Abstract
The phosphatases of regenerating liver (PRLs) are a unique family of plasma membrane-associated protein tyrosine phosphatases that have been hypothesized to be involved in metastatic cancer. How PRLs control cancer cell migration, invasion, and proliferation remains largely unknown. In the current study, we demonstrate a role for PRL-1 in the regulation of filamentous actin dynamics, which could promote cell metastatic processes. Human A549 non–small-cell lung cancer cells stably expressing wild-type PRL-1 exhibited a 60% increase in migration and a 3-fold increase in invasion. Cells expressing catalytic mutants of PRL-1 (C104S and D72A) lacked increased cell migration and invasion, indicating that these phenotypic changes required PRL-1 phosphatase activity. In contrast, PRL-1 small interfering RNA decreased in vitro lung cancer cell migration and invasion. The cadherin-catenin complex and dynamic filamentous actin are believed to control cellular invasiveness. Expression of wild-type PRL-1, but not phosphatase-inactive PRL-1 (C104S or D72A), decreased E-cadherin, vinculin, and paxillin expression. Ectopic expression of wild-type PRL-1 increased RhoA levels, which have an important role in actin filament assembly and stabilization of focal adhesion, and decreased activated Cdc42 and Rac. The Rho-associated protein kinase inhibitor, (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y-27632), decreased RhoA activity, actin filament levels, and cellular migration and invasion in PRL-1-expressing cells. These results suggest that PRL-1 could be a productive cancer therapeutic target and support further efforts to identify its substrates.
In the United States, lung cancer is the leading cause of cancer-related deaths in both men and women, accounting for >165,000 deaths per year, and it is predicted to become the leading cause of death worldwide (Jemal et al., 2008). The 5-year survival rate remains at <15%, even though there have been numerous attempts to control mortality from lung cancer (Jemal et al., 2008). The fundamental cause of patient death is the invasive and metastatic properties of tumors, processes about which we have incomplete understanding and for which we have no targeted drugs.
Tumor cell invasion and metastasis are dynamic cellular processes that extensively exploit phospho-relay signaling systems. The central role of abnormal protein tyrosine phosphorylation in human cancers is well accepted. The phosphatases of regenerating liver (PRLs) family represents a unique protein tyrosine phosphatase (PTP) subfamily with its three family members (PRL-1, PRL-2, and PRL-3) being the only PTPs that are subject to prenylation. Emerging evidence supports a role for PRLs in the basic biology of cancer cell development and metastasis (Stephens et al., 2005; Bessette et al., 2008).
PRL-1 (also known as PTP4A1 and PTPCAAX) was first identified as a low-molecular-weight immediate-early gene, the expression of which was induced in rat regenerating liver (Mohn et al., 1991). PRL-1 exploits a catalytic cysteine and an aspartic acid during protein dephosphorylation. Its catalytic pocket is unusually wide and shallow, suggesting the possibility of a broad range of phosphorylated substrates, all of which are currently unknown (Sun et al., 2005). PRLs are also unique because they can form trimers, at least in vitro (Jeong et al., 2005). Trimerization creates a large bipartite membrane-binding surface in which the exposed C-terminal basic residues could cooperate with the adjacent prenylated group to anchor the PRL on the acidic inner membrane and provide a docking site for other client proteins (Sun et al., 2007).
Involvement of PRL family members in cancer metastasis was first suggested with the observation that PRL-3 was overexpressed in metastatic colon cancer (Saha et al., 2001). PRLs appear to be elevated in a wide variety of human tumors (Stephens et al., 2005). In particular, PRL-1 is overexpressed in many histologically distinct, cultured, human tumor cell lines, including melanoma and pancreatic and lung cancer cells (Wang et al., 2002), compared with their normal counterpart such as normal lung bronchiolar epithelium (Diamond et al., 1996; Stephens et al., 2005). Cells expressing high levels of PRL-1 do exhibit an enhanced proliferation rate (Werner et al., 2003; Zeng et al., 2003). Chinese hamster ovary cells stably expressing PRL-1 have enhanced cell motility and invasiveness; cells with elevated PRL-1 have an increased capacity to produce metastatic tumors in mice (Zeng et al., 2003). Furthermore, pancreatic ductal epithelial cells stably overexpressing PRL-1 exhibit a transformed phenotype in culture and tumor growth in nude mice (Cates et al., 1996). Initially, the substrates for PRL-1 were thought to be nuclear because PRL-1 was originally described as a nuclear protein when ectopically expressed in transfected cells (Diamond et al., 1994). Subsequently, Zeng et al. (2000) reported that PRL phosphatases were localized to the plasma membrane and early endosomes and that the localization pattern was dependent on their post-translational farnesylation. Fiordalisi et al. (2006) reported that PRL-1 promoted motility and invasion in colon cancer cells by stimulating Rho signaling pathways using transfection approaches. Luo et al. (2009) observed that PRL-1 promotes cell migration and invasion by increasing matrix metalloproteinase expression, probably through activation of transcription factors AP1 and Sp1. Thus, additional studies into the molecular mechanism by which PRL-1 promotes cell proliferation, migration, and invasion are warranted.
Materials and Methods
Cells and Reagents.
A549 cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in basal medium Eagle (BME) supplemented with 10% fetal bovine serum and l-glutamine (Invitrogen, Carlsbad, CA). Cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C. We used specific antibodies for PRL-1 (Bethyl Laboratories, Montgomery, TX); Myc-Tag, Rac, and a Rho/Cdc42 activation assay kit (Millipore Bioscience Research Reagents, Temecula, CA); E-cadherin (BD Biosciences, San Jose, CA); vinculin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); RhoA, Cdc42, paxillin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Cell Signaling Technology (Danvers, MA); and (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y-26532) from Ascent Scientific LLC (Princeton, NJ).
Expression and Purification of Recombinant PRL-1.
Plasmid constructs for expressing recombinant wild-type (WT) PRL-1 and PRL-1 mutants with a C-terminal Myc-tag were generated. Full-length human PRL-1 cDNA was amplified by RT-PCR of human cDNA with PRL-1 primers based on GenBank accession number NM003463 (forward primer 5′-GCGAATTCACATGGCTCGAATGAACC-3′ and reverse primer 5′-GCGCTCGAGTTATTGAATGCAACAGTTG-3′) and cloned in-frame into the prokaryotic expression vector using pCMV-Myc-Tag vector (Clontech, Mountain View, CA). C104S and D72A mutants of PRL-1 were generated using a pCMV-Myc-PRL-1 construct as template DNA with a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Constructs were confirmed by DNA sequencing. To generate stable cell lines, we transfected A549 cells with pCMV-Myc-PRL-1 plasmids or empty vector or C104S and D72A mutant plasmids using Lipofectamine 2000, as recommended by the manufacturer. Twenty-four hours after transfection, cells were transferred into and maintained in a selection medium containing 1 mg/ml G418 for 2 weeks. Colonies were picked from each cell line and expanded. Protein expression was confirmed by Western blotting analysis for the Myc epitope tag.
Preparation of siRNA and Transfection.
PRL-1 siRNAs were prepared according to the Invitrogen Block-iT RNAi Designer. Two sets of oligonucleotides were chemically synthesized: for siRNA1, PRL-1 sense 5′-GGUAUCCAUGUUCUUGAUUGtt-3′ and PRL-1 antisense 5′-CAAUCAAGAACAUGGAUACCtt-3′; and for siRNA2, PRL-1 sense 5′-GAUUCCAACGGUCAUAGAAAtt-3′ and PRL-1 antisense 5′-UUUCUAUGACCGUUGGAAUCtt-3′. Stealth RNAi (negative control duplexes) from Invitrogen was used as scrambled control (SCR) siRNA. Cells in the exponential growth phase were plated in six-well plates containing serum-free medium and incubated overnight and then were transfected with siRNA using Lipofectamine 2000 and Opti-MEM I (Invitrogen), according to the manufacturer's protocol. The final concentration of siRNA was 50 nM. After 24 h, total proteins were extracted from cells for a Western blot.
mRNA Extraction and Reverse Transcription-PCR.
Total RNA was extracted with an RNeasy Mini Kit (QIAGEN, Valencia CA) according to the manufacturer's instructions. RT-PCR was conducted using a SuperScript III one-step RT-PCR System with Platinum Taq DNA Polymerase from Invitrogen. The sequences of the specific primers were as follows: PRL-1 forward 5′-GTGGAAGTCACATACAAGAAC-3′ and reverse 5′-GCAACAGTTGTTTCTATGACC-3′ and β-actin forward 5′-AAGAGAGGCATCCTCACCCT-3′ and reverse 5′-TACATGGCTGGGGTGTTGAA-3′.
Western Blot Analysis.
A549 cells were grown in BME for 24 h and then lysed in radioimmunoprecipitation assay buffer containing 200 mM sodium orthovanadate, 10 mM β-glycerol phosphate, and Roche complete EDTA-free protease inhibitor cocktail tablets. To analyze protein expression, we performed Western blotting by subjecting proteins (40 μg) to SDS-polyacrylamide gel electrophoresis and then transferring them onto a nitrocellulose membrane. Nonspecific binding to the membrane was blocked with Tris-buffered saline containing 5% (w/v) fetal bovine serum and 0.1% (v/v) Tween 20 (TBST) for 1 h. The membrane was incubated with an appropriate dilution of specific primary antibodies in TBST overnight at 4°C. Subsequently, the membrane was washed with TBST three times for 5 min and incubated with the appropriate horseradish peroxidase-conjugated IgG as secondary antibody for 1 h. After the membrane was washed three times for 5 min in TBST, the protein band was revealed by enhanced chemiluminescence using ECL Western blotting detection reagents. Protein expression was quantitatively determined by densitometry using Fujifilm Multi Gauge software (version 3.0; Fujifilm, Tokyo, Japan). In some studies, A549 cells were treated with 0.2% DMSO vehicle control or 5 or 15 μM Y-27632 for 12 h and harvested for Western blot analysis.
Morphological and Actin Immunofluorescence Staining.
Cells (1 × 105/ml) were plated and grown on 96-well plates overnight in a 37°C 5% CO2 incubator. In some studies, PRL-1-expressing cells were incubated with 5 μM Y-27632 for 30 min or 3 h in a 37°C 5% CO2 incubator. Cells were briefly rinsed twice with phosphate-buffered saline (PBS), fixed by incubation with 4% formalin in PBS for 10 min at room temperature, and rinsed twice with PBS. Samples were incubated for 20 min in 0.1% Triton X-100 in PBS and rinsed twice with PBS. Samples were stained by 10 μg/ml Hoechst 33342 for 10 min at dark room temperature and incubated with rhodamine phalloidin for 20 min at 4°C. Stained cells were analyzed by using a conventional fluorescence microscope or Arrayscan II.
Matrigel Invasion Assays.
The cell invasion assay was conducted using BD BioCoat Matrigel invasion chambers in 24-well plates with 8.0-μm pore size filters coated with extracellular matrix on the upper surface according to the protocol of the manufacturer (BD Biosciences). Control inserts were used for migration control. Cells (1 × 104/ml) in 0.5 ml of serum-free medium containing 0.2% DMSO alone or with 5 or 15 μM Y-27632 were placed in the upper chamber, and the lower chamber was loaded with 0.75 ml of medium containing 5% FBS. After incubation at 37°C for 24 h, cells were stained with 4 μg/ml calcein AM, and total cell invasion was determined. Three fields of each well were counted. Invasiveness was expressed as the percent invasion for each cell type through the Matrigel matrix and membrane relative to the migration through the control membrane.
Scratch Wound-Healing Motility Assays.
Cell migration was determined in a scratch wound-healing motility assay. A549 cells stably expressing PRL-1 (3 × 105/ml) were plated on 24-multiwell plates and allowed to grow to confluence. Growth medium was replaced with serum-free medium for an additional 18 h. Confluent monolayer was scratched with a sterile 200-μl pipette tip. Plates were washed once with sterile PBS, and fresh medium containing 0.5% FBS with 0.2% DMSO alone or 5 or 15 μM Y-27632 was added. Cells were photographed at low magnification (10× objective) for initial gap width measurements. Cells were additionally incubated for 0 to 72 h in fresh medium with 0.5% FBS-containing vehicle (0.2% DMSO). At the end of treatment cells were photographed again, and final wound size was determined. Gap width was defined as the cell-free area between wound edges and was measured in Adobe Photoshop at three chosen image sections along the wound edge as described previously (Vogt et al., 2002).
Rho Family GTPase Activation Assay.
Rho family activation assays were performed according to the manufacturer's instructions (Millipore, Billerica, MA). A549 cells stably expressing PRL-1 WT, C104S, D72A, or vector were plated on a 100-mm plate to approximately 85 to 90% confluence and rinsed twice with ice-cold PBS (pH 7.4), lysed for 10 min in ice-cold Mg2+ lysis/wash buffer containing 10% glycerol with phosphatase inhibitor and protein inhibitor, and scraped from plates into Microfuge tubes; cellular debris was removed by centrifugation at 14,000g for 5 min. Equal amounts of protein of each tube was added to 10 to 30 μg of the Rho family assay reagent, and the reaction mixture was incubated for 45 min at 4°C with gentle agitation. Beads were washed three times with Mg2+ lysis/wash buffer, and samples were prepared for Western blot analysis. GTP-bound RhoA was identified by blotting with anti-RhoA antibody. GTP-bound Cdc42 and GTP-bound Rac were identified by blotting with anti-Cdc42 and anti-Rac antibodies, respectively.
Statistical Analysis.
All data are presented as means ± S.D. of at least three independent experiments, each performed at least in triplicate and analyzed by Student's t test. Statistical differences are presented at probability levels of P < 0.05 (∗) or P < 0.01 (∗∗).
Results
PRL-1 Is Overexpressed in Human Lung Cancer Cells.
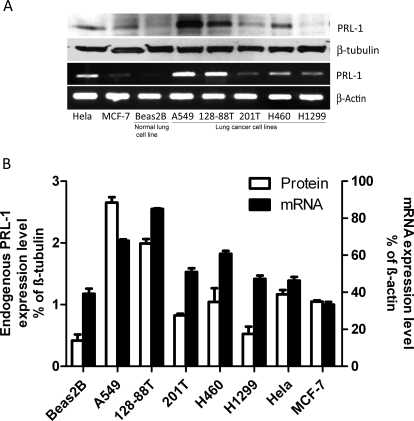
Because of the potential association of PRL-1 overexpression with the malignant phenotype (Wang et al., 2002; Achiwa and Lazo, 2007), we examined PRL-1 mRNA levels in several lung cancer cell lines and a normal lung cell line, Beas2B. As seen in Fig. 1, A and B, A549 and 128-88T cells exhibited the highest PRL-1 levels. Moreover, PRL-1 mRNA levels of the other three human lung cancer cell lines, 201T, H460, and H1299, were also higher than those found in Beas2B cells. In contrast, PRL-1 mRNA levels in MCF7 cells were lower than those in either Beas2B cells or the other lung cancer cell lines. Although there is no absolute concordance between PRL-1 mRNA and protein levels, PRL-1 protein levels were also elevated in most of the lung cancer cell lines. Therefore, these results support a further investigation into the role of PRL-1 in the malignant phenotype.
Fig. 1.
Expression of PRL-1 protein and mRNA in human cancer cell lines. A, endogenous PRL-1 protein expression was examined by Western blotting, and mRNA levels were determined by RT-PCR in seven cancer cell lines and one normal lung cell line, Beas2B. B, endogenous PRL-1 protein expression levels were normalized to β-tubulin protein levels that were determined by Western blotting. PRL-1 mRNA expression levels were normalized to β-actin mRNA levels that were determined with Fujifilm Multi Gauge software (version 3.0), as described under Materials and Methods. The quantitative assessments of protein and mRNA levels are expressed as mean ± S.D. of three independent experiments.
PRL-1 Promotes Cell Migration and Cell Invasion.
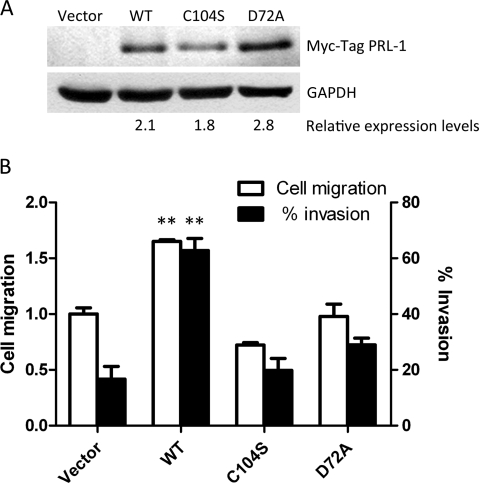
We selected A549 lung cancer cells for our subsequent cell migration and invasion studies, because A549 cells had among the highest PRL-1 expression in the lung tumor cells we examined (Fig. 1, A and B), they had low PRL-3 levels, they migrated well in culture, and they were studied previously by our group, providing a comparative foundation for any data analysis (Achiwa and Lazo, 2007). We established A549 lung cancer cell lines with stable ectopic expression of wild-type CMV-Myc-PRL-1 (WT), two catalytic mutant proteins [CMV-Myc-PRL-1 C104S (C104S) and CMV-Myc-PRL-1 D72A (D72A)], and empty vector. As illustrated in Fig. 2A, the stable transfectants expressed approximately similar levels of epitope-tagged WT and mutant proteins relative to a GAPDH control. To investigate the role of PRL-1 in tumor cell motility, we performed an in vitro scratch wound-healing assay with these cells. Expression of WT PRL-1 promoted a 60% increase in cell migration compared with the vector control (P < 0.01) (Fig. 2B). This migration enhancement required the catalytic Cys-104 and Asp-72. We next evaluated the role of phosphatase activity in cell invasion assays using Matrigel invasion chambers. Consistent with the scratch wound-healing assay, we found an increase in the migratory activity of cells expressing PRL-1 WT (Fig. 2B). In addition, PRL-1 WT enhanced cell invasion 3.8-fold compared with the control vector (P < 0.01) (Fig. 2B). Cells expressing PRL-1 with catalytic mutations (C104S and D72A) did not exhibit altered cell migration and invasion, indicating that the PRL-1 phosphatase activity was required.
Fig. 2.
PRL-1 promotes cell migration and invasion. A, based on Western blotting, the stable ectopic protein expression of Myc-tag PRL-1 WT and catalytic mutants in A549 cells was approximately the same as that for GAPDH. B, PRL-1 promotes cell motility in a scratch wound-healing assay. A monolayer of A549 cells stably expressing PRL-1 was scratched with a sterile micropipette tip. The number of cells in the denuded zone was determined after the indicated time (0 or 72 h) by inverted microscopy. Quantitative assessment of the mean number of cells in the denuded zone is expressed as the mean ± S.D. The experiments were repeated three times. ∗∗, P < 0.01 compared with vector control. B, PRL-1 promotes cell invasion and migration. Cell invasion of stably transfected A549 cells was assessed at 24 h using Matrigel invasion chambers, as described under Materials and Methods. Three fields in each well were counted and the mean percent invasion through the Matrigel matrix membrane was determined relative to the migration through the control membrane. The bar graph presents the mean relative values obtained from three independent determinations (±S.D.). ∗∗, P < 0.01 compared with the vector control.
Loss of PRL-1 by siRNA Inhibits Cell Migration and Invasion.
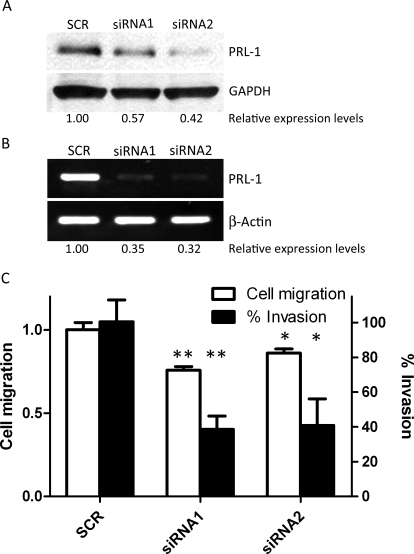
To examine further the role of PRL-1 in cell migration and invasion, we used two different 21-nucleotide siRNA sequences to reduce PRL-1 protein levels by 43 and 58%, respectively (Fig. 3A); siRNA1 and siRNA2 decreased PRL-1 mRNA by 65 and 68%, respectively (Fig. 3B). Twenty-four hours after transfection of PRL-1 siRNA or control siRNA (SCR), A549 cells were plated at high density in a 24-well plate, allowed to reach confluence overnight, and then serum-starved for additional 12 h. A 1-mm-wide wound was inflicted with a sterile pipette tip, and gap width was measured daily for 3 days. PRL-1 siRNA retarded cell migration compared with SCR siRNA-treated cells. Inhibition of PRL-1 expression by siRNA1 and siRNA2 prevented cell migration by 24 and 14%, respectively (siRNA1, P < 0.01; siRNA2, P < 0.05) (Fig. 3C). Furthermore, we evaluated cell invasion after PRL-1 siRNA using Matrigel invasion chambers. As shown in representative photographs, PRL-1 siRNA1 and siRNA2 significantly decreased cell invasion by 60% compared with SCR (siRNA1, P < 0.01; siRNA2, P < 0.05) (Fig. 3C). Thus, inhibition of PRL-1 expression by siRNA prevented cell migration and invasion.
Fig. 3.
Depletion of PRL-1 inhibits cell migration and invasion. A, Western blotting detects PRL-1 protein levels at 24 h after siRNA transfection. Protein expression levels are relative to GAPDH. B, reverse transcription-PCR detection of PRL-1 mRNA levels 24 h after siRNA transfection in A549 cells. mRNA levels are relative to β-actin. C, PRL-1 siRNA inhibits cell migration in the scratch wound-healing assay. Transiently siRNA transfected A549 cell monolayers were disrupted with a sterile micropipette tip. The number of cells in the denuded zone was determined at the indicated times (0 or 72 h) by inverted microscopy. Quantitative assessment of the mean number of cells in the denuded zone is expressed as mean ± S.D. The experiment was repeated three times. ∗, P < 0.05; ∗∗, P < 0.01, compared with scrambled control. B, PRL-1 siRNA inhibits cell invasion and migration. Cell invasion of transiently siRNA transfected A549 cells was assessed at 24 h using Matrigel invasion chambers, as described under Materials and Methods. Three fields in each well were counted, and the mean percent invasion through the Matrigel matrix membrane was determined relative to the migration through the control membrane. The bar graph presents the mean relative values obtained from three independent determinations (±S.D.). ∗, P < 0.05; ∗∗, P < 0.01, compared with the scrambled control.
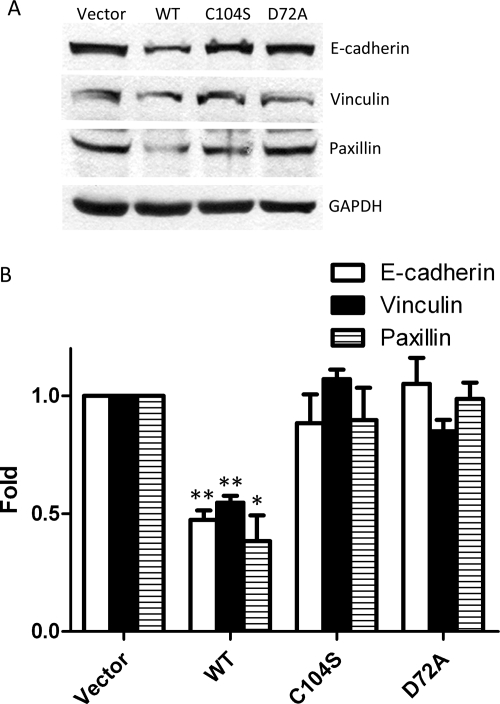
E-cadherin has been described as a tumor suppressor that prevents cell invasion through vinculin or a number of other molecules associated with the cadherin complex (Avizienyte and Frame, 2005). During tumor development, loss of E-cadherin function in epithelial cells results in cells with enhanced invasive and metastatic ability. We found that E-cadherin protein expression was decreased in cells expressing WT PRL-1 compared with vector and the C104S and D72A catalytic mutants. Furthermore, the loss of E-cadherin is accompanied by the loss of other intracellular adhesion molecules, vinculin and paxillin (Fig. 4, A and B).
Fig. 4.
PRL-1 regulates cell adhesion. A, cell lysates (40 μg) were analyzed by Western blotting in epithelial markers, E-cadherin, vinculin, and paxillin. GAPDH was used as a loading control. B, relative expression is average fold mean ± S.D. The protein level changes from three independent experiments normalized to vector. ∗, P < 0.05; ∗∗, P < 0.01, compared with vector control.
PRL-1 Regulates Cell Migration and Invasion through the Rho Family.
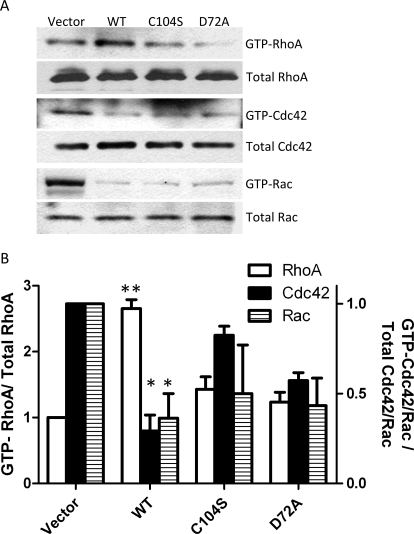
PRLs are thought to have important roles in regulating Rho family GTPases, including Rho, Cdc42, and Rac, which regulate cell motility (Fiordalisi et al., 2006). We hypothesized that PRL-1 activity may be associated with Rho family GTPases and regulate cell motility through disassembly and assembly of actins. Figure 5, A and B, shows that WT PRL-1 activated GTP-RhoA levels and inhibited GTP-Cdc42 and GTP-Rac levels. PRL-1 activation of GTP-RhoA required phosphatase-competent PRL-1 because the catalytically inactive C104S and D72A mutants did not increase GTP-RhoA activity. The catalytic inactive D72A mutant suppressed GTP-Cdc42 and GTP-Rac activity. This may be the result of substrate trapping or other, not yet known, nonphosphatase functions of PRL-1. However, our results demonstrate that PRL-1 promoted Rho family function, which has an important role in actin filament assembly and stabilization of focal adhesions.
Fig. 5.
PRL-1 regulates the GTP-Rho family. A, PRL-1-activated RhoA. A549 cells stably expressing PRL-1 (WT), catalytic mutant (C104S and D72A), and empty vector were assessed for the level of active RhoA using a Rho activation assay kit. PRL-1 inactivates GTP-Cdc42 and GTP-Rac. A549 cells stably expressing PRL-1 (WT), catalytic mutant (C104S and D72A), and empty vector were assessed for the level of GTP-Cdc42 or GTP-Rac using a Cdc42/Rac activation assay kit. B, PRL-1-expressing WT and catalytic mutants were compared with empty vector. The data are expressed as means ± S.D. of Rho and Cdc42/Rac activation assays of three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01, compared with the vector control.
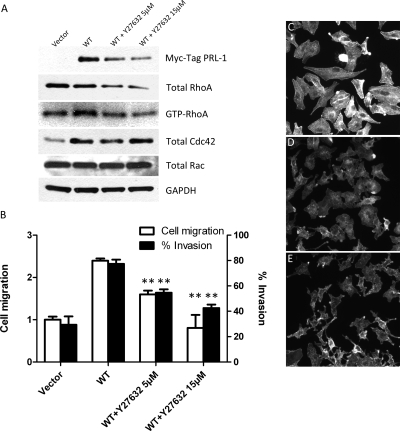
When cells migrate, dynamic reorganization of actin cytoskeleton occurs. Actin polymerizes at the leading edge of moving cells (Nomura et al., 2007). Therefore, we next tested whether the Rho-associated protein kinase (Rock) inhibitor, Y-27632, could block the PRL-1-mediated increase in RhoA activity. Incubation of A549 cells stably expressing Myc-tagged PRL-1 for 12 h with 5 or 15 μM Y-27632 decreased total and activated RhoA levels, although it did not markedly alter total Cdc42 and Rac levels (Fig. 6A). Y-27632 decreased Myc-tagged PRL-1 levels. Y-27632 is known to disrupt actin organization (Ishizaki et al., 2000). Therefore, we examined whether Y-27632 could block PRL-1-mediated alterations in cell migration and cell invasion. Indeed, both 5 and 15 μM Y-27632 inhibited PRL-1-induced cell migration by 30 and 60%, respectively (WT PRL-1 and 5 μM Y-27632, WT PRL-1 and 15 μM Y-27632; P < 0.01) (Fig. 6B ). Furthermore, 5 or 15 μM Y-27632 significantly decreased PRL-1-induced cell invasion in the Matrigel invasion chambers by 30 and 46% (WT PRL-1 and 5 μM Y-27632, WT PRL-1 and 15 μM Y-27632; P < 0.01) (Fig. 6B). Treatment of cells ectopically expressing WT PRL-1 with a 5 μM concentration of the Rock inhibitor Y-27632 for 0.5 or 3 h also decreased total actin stress fiber staining, resulting in cells with altered shape and size compared with similar cells treated with vehicle alone (Fig. 6, C–E). Taken together, these results support a role for PRL-1 in the regulation of cell motility, filamentous actin dynamics, and the Rho-Rock kinase pathway.
Fig. 6.
PRL-1 activity is interrupted by Y-27632. A, A549 cells stably expressing PRL-1 (WT) were cultured in BME with 10% FBS with either 0.2% DMSO alone or 5 or 15 μM Y-27632 for 12 h. The cellular extracts were analyzed by Western blotting using Myc-Tag PRL-1, RhoA, Cdc42, Rac, and GAPDH as indicated. The expression levels of GTP-RhoA were assessed using a Rho activation assay kit. GAPDH was used as a loading control. B, PRL-1 with Y-27632 inhibits cell migration in the scratch wound-healing assay and cell invasion using Matrigel invasion chambers. ∗∗, P < 0.01 compared with WT. C–E, PRL-1 effects on cell shape and actin stress fiber after treatment with Y-27632. A549 cells stably expressing WT PRL-1 were cultured in BME with 10% FBS for 24 h. The cells were incubated in a 5% CO2 incubator with either vehicle alone (C), 5 μM Y-27632 for 30 min (D), or 5 μM Y-27632 for 3 h at 37°C (E). The cells were then stained for actin stress fibers.
Discussion
Previous laboratory and clinical studies from our group (Wang et al., 2002; Achiwa and Lazo, 2007) and others (Diamond et al., 1996; Wang et al., 2002; Stephens et al., 2005) have implicated PRL-1 in cancer cell invasion and migration. We selected A549 cells for our current studies because they had the highest PRL-1 expression of any of the lung cancer cell lines we examined, because they migrate and invade well in culture, and because of our previous results with this cell line (Wang et al., 2002; Achiwa and Lazo, 2007). A549 cells do not, however, have the highest reported cellular expression levels (Wang et al., 2002), suggesting that it would be possible to express additional amounts in A549 cells. Using short hairpin RNA and A549 cells, we previously documented that loss of PRL-1 decreases invasiveness, Cdc42 activation, Rac activation, cell spreading, and cell membrane protrusions (Achiwa and Lazo, 2007). In this current extension of our investigations into the role of PRL-1 in lung cancer, we found that almost all of the human lung cancer cells we examined expressed higher PRL-1 mRNA and protein levels compared with those seen in nonmalignant immortalized human bronchial epithelial cells (Fig. 1, A and B). Reduction of PRL-1 mRNA after siRNA treatment clearly inhibited A549 cell invasion. Cell migration was less dramatically affected by siRNA (Fig. 3C), but the magnitude of the decrease in A549 cell migration was comparable to that reported previously with glutamate antagonists in these cells (Rzeski et al., 2001).
PRL-1 has a C-terminal polybasic region that binds to phosphoinositides and a C-terminal prenylation motif, which are required for plasma membrane localization (Sun et al., 2007). These two features position PRL-1 to control cell motility and the actin cytoskeleton. Consistent with this expectation, we found, using both depletion and repletion methods, that PRL-1 altered the actin stress fiber architecture. Among protein phosphatases, PRL-1 also has remarkably low intrinsic phosphatase activity (Sun et al., 2007). Even though PRL-1 does not have any obvious cognate docking sites or regulatory domains, it remains formally possible that PRL-1 participates in altering actin stress fiber architecture, not by its intrinsic phosphatase activity but by interacting with other intracellular macromolecules. However, our results with the catalytically inactive PRL-1 mutants suggested that alterations in cellular migration, invasion, E-cadherin, vinculin, and paxillin levels all required phosphatase activity.
Rho family members are critical regulators of actin organization associated with cell motility (Wennerber and Der, 2004). Growth factor receptors and integrins cooperate to control the activity of Rho GTPases, which results in the formation of distinct actin structures (Burridge and Wennerber, 2004). The subcellular localization of PRLs could enable these phosphatases to influence the Rho signaling pathway. Indeed, previous studies by Fiordalisi et al. (2006) with SW480 colon cancer cells suggested that elevated PRL-1 and PRL-3 expression can activate RhoA and RhoC. These authors also demonstrated with a pharmacological inhibitor that Rock was necessary for PRL-3-mediated invasion and motility. In the current study, we document the need for Rock in PRL-1-mediated invasion and motility using the same pharmacological inhibitor. We also found an increase in RhoA activity with ectopic expression of PRL-1 but not with the catalytic inactive protein, indicating that the phosphatase activity was essential for enhanced RhoA activity. We detected a decrease in Cdc42 and Rac activation with wild-type PRL-1 but, interestingly, also with the catalytic mutant forms of PRL-1. These observations seem to dissociate the changes in Cdc42 and Rac (Fig. 5, A and B) from a PRL-1-mediated increase in RhoA or loss of E-cadherin, vinculin, and paxillin (Fig. 4, A and B). It also seems to dissociate Cdc42 and Rac changes from the enhanced cell migration and invasion seen with PRL-1 (Fig. 2B).
PRL-1 clearly participates in the Rac/Cdc42/RhoA axis and in regulating actin stress fiber functionality. Nonetheless, there are many unresolved questions about the mechanism by which PRL-1 functions and its intracellular substrate or substrates. Our studies further validate PRL-1 as a participant in the lung cancer migration and invasion process and indicate that additional studies on this phosphatase are warranted.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA078039]; and the Fiske Drug Discovery Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167809.
- PRL
- phosphatase of regenerating liver
- PTP
- protein tyrosine phosphatase
- BME
- basal medium Eagle
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- WT
- wild type
- RT
- reverse transcription
- siRNA
- small interfering RNA
- SCR
- scrambled control
- TBST
- Tris-buffered saline with 5% fetal bovine serum and 0.1% Tween 20
- DMSO
- dimethyl sulfoxide
- Y-27632
- (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride
- PBS
- phosphate-buffered saline
- FBS
- fetal bovine serum
- Rock
- Rho-associated protein kinase
References
- Achiwa H, Lazo JS. (2007) PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res 67:643–650 [DOI] [PubMed] [Google Scholar]
- Avizienyte E, Frame MC. (2005) Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol 17:542–547 [DOI] [PubMed] [Google Scholar]
- Bessette DC, Qiu D, Pallen CJ. (2008) PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev 27:231–252 [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. (2004) Rho and Rac take center stage. Cell 116:167–179 [DOI] [PubMed] [Google Scholar]
- Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, Crowell PL, Crowell DN. (1996) Prenylation of oncogenic human PTPCAAX protein tyrosine phosphatases. Cancer Lett 110:49–55 [DOI] [PubMed] [Google Scholar]
- Diamond RH, Cressman DE, Laz TM, Abrams CS, Taub R. (1994) PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol 14:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond RH, Peters C, Jung SP, Greenbaum LE, Haber BA, Silberg DG, Traber PG, Taub R. (1996) Expression of PRL-1 nuclear PTPase is associated with proliferation in liver but with differentiation in intestine. Am J Physiol 271:G121–G129 [DOI] [PubMed] [Google Scholar]
- Fiordalisi JJ, Keller PJ, Cox AD. (2006) PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res 66:3153–3161 [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. (2000) Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57:976–983 [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Jeong DG, Kim SJ, Kim JH, Son JH, Park MR, Lim SM, Yoon TS, Ryu SE. (2005) Trimeric structure of PRL-1 phosphatase reveals an active enzyme conformation and regulation mechanisms. J Mol Biol 345:401–413 [DOI] [PubMed] [Google Scholar]
- Luo Y, Liang F, Zhang ZY. (2009) PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry 48:1838–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn KL, Melby AE, Tewari DS, Laz TM, Taub R. (1991) The gene encoding rat insulinlike growth factor-binding protein 1 is rapidly and highly induced in regenerating liver. Mol Cell Biol 11:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N, Nomura M, Sugiyama K, Hamada J. (2007) Phorbol 12-myristate 13-acetate (PMA)-induced migration of glioblastoma cells is mediated via p38MAPK/Hsp27 pathway. Biochem Pharmacol 74:690–6701 [DOI] [PubMed] [Google Scholar]
- Rzeski W, Turski L, Ikonomidou C. (2001) Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA 98:6372–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St Croix B, Romans KE, Choti MA, Lengauer C, Kinzler KW, et al. (2001) A phosphatase associated with metastasis of colorectal cancer. Science 294:1343–1346 [DOI] [PubMed] [Google Scholar]
- Stephens BJ, Han H, Gokhale V, Von Hoff DD. (2005) PRL phosphatases as potential molecular targets in cancer. Mol Cancer Ther 4:1653–1661 [DOI] [PubMed] [Google Scholar]
- Sun JP, Luo Y, Yu X, Wang WQ, Zhou B, Liang F, Zhang ZY. (2007) Phosphatase activity, trimerization, and the C-terminal polybasic region are all required for PRL1-mediated cell growth and migration. J Biol Chem 282:29043–29051 [DOI] [PubMed] [Google Scholar]
- Sun JP, Wang WQ, Yang H, Liu S, Liang F, Fedorov AA, Almo SC, Zhang ZY. (2005) Structure and biochemical properties of PRL-1, a phosphatase implicated in cell growth, differentiation, and tumor invasion. Biochemistry 44:12009–12021 [DOI] [PubMed] [Google Scholar]
- Vogt A, Pestell KE, Day BW, Lazo JS, Wipf P. (2002) The antisignaling agent SC-ααδ9,4-(benzyl-(2-[(2,5-diphenyloxazole-4-carbonyl)amino]ethyl)carbamoyl)-2-decanoylaminobutyric acid, is a structurally unique phospholipid analogue with phospholipase C inhibitory activity. Mol Cancer Ther 1:885–892 [PubMed] [Google Scholar]
- Wang J, Kirby CE, Herbst R. (2002) The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J Biol Chem 277:46659–46668 [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. (2004) Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell Sci 117:1301–1312 [DOI] [PubMed] [Google Scholar]
- Werner SR, Lee PA, DeCamp MW, Crowell DN, Randall SK, Crowell PL. (2003) Enhanced cell cycle progression and down regulation of p21(Cip1/Waf1) by PRL tyrosine phosphatases. Cancer Lett 202:201–211 [DOI] [PubMed] [Google Scholar]
- Zeng Q, Dong JM, Guo K, Li J, Tan HX, Koh V, Pallen CJ, Manser E, Hong W. (2003) PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res 63:2716–2722 [PubMed] [Google Scholar]
- Zeng Q, Si X, Horstmann H, Xu Y, Hong W, Pallen CJ. (2000) Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma membrane and the early endosome. J Biol Chem 275:21444–21452 [DOI] [PubMed] [Google Scholar]