Abstract
Ambulatory BP studies indicate that even small increases in BP, particularly nighttime BP levels, are associated with significant increases in cardiovascular morbidity and mortality. Accordingly, sleep-related diseases that induce increases in BP would be anticipated to substantially affect cardiovascular risk. Both sleep deprivation and insomnia have been linked to increases in incidence and prevalence of hypertension. Likewise, sleep disruption attributable to restless legs syndrome increases the likelihood of having hypertension. Observational studies demonstrate a strong correlation between the severity of obstructive sleep apnea (OSA) and the risk and severity of hypertension, whereas prospective studies of patients with OSA demonstrate a positive relationship between OSA and risk of incident hypertension. Intervention trials with continuous positive airway pressure (CPAP) indicate a modest, but inconsistent effect on BP in patients with severe OSA and a greater likelihood of benefit in patients with most CPAP adherence. Additional prospective studies are needed to reconcile observational studies suggesting that OSA is a strong risk factor for hypertension with the modest antihypertensive effects of CPAP observed in intervention studies.
Sleep alters autonomic nervous system function and other physiologic events that influence BP. Furthermore, sleep disorders alter the BP response and increase hypertension risk. Recent data on the effect of sleep and sleep disorders on BP and hypertension will be explored.
Sleep and Nocturnal BP
During normal sleep, there is a decrease in BP relative to wakefulness. This decrease is referred to as “nocturnal dipping” and partly is attributable to decreases in sympathetic output. Although arbitrary, a decrease of 10% to 20% in mean nocturnal BP (both systolic and diastolic) compared with mean daytime BP is considered normal. Conversely, an absence of nocturnal dipping, or nondipping, is designated as a < 10% decrease in nocturnal BP.
Lack or diminished nocturnal dipping of BP is a strong, independent predictor of cardiovascular risk. The Ohasama study noted that on average, each 5% deficiency in the normal decline in nocturnal BP was associated with an approximately 20% greater risk in cardiovascular mortality.1 Other studies have confirmed this finding.2-4 Many diseases are associated with diminished or absence of nocturnal dipping, including most secondary causes of hypertension, chronic kidney disease, diabetes, older age, resistant hypertension, and obstructive sleep apnea (OSA).
Large prospective studies have demonstrated that nocturnal BP is a better predictor of cardiovascular risk than is daytime BP. In the Dublin Outcome Study, 5,292 untreated patients with hypertension referred to a single hypertension clinic were prospectively followed for cardiovascular events.5 During a median follow-up period of 8.4 years, ambulatory BP measurements were superior to clinic BP measurements in predicting cardiovascular mortality, and nighttime BP was overall the strongest predictor of outcome. In this study, a 10-mm Hg increase in mean nighttime systolic BP was associated with a 21% increase in cardiovascular mortality. Other studies likewise have confirmed the superiority of nocturnal BP in predicting cardiovascular outcomes.6,7 Observational studies indicate that with aging, the cardiovascular risk attributable to office systolic BP increases, whereas the risk attributable to diastolic BP decreases. To what extent this interaction with aging is true of nocturnal hypertension has not yet been fully elucidated.
Poorly controlled hypertension remains a strong cause of cardiovascular morbidity and mortality worldwide. Even small changes in mean BP translate into potentially large decreases in cardiovascular complications. For example, data from observational studies and randomized trials suggest that a 2-mm Hg reduction in diastolic BP on a population basis results in a 17% decrease in hypertension prevalence, a 6% reduction in coronary heart disease risk, and a 15% reduction in the risk of stroke and transient ischemic attack.8 A metaanalysis of randomized trials of antihypertensive medications showed that a 10-mm Hg reduction in systolic BP or a 5-mm Hg reduction in diastolic BP reduces risk of coronary heart disease events by 22% and stroke by 41%.9
Taken together, these results demonstrate that even small changes in BP, especially nocturnal BP, can alter cardiovascular risk significantly. Accordingly, disease processes related to sleep that may affect BP have the potential to alter cardiovascular morbidity and mortality substantially.
Sleep Duration and Hypertension
Habitual sleep duration over the past 50 years has decreased by 1.5 to 2 h/day, and > 30% of Americans report sleeping less than 6 h/night.10 In the Sleep Heart Health Study, subjects sleeping ≤ 5 h/night had a higher frequency of prevalent hypertension (adjusted odds ratio [OR], 1.66; 95% CI, 1.35-2.04), after adjusting for multiple confounders.11 The Whitehall II Study examined cross-sectional and prospective associations of sleep duration with prevalent and incident hypertension in a cohort of 10,308 British civil servants aged 35 to 55 years.12 At baseline, no association was noted in men; however, women (n = 1,567) sleeping ≤ 5 h/night had a higher risk of hypertension compared with those sleeping 7 h/night (OR, 1.72; 95% CI, 1.07-2.74; P = .037), independent of confounders. In the prospective analysis, the incident hypertension risk was attenuated after confounding for cardiovascular (OR, 1.42; 95% CI, 0.94-2.15) and psychiatric (OR, 1.31; 95% CI, 0.65-2.63) comorbidities, emphasizing the importance of extensive evaluation of confounders in assessing this association.
In the first National Health and Nutrition Examination Survey of 4,810 middle-aged (32-59 years) Americans in fully adjusted models, short sleep duration (≤ 5 h/night) was associated with a 60% higher risk of self-reported incident hypertension over an 8- to 10-year follow-up period (hazard ratio, 2.10; 95% CI, 1.58-2.79).13 No association was found in individuals aged ≥ 60 years.
Sleep duration and hypertension may not be associated in persons aged > 58 years.14 In the 5,058 participants of the population-based Rotterdam study,14 and a Spanish prospective cohort study of 3,686 persons,15 no association was found in prevalent or incident hypertension. Note that most of these cross-sectional population studies use subjective reports of sleep duration and not objective data, such as that obtained from prolonged actigraphy monitoring. Subjective reports of sleep duration may not be accurate.
The association between short sleep duration and hypertension appears to be most significant during middle age. The Coronary Artery Risk Development in Young Adults cohort examined objective sleep duration by measuring 3-day wrist actigraphy twice between 2003 and 2005, sleep quality, 5-year incidence of hypertension, and changes in systolic and diastolic pressure in 578 Americans aged 33 to 45 years at baseline.16 Short sleep duration predicted increased odds of incident hypertension (OR, 1.37; 95% CI, 1.05-1.78). Each hour of reduced sleep was associated with a 37% increase in the odds of incident hypertension.
In a sample of 238 adolescents without sleep apnea or severe comorbidities from the Cleveland Children’s Sleep and Heart Study, children with short sleep duration (≤ 6.5 h/night) had an adjusted OR of prehypertension of 2.54 (95% CI, 0.93-6.90).17 Furthermore, poor sleep efficiency (< 85%) on overnight polysomnography was associated with an average adjusted increase in systolic BP of 4 mm Hg, and the odds of prehypertension increased 3.5-fold (95% CI, 1.5-8.0). The researchers defined prehypertension as systolic or diastolic BP ≥ 90th percentile for age, sex, and height as noted by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.
Furthermore, the Sleep Heart Health Study noted that long sleep duration (≥ 9 h) is associated with prevalent hypertension (OR, 1.30; 95% CI, 1.04-1.62) compared with individuals sleeping 7 to 8 h.11 Friedman et al18 assessed the relationship between self-reported sleep duration and 24-h ambulatory BP monitoring in 108 subjects with normal BP and 417 subjects with hypertension. Assessing both nondipping status and elevated morning BP surge, a 1-h decrease in sleep duration was associated with nondipping (nocturnal BP fall, < 10%; OR, 1.12; P = .04) without an elevated morning BP surge. However, long sleep duration was associated with a morning BP surge and less nondipping.
Insomnia and Hypertension
Activation of the hypothalmic-pituitary-adrenal axis and the sympathetic nervous system as seen in insomnia may predispose to hypertension development.19 Phillips and Mannino20 examined the 8,757 participants in the Atherosclerosis Risk in Communities study over 6 years to determine whether they reported insomnia at baseline. The combination of difficulty falling asleep, staying asleep, and having nonrestorative sleep was not associated with an increased risk of hypertension; however, participants reporting difficulty falling asleep or sleep continuity problems had a slightly increased risk of hypertension at follow-up (OR, 1.2; 95% CI, 1.03-1.3), even after controlling for confounders, so, the effects are somewhat inconsistent.
If insomnia is associated with an increased risk of hypertension, it does not appear to be the case in older adults. In the Cardiovascular Health Study, a prospective cohort study of 1,419 older persons aged 73 years at baseline with a 6-year follow-up, insomnia complaints did not predict incident hypertension.21
Lanfranchi et al22 examined 13 subjects with normal BP but with chronic primary insomnia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria) and 13 sex- and age-matched good sleepers using 24-h beat-to-beat BP along with electroencephalography spectral analysis. The subjects with insomnia had higher nighttime systolic BP and a decrease in the day-to-night systolic BP dipping compared with the good sleepers (both P = .01). Daytime diastolic BP (P = .02) and nighttime diastolic BP (P = .01) were higher in the subjects with insomnia, whereas the day-to-night diastolic BP dipping did not differ between the groups. A borderline association (r = 0.38; P = .08) was noted between nighttime systolic BP and electroencephalography activity in the β frequency.
Another confounder when examining the association between insomnia and hypertension risk is that insomnia can lead to short sleep duration, and short sleep duration affects hypertension risk. Vgontzas et al23 examined the joint effect of insomnia and objective short sleep duration on hypertension in a cross-sectional, population-based sample of 1,741 randomly selected adults from Pennsylvania. Insomnia was associated with a significantly higher risk for hypertension and when confounding variables were adjusted for (OR, 2.41; 95% CI, 1.6-3.7; P < .05). A sleep duration of ≤ 5 h increased hypertension risk (OR, 1.56; 95% CI, 1.1-2.1; P < .05) compared with the group sleeping > 6 h. Using logistic regression analysis, they examined the joint effect of insomnia and objective sleep duration on hypertension. The presence of both insomnia and an objective sleep duration of ≤ 5 h increased hypertension risk (OR, 5.12; 95% CI, 2.2-11.8) compared with sleeping > 6 h. On the basis of these findings, approximately 50% of persons with chronic insomnia run a significant risk for hypertension. Additionally, controlling for the presence of depression did not diminish the association. These data need to be taken seriously because they are from the first large population-based study examining polysomnographic variables linking insomnia with short sleep duration and hypertension.19 Note that participants with insomnia who slept > 6 h did not show an increased risk for hypertension compared with control subjects.
Restless Legs Syndrome and Periodic Limb Movements in Sleep and Hypertension
Epidemiologic studies have suggested that a relationship may exist between self-reported restless legs syndrome (RLS) and hypertension.24 Ohayon and Roth25 examined RLS prevalence in a cross-sectional population study of 18,980 subjects aged ≥ 15 years in five European countries through a telephone interview. Hypertension (treated or untreated) was significantly associated with RLS (P < .001) and made an independent significant contribution to RLS (OR, 1.36; 95% CI, 1.14-1.61; P < .001) but not to periodic limb movements in sleep (PLMS).25 Of note, the diagnosis of PLMS was not made by polysomnography but by the validated Sleep-EVAL system questionnaire, which has a κ for diagnosing PLMS of 0.84.25 Likewise, Phillips et al26 examined RLS prevalence and correlates as part of the 2005 National Sleep Foundation Poll, a telephone interview of 1,506 randomly selected adults in the United States. Hypertension was associated with RLS (P < .05). Ulfberg et al27 examined by questionnaire a random population sample of 4,000 men living in central Sweden, finding that subjects with reported RLS symptoms more frequently reported hypertension (OR, 1.15; 95% CI, 0.9-2.4). Examining the 3,433 men and women enrolled in the Sleep Heart Health Study, Winkelman et al28 also noted only a weak association of RLS with hypertension (OR, 1.30; 95% CI, 0.92-1.82) after adjusting for age, sex, race, and BMI.
However, conflicting data come from three population-based studies that assessed both prevalence of and risk factors for RLS: one in an elderly population of 731 subjects in northern Italy, another of 701 subjects from the general community in Austria, and a third of 2,821 subjects from the Wisconsin Sleep Cohort Study.29-31 These studies did not find an association between RLS and hypertension. Potentially, the age of the study participants enrolled in these cohorts may be an important confounding factor. This possible association needs further careful study before definitive conclusions can be made.
PLMS also may be associated with hypertension, with movements temporally associated with sympathetic activation.24 Pennestri et al32 examined the temporal association between PLMS and beat-to-beat BP monitoring in 10 patients with RLS undergoing polysomnography. Using a 25-beat temporal window comprising 10 beats before and 15 beats after onset of each movement, systolic BP increased 22 mm Hg and diastolic BP increased 11 mm Hg in association with PLMS. Furthermore, the BP response for PLMS associated with microarousals were greater than PLMS not associated with arousals (P < .05). This BP response also was greater with increasing age (systolic r = 0.76; P = .02) and duration of RLS symptoms (systolic r = 0.76; P = .02). Another investigation using a similar study design confirmed these findings in eight subjects with RLS.33 PLMS during wakefulness was associated with a systolic BP elevation of 11.7 ± 7.6 mm Hg. PLMS associated with microarousals during sleep were associated with a systolic BP elevation of 16.7 ± 9.4 mm Hg, and in PLMS not associated with an arousal, the systolic BP increased 11.2 ± 8.7 mm Hg. Fake PLMS during wakefulness served as another control and was associated with a mean systolic BP increase of 3.2 ± 3.1 mm Hg. These results confirm that individual movements are associated with significant elevations of systolic and diastolic BP, and these elevations are greater if the PLMS is associated with a cortical arousal.
Data are emerging that look at PLMS and hypertension. A recent study abstract reported that in an Icelandic cohort of 861 subjects enriched for RLS and objectively monitored for PLMS, hypertension likelihood increased with PLMS severity.34 For instance, hypertension risk was twice as high for a PLMS index > 30 (OR, 2.26; 95% CI, 1.28-3.99), even after controlling for confounders.
OSA and Prevalence of Hypertension
OSA and hypertension commonly coexist. Approximately 50% of patients with OSA are hypertensive, and an estimated 30% to 40% of patients with hypertension have OSA.35-38 Cross-sectional studies have been consistent in demonstrating that moderate-severe OSA (apnea-hypopnea index [AHI] > 15 events/h) is significantly associated with risk of having arterial hypertension.37 In general, there is a linear relationship between AHI and prevalence and severity of hypertension, that is, the more severe the OSA, the higher the risk of hypertension of increasing severity.
In two studies, Grunstein et al39,40 reported that a high AHI was associated with an increased likelihood of having hypertension, even after correcting for confounding variables, including age and obesity. In a study of 2,677 adult subjects with suspected OSA, Lavie et al41 noted that as indexed by the AHI, both the prevalence and the severity of hypertension increased as OSA severity increased. Overall, the AHI significantly predicted both systolic and diastolic BP independent of age, BMI, and sex. For each 1-event increase in the AHI, there was a 1% higher risk of having hypertension. This finding also was confirmed in the Wisconsin Sleep Cohort Study, which found that an AHI of 15 (compared with 0) was associated with an OR of having hypertension of 1.8 (95% CI, 1.3-2.4).42 The hypertension risk increased in a dose-dependent fashion in relation to increasing OSA severity. In this same cohort, a linear relationship between AHI and hypertension severity also was observed such that increasing AHI was associated with progressively higher 24-h ambulatory BP levels.43
In a study of 1,741 subjects aged 20 to 100 years, Bixler et al44 found that an AHI ≥ 15 (compared with 0) was significantly associated with hypertension risk; however, the strength of this association decreased with age. Another study of 2,148 subjects aged 30 to 70 years and with an AHI ≥ 15 had an OR for hypertension risk of 2.28 (95% CI, 0.92-5.66), after adjusting for confounders such as BMI, neck circumference, and alcohol use.45 In this analysis, an increase in the AHI of 5 events/h increased the risk of having hypertension by 1.25%. In the Sleep Heart Health Study, which included 6,123 subjects aged > 40 years, an AHI ≥ 30 compared with < 1.5 was associated with an OR for prevalent hypertension of 1.37 (95% CI, 1.03-1.83).46 These data demonstrate that the presence of moderate-severe OSA is positively related to both the prevalence and the severity of hypertension.
OSA and Risk of Incident Hypertension
Two large observational longitudinal studies assessed the relationship between OSA severity and subsequent risk of incident hypertension in normotensive cohorts at baseline. In the Wisconsin Sleep Cohort Study, Peppard et al47,48 followed 709 subjects with normal BP for 4 years after evaluation by overnight polysomnography. Subjects with moderate-severe OSA (AHI ≥ 15 events/h) had a 3.2-fold increased odds of developing hypertension relative to subjects without OSA. In contrast, results from the recent Sleep Heart Health Study analysis of 2,470 subjects with normal BP at 5-year follow-up noted no increased risk of incident hypertension, even in patients with severe OSA (AHI ≥ 15), after adjusting for BMI.49 These disparate results may be related to methodological differences, including differences in the cohort size and diversity.50 For example, participants in the Sleep Heart Health Study were, on average, considerably older than participants in the Wisconsin Sleep Cohort Study (60 years vs 47 years, respectively) and, therefore, perhaps not as sensitive to hypertensive effects of untreated OSA. In addition, the observed risk may have been blunted because both studies selected patients with normal BP at baseline despite having OSA. That is, patients with OSA at highest risk of developing hypertension may have been excluded because they were already hypertensive at the start of the study, whereas eligible subjects who remained normotensive were somehow more resistant to the hypertensive effects of OSA. Nonetheless, although the longitudinal results of the Wisconsin Sleep Cohort Study are consistent with the large body of observational evidence linking OSA to risk of having hypertension, additional prospective studies are needed to reconcile those positive results with the negative results of the Sleep Heart Health Study.
Effect of Continuous Positive Airway Pressure on BP
If OSA contributes to hypertension development or progression, then effective OSA treatment with continuous positive airway pressure (CPAP) should lower BP. However, reports are conflicting. This lack of a consistent treatment effect may be related to multiple variables, including differences in study design, type and size of cohorts, degree of CPAP compliance, treatment duration, and accuracy of BP assessments.38
Recently, four metaanalyses of randomized controlled trials on CPAP use have been published (Table 1). Bazzano et al51 analyzed 16 randomized clinical trials published between 1980 and 2006, representing 818 participants, that compared participants treated with CPAP with control subjects, that had a minimum treatment duration of 2 weeks, and that reported BP changes during the intervention and control period. Mean net change in systolic BP for participants treated with CPAP vs control subjects was −2.46 mm Hg (95% CI, −4.31 to −0.62 mm Hg); mean net change in diastolic BP, −1.83 mm Hg (95% CI, −3.05 to −0.61 mm Hg); and mean net change in mean arterial pressure, −2.22 mm Hg (95% CI, −4.38 to −0.05 mm Hg). The authors concluded that their analysis provided evidence that effective CPAP treatment reduces BP.
Table 1.
—Summary of Metaanalyses of Randomized Controlled Trials of Continuous Positive Airway Pressure Use
| Reference | No. of Trials (Patients) | BP End Point | Minimum CPAP Duration, wk | Outcome |
| Bazzano et al51 | 16 (818) | Office/ambulatory | 2 | SBP –2.46 mm Hg |
| DBP –1.83 mm Hg | ||||
| More benefit in patients with higher baseline BP, higher BMI, and more severe OSA | ||||
| Alajmi et al52 | 10 (587) | Office/ambulatory | 4 | SBP –1.38 mm Hg (not significant) |
| DBP –1.52 mm Hg (not significant) | ||||
| More benefit in more severe OSA; trend for better SBP reduction with better CPAP adherence | ||||
| Mo and He53 | 7 (471) | Ambulatory | 4 | 24-h SBP –0.95 mm Hg (not significant) |
| 24-h DBP –1.78 mm Hg | ||||
| Haentjens et al54 | 12 (572) | Ambulatory | 1 | 24-h SBP –1.64 mm Hg |
| 24-h DBP –1.48 mm Hg | ||||
| More benefit in more severe OSA and with better CPAP adherence | ||||
CPAP = continuous positive airway pressure; DBP = diastolic BP; OSA = obstructive sleep apnea; SBP = systolic BP.
Alajmi et al52 identified 10 randomized controlled trials up through July 2006 that included an appropriate control group and reported systolic and diastolic BP before and after CPAP treatment and a control condition; data from 587 subjects were included. Overall, the effects of CPAP were modest and not significant. CPAP treatment compared with the control condition reduced systolic BP by 1.38 mm Hg (95% CI, 3.6 to −0.88 mm Hg) and diastolic BP by 1.52 mm Hg (95% CI, 3.1 to −0.07 mm Hg). Reductions in BP tended to be larger in patients with severe OSA (AHI > 30), and a trend for systolic BP reduction was associated with higher CPAP adherence.
Mo and He53 analyzed randomized controlled trials published between 2000 and 2006. Inclusion criteria included a treatment duration of ≥ 4 weeks and measurement of 24-h ambulatory BP before and after CPAP and non-CPAP treatment. Seven studies with 471 participants were included. Overall, CPAP reduced 24-h systolic BP by 0.95 mm Hg (95% CI, −2.85-0.94 mm Hg), 24-h diastolic BP by 1.78 mm Hg (95% CI, −3.34 to −0.22 mm Hg), and 24-h mean BP by 1.25 mm Hg (95% CI, −4.00-1.49 mm Hg). The overall treatment effects were modest and significant only for 24-h diastolic BP.
Haentjens et al54 also limited their analysis to studies that had measured 24-h ambulatory BP, which included 572 participants from 12 randomized placebo-controlled trials. The CPAP treatment condition compared with placebo reduced 24-h systolic BP by 1.64 mm Hg (95% CI, −2.67 to −0.60 mm Hg) and 24-h diastolic BP by 1.48 mm Hg (95% CI, −2.18 to −0.78 mm Hg). The effect size was larger for daytime BP, with only the change in mean and systolic BP being significant at nighttime. In a prespecified metaregression analysis, greater CPAP treatment-related reduction in 24-h mean BP was observed in participants with more severe OSA and in those with the most CPAP adherence.
Overall, these four metaanalyses indicate, at best, a modest antihypertensive effect of CPAP. There is evidence that individual variation in patients with more severe OSA and patients most adherent with CPAP use manifest greater benefit, but this overall small treatment effect raises the question of why effective use of CPAP does not lower BP better. Even small reductions in BP can result in substantial reductions in cardiovascular risk such that small observed effects should not be discounted. Why the treatment effects, however, are not larger is an important clinical question that at this point remains an area of conjecture. Although multiple possible explanations need exploration, two issues may be particularly relevant. The first is the level of CPAP adherence needed to obtain maximum vascular and hemodynamic benefit. Adherence with CPAP use often is low, particularly in less symptomatic patients. Even in clinical trials, CPAP adherence often has averaged < 4 to 5 h/night,53 meaning that many patients are untreated for several hours a night. It may be that with full-night CPAP treatment there is a more-pronounced BP effect. Data suggest that patients who are most adherent with CPAP use manifest the largest decrease in BP.54
The other consideration is the duration of treatment. Most of the randomized clinical trials of CPAP have been short, usually ≤ 12 weeks in duration.54 Given that OSA has been present in most cases for an extended period before being diagnosed, it may be that mechanisms of OSA-induced hypertension cannot be reversed quickly or completely. Although studies have indicated that changes in sympathetic activation, inflammation, and oxidative stress can be reduced relatively quickly with CPAP use, associated vascular fibrotic changes may be more recalcitrant to treatment or even permanent. In the former case, longer treatment periods may be necessary to maximize an antihypertensive effect, and in the latter case, CPAP may be more effective in preventing hypertension or the progression of hypertension than in lowering BP.
Potential Mechanisms of OSA-Induced Hypertension
OSA-induced increases in sympathetic activation contribute to increased BP. This effect is not limited to the sleep period because there is a sustained increase in sympathetic activation noted during wakefulness in patients with untreated OSA.55 Heightened sympathetic activity increases BP by increasing vascular resistance and cardiac output and, possibly, by stimulating the renin-angiotensin-aldosterone system. Effective OSA treatment with CPAP suppresses this sympathetic activation.56
Other pathophysiologic mechanisms, including proinflammatory mediator effects, increased oxidative stress, and increased vascular stiffness also may play a role (Fig 1). Small CPAP intervention trials suggest that each of these effects can be reduced with effective CPAP use, often quite rapidly.57-61
Figure 1.
Pathophysiologic mechanisms involved in the etiology of OSA-induced hypertension. OSA = obstructive sleep apnea.
OSA and Resistant Hypertension
OSA is common in patients with resistant hypertension, which is defined as BP that remains uncontrolled with three or more medications. In a prospective evaluation of 41 patients with resistant hypertension, Logan et al62 found that 96% of the men and 65% of the women had significant OSA (AHI ≥ 10 events/h). In 71 consecutive subjects referred to the hypertension clinic at the University of Alabama at Birmingham for resistant hypertension, we found that 90% of the men and 77% of the women had OSA (AHI > 5 events/h).63 As OSA severity increases, there is an increased need for additional BP medications; that is, the more severe the OSA, the less likely BP is controlled with pharmacologic therapy.64-67 A prospective, but uncontrolled CPAP trial demonstrated that CPAP use can have substantial antihypertensive benefit in patients with resistant hypertension. Logan et al68 reported that CPAP use after 2-month follow-up in 11 patients with resistant hypertension lowered nighttime systolic BP by 14.4 ± 4.4 mm Hg and diastolic BP by 7.8 ± 3.0 mm Hg.
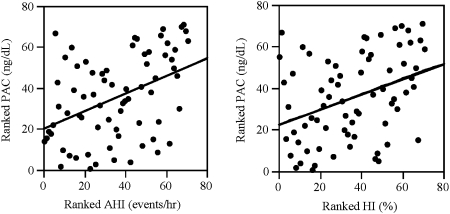
The explanation of the extraordinarily high prevalence of OSA in patients with resistant hypertension remains obscure. Data from our laboratory, however, suggest that it may be linked to the high occurrence of hyperaldosteronism in patients with resistant hypertension. In an evaluation of 114 patients with resistant hypertension, we found that patients at high risk for OSA (based on their responses to the Berlin Questionnaire) had significantly greater 24-h urinary excretion of aldosterone and were almost twice as likely to be diagnosed with primary aldosteronism compared with control subjects with resistant hypertension who were at low risk for OSA.63 In a subsequent study, we reported that plasma aldosterone levels in patients with resistant hypertension are positively correlated with severity of OSA (AHI and hypoxic index), that is, the higher the plasma aldosterone level the more severe the OSA (Fig 2).64
Figure 2.
Plasma aldosterone concentration positively correlates with apnea-hypopnea index and hypoxic index in patients with obstructive sleep apnea and resistant hypertension. AHI = apnea-hypopnea index; HI = hypoxic index; PAC = plasma aldosterone concentration. Reprinted with permission from Pratt-Ubunama et al.64
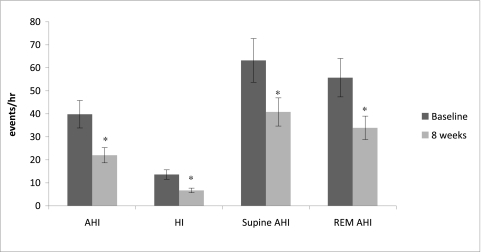
We hypothesize that the positive correlation between aldosterone levels and increasing severity of OSA in patients with resistant hypertension is secondary to aldosterone-induced fluid retention that leads to an increase in upper airway resistance due to greater parapharyngeal edema. Such an increase in upper airway resistance attributable to increases in intravascular fluid expansion has been described in healthy volunteers subjected to acute lower-body positive pressure.69 In addition, decreases in airway resistance and associated improvements in severity of OSA are observed in patients acutely diuresed for exacerbations of congestive heart failure.70 We believe it likely that the same is occurring in patients with resistant hypertension but chronically; that is, persistent intravascular fluid retention worsens OSA through increased upper airway resistance due to increased parapharyngeal edema. If so, effective diuresis, particularly with use of aldosterone antagonists, would be expected to lessen the severity of OSA in patients with resistant hypertension. Support for such an effect is provided by a recently completed study in which we observed an almost 50% reduction in OSA severity in patients with resistant hypertension who were treated with spironolactone for 3 months (Fig 3).71 Not known at this point is whether the same benefit could be achieved with other types of diuretics.
Figure 3.
Effects of 8 weeks of treatment with spironolactone on apnea-hypopnea index (AHI); hypoxic index; supine AHI; and rapid eye movement sleep AHI at 8 weeks (light gray bars) compared with baseline (dark gray bars) in patients with resistant hypertension. REM = rapid eye movement. See Figure 2 legend for expansion of other abbreviations. *Different compared with baseline (P < .05). Reprinted with permission from Gaddam et al.71
Conclusion
BP decreases during sleep, and reduced dipping of BP during sleep increases cardiovascular risk. Habitual short sleep duration is associated with hypertension, especially during middle age. Insomnia with objective short sleep duration also is associated with increased hypertension risk. RLS has a weak association with hypertension; however, PLMS increases BP, especially when associated with arousals.
Moderate to severe OSA is associated with prevalent hypertension; however, there are conflicting results examining incident hypertension. Metaanalyses show that CPAP use reduces systolic and diastolic BP only modestly. OSA is present in up to 90% of patients with resistant hypertension, and data suggest that it may be linked to hyperaldosteronism. More research is needed to determine to what degree increased sleep duration or treating sleep disorders affects BP.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Arren Graf for his editorial assistance in the preparation of this article.
Abbreviations
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- OR
odds ratio
- OSA
obstructive sleep apnea
- PLMS
periodic limb movements in sleep
- RLS
restless legs syndrome
Footnotes
Funding/Support: This study was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute [Grant 2R01–HL075614-5, “Etiology of Sleep Apnea-Related Hyperaldosteronism,” David A. Calhoun, Principal Investigator, and Susan M. Harding, Co-investigator].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens. 2000;18(7):847–854. doi: 10.1097/00004872-200018070-00005. [DOI] [PubMed] [Google Scholar]
- 2.Dolan E, Stanton AV, Thom S, et al. ASCOT Investigators Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients—an Anglo-Scandinavian cardiac outcomes trial substudy. J Hypertens. 2009;27(4):876–885. doi: 10.1097/HJH.0b013e328322cd62. [DOI] [PubMed] [Google Scholar]
- 3.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38(4):852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49(6):1235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262. [DOI] [PubMed] [Google Scholar]
- 5.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen OL, Mancia G, Pickering T, et al. VALUE Trial Group. Ambulatory blood pressure monitoring after 1 year on valsartan or amlodipine-perindopril treatment: a VALUE substudy. J Hypertens. 2007;25(3):707–712. doi: 10.1097/HJH.0b013e3280147119. [DOI] [PubMed] [Google Scholar]
- 7.Salles GF, Cardoso CRL, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–709. [PubMed] [Google Scholar]
- 9.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338(191):1245–1253. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics QuickStats: Percentage of adults who reported an average of 6 hours of sleep per 24-hour period, by sex and age group - United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep. 2005;54(37):933. [Google Scholar]
- 11.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [Erratum: Hypertension 2007;50(5): e170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangwisch JE, Heymsfield SB, Boden-Albala B. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg JF, Tulen JHM, Neven AK, et al. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50(3):585–589. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Garcia E, Faubel R, Guallar-Castillon P, Leon-Muñoz L, Banegas JR, Rodriguez-Artalejo F. Self-reported sleep duration and hypertension in older Spanish adults. J Am Geriatr Soc. 2009;57(4):663–668. doi: 10.1111/j.1532-5415.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169(11):1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman O, Shukla Y, Logan AG. Relationship between self-reported sleep duration and changes in circadian blood pressure. Am J Hypertens. 2009;22(11):1205–1211. doi: 10.1038/ajh.2009.165. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MH. Evidence for the pathophysiology of insomnia. Sleep. 2009;32(4):441–442. doi: 10.1093/sleep/32.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3(5):489–494. [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips B, Bůzková P, Enright P. Cardiovascular Health Study Research Group Insomnia did not predict incident hypertension in older adults in the cardiovascular health study. Sleep. 2009;32(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 22.Lanfranchi PA, Pennestri MH, Fradette L, et al. Nighttime blood pressure in normotensive subjects with chronic insomnia: implication for cardiovascular risk. Sleep. 2009;32(6):760–766. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 26.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129(1):76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 27.Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16(6):1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 28.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 29.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54(5):1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 30.Högl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64(11):1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 31.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7(7):545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68(15):1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118(9):1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Billars L, Hicks A, Bliwise D, et al. Hypertension risk and PLMS in restless legs syndrome [abstract] Sleep. 2007;30(suppl):A297–A298. [Google Scholar]
- 35.Fletcher ED, DeBehnke RD, Lovoi MS, et al. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103(2):190–195. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- 36.Lavie P, Ben-Yosef R, Rubin AE. Prevalence of sleep apnea syndrome among patients with essential hypertension. Am Heart J. 1984;108(2):373–376. doi: 10.1016/0002-8703(84)90628-8. [DOI] [PubMed] [Google Scholar]
- 37.Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157(1):111–115. doi: 10.1164/ajrccm.157.1.9609063. [DOI] [PubMed] [Google Scholar]
- 38.Durán-Cantolla J, Aizpuru F, Martínez-Null C, Barbé-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13(5):323–331. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1994;17(9):533–540. [PubMed] [Google Scholar]
- 40.Grunstein RR, Stenlöf K, Hedner J, Sjöström L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int J Obes Relat Metab Disord. 1995;19(6):410–418. [PubMed] [Google Scholar]
- 41.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 43.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120(5):382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 44.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160(15):2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 45.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 46.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. J Am Med Assoc. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 47.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 48.Peppard PE, Young T. Sleep-disordered breathing and hypertension [Reply] N Engl J Med. 2000;343(13):967. doi: 10.1056/NEJM200009283431310. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of the association between sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peppard PE. Is obstructive sleep apnea a risk factor for hypertension?—differences between the Wisconsin Sleep Cohort and the Sleep Heart Health Study. J Clin Sleep Med. 2009;5(5):404–405. [PMC free article] [PubMed] [Google Scholar]
- 51.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 52.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185(2):67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 53.Mo L, He QY. Effect of long-term continuous positive airway pressure ventilation on blood pressure in patients with obstructive sleep apnea hypopnea syndrome: a meta-analysis of clinical trials [in Chinese] Zhonghua Yi Xue Za Zhi. 2007;87(17):1177–1180. [PubMed] [Google Scholar]
- 54.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 55.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100(23):2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 57.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136(1):125–129. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Lima AM, Franco CM, de Castrol CM, Bezerra AD, Ataíde L, Jr,, Halpern A. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration. 2010;79(5):370–376. doi: 10.1159/000227800. [DOI] [PubMed] [Google Scholar]
- 59.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16(2):217–225. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 60.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 61.Kitahara Y, Hattori N, Yokoyama A, Nakajima M, Kohno N. Effect of CPAP on brachial-ankle pulse wave velocity in patients with OSAHS: an open-labelled study. Respir Med. 2006;100(12):2160–2169. doi: 10.1016/j.rmed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 63.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125(1):112–117. doi: 10.1378/chest.125.1.112. [DOI] [PubMed] [Google Scholar]
- 64.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131(2):453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 65.Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18(6):679–685. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- 66.Lavie P, Hoffstein V. Sleep apnea syndrome: a possible contributing factor to resistant. Sleep. 2001;24(6):721–725. doi: 10.1093/sleep/24.6.721. [DOI] [PubMed] [Google Scholar]
- 67.Elias RM, Castro MCM, de Queiroz EL, Abensur H, Romão JE, Jr,, Lorenzi-Filho G. Obstructive sleep apnea in patients on conventional and short daily hemodialysis. Am J Nephrol. 2009;29(6):493–500. doi: 10.1159/000178941. [DOI] [PubMed] [Google Scholar]
- 68.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21(2):241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 69.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body airway cross-sectional increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174(12):1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 70.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132(2):440–446. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 71.Gaddam K, Pimenta E, Thomas SJ. Spironolactone reduces severity of obstructive sleep apnea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2009 doi: 10.1038/jhh.2009.96. doi:10.1038/jhh.2009.96 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]