to transport or social stress.
Acknowledgments
The authors gratefully acknowledge the support of the Biotechnology & Biological Sciences Research Council, United Kingdom (grant number BB/C500822/1).
References
- 1.Alverdy J., Holbrook C., Rocha F., Seiden L., Wu R.L., Musch M., et al., Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa, Ann. Surg. (2000) 232:480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous, Zoonoses Report, United Kingdom 2008, Department for Environment, Food and Rural Affairs Publications, London, 2010 [Google Scholar]
- 3.Bailey M.T., Dowd S.E., Parry N.M., Galley J.D., Schauer D.B., Lyte M., Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium, Infect. Immun. (2010) 78:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthel M., Hapfelmeier S., Quintanilla-Martínez L., Kremer M., Rohde M., Hogardt M., et al., Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host, Infect. Immun. (2003) 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearson B.L., Bearson S.M., The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium, Microb. Pathog. (2008) 44:271–278 [DOI] [PubMed] [Google Scholar]
- 6.Bearson B.L., Bearson S.M., Lee I.S., Brunelle B.W., The Salmonella enterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase, Microb. Pathog. (2010) 48:214–219 [DOI] [PubMed] [Google Scholar]
- 7.Berends B.R., Urlings H.A., Snijders J.M., Van Knapen F., Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs, Int. J. Food Microbiol. (1996) 30:37–53 [DOI] [PubMed] [Google Scholar]
- 8.Callaway T.R., Morrow J.L., Edrington T.S., Genovese K.J., Dowd S., Carroll J., et al., Social stress increases fecal shedding of Salmonella typhimurium by early weaned piglets, Curr. Issues Intest. Microbiol. (2006) 7:65–71 [PubMed] [Google Scholar]
- 9.Clarke M.B., Hughes D.T., Zhu C., Boedeker E.C., Sperandio V., The QseC sensor kinase: a bacterial adrenergic receptor, Proc. Natl. Acad. Sci. USA (2006) 103:10420–10425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies R.H., Dalziel R., Gibbens J.C., Wilesmith J.W., Ryan J.M., Evans S.J., et al., National survey for Salmonella in pigs, cattle and sheep at slaughter in Great Britain, (1999–2000), J. Appl. Microbiol. (2004) 96:750–760 [DOI] [PubMed] [Google Scholar]
- 11.de Champlain J., Degeneration and regrowth of adrenergic nerve fibers in the rat peripheral tissues after 6-hydroxydopamine, Can. J. Physiol. Pharmacol. (1971) 4:345–355 [DOI] [PubMed] [Google Scholar]
- 12.Dowd S.E., Callaway T.R., Morrow-Tesch J., Handling may cause increased shedding of Escherichia coli and total coliforms in pigs, Foodborne Pathog. Dis. (2007) 4:99–102 [DOI] [PubMed] [Google Scholar]
- 13.Ellermeier J.R., Slauch J.M., Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD, J. Bacteriol. (2008) 190:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster N., Lovell M.A., Marston K.L., Hulme S.D., Frost A.J., Bland P., Barrow P.A., Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica, Infect. Immun. (2003) 71:2182–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freestone P.P., Haigh R.D., Lyte M., Specificity of catecholamine-induced growth in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica, FEMS Microbiol. Lett. (2007) 269:221–228 [DOI] [PubMed] [Google Scholar]
- 16.Freestone P.P., Lyte M., Microbial endocrinology: experimental design issues in the study of interkingdom signalling in infectious disease, Adv. Appl. Microbiol. (2008) 64:75–105 [DOI] [PubMed] [Google Scholar]
- 17.Freestone P.P., Sandrini S.M., Haigh R.D., Lyte M., Microbial endocrinology: how stress influences susceptibility to infection, Trends Microbiol. (2008) 16:55–64 [DOI] [PubMed] [Google Scholar]
- 18.Garner C.D., Antonopoulos D.A., Wagner B., Duhamel G.E., Keresztes I., Ross D.A., et al., Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar Typhimurium murine model of infection, Infect. Immun. (2009) 77:2691–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoiseth S.K., Stocker B.A., Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines, Nature (1981) 291:238–239 [DOI] [PubMed] [Google Scholar]
- 20.Hurd H.S., McKean J.D., Griffith R.W., Wesley I.V., Rostagno M.H., Salmonella enterica infections in market swine with and without transport and holding, Appl. Environ. Microbiol. (2002) 68:2376–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaacson R.E., Firkins L.D., Weigel R.M., Zuckermann F.A., DiPietro J.A., Effect of transportation and feed withdrawal on shedding of Salmonella typhimurium among experimentally infected pigs, Am. J. Vet. Res. (1999) 60:1155–1158 [PubMed] [Google Scholar]
- 22.Jones P.H., Roe J.M., Miller B.G., Effects of stressors on immune parameters and on the faecal shedding of enterotoxigenic Escherichia coli in piglets following experimental inoculation, Res. Vet. Sci. (2001) 70:9–17 [DOI] [PubMed] [Google Scholar]
- 23.Karavolos M.H., Spencer H., Bulmer D.M., Thompson A., Winzer K., Williams P., et al., Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses, BMC Genomics (2008) 9:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughlin R.S., Musch M.W., Hollbrook C.J., Rocha F.M., Chang E.B., Alverdy J.C., The key role of Pseudomonas aeruginosa PA-I lectin on experimental gut-derived sepsis, Ann. Surg. (2000) 232:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyte M., Bailey M.T., Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma, J. Surg. Res. (1997) 70:195–201 [DOI] [PubMed] [Google Scholar]
- 26.McCuddin Z.P., Carlson S.A., Sharma V.K., Experimental reproduction of bovine Salmonella encephalopathy using a norepinephrine-based stress model, Vet. J. (2008) 175:82–88 [DOI] [PubMed] [Google Scholar]
- 27.Methner U., Rabsch W., Reissbrodt R., Williams P.H., Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products, Int. J. Med. Microbiol. (2008) 298:429–439 [DOI] [PubMed] [Google Scholar]
- 28.Moreira C.G., Weinshenker D., Sperandio V., QseC mediates Salmonella enterica serovar Typhimurium virulence in vitro and in vivo, Infect. Immun. (2010) 78:914–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan I.R., Krautil F.L., Craven J.A., Effect of time in lairage on caecal and carcass Salmonella contamination of slaughter pigs, Epidemiol. Infect. (1987) 98:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulin S.M., Jagannathan A., Campbell J., Wallis T.S., Stevens M.P., Net replication of Salmonella enterica serovars Typhimurium and Choleraesuis in porcine intestinal mucosa and nodes is associated with their differential virulence, Infect. Immun. (2007) 75:3950–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pullinger G.D., Carnell S.C., Sharaff F.F., van Diemen P.M., Dziva F., Morgan E., et al., Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE, Infect. Immun. (2010) 78:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasko D.A., Moreira C.G., Li de R., Reading N.C., Ritchie J.M., Waldor M.K., et al., Targeting QseC signaling and virulence for antibiotic development, Science (2008) 321:1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasko D.A., Sperandio V., Anti-virulence strategies to combat bacteria-mediated disease, Nat. Rev. Drug Discov. (2010) 9:117–128 [DOI] [PubMed] [Google Scholar]
- 34.Reading N.C., Rasko D.A., Torres A.G., Sperandio V., The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis, Proc. Natl. Acad. Sci. USA (2009) 106:5889–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reissbrodt R., Rienaecker I., Romanova J.M., Freestone P.P., Haigh R.D., Lyte M., et al., Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer, Appl. Environ. Microbiol. (2002) 68:4788–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandrini S.M., Shergill R., Woodward J., Muralikuttan R., Haigh R.D., Lyte M., Freestone P.P., Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin, J. Bacteriol. (2010) 192:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekirov I., Tam N.M., Jogova M., Robertson M.L., Li Y., Lupp C., Finlay B.B., Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection, Infect. Immun. (2008) 76:4726–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer H., Karavolos M.H., Bulmer D.M., Aldridge P., Chhabra S.R., Winzer K., et al., Genome-wide transposon mutagenesis identifies a role for host neuroendocrine stress hormones in regulating the expression of virulence genes in Salmonella, J. Bacteriol. (2010) 192:714–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stabel T.J., Evaluation of 2-deoxy-D-glucose for induction of a stress response in pigs, Am. J. Vet. Res. (1999) 60:708–713 [PubMed] [Google Scholar]
- 40.Stabel T.J., Fedorka-Cray P.J., Effect of 2-deoxy-D-glucose induced stress on Salmonella choleraesuis shedding and persistence in swine, Res. Vet. Sci. (2004) 76:187–194 [DOI] [PubMed] [Google Scholar]
- 41.Stevens M.P., Modulation of the interaction of enteric bacteria with intestinal mucosa by stress-related catecholamines, in: Lyte M., Freestone P. (Eds.), Microbial Endocrinology, Springer Press, London, 2010, pp. 111–134 [DOI] [PubMed] [Google Scholar]
- 42.Thomas G.D., O’Hagan K.P., Zambraski E.J., Effects of 6-hydroxydopamine in hypertensive adult miniature swine, Clin. Exp. Hypertens. A (1990) 12:647–661 [DOI] [PubMed] [Google Scholar]
- 43.Toscano M.J., Stabel T.J., Bearson S.M.D., Bearson B.L., Lay D.C., Cultivation of Salmonella enterica serovar Typhimurium in a norepinephrine-containing medium alters in vivo tissue prevalence in swine, J. Exp. Anim. Sci. (2007) 43:329–338 [Google Scholar]
- 44.Vlisidou I., Lyte M., van Diemen P.M., Hawes P., Monaghan P., Wallis T.S., Stevens M.P., The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection, Infect. Immun. (2004) 72:5446–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson P.R., Paulin S.M., Jones P.W., Wallis T.S., Interaction of Salmonella serotypes with porcine macrophages in vitro does not correlate with virulence, Microbiology (2000) 146:1639–1649 [DOI] [PubMed] [Google Scholar]
- 46.Williams P.H., Rabsch W., Methner U., Voigt W., Tschäpe H., Reissbrodt R., Catecholate receptor proteins in Salmonella enterica: role in virulence and implications for vaccine development, Vaccine (2006) 24:3840–3844 [DOI] [PubMed] [Google Scholar]