Abstract
Dopamine (DA) D3 and D2 receptor mechanisms are implicated in cocaine's abuse-related behavioral effects, but the relative contribution of the two receptor subtypes is only partially characterized. This study investigated the role of D3 and D2 subtype mechanisms by determining the degree to which the D3-preferring antagonist PG01037 [N-{4-[4-(2,3-dichlorophenyl)-piperazin- 1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide HCl] and the D2-preferring antagonist L-741626 [3-[4-(4-chlorophenyl)-4- hydroxypiperidin-1-yl]methyl-1H-indole] attenuated several behavioral effects of cocaine in squirrel monkeys. Quantitative observational studies established doses of each antagonist that did not produce untoward effects, which were used in subsequent comparisons. In addition, the ability of the D3-preferring agonist PD128907 [(R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4-oxazin-9-ol)] and the D2-preferring agonist sumanirole [(R)-5,6-dihydro-5-(methylamino)-4H- imidazo[4,5,1-ij]quinolin-2(1H)-one(Z)-2-butenedioate] to reproduce cocaine's discriminative stimulus (DS) and priming effects were compared. In monkeys trained to discriminate cocaine from vehicle, both DA antagonists attenuated and both DA agonists partially reproduced cocaine's DS effects. PG01037 also selectively attenuated the cocaine-like DS effects of PD128907, whereas L-741626 attenuated the cocaine-like DS effects of both agonists. In self-administration studies, L-741626 nonselectively reduced cocaine- and food-maintained responding, whereas PG01037 was ineffective against either reinforcer. In studies involving reinstatement of extinguished cocaine seeking, both antagonists attenuated cocaine-induced reinstatement of responding, and both agonists induced at least partial reinstatement of cocaine seeking. L-741626 also attenuated sumanirole-induced, but not PD128907-induced, reinstatement of responding, whereas PG01037 was ineffective against either DA agonist. The results are consistent with a role for D3 and D2 receptor mechanisms in cocaine's DS effects and cocaine-induced reinstatement of drug seeking, but provide no evidence for a major role of D3 receptors in the direct reinforcing effects of cocaine.
Cocaine inhibits the reuptake of DA into presynaptic terminals, resulting in stimulation of DA receptors primarily located in motor and limbic brain systems. Within the D2 family of DA receptors, there is substantial evidence for the role of the D2 receptor subtype in cocaine-induced behaviors; however, this research has not generated successful candidates for the treatment of cocaine addiction (see Xi and Gardner, 2008 for review). Considerably less is understood about the function of the D3 receptor subtype, primarily because of a lack of selective pharmacological probes that distinguish between the D2 and D3 receptor subtypes (Boeckler and Gmeiner, 2007). One of the major differences between D2 and D3 receptor subtypes is their brain distribution. In situ hybridization studies have shown that unlike D2 receptors, which are expressed in both mesolimbic and nigrostriatal pathways, D3 receptors are expressed predominantly in the nucleus accumbens (NAc) in humans and nonhuman primates (Murray et al., 1994; Rabiner et al., 2009). This relatively restricted distribution of D3 receptors has prompted speculation that these receptors may have unique potential as a pharmacological target for cocaine addiction treatment without induction of extrapyramidal side effects (Heidbreder and Newman, 2010).
There is mounting cellular and behavioral evidence suggesting that some of cocaine's effects are mediated, at least in part, by D3 receptor activity. In particular, chronic cocaine exposure results in the long-term elevation of D3 protein markers (Staley and Mash, 1996; Le Foll et al., 2002) but not D2 protein markers (Meador-Woodruff et al., 1993) in the NAc of humans and rodents. Furthermore, several mechanisms have been proposed that specifically implicate D3 activity in cocaine's long-term effects. For example, D3 expression in the NAc is regulated by brain-derived neurotrophic factor (Le Foll et al., 2002), a growth factor involved in synaptic plasticity and thought to contribute to cocaine-conditioned associations. In addition, a recent finding suggests that behavioral sensitization to cocaine's stimulant effects occurs via inhibition of D3 activity by calcium/calmodulin-dependent protein kinase II, an enzyme critical in learning and memory (Liu et al., 2009). Mice lacking D3 receptors also display altered sensitivity to the conditioned reinforcing effects of cocaine (Xu et al., 1997), whereas D2 mutants do not (Welter et al., 2007). Moreover, it has been suggested that DA receptor dysfunction in the NAc may be associated with impulsivity, a vulnerability marker for persistent cocaine-seeking behavior (see Dalley and Everitt, 2009 for review). Recently, it has been shown that antagonism of D3 receptors in the NAc shell using the D3-preferential antagonist nafadotride increased levels of impulsivity in highly impulsive rats, whereas antagonism in the NAc core had the opposite effect (Besson et al., 2010). Finally, it has been suggested that D3 mechanisms play an integral role in the transduction of cocaine's discriminative stimulus (DS) effects based on findings by Acri et al. (1995) and Spealman (1996) in which drugs acting as preferential agonists at D3 receptors, such as PD128907 [R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4-oxazin-9-ol)] and 7-OH-DPAT [7-hydroxy-N,N-dipropyl-2-aminotetralin], partially reproduced cocaine's DS effects in rats and monkeys. Conversely, blocking D3 activity with D3-preferring antagonists or partial agonists, such as NGB2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamidehave] has been shown in some cases to attenuate the DS, priming or reinforcing effects of cocaine (Xi et al., 2006; Martelle et al., 2007; Achat-Mendes et al., 2009). Collectively, these observations suggest that there may be specific functions of D3 receptors, which are distinct from or interact with D2 receptor activity, in mediating cocaine's abuse-related effects.
The goal of the present study was to help differentiate the contributions of D3 and D2 receptors to cocaine's abuse-related effects by comparing the modulation of cocaine-induced behaviors by the novel D3-preferring antagonist PG01037 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-trans-but-2-enyl)-4-pyridine-2-ylbenzamide)] (Grundt et al., 2005) and the D2-preferring antagonist L-741626 [3-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]methyl-1H-indole] (Grundt et al., 2007a). PG01037 preferentially occupies D3-rich regions over D2-rich regions as illustrated by pharmacologic magnetic resonance imaging (Grundt et al., 2007b) and is at least twice as D3-selective compared with other antagonists such as NGB2904. To further investigate the contribution of these receptor subtypes, the capacities of the D3-preferring agonist PD128907 (∼13-fold D3/D2 selectivity; Pugsley et al., 1995) and the D2-preferring agonist sumanirole [(R)-5,6-dihydro-5-(methylamino)-4H-imidazo[4,5,1-ij]quinolin- 2(1H)-one(Z)-2-butenedioate] (∼200-fold D2/D3 selectivity; Heier et al., 1997) to substitute for the DS and priming effects of cocaine were compared. The effects of PG01037 and L-741626 on cocaine and these selective DA agonists were evaluated in squirrel monkeys trained to: 1) discriminate cocaine from saline; 2) self-administer cocaine or food under a second-order fixed-interval (FI), fixed-ratio (FR) schedule of reinforcement; and 3) reinstate cocaine-seeking behavior by cocaine priming after extinction. The effects of PG01037 and L-741626 on unconditioned behaviors of squirrel monkeys were also compared.
Materials and Methods
Subjects.
Twenty-seven adult squirrel monkeys (Saimiri sciureus), weighing 700 to 1100 g, were studied in daily experimental sessions (Monday to Friday). Each monkey had unlimited access to water in its home cage. Monkeys participating in observational and cocaine self-administration studies also had unrestricted access to food (Teklad Monkey Diet; Harlan Teklad, Madison, WI; supplemented with fresh fruit). Monkeys participating in drug discrimination and food self-administration studies were maintained at approximately 90% of their free-feeding body weight by adjusting their access to food in the home cage. All monkeys were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, DC, 1996). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Surgical Procedures.
Monkeys in the cocaine self-administration and reinstatement studies were prepared with a chronic indwelling venous catheter (polyvinyl chloride; inside diameter,0.015 mm; outside diameter, 0.035 mm) using the surgical procedures described by Platt et al., (2005). In brief, under isoflurane anesthesia and aseptic conditions, one end of a catheter was passed to the level of the right atrium by way of a femoral or jugular vein. The distal end of the catheter was passed subcutaneously and exited the skin in the midscapular region. Catheters were flushed daily with 0.9% saline solution and sealed with stainless-steel obturators when not in use. Monkeys wore nylon-mesh jackets (Lomir Biomedical, Toronto, Canada) at all times to protect the catheter.
Apparatus.
In studies involving cocaine discrimination, cocaine and food self-administration, and cocaine-induced reinstatement of drug seeking, daily sessions were conducted in ventilated, sound-attenuated chambers with white background noise (MED Associates, St. Albans, VT). Within the chambers, monkeys sat in Plexiglas chairs (MED Associates) facing a panel equipped with response levers and red and white stimulus lights. In experiments involving catheterized subjects, catheters were connected to syringe pumps (MED Associates) located outside of the chamber. The pumps were programmed to deliver drug or vehicle solutions into the catheter at a rate of 0.18 ml/s for 1 s. In cocaine discrimination and food self-administration experiments, 190-mg sucrose pellets (Bioserve Biotechnologies, Laurel, MD) were delivered to a receptacle in the front panel of the chair. Experiments were controlled, and data were recorded via interfaces (Med Associates) and PC-compatible computers located in an adjacent room. Behavioral observation studies were conducted in a ventilated, transparent Plexiglas arena (114 × 122 × 213 cm) situated in a lighted room, separate from other animals (cf. Platt et al., 2003). The arena was equipped with perches, suspended plastic chains, manipulable objects, and a wood-chip substrate to permit a range of species-typical behaviors. A digital video camera was positioned 1 m in front of the chamber to record a subject's behavior during the session.
Observation Studies.
To determine the doses of PG01037 and L-741626 to be used in subsequent experiments, quantitative behavioral observations were first conducted in a group of four monkeys, which established the highest dose of each drug that did not produce significant untoward effects. A range of doses of PG01037 (10–100 mg/kg) and L-741626 (0.1–1.0 mg/kg) and their vehicles were administered intramuscularly 5 or 60 min, respectively, before a 30-min observation session. These pretreatment times were determined on the basis of initial studies in a subgroup of subjects during which subjects were observed for up to 8 h after drug injection. During the 30-min observation session, the animal's behavior was videotaped to provide an archival record of data, which was subsequently analyzed by a trained observer who was not informed about the drugs under investigation. The behavioral scoring system (cf. Platt et al., 2003) included 10 categories that were scored by recording the presence or absence of each behavior in 15-s intervals during three 5-min observation periods, spaced at regular intervals across the session. Modified frequency scores were calculated from these data as the proportion of 15-s intervals in which a particular behavior was observed. In addition, the categories locomotion, object manipulation, and foraging were combined into the more general category of environment-directed behavior, and self-grooming and scratching were combined into the more general category of self-directed behavior. Lastly, the ability of test drugs to induce catalepsy (defined as static posture accompanied by increased muscle resistance) was evaluated by scoring of muscle resistance at three time points across each session. The monkeys were removed from the observation arena by a trained handler and evaluated for muscle rigidity defined as increased resistance to hind-limb extension and/or rigid grasping of the grid floor. Muscle rigidity scores ranged from 0 (indicating no increased rigidity) to 2 (indicating strong resistance to hind-limb extension and clinging to the grid floor). Total scores for each behavioral category were calculated by adding the scores from each of the three assessments made during a single test session. Drug test sessions were conducted once or twice per week, with saline control sessions on intervening days.
Cocaine Discrimination.
Six monkeys were trained to discriminate cocaine from saline by using procedures described previously (Spealman et al., 1996). Initially, each monkey was trained to respond under a FR10 schedule of food presentation, with either the left or right lever available during alternate sessions. After responses were maintained consistently under the FR schedule, monkeys were trained to respond differentially on the left and right levers (both present) depending on whether they had received an intramuscular injection of cocaine (0.3–0.42 mg/kg, depending on the monkey) or an identical volume of vehicle (0.3 ml/kg; 0.9% saline solution). After a cocaine injection, 10 consecutive responses on one lever (left for three monkeys; right for the other three monkeys) produced a sucrose pellet, whereas after a saline injection 10 consecutive responses on the alternate lever produced a sucrose pellet. Responses on the inappropriate lever (e.g., the saline lever when cocaine was injected) reset the FR requirement. Training sessions consisted of a variable number of components (n = 1–4) of the FR schedule. The number of components per session was randomized, with the restriction that each number occur equally often within a block of 20 sessions. Each component of the session ended after completion of 10 FRs or 10 min, whichever occurred first. A 10-min timeout (TO) period, during which the lights were off and responses had no programmed consequences, preceded each component. Cocaine or saline injections were administered in the thigh or calf muscle of either leg during the fifth minute of this 10-min TO period. During most training sessions, saline was administered during TO periods preceding the first one to three components, and cocaine was administered during the last component of the session. Periodically, saline was injected before all four components of a training session or cocaine was administered before the first (and only) component of a session to prevent an invariant association between the first component and vehicle and the fourth component and cocaine. Discrimination training continued until a criterion of ≥90% responses on the injection-appropriate lever was met for at least three consecutive sessions.
Drug test sessions typically were conducted once per week and consisted of three or four FR components in which responding on either lever produced food. Dose–response functions for cocaine, PD128907, and sumanirole were determined by using a cumulative-dosing procedure in which incremental doses of a test drug (0.25–0.5 log unit increments) were injected during the TO periods that preceded sequential components of a test session, resulting in a three- or four-point cumulative dose–response function determined in a single session, as described by Achat-Mendes et al., (2009). Experiments involving combinations of a DA antagonist with either cocaine or a DA agonist were conducted by administering PG01037 (10 or 30 mg/kg) or L-741626 (0.1 or 0.3 mg/kg) before the session (5 min for PG01037; 60 min for L-741626), followed by cumulative doses of cocaine, PD128907, or sumanirole during the session, as described above. Pretreatment times for the DA antagonists were chosen on the basis of time courses of action (determined during the observation studies described above and by evaluating the effects at different pretreatment times in preliminary drug discrimination experiments with a subgroup of subjects).
Cocaine Self-Administration.
A group of six monkeys was trained to self-administer cocaine under a second-order FI (FR) schedule of intravenous cocaine injection using the procedures described by Achat-Mendes et al., (2009). Each monkey self-administered cocaine under this schedule for at least 8 months before testing began. Under the second-order schedule, a white light indicated the start of the session, and completion of a FR10 extinguished the white light and simultaneously illuminated a red light for 2 s. This cycle was repeated throughout a 5-min FI, and the first FR10 completed after the expiration of the FI produced the 2-s light change paired with an intravenous injection of cocaine. Each cocaine injection was followed by a 60-s TO period during which time all lights were off and responses had no scheduled consequences. If the FR requirement was not completed within 8 min after the expiration of the FI (limited hold), the TO period was started automatically without an injection. Daily sessions ended after 10 cycles of the second-order schedule or a maximum session length of 90 min. Once baseline performance stabilized (no monotonic trend in response rate over at least three consecutive sessions), the dose of self-administered cocaine was varied over a 10-fold range (0.03–0.3 mg/kg/injection), with each dose tested for at least five consecutive sessions and until responding was stable. This dose-variation procedure was then repeated after daily intramuscular pretreatment with PG01037 (30.0 mg/kg, 5 min before the session), L-741626 (0.3 mg/kg, 60 min before the session), or vehicle using corresponding pretreatment times. Doses of cocaine and each DA antagonist were administered in a counterbalanced order among subjects with the exception of the highest dose of PG01037 (100 mg/kg), which was tested last in all subjects. In addition, each subject received at least two of the following three noncocaine control conditions: 1) extinction, in which responses did not produce cocaine or the cocaine-paired stimulus; 2) noninjection control in which responses produced the cocaine-paired stimulus but no injection; and 3) saline control in which responses produced the cocaine-paired stimulus and an injection of 0.9% saline. Because each type of control procedure induced very low levels of responding (<10% of the rate maintained by cocaine self-administration), data were averaged across conditions for individual subjects.
Food-Maintained Behavior.
Five monkeys were trained to respond under a second-order FI (FR) schedule of food pellet delivery with schedule parameters identical to the second-order schedule described above for cocaine self-administration, except that completion of every FR10 after expiration of the 5-min FI produced three food pellets instead of a cocaine injection (Platt et al., 2003). Drug testing began once stable food-maintained behavior (i.e., no monotonic trend in response rate over at least three consecutive sessions) was observed under the baseline conditions. Monkeys were pretreated with intramuscular injections of PG01037 (30 mg/kg, 5 min before the session), L-741626 (0.3 mg/kg, 60 min before the session), or vehicle at corresponding pretreatment times for at least five consecutive sessions.
Reinstatement of Cocaine Seeking.
A final group of six monkeys was used to evaluate the effects of PG01037 and L-741626 on the reinstatement of extinguished cocaine seeking by using procedures similar to those described by Khroyan et al., (2000). Subjects were first trained under a second-order FI (FR) schedule of intravenous cocaine self-administration similar to the one described previously except that the FI was 10 min in length and a session consisted of five cycles of the second-order schedule. After a period of stable responding (defined above), cocaine seeking was extinguished by substituting saline for cocaine injections and omitting presentations of the cocaine-paired stimulus. Extinction sessions were conducted daily until response rates declined to 10% or less of the rate maintained by cocaine self-administration. Reinstatement tests began once this criterion was met for at least three consecutive sessions. Reinstatement tests used the same schedule parameters as the second-order schedule for self-administration described above, except that vehicle rather than cocaine was available for self-administration. On reinstatement test days, monkeys received an intramuscular pretreatment of PG01037 (30.0 mg/kg, 5 min before the session), L-741626 (0.3 mg/kg, 60 min before the session), or their respective vehicles at corresponding pretreatment times followed by an intravenous priming injection of cocaine (0–1.0 mg/kg), sumanirole (0.3–3.0 mg/kg), or PD128907 (0.03–0.3 mg/kg), immediately before the session. To separate the effects of PG01037 and L-741626 on reinstatement of drug seeking induced by the cocaine-paired stimulus versus cocaine priming, additional tests were conducted in which subjects were pretreated with either PG01037 (30.0 mg/kg) or L-741626 (0.3 mg/kg) before a vehicle rather than a cocaine prime. Experiments in which a different DA agonist was used for priming were separated by cycles of cocaine self-administration and extinction sessions to re-establish stable baselines of cocaine self-administration (cf. Khroyan et al., 2000).
Drugs.
Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO), PD128907 hydrochloride (Sigma-Aldrich), and sumanirole maleate (Pfizer Inc., Groton, CT and Medicinal Chemistry Section, National Institute on Drug Abuse-Intramural Research Program, Baltimore MD) were dissolved in 0.9% saline solution. L-741626 (Tocris Bioscience, Ellisville, MO) was dissolved in sterile distilled water containing 20% dimethyl sulfoxide. PG01037 was synthesized at the Medicinal Chemistry Section, National Institute on Drug Abuse-Intramural Research Program by using methods described in Grundt et al., (2005) and dissolved in sterile water. Injection volumes did not exceed 0.3 ml per intramuscular injection or 0.18 ml per intravenous injection.
Analysis of Drug Effects.
In drug discrimination studies, responses on the cocaine and saline levers during each component of the session were recorded and used to calculate the percentage of responses on the cocaine lever. The rate of responding in each component of a session was computed by dividing the total number of responses, regardless of lever, by the total component duration When appropriate, the dose estimated to engender 50% cocaine-lever responding (ED50) was determined for individual subjects by linear interpolation of the log dose–response functions as described previously (Spealman et al., 1996). In studies involving cocaine and food-maintained behavior and cocaine-induced reinstatement of drug seeking, rates of responding under the second-order schedules were computed for each session by dividing the total number of responses by the total elapsed time (excluding timeout periods) for individual subjects across sequential components of the session and the entire session. In each type of experiment, data from individual subjects were averaged to obtain group means. In observational experiments, individual scores for each behavior were averaged across the three 5-min observation periods, and statistical significance was determined by one-way repeated measures (RM) ANOVA. Statistical significance of antagonist pretreatments was analyzed with two-way RM ANOVAs for pretreatment dose and agonist dose. Significant main effects were followed by pairwise comparisons by using Bonferroni t tests. Criterion for significance was p < 0.05 for all analyses.
Results
Observation Studies.
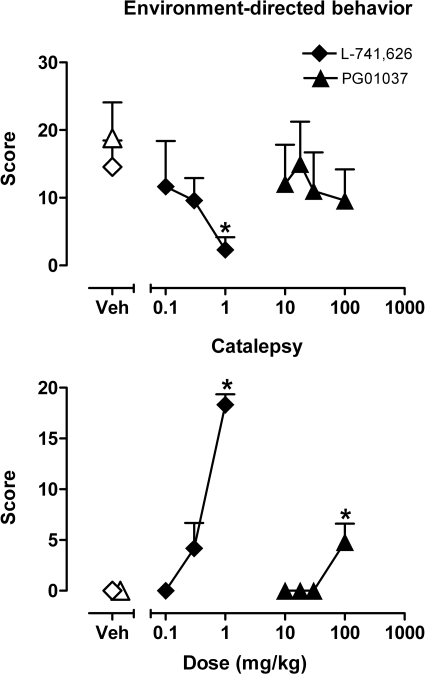
Quantitative analysis of unconditioned behaviors revealed that, compared with vehicle, administration of PG01037 at doses ≤30 mg/kg had no effect on any behaviors studied. However, as illustrated in Fig. 1, administration of 100 mg/kg induced a significant increase in catalepsy (defined by static posture and muscle rigidity) and had no effect on environment-directed behaviors (including locomotion, foraging, and object manipulation) or self-directed behaviors. One-way RM ANOVA revealed a significant effect of PG01037 (100 mg/kg) on catalepsy compared with vehicle (F3,12 = 7.4, p < 0.01; p < 0.05, Bonferroni t tests). Analysis of the effects of L-741626 revealed that compared with vehicle doses ≤0.3 mg/kg produced no significant changes in any of the behaviors studied. However, a higher dose of L-741626 (1.0 mg/kg) significantly reduced environment-directed behaviors (F3,9 = 9.4, p < 0.01; p < 0.05, Bonferroni t tests) and increased signs of catalepsy (F3,9 = 49.3, p < 0.001; p < 0.05, Bonferroni t test). Based on these results, doses of up to 30.0 mg/kg PG01037 and up to 0.3 mg/kg L-741626 were tested in all subsequent experiments except where noted.
Fig. 1.
Effects of PG01037 and L-741626 on species-typical behaviors. Environment-directed behavior includes locomotion, object manipulation, and foraging. Catalepsy indicates static posture and muscle rigidity. ∗, p < 0.05 comparing scores after pretreatments with drug and vehicle, Bonferroni t test. Data are means ± S.E.M. (n = 4).
Discriminative Stimulus Effects of Cocaine.
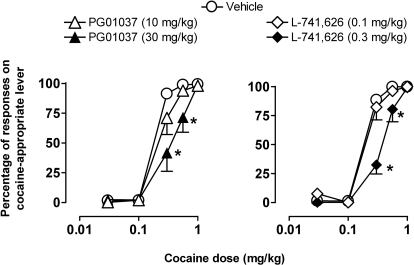
During training, administration of vehicle engendered responding primarily on the saline lever (mean percentage of saline-appropriate responding: 98.1 ± 0.8), and administration of the training dose of cocaine (0.3–0.42 mg/kg) resulted in responding primarily on the cocaine lever (mean percentage of cocaine-appropriate responding: 98.8 ± 0.3). Rate of responding was not significantly different between administration of saline and cocaine (0.53 ± 0.08 and 0.68 ± 0.16 responses/s, respectively). During testing, cumulative doses of cocaine engendered a dose-dependent increase in cocaine-lever responding (Fig. 2, ○) without significantly affecting response rates (not shown).
Fig. 2.
Percentage of responses on the cocaine lever as a function of cumulative dose of cocaine after intramuscular pretreatment with DA antagonists or their respective vehicles in squirrel monkeys trained to discriminate cocaine from vehicle. Points are means ± S.E.M. (n = 6). ∗, p < 0.05 comparing percentage of cocaine-lever responding after pretreatments with drug and vehicle, Bonferroni t test.
Pretreatment with 30.0 mg/kg PG01037, but not a lower dose of 10.0 mg/kg, produced a rightward shift in the cocaine cumulative dose–response curve (Fig. 2, left). Two-way RM ANOVA revealed a main effect of cocaine (F4,39 = 130; p < 0.001), PG01037 (F2,39 = 8; p < 0.05), and a significant cocaine × PG01037 interaction (F8,39 = 3; p < 0.05). Likewise, pretreatment with 0.3 mg/kg L-741626, but not a lower dose of 0.1 mg/kg, resulted in a rightward shift in the cocaine cumulative dose–response curve (Fig. 2, right). Two-way RM ANOVA revealed a significant main effect of cocaine (F4,38 = 267; p < 0.001), L-741626 (F2,38 = 9; p < 0.05), and a significant cocaine × L-741626 interaction (F10,38 = 8; p < 0.001). Compared with vehicle pretreatment, both 30.0 mg/kg PG01037 and 0.3 mg/kg L-741626 significantly reduced the percentage of cocaine-lever responding engendered by 0.3 and 0.56 mg/kg cocaine, respectively (Bonferroni t test, p < 0.05) and increased the ED50 ∼2-fold. The rate of responding during cocaine testing was not altered significantly by pretreatment with either PG01037 or L-741626 compared with vehicle (not shown).
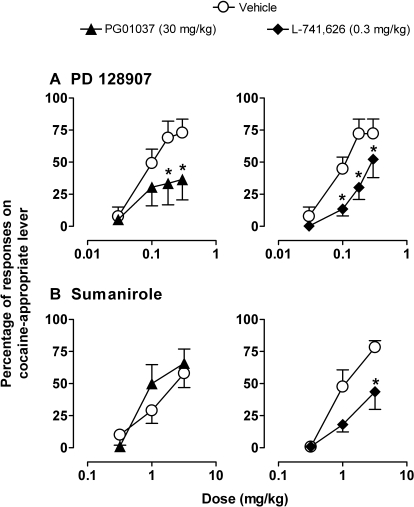
When the D3-preferring agonist PD 128097 was substituted for cocaine, cocaine-lever responding increased in a dose-dependent fashion, reaching a mean maximum of 73% (±10) at 0.3 mg/kg PD128907 (Fig. 3, top, ○). Substitution of cocaine with the D2-preferring agonist sumanirole also produced dose-dependent increase in cocaine-lever responding, reaching a mean maximum of 78% (±5) at 3.0 mg/kg sumanirole (Fig. 3, bottom, ○). Higher doses of PD128907 or sumanirole were not tested systematically because in initial studies with a subgroup of animals doses of PD128907 > 0.3 mg/kg resulted in agitation, excessive salivation, and/or hair pulling, and doses of sumanirole >3.0 mg/kg caused excessive scratching and hair pulling, accompanied by emesis in some cases .
Fig. 3.
Percentage of responses on the cocaine lever as a function of cumulative dose of DA agonists PD128907 (A) or sumanirole (B) after intramuscular pretreatment with PG01037, L-741626, or their respective vehicles in squirrel monkeys trained to discriminate cocaine from vehicle. Points are means ± S.E.M. (n = 6). ∗, p < 0.05 comparing percentage of cocaine-lever responding after pretreatments with drug and vehicle, Bonferroni t test.
Pretreatment with the dose of PG01037 that attenuated the DS effects of cocaine (30 mg/kg) also significantly attenuated the cocaine-like DS effects of PD128907, but did not significantly alter the cocaine-like DS effects of sumanirole (Fig. 3, left, closed symbols). Two-way RM ANOVA revealed a significant effect of PD128907 (F3,15 = 17; p < 0.001) and PG01037 (F1,15 = 5; p < 0.05) on the percentage of cocaine-lever responses. Additional analysis showed that compared with vehicle pretreatment PG01037 (30 mg/kg) significantly reduced the percentage of cocaine-lever responses engendered by the two highest doses of PD128907 (p < 0.05; Bonferroni t test). Pretreatment with PG01037 did not produce significant changes in the rate of responding during PD128907 testing compared with pretreatment with vehicle (not shown).
In contrast to the findings with PG01037, pretreatment with a dose of L-741626 that attenuated the DS effects of cocaine (0.3 mg/kg) also significantly attenuated the cocaine-like DS effects of both PD128907 and sumanirole (Fig. 3, right, closed symbols). Two-way RM ANOVA revealed a significant effect of PD128907 (F3,15 = 25; p < 0.001) and L-741626 (F1,15 = 376; p < 0.001) on the percentage of cocaine-lever responses. Bonferroni t tests showed that L-741626 significantly reduced cocaine-lever responding engendered by all except the lowest dose of PD128907. Two-way RM ANOVA also revealed a significant effect of sumanirole (F2,10 = 26; p < 0.001) and L-741626 (F1,10 = 7; p < 0.05) on the percentage of cocaine-lever responses. Bonferroni t tests showed that compared with vehicle pretreatment L-741626 significantly reduced cocaine-lever responding at the highest dose of sumanirole tested (3.0 mg/kg). L-741626 pretreatments did not produce any alterations in the rate of responding during PD128907 or sumanirole testing compared with vehicle pretreatment (not shown).
Cocaine Self-Administration.
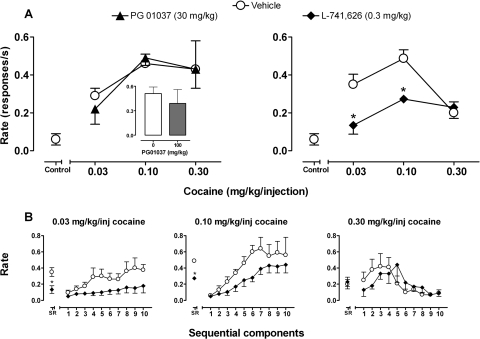
In the absence of drug pretreatments, the mean rate of responding maintained by self-administered cocaine reached a maximum of 0.58 ± 0.1 responses/s at a dose of 0.1 mg/kg/injection for a total of 10 injections/session. Both the lower dose (0.03 mg/kg/injection) and higher dose (0.3 mg/kg/injection) of cocaine engendered lower response rates, resulting in an inverted-U shaped dose–response function. Under control conditions in which lever pressing did not result in cocaine injections (see Materials and Methods), response rates declined to ∼10% of the mean rate of responding during cocaine self-administration (Fig. 4A, Control) and the number of self-administered injections decreased from 10 to an average of six injections/sessions. Compared with vehicle, pretreatment with PG01037 (30 mg/kg) did not markedly alter the shape or position of the cocaine dose–response curve (Fig. 4A, left) and did not decrease the number of self-administered injections. Two-way RM ANOVA and Bonferroni t tests showed no significant effect of PG01037 on the average rate of cocaine-maintained behavior at any cocaine dose. Pretreatment with PG01037 also did not significantly alter the rate of cocaine-maintained responding during any of the 10 sequential components of the daily sessions (not shown). Daily pretreatment with 100 mg/kg PG01037 (administered either as one or three intramuscular injections) caused tissue irritation that resulted in swelling and knotting in the muscle around the site of injection, precluding systematic testing for 5 days with this dose. However, when tested for 3 or 4 consecutive days in four subjects, there was no effect of PG01037 (100 mg/kg) on response rates during cocaine (0.10 mg/kg/injection) self-administration compared with vehicle pretreatment (Fig. 4A, left, inset).
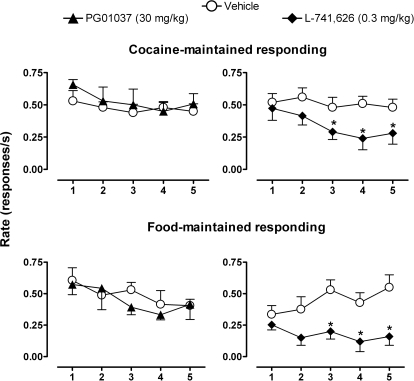
Fig. 4.
A, effects of pretreatment with DA antagonists on the overall rate of responding under the second-order schedule of intravenous cocaine self-administration as a function of cocaine dose. Data presented are averaged over the last 3 days of treatment. Points above Control indicate the rate of responding in the absence of cocaine injections. Inset, comparison of the effects of pretreatment with 100 mg/kg PG01037 versus vehicle during self-administration of cocaine (0.10 mg/kg/injection). ∗, p < 0.05 comparing response rates after pretreatments with drug and vehicle, Bonferroni t test. B, effects of pretreatment with L-741626 as a function of sequential components during the cocaine self-administration session; points above SR indicate overall session rates. Data are means ± S.E.M. (n = 4–5).
In contrast to PG01037, pretreatment with L-741626 (0.3 mg/kg) produced a downward shift of the ascending limb of the cocaine dose–response curve (Fig. 4A, right). Two-way RM ANOVA revealed a main effect of L-741626 (F1,8 = 26; p < 0.05) and a significant L-741626 × cocaine interaction (F2,8 = 11; p < 0.05) on the rate of responding during cocaine self-administration. Bonferroni t tests showed that L-741626 significantly attenuated the rate of cocaine-maintained responding at the low and intermediate cocaine doses (0.03 and 1.0 mg/kg; p < 0.05). Within-session analysis revealed that L-741626 reduced the rate of responding over sequential components of the session when either 0.03 or 0.10 mg/kg cocaine was available for self-administration and shifted the time-effect curve rightward during 0.3 mg/kg cocaine availability compared with vehicle pretreatment (Fig. 4B).
Food-Maintained Responding.
Under baseline conditions, the average rate of responding maintained by food presentation was 0.53 (± 0.04) responses/s, which was comparable with the average rate maintained by the maximally effective dose of cocaine. As illustrated in Fig. 5, left, daily pretreatment with PG01037 (30 mg/kg) before each of five consecutive test sessions had no effect on the average response rates maintained by either food or cocaine (0.1 mg/kg/injection) compared with vehicle pretreatment. Daily pretreatment with L-741626 (0.3 mg/kg), however, resulted in a decline in the rate of responding maintained by both food presentation and cocaine (0.1 mg/kg/injections) over the 5-day test period (Fig. 5, right). Two-way RM ANOVA revealed an effect of L-741626 treatment on food-maintained responding that approached significance (F1,3 = 7.3; p = 0.07), and Bonferroni t tests revealed significant differences in average response rates on days 3 to 5 compared with vehicle pretreatment on these days (p < 0.05). Likewise, two-way RM ANOVA of the effect of L-741626 on cocaine self-administration (0.1 mg/kg/injection) showed a significant effect of L-741626 pretreatment (F1,16 = 23; p < 0.05), and Bonferroni t tests revealed significant differences in average response rates on days 3 to 5 compared with vehicle (p < 0.05).
Fig. 5.
Comparison of the effects of DA antagonists on behavior maintained by self-administered cocaine (0.10 mg/kg/injection) (A) and food under a second-order schedule over a 5-day test period (B). Data are means ± S.E.M. (n = 5). ∗, p < 0.05 comparing response rates for the corresponding days after pretreatments with drug and vehicle, Bonferroni t test.
Reinstatement of Cocaine Seeking.
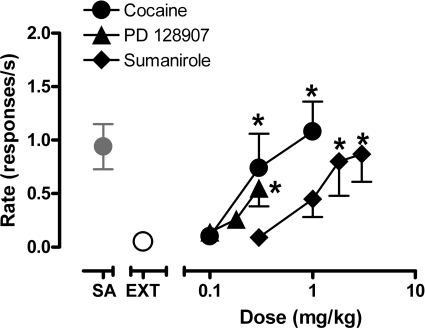
As illustrated in Fig. 6, extinction produced a decline in response rate to <3% of the mean rate of responding during cocaine self-administration (0.03 ± 0.01 versus 0.94 ± 0.21 responses/s). Priming with cocaine combined with restoration of the cocaine-paired stimulus induced a dose-dependent reinstatement of cocaine seeking and yielded a maximum response rate of 1.10 ± 0.28 responses/s. Priming with the D3-preferring agonist PD128907 or the D2-preferring agonist sumanirole also reinstated cocaine-seeking behavior in a dose-dependent fashion. The maximum rate of responding induced by sumanirole priming (0.87 ± 0.26 responses/s at 3.0 mg/kg) approached the maximum rate induced by cocaine, whereas the maximum rate induced by priming with PD128907 (0.55 ± 0.17 responses/s at 0.3 mg/kg) was approximately half that induced by cocaine. Because preliminary observations revealed that higher doses of PD128907 induced marked behavioral side effects (see above), doses >0.3 mg/kg were not tested. Analysis of the effects of priming with DA agonists by one-way RM ANOVAs revealed that compared with response rates during extinction there was a significant effect of priming with cocaine (F3,12 = 12; p < 0.001), PD128907 (F3,12 = 7; p < 0.05), and sumanirole (F4,16 = 7; p < 0.05) on response rates. Bonferroni t tests showed a significant increase in the rate of responding compared with extinction after priming with cocaine (0.3 and 1.0 mg/kg), PD128907 (0.3 mg/kg), or sumanirole (1.8 and 3.0 mg/kg; p < 0.05).
Fig. 6.
Rate of responding during reinstatement of extinguished drug-seeking behavior induced by priming with cocaine, PD128907 and sumanirole. Point above SA is the response rate during baseline cocaine self-administration; point above EXT is the response rate in the absence of cocaine and cocaine-paired stimulus. Data are means ± S.E.M. (n = 5). ∗, p < 0.05 comparing response rates induced by drug priming to response rate during extinction, Bonferroni t test.
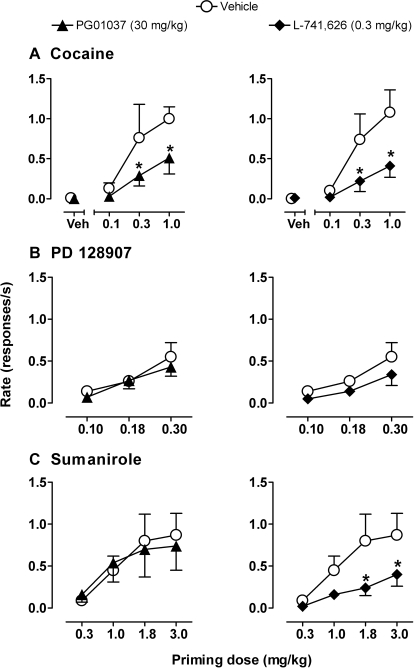
Pretreatment with PG01037 (30 mg/kg) resulted in a downward shift in the dose–response function for cocaine priming compared with vehicle pretreatment, (Fig. 7A, left). Two-way RM ANOVA revealed a main effect of cocaine (F2,6 = 9; p < 0.05) and PG01037 pretreatment (F1,6 = 13; p < 0.05). Bonferroni t test showed that PG01037 significantly reduced the rate of responding induced by the two highest priming doses of cocaine (0.3 and 1.0 mg/kg; p < 0.05). Pretreatment with L-741626 (0.3 mg/kg) produced effects similar to those of PG01037 (Fig. 7A, right). Two-way RM ANOVA revealed a significant effect of cocaine dose (F2,8 = 13; p < 0.05) and a significant cocaine dose × L-741626 interaction (F2,8 = 5; p < 0.05). Bonferroni t tests showed that L-741626 significantly attenuated the reinstatement of drug seeking induced by the two higher doses of cocaine (0.3 and 1.0 mg/kg; p < 0.05).
Fig. 7.
Effects of DA antagonists on reinstatement of drug-seeking behavior induced by priming with cocaine (A), PD128907 (B), and sumanirole (C). Points above Veh indicate the effect of DA antagonists on reinstatement induced by the cocaine-paired stimulus after vehicle priming. Data are means ± S.E.M. (n = 5). ∗, p < 0.05 comparing response rates after pretreatments with drug and vehicle, Bonferroni t test.
When reinstatement was induced by the cocaine-paired stimulus in the absence of cocaine (i.e., restoration of the stimulus after priming with vehicle), the mean number of responses/session was approximately twice as high as that seen during extinction, when the cocaine-paired stimulus was omitted (38.3 ± 23.2 versus 19 ± 18.5 responses during the 90-min session, respectively). Pretreatment with PG01037 (30 mg/kg) reduced the number of responses induced by the cocaine-paired stimulus to approximately one-quarter of the level engendered by the cocaine-paired stimulus after vehicle pretreatment (9.7 ± 5.0 responses), whereas pretreatment with L-741626 (0.3 mg/kg) had little effect (45.3 ± 31.9 responses). However, because of high intersubject variability, none of these changes was statistically significant whether analyzed in terms of total responses (above) or response rate (Fig. 7A , points above Veh).
The PD128907 dose–response curve was not altered after pretreatment with either PG01037 or L-741626 compared with vehicle pretreatment (Fig. 7B, left and right, respectively). Two-way RM ANOVA revealed a main effect of PD128907 dose only (F2,8 = 18; p < 0.05). Pretreatment with PG01037 also produced no changes in the sumanirole dose–response curve compared with vehicle (Fig. 7C , left). In contrast, pretreatment with L-741626 produced a selective downward shift in the sumanirole, but not the PD128907 dose–response curve (Fig. 7C , right). Two-way RM ANOVA revealed a significant effect of sumanirole (F3,12 = 6; p < 0.05), L-741626 (F1,12 = 8; p < 0.05), and a sumanirole × L-741626 interaction (F3,12 = 5; p < 0.05). Bonferroni t tests showed that L-741626 significantly attenuated responding induced by priming with the two higher doses of sumanirole (1.8 and 3.0 mg/kg; p < 0.05).
Discussion
The effects of pharmacological manipulation by preferential D3 and D2 DA antagonists on cocaine discrimination, cocaine self-administration, and reinstatement of extinguished drug-seeking behavior were studied to investigate the contributions of these receptor subtypes to the behavioral effects of cocaine in monkeys. To the extent that the doses of the antagonists used in this study act preferentially on D3 or D2 receptors in vivo as predicted by their in vitro binding data, the results support a role for D3 receptor mechanisms in cocaine's DS effects and cocaine-induced reinstatement of drug seeking, but provide no evidence of a role for D3 receptor mechanisms in the direct reinforcing effects of cocaine. In contrast, D2 receptor mechanisms seem to play a role in all three behavioral effects of cocaine.
In the cocaine discrimination study, results from antagonism and agonist substitution experiments indicate that both D3 and D2 receptor subtypes contribute significantly to cocaine's DS effects. PG01037 and L-741626 both attenuated cocaine's DS effects by the same degree (∼2-fold increase in ED50), consistent with previous reports of antagonism of the DS effects of cocaine by other D3- and D2-preferring antagonists and partial agonists (Costanza et al., 2001; Martelle et al., 2007; Achat-Mendes et al., 2009). In addition, PD128907 and sumanirole substituted for cocaine to comparable degrees (73 and 78% cocaine-lever responding, respectively) in agreement with earlier research showing that other D3- and D2-preferring agonists can partially mimic cocaine's DS effects (Acri et al., 1995; Spealman 1996; Platt et al., 2002). These results collectively support the hypothesis that multiple DA receptor subtypes contribute to mediate the DS effects of cocaine.
The antagonist effects of PG01037 were selective to the DS effects of cocaine and PD128907, having no significant effect on the cocaine-like DS effects of sumanirole, suggesting that PG01037 acted as a selective D3 antagonist in vivo. Consistent with our findings, PG01037 has been found to localize primarily in D3-rich brain regions as determined by pharmacological magnetic resonance imaging (Grundt et al., 2007b) and selectively attenuate the D3-mediated component of quinpirole-induced yawning in rats (Baladi et al., 2010). Unlike PG01037, L-741626 attenuated the cocaine-like DS effects of both sumanirole and PD128907, suggesting less selective effects consistent with previous studies in which L-741626 attenuated the DS effects of another D3 agonist, S32504 [(+)-trans-3,4,4a,5,6, 10b-hexahydro-9-carbamoyl-4-propyl-2H-naphth[1,2-b]-1,4-oxazine] (Millan et al., 2007). Given the modest in vitro receptor subtype selectivity of both L-741626 (∼15-fold D2/D3; Grundt et al., 2007a) and PD128907 (∼13-fold D3/D2; Pugsley et al., 1995), it is possible that L-741626 and/or PD128907 were active at both receptor subtypes at the doses tested. Another possibility is that the apparent cross-reactivity between ligands and receptors of different classes in our study was caused by interactions between D2 and D3 receptor systems. For example, it has been proposed that D2 and D3 receptors cooperate to modulate DA transmission (Zapata and Shippenberg, 2005) and mediate DA-dependent behaviors via downstream molecules that converge at the Akt/glycogen synthase kinase-3 signaling cascade in DA neurons (Beaulieu et al., 2007).
In cocaine self-administration studies, analysis of behavior averaged during individual components and over the entire session showed that L-741626 served as a functional antagonist of the reinforcing effects of cocaine. This is in agreement with previous outcomes using other preferential and nonpreferential D2 receptor antagonists during cocaine self-administration in monkeys (Platt et al., 2002, 2003). In contrast to L-741626, PG01037 did not alter responding maintained by any dose of self-administered cocaine, and the highest dose of 100 mg/kg did not affect self-administration of the cocaine dose that maintained maximum response rates. The absence of effect of D3 receptor antagonism on cocaine self-administration is consistent with previous findings using another D3 receptor antagonist, NGB2904, in rhesus monkeys (Martelle et al., 2007), and results with NGB2904 and PG01037 in rats responding under a FR schedule of cocaine or methamphetamine self-administration (Xi et al., 2006; Higley et al., 2010). In the latter reports, however, NGB2904 and PG01037 attenuated cocaine or methamphetamine self-administration under a progressive ratio schedule of drug injection in rats. These seemingly contradictory findings could be caused by the different schedules of drug self-administration. Progressive ratio schedules, in which the response requirement for obtaining drug increases throughout the session, may be more sensitive in revealing changes in the reinforcing effects of a drug compared with fixed ratio or second-order schedules. Alternatively, differences in our results with PG01037 compared with those of Higley et al., (2010) could reflect the use of different species (monkeys versus rats), different self-administered drugs (cocaine versus methamphetamine), or history of drug self-administration. In our study, monkeys self-administered cocaine for ≥8 months before testing, whereas rats in the other studies typically underwent training for a maximum of 1 month before testing. A shorter drug self-administration history could render the self-administration behavior or the reinforcing effects of a drug more susceptible to inhibition.
Antagonism of the reinforcing effects of cocaine by L-741626 was most evident at lower cocaine doses. Despite the apparent lack of effect of L-741626 on self-administration of the highest dose of cocaine using whole-session averages, the within-session analysis revealed that L-741626 pretreatment shifted the time–effect function to the right, consistent with the concept of surmountable antagonism. Further analysis showed that L-741626 produced a gradual decline in cocaine-maintained responding over days. This extinction-like pattern suggests that the effect of L-741626 on cocaine self-administration was not simply caused by impaired motor function, which would be expected to interfere with lever pressing (also see observation studies), but rather to antagonism of cocaine's reinforcing effects. As in the case of cocaine self-administration, L-741626, but not PG01037, reduced the rate of food-maintained behavior over days, suggesting a generalized effect of L-741626 on both cocaine- and food-reinforced behavior rather than a selective effect on cocaine self-administration.
In the reinstatement experiments, drug seeking induced by cocaine priming was attenuated by both PG01037 and L-741626, consistent with previous studies using other D3 and D2 ligands to antagonize cocaine's priming effects (Khroyan et al., 2000; Xi et al., 2006). We reported previously, however, that the D3-preferring partial agonist, CJB 090 did not attenuate cocaine-priming reinstatement of drug seeking (Achat-Mendes et al., 2009) under the same conditions in which PG01037 attenuated cocaine seeking in this study. It is possible that the ineffectiveness of CJB 090 compared with PG01037 reflects the former drug's weak agonist properties, which may have limited expression of its antagonist effects. Our reinstatement data further reveal that sumanirole-induced reinstatement was selectively attenuated by L-741626, whereas PD128907-induced reinstatement was not attenuated by either DA antagonist. This might be caused by the comparatively low rates of reinstated cocaine seeking induced by PD128907 (40–50% lower than the response rates induced by either cocaine or sumanirole), which may have obscured potential attenuation by PG01037 or L-741626. Alternatively, low levels of reinstatement induced by PD12907 and the failure of PG01037 to antagonize PD128907-induced reinstatement could reflect the partial agonist efficacy of PD128907 at D3 receptors (Vanhauwe et al., 1999; van Vliet et al., 2000).
There are currently no effective medications to treat cocaine addiction and, consequently, no validated animal models for predicting the utility of candidate pharmacotherapies for this indication. To the extent that our findings in monkeys can be generalized to humans, however, the failure of PG01037 to inhibit cocaine self-administration and the similar findings with another D3-preferring antagonist, NGB2904, in rhesus monkeys (Martelle et al., 2007) suggest that antagonists acting selectively at D3 receptors would not decrease cocaine use in active drug users. Unlike PG01037 and NGB2904, the D2-preferential antagonist L-741626 and other D2-like receptor antagonists (Platt et al., 2002, 2003) have been found to reduce cocaine self-administration in monkeys, which might suggest a more promising role for D2 than D3 receptor antagonists in reducing active cocaine use. D2 antagonists have not, however, been developed successfully for cocaine addiction treatment for a number of reasons (Xi and Gardner, 2008), including the observation that many of these drugs, such as L-741626, produce extrapyramidal side effects and reduce food-maintained operant behavior at doses similar to or slightly higher than those needed to reduce cocaine self-administration (present study; Platt et al., 2002, 2003).
Recent studies on neurobiological mechanisms underlying cocaine addiction have shifted emphasis from the positive reinforcing effects of abused drugs to investigating neural systems that might become dysfunctional because of repeated drug use and thus promote compulsive drug taking (Dalley and Everitt, 2009). These systems seem to be involved in persistent drug-seeking behavior and may play a significant role in cocaine's subjective effects and relapse. Based on the present findings and others (see review by Heidbreder and Newman, 2010), D3-preferring antagonists might be expected to blunt some aspects of cocaine's subjective effects and also forestall relapse in abstinent cocaine abusers. Currently, however, the roles of different DA receptor subtypes in the neurobiology of addiction remain only partially resolved, and the discovery of novel D2- and D3-preferring ligands for in vivo investigation could help guide development of effective medications for the treatment of cocaine addiction and relapse.
Acknowledgments
We thank Shana Langer and Rebecca Smith for technical assistance and Dr. Mu-Fa Zou, Medicinal Chemistry Section, National Institute on Drug Abuse-Intramural Research Program, Baltimore, MD for synthesis of sumanirole maleate.
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse; the National Institutes of Health National Institute on Drug Abuse [Grants DA011054, DA01770]; the National Institutes of Health National Center for Research Resources [Grant RR00168].
Portions of this work were previously presented: Achat-Mendes C, Platt DM, Newman AH, Grundt P, and Spealman RD (2008) Comparison of selective dopamine D2 and D3 receptor antagonists on cocaine's abuse-related behaviors in squirrel monkeys. Society for Neuroscience Meeting; 2008, Nov 15–19; Washington, DC. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167619.
- DA
- dopamine
- CJB 090
- N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide
- D1
- dopamine receptor subtype 1
- D2
- dopamine receptor subtype 2
- D3
- dopamine receptor subtype 3
- DS
- discriminative stimulus
- FR
- fixed ratio
- FI
- fixed interval
- L-741626
- 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]methyl-1H-indole
- NGB2904
- N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide
- PG01037
- N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-trans-but-2-enyl)-4-pyridine-2-ylbenzamide)
- PD128907
- R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4-oxazin-9-ol)
- sumanirole
- (R)-5,6-dihydro-5-(methylamino)-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one(Z)-2-butenedioate
- RM
- repeated measures
- ANOVA
- analysis of variance
- NAc
- nucleus accumbens
- TO
- timeout
- S32504
- (+)-trans-3,4,4a,5,6, 10b-hexahydro-9-carbamoyl-4-propyl-2H-naphth[1,2-b]-1,4-oxazine.
References
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. (2009) The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology (Berl) 206:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, Katz JL, Witkin JM. (1995) Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol 281:R7–R9 [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. (2010) Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther 332:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. (2007) Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 27:881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, et al. (2010) Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology 35:560–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckler F, Gmeiner P. (2007) Dopamine D3 receptor ligands: recent advances in the control of subtype selectivity and intrinsic activity. Biochim Biophys Acta 1768:871–887 [DOI] [PubMed] [Google Scholar]
- Costanza RM, Barber DJ, Terry P. (2001) Antagonism of the discriminative stimulus effects of cocaine at two training doses by dopamine D2-like receptor antagonists. Psychopharmacology (Berl) 158:146–153 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ. (2009) Dopamine receptors in the learning, memory and drug reward circuitry. Semin Cell Dev Biol 20:403–410 [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. (2005) Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem 48:839–848 [DOI] [PubMed] [Google Scholar]
- Grundt P, Husband SL, Luedtke RR, Taylor M, Newman AH. (2007a) Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR. Bioorg Med Chem Lett 17:745–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. (2007b) Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem 50:4135–4146 [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. (2010) Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci 1187:4–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier RF, Dolak LA, Duncan JN, Hyslop DK, Lipton MF, Martin IJ, Mauragis MA, Piercey MF, Nichols NF, Schreur PJ, et al. (1997) Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem 40:639–646 [DOI] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZZ, Gardner EL. (2010) PG01037, a novel dopamine D3 receptor antagonist inhibits the effects of methamphetamine in rats. J Psychopharmacol doi:10.1177/0269881109358201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. (2000) Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther 294:680–687 [PubMed] [Google Scholar]
- Le Foll B, Francès H, Diaz J, Schwartz JC, Sokoloff P. (2002) Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci 15:2016–2026 [DOI] [PubMed] [Google Scholar]
- Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, Zhou HF, Xu M, Wang JQ. (2009) Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron 61:425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. (2007) Effects of two novel D3-selective compounds, NGB2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 321:573–582 [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Little KY, Damask SP, Mansour A, Watson SJ. (1993) Effects of cocaine on dopamine receptor gene expression: a study in the postmortem human brain. Biol Psychiatry 34:348–355 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Iob L, Péglion JL, Dekeyne A. (2007) Discriminative stimulus properties of S32504, a novel D3/D2 receptor agonist and antiparkinson agent, in rats: attenuation by the antipsychotics, aripiprazole, bifeprunox, N-desmethylclozapine, and by selective antagonists at dopamine D2 but not D3 receptors. Psychopharmacology (Berl) 191:767–782 [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. (1994) Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA 91:11271–11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. (2005) Intravenous self-administration techniques in monkeys. Curr Protoc Neurosci, Chapter 9, Unit 9.21 [DOI] [PubMed] [Google Scholar]
- Platt DM, Rodefer JS, Rowlett JK, Spealman RD. (2003) Suppression of cocaine- and food-maintained behavior by the D2-like receptor partial agonist terguride in squirrel monkeys. Psychopharmacology (Berl) 166:298–305 [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282 [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW. (1995) Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther 275:1355–1366 [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, et al. (2009) In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse 63:782–793 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1996) Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 278:1128–1137 [PubMed] [Google Scholar]
- Staley JK, Mash DC. (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhauwe JF, Fraeyman N, Francken BJ, Luyten WH, Leysen JE. (1999) Comparison of the ligand binding and signaling properties of human dopamine D(2) and D(3) receptors in Chinese hamster ovary cells. J Pharmacol Exp Ther 290:908–916 [PubMed] [Google Scholar]
- van Vliet LA, Rodenhuis N, Dijkstra D, Wikström H, Pugsley TA, Serpa KA, Meltzer LT, Heffner TG, Wise LD, Lajiness ME, et al. (2000) Synthesis and pharmacological evaluation of thiopyran analogues of the dopamine D3 receptor-selective agonist (4aR,10bR)-(+)-trans-3,4,4a,10b-tetrahydro-4-n-propyl-2H,5H [1]benzopyrano[4,3-b]-1,4-oxazin-9-ol (PD 128907). J Med Chem 43:2871–2882 [DOI] [PubMed] [Google Scholar]
- Welter M, Vallone D, Samad TA, Meziane H, Usiello A, Borrelli E. (2007) Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA 104:6840–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. (2008) Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev 1:303–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 31:1393–1405 [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. (1997) Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19:837–848 [DOI] [PubMed] [Google Scholar]
- Zapata A, Shippenberg TS. (2005) Lack of functional D2 receptors prevents the effects of the D3-preferring agonist (+)-PD 128907 on dialysate dopamine levels. Neuropharmacology 48:43–50 [DOI] [PubMed] [Google Scholar]