Abstract
Clinical and preclinical studies suggest that nicotinic acetylcholine receptors are involved in affective disorders; therefore, the potential therapeutic value of nicotinic partial agonists as treatments of these disorders is of growing interest. This study evaluated the effects of acute and chronic administration of nicotine and the α4β2 nicotinic partial agonists varenicline and sazetidine-A in mouse models of anxiety and depression. Acutely, only nicotine and varenicline had anxiolytic effects in the marble-burying test and in the novelty-induced hypophagia (NIH) test. In contrast, in animal models of antidepressant efficacy, such as the forced swim and the tail suspension test, only acute sazetidine-A had significant antidepressant-like effects. The NIH test provides an anxiety-related measure that is sensitive to the effects of chronic but not acute antidepressant treatment. Chronic nicotine and chronic sazetidine-A treatment were effective in this paradigm, but varenicline was ineffective. These results suggest that the partial agonists varenicline and sazetidine-A may have diverse therapeutic benefits in affective disorders.
Depression and anxiety are both highly comorbid with nicotine dependence (Paperwalla et al., 2004), and nicotine use may be an attempt at self-medication in these conditions (Markou et al., 1998). Currently, antidepressant (AD) medications are the predominant form of treatment for both depression and some forms of anxiety. These drugs, including tricyclic antidepressants, selective serotonin reuptake inhibitors, and norepinephrine reuptake inhibitors, have been shown to act as noncompetitive nicotinic receptor (nAChR) antagonists on the cellular, physiologic, and behavioral level (Hennings et al., 1997; Fryer and Lukas, 1999; López-Valdés and García-Colunga, 2001). In addition, in preclinical paradigms used to predict antidepressant response, the effects of AD medications can be augmented by administration of nicotinic drugs (Caldarone et al., 2004; Rollema et al., 2009a).
Acute treatment with nicotinic antagonists, such as mecamylamine, but not acute nicotine, results in antidepressant-like effects in the forced swim test (FST) and the tail suspension test (TST) (Caldarone et al., 2004; Rabenstein et al., 2006). Furthermore, chronic, but not acute, administration of nicotine elicits antidepressant-like effects in the learned helplessness model of depression (Ferguson et al., 2000). As a time-averaged antagonist, nicotine administration can result in prolonged periods of receptor desensitization (Hulihan-Giblin et al., 1990). This property of nicotine may explain its apparent dichotomy in behavioral paradigms sensitive to antidepressant-like effects. However, mechanisms underlying acute and chronic effects of nicotine and other nicotinic drugs in models of anxiety and depression are not well characterized (Picciotto et al., 2008).
Varenicline (Chantix; Pfizer, Groton, CT) is a potent partial agonist at α4β2 nAChRs, with 40 to 60% of the agonist efficacy of nicotine (Rollema et al., 2007), and it is a less potent partial agonist at α3β4 and a full agonist at α7 nAChRs (Mihalak et al., 2006; Rollema et al., 2007, 2009b). Clinically, varenicline has demonstrated enhancement of both positive affect and cognitive function during smoking cessation (Patterson et al., 2009), and it was recently shown to augment the effects of antidepressants in depressed smokers (Philip et al., 2009). Despite its clinical success in smoking cessation (Nides et al., 2008), there have been a limited number of studies evaluating the behavioral effects of varenicline in animal models of depression (Rollema et al., 2009a) and none examining its effects on anxiety.
A newly developed nicotinic partial agonist, sazetidine-A, potently and selectively desensitizes α4β2 nicotinic acetylcholine receptors (Xiao et al., 2006), but it has very low affinities for all other nAChR subtypes (Xiao et al., 2006; Zwart et al., 2008). A recent in vivo study using sazetidine-A found it to be an effective analgesic (Cucchiaro et al., 2008). However, the activity of sazetidine-A in behavioral models of anxiety or depression has not been investigated. Therefore, we conducted a complementary series of experiments examining the acute and chronic effects of sazetidine-A, varenicline, and nicotine in models of anxiety and depression. Findings from these experiments show that acute nicotine and varenicline are efficacious in two models of anxiety; the novelty-induced hypophagia (NIH) test and the marble-burying test. In contrast, sazetidine-A was anxiogenic in the NIH test but did have AD-like effects in acute tests of AD efficacy. Furthermore, sazetidine-A, as well as nicotine, was effective when administered chronically in the NIH test, a profile consistent with clinically effective antidepressants. These results suggest that the development of novel nicotinic ligands with differential subtype selectivity presents a new therapeutic avenue for the treatment of affective disorders.
Materials and Methods
Animals.
Male 129SvJ;C57BL/6J F1 hybrid mice (6–12 weeks of age; 25–35 g) were bred, group-housed, and maintained on a 12-h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. The C57BL/6 and 129SvEv strains are commonly used for the development of knockout mouse models; therefore, information regarding nicotine response in a variety of behaviors in this strain is of value for future studies aimed at investigating underlying genetic mechanisms. For the NIH paradigm, mice were housed in groups of two. All experimental testing sessions were conducted between 9:00 AM and 3:00 PM, with animals randomly assigned to treatment conditions and tested in counterbalanced order.
Drugs.
Doses of nicotine tartrate (Sigma-Aldrich, St. Louis, MO), varenicline tartrate, and sazetidine-A tartrate are reported as free base weight, whereas desipramine (DMI) (Sigma-Aldrich) and chlordiazepoxide (CDP) (Sigma-Aldrich) were calculated as milligrams per kilogram of the salt form. For injection studies, all drugs were prepared immediately before use in 0.9% saline and injected intraperitoneally. Sazetidine-A tartrate was kindly synthesized by Drs. Milton L. Brown, Mikell A. Paige, and Brian E. McDowell (Georgetown University) and provided by Drs. Ken Kellar and Yingxian Xiao (Georgetown University, Washington, DC). The chemical structure of this compound has been published previously (Xiao et al., 2006). Varenicline tartrate was provided by Pfizer Global Research and Development (Groton, CT). The chemical structure of this compound has been published previously (Mihalak et al., 2006).
Osmotic Minipumps.
Nicotine tartrate, sazetidine-A tartrate, and varenicline tartrate were dissolved in sterile 0.9% saline solution and infused through subcutaneous osmotic minipumps for 14 days (model 2002; Alzet, Cupertino, CA). Mice were anesthetized with an isoflurane/oxygen vapor mixture (1–3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the wound. The wound was closed with 7-mm stainless steel wound clips (Reflex; Cellpoint Scientific, Gaithersburg, MD).
Elevated-Zero Maze Test.
Testing on the elevated-zero maze (EZM) was conducted as described previously (Gur et al., 2007). In brief, the zero maze (Stoelting, Wood Dale, IL) was elevated 61 cm (24 in) from the ground and consisted of two open areas and two closed areas. After a 1-h acclimation period before testing, mice were injected intraperitoneally with saline, nicotine, sazetidine-A, varenicline, or CDP. Ten minutes later, each mouse was initially placed in the closed area and tested for 300 s. The ViewPoint Tracking System (ViewPoint, Champagne au Mont d'Or, France) was used to record and register the time spent in the open areas and the locomotor activity of the animal.
Locomotor Activity.
Locomotor activity in response to intraperitoneal drug administration was analyzed in a “home cage” activity monitoring system (MED Associates, St. Albans, VT). The home cage (28.9 × 17.8 × 12 cm) was placed in a photobeam frame (30 × 24 × 8 cm) with sensors arranged in an eight-beam array strip. For dose studies, mice were injected intraperitoneally with saline or drug. Ten minutes after drug administration, the mice were individually placed in the cages. Beam break data were monitored and recorded for 60 min.
Marble-Burying Test.
After a period of acclimation (1 h), mice (n = 6–10/group) were injected intraperitoneally with saline, nicotine, sazetidine-A, varenicline, or CDP at the doses indicated. Ten minutes later, the mice were placed individually in small cages (26 × 20 × 14 cm), in which 20 marbles had been equally distributed on top of mouse bedding (5 cm in depth), and a wire lid was placed on top of the cage. Mice were left undisturbed for 15 min, after which time the number of buried marbles (i.e., those covered by bedding three quarters or more) was counted.
NIH Test.
For 1 week before the training period and for the duration of the experiment, mice were housed in groups of two. Training consisted of daily sessions in which mice were exposed to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) in a clear plastic dish. Plastic dividers (dividing the standard mouse cage lengthwise) were placed inside each cage to separate the mice during the training and home cage testing periods. Mice were acclimated to the barriers for 1 h before placement of food. Food was placed in the cage for 15 min, and latency to consume was measured. By the 12th day, a baseline latency to approach and consume the food was reached such that there was <20% variability between mice. In the chronic treatment studies, mice (n = 10/group) were implanted with 14-day osmotic minipumps filled with nicotine (18 mg/kg/day), sazetidine-A (1.8 mg/kg/day), varenicline (1.8 mg/kg/day), or 0.9% saline. As a positive control, mice were injected chronically twice a day for 28 days with either DMI or 0.9% saline. No differences in behavior were observed between minipump and injection saline groups in the chronic NIH test; therefore, these groups were combined for analysis. Testing in the home cage (home day 1), novel environment, and home cage (home day 2) occurred on the last 3 days of minipump viability. For testing in the novel environment, mice were removed from the home cage and placed in an empty standard cage with no bedding. The cage was wiped with a cleanser (Pine Sol; 1:10 dilution) to emit a novel odor and placed in a white box with bright light illumination (2150 lux). Latency to consume was recorded. Mice were tested again in the home cage on the following day (home day 2), under the same conditions as the first home test day (home day 1). On both home test days, the amount consumed was recorded as grams peanut butter chips. In the acute treatment experiment, training was performed as described above. Ten minutes after intraperitoneal drug administration, the mice (n = 10/group) were tested in the home environment on day 1 (saline only), in the novel environment on day 2 (saline or drug), and once again in the home environment on day 3 (saline or drug). Thus, in the acute studies, the animals received no drugs until 10 min before novel test day.
Forced Swim Test.
The FST was conducted as described previously (Cryan et al., 2005). In brief, 10 or 30 min after intraperitoneal injection with saline, nicotine, sazetidine-A, varenicline, or DMI, mice were placed into Plexiglas cylinders filled with water (25°C) for 6 min while being videotaped. The forced swim score for the entire 6-min test was assessed using the ViewPoint videotracking system (ViewPoint) and confirmed with visual scoring by a trained observer. A mouse was judged to be immobile when making only those movements necessary to keep its head above water.
Tail Suspension Test.
Ten minutes after intraperitoneal injection of saline, nicotine, sazetidine-A, or varenicline or 30 min after intraperitoneal injection of DMI, mice were tested in an automated TST device (MED Associates). Mice were suspended by their tails with tape from an aluminum bar connected to a strain gauge for 6 min. The duration of immobility was calculated as the time the force of the animal's movements was below a preset threshold (breathing only). Optimal thresholds were originally determined by comparing manually scored videotapes with automated scores (Crowley et al., 2006).
Data Analysis.
Using the GraphPad Prism 5.0 software package (GraphPad Software Inc., San Diego, CA), statistical analyses of the differences between groups were assessed using one- or two-way analysis of variance followed by Bonferroni's multiple comparison test.
Results
Nicotine, Sazetidine-A, and Varenicline Have No Effect in the Elevated-Zero Maze.
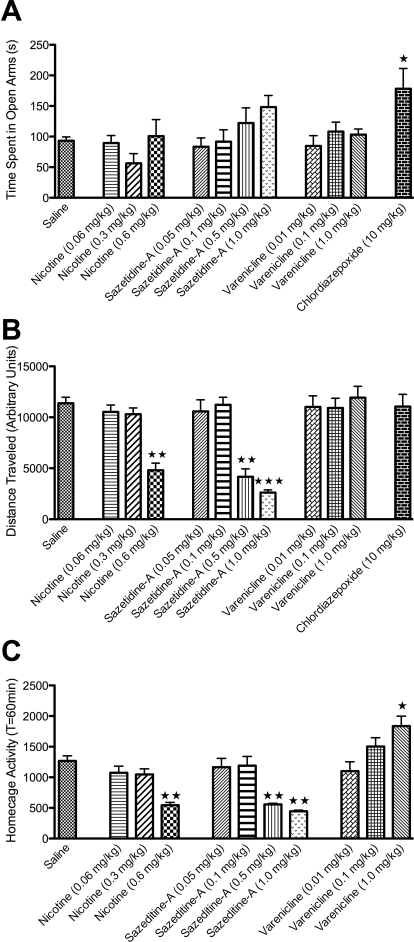
The elevated-zero maze is a modification of the elevated-plus maze model of anxiety in rodents and has been found to be a less ambiguous model to study anxiolytic activity compared with elevated-plus maze and mirror chamber (Kulkarni et al., 2007). CDP, a benzodiazepine with proven efficacy in this paradigm (Ring et al., 2006), significantly increased time spent in the open arms (Fig. 1 A), indicative of reduced anxiety. In contrast, nicotine, sazetidine-A, and varenicline did not significantly affect time spent in the open arms of the elevated-zero maze (Fig. 1A). In addition, the highest dose of nicotine (0.6 mg/kg) and the two highest doses of sazetidine-A (0.5 and 1.0 mg/kg) resulted in profound hypolocomotion in this test (Fig. 1B) as well as in homecage activity (Fig. 1C). Although varenicline treatment did not significantly alter locomotor activity in the elevated-zero maze (Fig. 1B ), the highest dose of varenicline used (1 mg/kg) resulted in significant hyperlocomotion in the homecage (Fig. 1C). Although altered activity in the zero maze may confound results at these higher doses, at lower doses where no impairment in locomotion is observed, animals still do not spend more time in the open arm.
Fig. 1.
No effect of acute nicotine, sazetidine-A, or varenicline in the elevated-zero maze. Mice received intraperitoneal injections of saline or drug 10 min before testing. A, the mean (±S.E.M.) amount of time spent the open arm is shown. Chlordiazepoxide (10 mg/kg) treatment resulted in significantly increased time spent in the open arm (∗, p = 0.01). No effect of nicotine, sazetidine-A, or varenicline was observed. B, the mean (±S.E.M.) amount of distance traveled over 5 min is shown. The highest dose of nicotine (0.6 mg/kg; ∗∗∗, p = 0.0001) and the two highest doses of sazetidine-A (0.5 and 1.0 mg/kg; ∗∗∗, p = 0.0001) significantly reduced the total distance traveled in the EZM. No other significant differences were observed. C, the mean (±S.E.M.) homecage activity over 60 min is shown. The highest dose of nicotine (0.6 mg/kg; ∗∗, p = 0.001) and the two highest doses of sazetidine-A (0.5 and 1.0 mg/kg; ∗∗, p = 0.001) significantly reduced homecage activity. In addition, the highest dose of varenicline (1.0 mg/kg; ∗, p = 0.01) significantly increased homecage activity. ∗, ∗∗, and ∗∗∗, indicate significant differences compared with saline controls (n = 6–12).
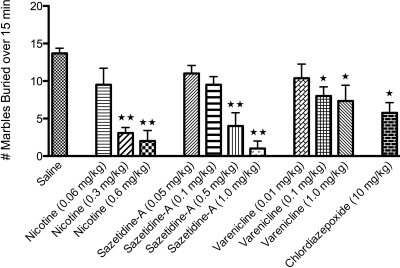
Nicotine and Varenicline Are Anxiolytic in the Marble-Burying Test.
Studies examining the effects of nicotinic compounds in the elevated-zero maze, as well as the elevated-plus maze and the mirror chamber, have yielded diverse and conflicting results (Picciotto et al., 2002). An additional paradigm used to evaluate putative anxiolytic compounds is the marble-burying test (Nicolas et al., 2006). This test has high predictive value to detect anxiolytic-like activity of acutely administered drugs (Nicolas et al., 2006), which is demonstrated by the positive result of CDP (10 mg/kg) in this test (Fig. 2). In this paradigm, both 0.3 and 0.6 mg/kg nicotine reduced the number of marbles buried (Fig. 2). When controlling for locomotor activity, the nonsedating dose of nicotine (0.3 mg/kg) still reduces the total number of marbles buried, indicating an anxiolytic effect. Treatment with varenicline had a small but significant effect in reducing the number of marbles buried at both the hyperlocomoting (1.0 mg/kg) and nonhyperlocomoting (0.1 mg/kg) doses (Fig. 2). In addition, treatment with sazetidine-A (0.5 and 1.0 mg/kg) also reduced marble burying behavior, but only at doses that severely reduce activity on the elevated-zero maze (Figs. 1, B and C, and 2).
Fig. 2.
Effect of nicotine, sazetidine-A, and varenicline in the marble-burying test. Mice received intraperitoneal injections of saline or drug 10 min before testing. The mean (±S.E.M.) number of marbles buried over 15 min is shown. Chlordiazepoxide (10-mg/kg) treatment significantly reduced the number of marbles buried (∗, p = 0.01). Nicotine treatment at the higher doses (0.3 and 0.6 mg/kg), but not the lowest dose (0.06 mg/kg), resulted in significantly fewer marbles buried (∗∗, p = 0.001), indicated an anxiolytic response. In addition, the higher doses of sazetidine-A (0.5 and 1.0 mg/kg; ∗∗, p = 0.001) and varenicline (0.1 and 1.0 mg/kg; ∗, p = 0.01) also significantly affected marble-burying behavior. ∗ and ∗∗ indicate significant differences compared with saline controls (n = 6–14)).
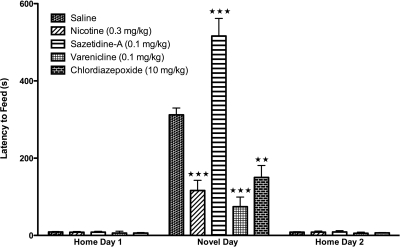
Acute Nicotine and Acute Varenicline, but Not Acute Sazetidine-A, Are Anxiolytic in the NIH Test.
In the NIH test, the reduction in feeding in response to a novel environment is a well established measure for anxiety-related behaviors (Dulawa and Hen, 2005) and is sensitive to acute benzodiazepine administration, such as CDP (Merali et al., 2003). In contrast to the marble-burying test where an active behavior indicates an anxiety response (the number of marbles actively buried), a passive behavior in the NIH test (not approaching and consuming a highly palatable food) indicates an anxiety response. As shown in Fig. 3, CDP (10 mg/kg) significantly reduced the latency to consume the food in a novel environment. Because altered locomotor activity can have a negative effect on this behavior by yielding a false result (by increasing or decreasing latency to consume food), the choice of drug dosage for nicotine, varenicline, and sazetidine-A in these experiments was based on a dose that did not affect locomotor activity in the homecage (Fig. 1C). Acute administration of 0.3 mg/kg nicotine, which was anxiolytic in the marble-burying test, had a similar effect in the NIH test by reducing the latency to consume food in the novel environment. Acute varenicline (0.1 mg/kg), which was also anxiolytic in the marble-burying test, also reduced the latency to consume food in the novel environment. In contrast, acute administration of sazetidine-A (0.1 mg/kg) significantly increased the time required to investigate and consume the palatable food, reflecting an anxiogenic effect compared with saline. None of the treatments resulted in significant differences in latency to consume the food on home day 1 or 2 (Fig. 3) or in total food consumption on homecage test days (Table 1).
Fig. 3.
Nicotine and varenicline are anxiolytic in the NIH test on novel test day. Mice received intraperitoneal injections of saline or drug 10 min before testing. Latency to approach and consume food is shown as seconds ± S.E.M. Treatment with chlordiazepoxide (10 mg/kg), a benzodiazepine, significantly reduced the latency to consume food in a novel environment (∗∗, p = 0.001). Similarly, acute nicotine treatment (0.3 mg/kg) significantly reduced the amount of time required to investigate and consume food in a novel environment relative to saline controls (∗∗∗, p = 0.0001). Likewise, acute varenicline (0.1 mg/kg) significantly reduced the time required to investigate and consume food in a novel environment compared with saline controls (∗∗∗, p = 0.0001). However, acute treatment with sazetidine-A resulted in significantly increased latencies to approach and consume food in a novel environment compared with saline controls (∗∗∗, p = 0.0001). No significant treatment effects were observed in the home environment (n = 10).
TABLE 1.
Food consumption and body weight in the acute or chronic NIH tests
Data are from n = 10 mice.
| NIH Paradigm | Avg. Amount Consumed |
Avg. Body Weight |
||
|---|---|---|---|---|
| Home Day 1 | Home Day 2 | End of Training | End of Experiment | |
| g± S.D. | ||||
| Acute | ||||
| Saline | 0.36 ± 0.11 | 0.35 ± 0.12 | N.A. | 25.6 ± 2.9 |
| Nicotine | 0.37 ± 0.15 | 0.39-±0.12 | N.A. | 26.2 ± 2.7 |
| Sazetidine-A | 0.37 ± 0.16 | 0.40 ± 0.15 | N.A. | 24.9 ± 3.4 |
| Varenicline | 0.37 ± 0.12 | 0.40 ± 0.20 | N.A. | 25.9 ± 2.5 |
| CDP | 0.37 ± 0.20 | 0.40 ± 0.22 | N.A. | 25.7 ± 2.6 |
| Chronic | ||||
| Saline | 0.37 ± 0.20 | 0.35 ± 0.10 | 26.9 ± 2.0 | 27.6 ± 2.5 |
| Nicotine | 0.40 ± 0.20 | 0.38-±0.15 | 27.0 ± 2.5 | 27.8 ± 2.1 |
| Sazetidine-A | 0.41 ± 0.20 | 0.35 ± 0.11 | 27.3 ± 2.4 | 28.2 ± 2.2 |
| Varenicline | 0.40 ± 0.15 | 0.40 ± 0.25 | 27.6 ± 2.1 | 28.0 ± 2.9 |
| DMI | 0.39 ± 0.12 | 0.38 ± 0.15 | 26.7 ± 2.1 | 27.2 ± 2.4 |
N.A., not applicable.
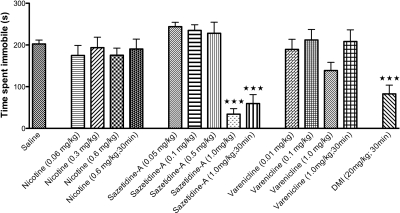
Sazetidine-A, but Not Nicotine or Varenicline, Has Significant Effects in the FST.
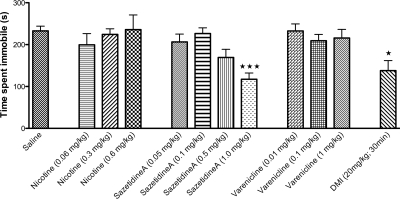
The forced swim test is the most widely used model for assessing antidepressant-like activity in rodents. In this test, acute administration of an antidepressant, which is often accompanied by hypolocomotion in the home cage, results in increased activity or reduced immobility in the FST (Cryan et al., 2005). As demonstrated in Fig. 4, a 30-min pretreatment with DMI, a tricyclic antidepressant, significantly reduced immobility. For direct comparison with DMI, as well as with other published work with these nicotinic compounds in the FST (Rollema et al., 2009a), we examined behavioral responses for all compounds at 10- and 30-min time points. The 1.0 mg/kg dose of sazetidine-A at either the 10- or 30-min time point resulted in reduced immobility, indicating an antidepressant-like response. However, neither nicotine nor varenicline had a significant effect on immobility at any of the doses or time points (Fig. 4).
Fig. 4.
Acute treatment with sazetidine-A has antidepressant effects in the forced swim test. Mice received intraperitoneal injections of saline or drug 10 or 30 min before testing. Time spent immobile over the total 6 min is shown as seconds ± S.E.M. Treatment with DMI (20 mg/kg), a tricyclic antidepressant, significantly reduced immobility over 6 min in the forced swim test (∗∗∗, p = 0.0001). Acute administration of nicotine or varenicline did not significantly alter immobility in this test. However, acute treatment with sazetidine-A (1.0 mg/kg at 10 and 30 min) resulted in significantly reduced immobility (∗∗∗, p = 0.0001). ∗∗∗ indicates significant difference compared with saline controls (n = 6–12).
Sazetidine-A, but Not Nicotine or Varenicline, Has Significant Effects in the TST.
Similar to the FST, the TST measures alterations in escape-oriented movements and immobility when placed in an inescapable stressful situation (Cryan et al., 2005). One major difference between the FST and the TST is that the FST does not consistently detect SSRI activity (Cryan et al., 2005). Thus, to more fully characterize the effects of nicotine, varenicline, and sazetidine-A, we also tested these compounds in the TST. As shown in Fig. 5, DMI significantly reduced immobility in the TST. In addition, sazetidine-A displayed an antidepressant-like response in this paradigm at the 1.0 mg/kg dose. In contrast, neither nicotine nor varenicline reduced immobility in the TST at any of the doses tested (Fig. 5).
Fig. 5.
Acute treatment with sazetidine-A has antidepressant effects in the tail suspension test. Mice received intraperitoneal injections of saline or drug 10 or 30 min before testing. Time spent immobile over the total 6 min is shown as seconds ± S.E.M. Treatment with DMI (20 mg/kg), a tricyclic antidepressant, significantly reduced immobility over 6 min in the tail suspension test (∗, p = 0.05). In addition, acute treatment with sazetidine-A (1.0 mg/kg at 10 and 30 min) resulted in significantly reduced immobility (∗∗∗, p = 0.0001). However, acute administration of nicotine or varenicline did not significantly alter immobility in this test. ∗ and ∗∗∗ indicate significant differences compared with saline controls (n = 6–12).
Chronic Administration of Nicotine and Sazetidine-A Is Anxiolytic, but Varenicline Has No Effect, in the NIH Paradigm.
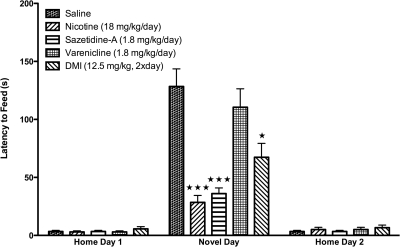
The NIH test is an effective tool to study mechanisms of antidepressant response, because the time course of the anxiolytic effects of antidepressants is consistent with a therapeutic time course (Dulawa and Hen, 2005). Because the NIH paradigm is sensitive to chronic, but not acute, antidepressant treatment, we tested whether chronic administration of nicotine, sazetidine-A, or varenicline was effective in the NIH paradigm. Mice metabolize nicotine very quickly, making chronic daily injections ineffective (Matta et al., 2007); therefore, mice were implanted with osmotic minipumps filled with nicotine, sazetidine-A, or varenicline, and tested in the NIH paradigm during the last 3 days of minipump viability. A moderate nicotine dose was chosen based on past studies examining the cognitive effects of nicotine and nicotine withdrawal in mice (Stoker et al., 2008). Both sazetidine-A and varenicline were administered at a one log lower dose compared with nicotine based on the higher affinity of these drugs for α4β2 nAChRs (α4β2 Ki for nicotine, ∼2–6 nM; for sazetidine-A and varenicline, ∼0.2–0.4 nM) (Xiao et al., 2006; Rollema et al., 2007, 2009b; Zwart et al., 2008). As shown in Fig. 6, chronic treatment with the tricyclic antidepressant DMI resulted in significantly reduced latencies in the NIH test. In addition, chronic administration of both nicotine (18 mg/kg/day) and sazetidine-A (1.8 mg/kg/day) significantly reduced the amount of time required to approach and consume food. However, chronic administration of varenicline (1.8 mg/kg/day) had no significant effect on the latency to consume food in the novel environment. None of the treatments resulted in significant differences in latency to consume the food on home day 1 or 2 (Fig. 6) or in food consumption on home cage test days or in mean body weights (Table 1).
Fig. 6.
Effects of chronic treatment of nicotine, sazetidine-A, and varenicline on novel test day in the NIH test. Mice were implanted with 14-day osmotic minipumps filled with saline, nicotine, sazetidine-A, or varenicline. A parallel cohort of mice were injected twice daily with either saline or DMI. No differences were observed between saline minipump and saline injected groups. After chronic treatment, mice were tested in the home environment on home days 1 and 2 and the novel environment on novel day in the NIH paradigm. Latency to approach and consume food is shown as seconds ± S.E.M. Treatment with DMI (12.5 mg/kg, 2 times a day), a tricyclic antidepressant, significantly reduced the latency to consume food in a novel environment (∗, p = 0.01). Chronic treatment with both nicotine (18 mg/kg/day) and sazetidine-A (1.8 mg/kg/day) resulted in significantly lower latencies to approach and consume food on novel test day in the NIH test compared with saline controls (∗∗∗, p = 0.0001). In contrast, chronic treatment with varenicline (1.8 mg/kg/day) did not significantly alter the latency to consume food in a novel environment compared with saline-treated animals. No treatment effects were observed on home day 1 or 2 (n = 10)
Discussion
The potential utility of nicotinic drugs in the treatment of affective disorders has gained interest in recent years (Picciotto et al., 2002; Caldarone et al., 2004). Cytisine, a nicotinic partial agonist, exhibits antidepressant-like activity in the FST and the TST, two tests sensitive to the effects of antidepressant compounds (Mineur et al., 2007, 2009). In addition, nicotine has significant anxiolytic effects in the approach-avoidance conflict paradigm, which is a preclinical model of anxiety (Cohen et al., 2009). As a consequence of the utility of the nicotinic partial agonist varenicline, an effective and relatively well tolerated smoking cessation therapy, an increasing number of nicotinic compounds are becoming available for testing in models of affective disorders.
In the present study, we directly compare the effects of nicotine and two novel nicotinic partial agonists with differing activity profiles, sazetidine-A and varenicline, in a variety of behavioral paradigms. Although the anxiogenic effects of nicotine withdrawal have been described in detail (Malin and Goyarzu, 2009), the effects of chronic administration of nicotinic drugs in models of anxiety and depression have not been well established. Our findings evaluating the acute and chronic effects of nicotine, sazetidine-A, and varenicline are the first to demonstrate distinct anxiolytic and antidepressant behavioral effects of these nicotinic compounds, which may be related to their dissimilar subtype selectivity in vivo.
Sazetidine-A is a high-affinity, highly selective partial agonist of α4β2 nAChRs (Xiao et al., 2006; Cucchiaro et al., 2008). Although the Ki values for sazetidine-A and varenicline at α4β2 nAChRs are very similar (0.4 and 0.2 nM, respectively) (Xiao et al., 2006; Rollema et al., 2007), the high-affinity α4β2 partial agonist varenicline also has moderate affinity for other nAChRs and acts as a partial agonist at α3β4 and a full agonist at α7 nAChRs (Mihalak et al., 2006; Rollema et al., 2007). The partial agonist activity of varenicline at α4β2 nAChRs as well as accompanying α7 and α3β4 activities, may underlie its similarity to nicotine treatment when administered acutely; however, its contrasting effects compared with nicotine and sazetidine-A in chronic studies suggest other mechanisms may be engaged after prolonged exposure to these drugs.
The effects of nicotine, sazetidine-A, and varenicline were examined in three tests of anxiety, the elevated-zero maze, the marble-burying test, and the novelty-induced hypophagia test, and they were compared with the effects of a standard antianxiety drug, CDP. None of the nicotinic compounds had significant effects in the elevated-zero maze. Sazetidine-A treatment had an apparent trend toward increased time in the open arm in the elevated-zero maze, but this result is most likely related to less distance traveled and the reduced locomotion of animals treated with higher concentrations of sazetidine-A rather than a positive anxiolytic effect in this test. However, the elevated-zero maze has been found to yield diverse and conflicting results with nicotinic compounds (Picciotto et al., 2002). Therefore, we tested these drugs in two other established models of anxiety, the NIH test and the marble-burying test, which possess complementary assessments of anxiety behavior. The NIH paradigm is a test in which a normal exploratory response, which is rewarded by consumption of a palatable food, is reduced due to a novel and potentially aversive environment. This behavior is quantified by measuring the length of time it takes the animal to eventually venture into an open space and consume food. Thus, a passive behavior (not approaching the food) is taken as the measure of anxiety. In contrast, the marble-burying test measures the ability of an aversive environment to induce an anxiety-like state resulting in an active response. Thus, the active behavior (burying) is taken as a measure of heightened anxiety. Our use of these complementary tests allows for the assessment of the generalized anxiolytic-like effects of nicotinic drugs in combination with determination of effects on active versus passive behavioral strategies (Bechtholt et al., 2007). In both the marble-burying and the NIH tests, acute administration of nicotine and varenicline resulted in significant anxiolytic effects (Figs. 2 and 3). Although higher doses of sazetidine-A (0.5 and 1.0 mg/kg) resulted in anxiolytic effects in the marble-burying test (Fig. 2 ), this could be a false positive due to the decreased locomotor activity observed with these doses (Fig. 1, B and C). Furthermore, acute administration of sazetidine-A in the NIH test at a dose that does not depress locomotor activity increases latency to approach and consume the food, suggestive of an increased anxiety state.
Sazetidine-A decreased immobility in both the FST and the TST, two tests that predict antidepressant efficacy. Of interest, the magnitude of this response was equivalent to that observed with the standard antidepressant drug, DMI. In contrast, neither nicotine nor varenicline had significant effects in these tests. A recent study from Rollema et al. (2009a) observed an antidepressant response after varenicline administration in the FST. However, the magnitude of the effect of varenicline varied greatly in the two mouse strains used in these studies (C57BL/6J and CD-1), suggesting that this effect may be strain specific. Our use of 129SvJ;C57BL/6J F1 hybrid mice contributes to the literature in evaluating these compounds in a novel strain. In addition, because these mice are from a genetically diverse background, the results from these studies may be more generalizable than results from exclusively inbred strains.
Both nicotine and sazetidine-A were found to have antidepressant-like effects after chronic exposure in the NIH paradigm, suggesting that selective reduction of α4β2 nAChR activity through receptor desensitization may be sufficient to elicit these responses after chronic treatment. This may explain why nicotine does not show antidepressant-like effects acutely in the FST or TST, but its efficacy as an antidepressant is observed chronically in the NIH paradigm. Because the desensitization properties of nicotine occur over time, an acute administration may not be sufficient to elicit this response, whereas sazetidine-A, which is a rapid desensitizer, would effectively desensitize receptors that may account for antidepressant-like behaviors observed in these tests after an acute dose. However, further functional studies examining the differential chronic effects of these drugs on receptor properties are needed to clarify the relevance of nAChR activation and desensitization in these behavioral models.
Recent studies have described the effects of another nicotinic agonist, cytisine, in a paradigm similar to the NIH test, the novelty-suppressed feeding (NSF) paradigm. Cytisine is a partial agonist of β2* receptors and a full agonist of β4* receptor. Mineur et al. (2007) found that chronic, but not acute, cytisine treatment significantly reduced anxiety in the NSF paradigm, which parallels our findings with sazetidine-A. Although varenicline, like cytisine, is a potent and selective α4β2 partial agonist (Rollema et al., 2007), we did not observe antidepressant effects after chronic administration in the NIH paradigm. In addition, a recent study by Vieyra-Reyes et al. (2008) did not observe any behavioral changes in the NSF paradigm after chronic oral administration of nicotine, whereas our data indicate that chronic nicotine decreases the latency to feed in the NIH paradigm. These dissimilar findings may be due to differences in dosing regime (oral versus minipumps) or differences between the novelty-suppressed feeding paradigm and the novelty-induced hypophagia test. One significant difference between the paradigms is that the NSF test includes food deprivation, which is itself a stressor, and may present a confounding factor in the determination of anxiogenic responses arising from a novel environment (Merali et al., 2003).
Nicotine dependence is a chronic, relapsing disorder. Relapse curves for self-quitters are striking, with up to 75% of smokers relapsing within the 1st week of a quit-attempt (Shiffman, 2006). The ability of smokers to quit has been aided by new medications and counseling. The most effective medication for smoking cessation currently available is varenicline. In clinical studies, administration of varenicline during smoking cessation results in the improvement of both positive affect and cognitive function (Patterson et al., 2009). In addition, coadministration of varenicline was shown to positively enhance the effects of antidepressants in depressed smokers in a small open label study (Philip et al., 2009). However, despite its success, less than half of those using varenicline were able to quit smoking at a 3-month follow-up period (Garrison and Dugan, 2009). Our findings suggest that perhaps the lack of long-term antidepressant effects of varenicline may underlie this low success rate. Thus, development of newer nicotinic drugs, such as sazetidine-A, which has antidepressant-like activity and utility as a smoking cessation therapy (Levin et al., 2010), would lead to more beneficial treatments for smoking cessation.
Acknowledgments
We thank Drs. Ken Kellar, Yingxian Xiao, and Irwin Lucki for helpful discussions of data. We thank Drs. Milton L. Brown, Mikell A. Paige, and Brian E. McDowell for synthesizing sazetidine-A. We also thank Dr. Hans Rollema (Pfizer, Groton, CT) for providing a sample and pharmacological details of varenicline.
This work was supported by the National Institutes of Health National Cancer Institute [Grant P50-CA143187] and the National Institutes of Health National Institute on Drug Abuse [Grant 1-F32-DA026236-01A1].
Some of this work was presented previously in Turner JR, Hodes G, Lucki I, and Blendy J (2008) Nicotine exhibits antidepressant-like effects in models of anxiety and increases cell proliferation. Proceedings of the 38th Annual Meeting of the Society for Neuroscience; 2008 Nov 15–19; Washington, DC. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166280.
- AD
- antidepressant
- nAChR
- neuronal nicotine acetylcholine receptor
- FST
- forced swim test
- TST
- tail suspension test
- NIH
- novelty-induced hypophagia
- DMI
- desipramine
- CDP
- chlordiazepoxide
- EZM
- elevated-zero maze
- NSF
- novelty-suppressed feeding.
References
- Bechtholt AJ, Hill TE, Lucki I. (2007) Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl) 190:531–540 [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. (2004) High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry 56:657–664 [DOI] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. (2009) Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology (Berl) 204:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. (2006) Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology 31:2433–2442 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. (2005) Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 182:335–344 [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. (2008) Analgesic effects of sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology 109:512–519 [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. (2005) Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783 [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. (2000) Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmocology (Berl) 152:295–303 [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. (1999) Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem 72:1117–1124 [DOI] [PubMed] [Google Scholar]
- Garrison GD, Dugan SE. (2009) Varenicline: a first-line treatment option for smoking cessation. Clin Ther 31:463–491 [DOI] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. (2007) cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci 27:7860–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings EC, Kiss JP, Vizi ES. (1997) Nicotinic acetylcholine receptor antagonist effect of fluoxetine in rat hippocampal slices. Brain Res 759:292–294 [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. (1990) Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of a single injection of nicotine. J Pharmacol Exp Ther 252:15–20 [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M. (2007) Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol 29:343–348 [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells C, Hampton D, Petro A, Rose JE, Brown ML, et al. (2010) Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. J Pharmacol Exp Ther 332:933–939 [DOI] [PubMed] [Google Scholar]
- López-Valdés HE, García-Colunga J. (2001) Antagonism of nicotinic acetylcholine receptors by inhibitors of monoamine uptake. Mol Psychiatry 6:511–519 [DOI] [PubMed] [Google Scholar]
- Malin DH, Goyarzu P. (2009) Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol 192:401–434 [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18:135–174 [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319 [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. (2003) Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry 54:552–565 [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gündisch D, Picciotto MR. (2009) Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther 329:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. (2007) Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology 52:1256–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EP. (2006) A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 547:106–115 [DOI] [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, Jr, Williams KE. (2008) Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav 32:664–675 [DOI] [PubMed] [Google Scholar]
- Paperwalla KN, Levin TT, Weiner J, Saravay SM.(2004)Smoking and depression. Med Clin North Am 88:1483–1494, x-xi [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. (2009) Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH. (2009) Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatry 70:1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. (2002) Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 13:1097–1106 [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. (2006) The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology 189:395–401 [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. (2006) Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 185:218–225 [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. (2007) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994 [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, Picciotto MR. (2009a) Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol 605:114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Hajós M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, et al. (2009b)Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol 78:813–824 [DOI] [PubMed] [Google Scholar]
- Shiffman S. (2006) Reflections on smoking relapse research. Drug Alcohol Rev 25:15–20 [DOI] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. (2008) Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54:1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieyra-Reyes P, Picciotto MR, Mineur YS. (2008) Voluntary oral nicotine intake in mice down-regulates GluR2 but does not modulate depression-like behaviors. Neurosci Lett 434:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. (2006) Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460 [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol 73:1838–1843 [DOI] [PubMed] [Google Scholar]