Abstract
Human populations exhibit genetic polymorphism in N-acetylation capacity, catalyzed by N-acetyltransferase 2 (NAT2). We investigated the relationship between NAT2 acetylator genotype and phenotype in cryopreserved human hepatocytes. NAT2 genotypes determined in 256 human samples were assigned as rapid (two rapid alleles), intermediate (one rapid and one slow allele), or slow (two slow alleles) acetylator phenotypes based on functional characterization of the NAT2 alleles reported previously in recombinant expression systems. A robust and significant relationship was observed between deduced NAT2 phenotype (rapid, intermediate, or slow) and N-acetyltransferase activity toward sulfamethazine (p < 0.0001) and 4-aminobiphenyl (p < 0.0001) and for O-acetyltransferase-catalyzed metabolic activation of N-hydroxy-4-aminobiphenyl (p < 0.0001), N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline (p < 0.01), and N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (p < 0.0001). NAT2-specific protein levels also significantly associated with the rapid, intermediate, and slow NAT2 acetylator phenotypes (p < 0.0001). As a negative control, p-aminobenzoic acid (an N-acetyltransferase 1-selective substrate) N-acetyltransferase activities from the same samples did not correlate with the three NAT2 acetylator phenotypes (p > 0.05). These results clearly document codominant expression of human NAT2 alleles resulting in rapid, intermediate, and slow acetylator phenotypes. The three phenotypes reflect levels of NAT2 protein catalyzing both N- and O-acetylation. Our results suggest a significant role of NAT2 acetylation polymorphism in arylamine-induced cancers and are consistent with differential cancer risk and/or drug efficacy/toxicity in intermediate compared with rapid or slow NAT2 acetylator phenotypes.
The N-acetylation polymorphism was discovered more than 50 years ago when individual variability in isoniazid neurotoxicity was attributed to genetic variability in N-acetylation capacity identified as rapid and slow acetylators (Evans et al., 1960). In addition to isoniazid, many aromatic amine drugs such as sulfamethazine (SMZ) are subject to the acetylation polymorphism thus affecting therapeutic efficacy and toxicity for many therapeutic drugs (reviewed by Weber and Hein, 1985). Whereas the N-acetylation of isoniazid and SMZ divided human populations into rapid and slow acetylator phenotypes, the N-acetylation of drugs such as p-aminosalicylic acid yielded apparently unimodal distributions of individuals (reviewed in Weber and Hein, 1985). The biochemical basis relates to substrate specificity and molecular genetics of two distinct N-acetyltransferase isozymes, subsequently identified as N-acetyltransferase 1 (NAT1) and N-acetyltransferase 2 (NAT2) (reviewed by Grant, 2008).
Arylamine carcinogens undergo N-acetylation, and N-hydroxy-arylamine carcinogens undergo O-acetylation in human liver cytosol (reviewed by Hein, 1988). The N-acetylation of arylamines and the O-acetylation of N-hydroxy-arylamine amines are catalyzed by both human NAT1 and NAT2 (Minchin et al., 1992; Hein et al., 1993). Thus, a role for NAT2 acetylation polymorphism in individual risk to various cancers in which arylamines play an etiologic role is biologically plausible and has been the subject of numerous studies (reviewed by Agúndez, 2008).
An early review (Hein, 1988) describing the role of the acetylator genotype in arylamine-induced cancers proposed codominant expression of human N-acetyltransferase genotypes resulting in rapid, intermediate, and slow acetylator phenotypes. This study was designed to test this hypothesis for both arylamine N-acetylation and N-hydroxy-arylamine O-acetylation in cryopreserved human hepatocytes.
Materials and Methods
Cryopreserved Human Hepatocytes.
Cryopreserved human hepatocyte samples (256) were received from Celsis In Vitro Technologies (Baltimore, MD) and stored in liquid nitrogen until use. Upon removal from the liquid nitrogen, the hepatocytes were thawed and centrifuged as described previously (Martin et al., 2009). Information on gender, age, race, cause of death, substance abuse, serology, and other enzyme activities for the hepatocytes was accessed at http://www.celsis.com/ivt/characterization-tables. Ethnicity was available for more than 99% of the samples. The ethnic frequencies in these samples were 79.3% white, 10.55% African-American, 7.03% Hispanic, 1.17% Asian, 1.17% Polynesian, and 0.78% unknown.
NAT2 Acetylator Genotyping Assay.
Genomic DNA was isolated from pelleted cells prepared as described above by using the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. NAT2 haplotypes, genotypes, and deduced phenotypes were determined as described previously (Doll and Hein, 2001; Hein, 2006). In brief, single-nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes were designed by using Primer Express (Applied Biosystems, Foster City, CA). The fluorogenic probes were labeled with a reporter dye (either FAM or VIC) and were specific for one of the two possible bases identified at seven single-nucleotide polymorphisms in the NAT2 coding region. Controls (no DNA template) were run to ensure that there was no amplification of contaminating DNA. Subjects were classified as rapid, intermediate, and slow acetylator phenotypes. Individuals possessing two of the NAT2 alleles associated with rapid acetylation activity (NAT2*4, NAT2*12, and NAT2*13) were classified as rapid acetylators; individuals possessing one of these alleles and one allele associated with slow acetylation (NAT2*5, NAT2*6, NAT2*7, and NAT2*14) were classified as intermediate acetylators, and those individuals that possessed two slow acetylation alleles were classified as slow acetylators.
Lysate Preparation.
Hepatocytes were lysed in 20 mM sodium phosphate (pH 7.4), 1 mM EDTA, 1 mM dithiothreitol, 100 μM phenylmethanesulfonyl fluoride, 1 μg/ml aprotinin, and 1 μM pepstatin A by three rounds of freeze (−70°C)/thawing (37°C). Lysates were centrifuged at 15,000g for 20 min at 4°C, and the resulting supernatant was assayed for protein and enzymatic activity as described below.
N-Acetyltransferase Assays.
N-acetyltransferase assays with SMZ, 4-aminobiphenyl (ABP), or p-aminobenzoic acid (PABA) as arylamine substrate were conducted as described previously (Hein et al., 2006). In brief, reactions containing supernatant of hepatocyte lysate (<2 mg of protein/ml), arylamine substrate (300 μM PABA or SMZ; 1 mM ABP), and 1 mM acetyl coenzyme A were incubated at 37°C. Reactions were terminated by the addition of 1/10 volume of 1 M acetic acid. The reaction tubes were centrifuged to precipitate protein. Reactants and products were separated and quantified by reverse-phase high-performance liquid chromatography (HPLC).
O-Acetyltransferase Assays.
Assays to measure the metabolic activation (via O-acetylation) of N-hydroxy-ABP (N-OH-ABP), N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline (N-OH-MeIQx), and N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (N-OH-PhIP) to DNA adducts were performed by HPLC separation and measurement of the respective arylamine-C8-deoxyguanosine (dG) adduct as described previously (Hein et al., 2006; Bendaly et al., 2007; Metry et al., 2007). In brief, supernatants of hepatocyte lysate were incubated with 1 mM acetyl coenzyme A, 1 mg/ml dG, and 0.1 mM N-OH-ABP, N-OH-MeIQx, or N-OH-PhIP at 37°C. Reactions were stopped by the addition of equal volume of water-saturated ethyl acetate and centrifuged for 10 min, and the organic phase was transferred, evaporated to dryness, and resuspended in 100 μl of 10% acetonitrile. dG-C8-ABP, dG-C8-MeIQx, and dG-C8-PhIP adducts were isolated and quantified against authentic standards by HPLC as described previously.
Quantitation of Protein.
Total protein concentrations were determined by using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). NAT2-specific protein was measured by Western blotting with primary antibody that specifically binds to a 13-amino acid region (FLNSHLLPKKKHQ) of human NAT2 as described previously (Zang et al., 2004). The NAT2-specific bands were quantified by densitometry and expressed as density units.
Data Analysis.
Statistical analyses were performed by using Prism 5.03 (GraphPad Software Inc., San Diego, CA). Differences of catalytic activities and NAT2 protein levels among the three NAT2 genotype groups were analyzed by one-way analysis of variance. Correlations between N-acetylation and O-acetylation catalytic activities and between NAT2 protein and catalytic activity were analyzed by the Pearson correlation coefficient. Differences or correlations were considered significant when p < 0.05.
Results
NAT2 Genotypes and Deduced Phenotypes.
Seventeen NAT2 genotypes were identified among the 256 individual cryopreserved human hepatocyte samples. Eighteen (7%) of the samples had rapid acetylator genotypes (NAT2*4/*4, NAT2*4/*12, NAT2*4/*13), 114 (45%) of the samples had intermediate acetylator genotypes (NAT2*4/*5, NAT2*4/*6, NAT2*4/*7, NAT2*4/*14, NAT2*5/*12, NAT2*5/*13, NAT2*6/NAT2*12, NAT2*6/*13), and 124 (48%) had slow acetylator genotypes (NAT2*5/*5, NAT2*5/*6, NAT2*5/*7, NAT2*6/*6, NAT2*6/*7, NAT2*6/*14, NAT2*7/*14).
N-Acetyltransferase Catalytic Activities.
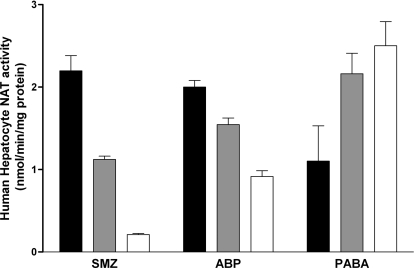
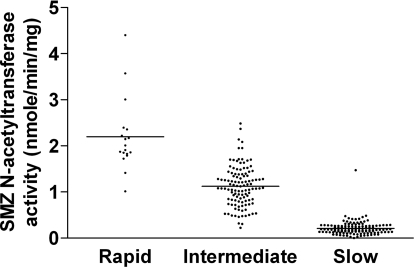
As illustrated in Fig. 1, SMZ N-acetyltransferase catalytic activities differed significantly with respect to NAT2 acetylator genotype (p < 0.0001), whereas PABA N-acetyltransferase catalytic activities did not (p > 0.05). It is noteworthy that PABA N-acetyltransferase activities were actually highest in the slow acetylators and lowest in the rapid acetylators, although this trend was not significant (p > 0.05). SMZ N-acetyltransferase activity in the intermediate acetylator genotype (1.12 nmol/min/mg) matched precisely with the arithmetic mean of the N-acetyltransferase activities in the rapid (2.20 nmol/min/mg) and slow (0.21 min/min/mg) acetylator genotypes. Despite the codominant expression of N-acetyltransferase activity, scatter plots showed some limited overlap between the N-acetyltransferase activities in the three acetylator genotypes (Fig. 2). A similar finding was observed in a subset of 8 rapid, 65 intermediate, and 77 slow acetylator genotypes for ABP N-acetyltransferase activities (p < 0.0001), except that the magnitude of the acetylator genotype-dependent differences was smaller than observed for SMZ (Fig. 1). No significant differences (p > 0.05) were observed in NAT2 catalytic activities among individual genotypes within the rapid (NAT2*4/*4, NAT2*4/*12 or *13), intermediate (NAT2*4/*5, NAT2*4/*6, NAT2*4/*7, NAT2*4/*14, NAT2*5/*12 or *13, NAT2*6/NAT2*12 or *13), or slow (NAT2*5/*5, NAT2*5/*6, NAT2*5/*7, NAT2*6/*6, NAT2*6/*7, NAT2*6/*14, NAT2*7/*14) acetylator genotype groups.
Fig. 1.
N-acetyltransferase catalytic activities toward SMZ (NAT2-specific), PABA (NAT1-specific), and ABP in cryopreserved human hepatocyte samples. Black bars illustrate mean ± S.E.M. activities in rapid acetylator genotypes (n = 18 for SMZ and PABA, 8 for ABP), gray bars illustrate mean ± S.E.M. activities in intermediate acetylator genotypes (n = 114 for SMZ and PABA, 65 for ABP), and white bars illustrate mean ± S.E.M. activities in slow acetylator genotypes (n = 124 for SMZ and PABA, 77 for ABP). SMZ and ABP N-acetyltransferase activities differed significantly (p < 0.0001) with respect to NAT2 acetylator genotype, whereas PABA NAT activities did not (p > 0.05). After post hoc Tukey-Kramer multiple comparison tests, the SMZ NAT2 activities in the intermediate acetylator phenotype differed significantly (p < 0.001) from both the rapid and the slow acetylator phenotypes.
Fig. 2.
Scatter plot of SMZ N-acetyltransferase activities in cryopreserved human hepatocytes. Each dot represents SMZ N-acetyltransferase activity in each of the 256 human hepatocyte samples stratified by rapid, intermediate, or slow acetylator NAT2 genotype. Lines illustrate mean activity in each genotype.
O-Acetyltransferase Catalytic Activities.
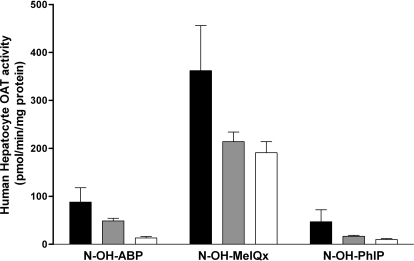
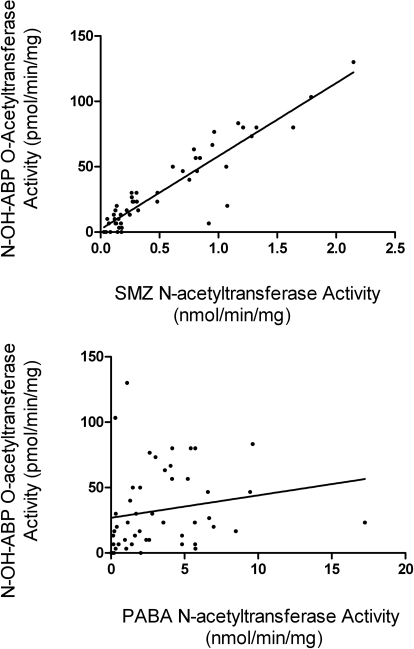
As illustrated in Fig. 3, metabolic activation by O-acetylation differed significantly with respect to NAT2 acetylator genotype toward N-OH-ABP (p < 0.0001), N-OH-MeIQx (p < 0.01), and N-OH-PhIP (p < 0.0001) in a subset of 3 rapid, 20 intermediate, and 26 slow acetylator samples. As shown in Fig. 4, N-OH-ABP O-acetyltransferase activities correlated very highly with SMZ (NAT2-specific) N-acetyltransferase activities (p < 0.0001) but not with PABA (NAT1-specific) N-acetyltransferase activities (p > 0.05).
Fig. 3.
O-acetyltransferase catalytic activities in cryopreserved human hepatocyte samples. Black bars illustrate mean ± S.E.M. activities in rapid acetylator genotypes (n = 3), gray bars illustrate mean ± S.E.M. activities in intermediate acetylator genotypes (n = 20), and white bars illustrate mean ± S.E.M. activities in slow acetylator genotypes (n = 26). O-acetyltransferase activities differed significantly with respect to NAT2 acetylator genotype for N-OH-ABP (p < 0.0001), N-OH-MeIQx (p < 0.01), and N-OH-PhIP (p < 0.0001). After post hoc Tukey-Kramer multiple comparison tests, the N-OH-ABP O-acetyltransferase activities in the intermediate acetylator phenotype differed significantly (p < 0.001) from both the rapid and the slow acetylator phenotypes.
Fig. 4.
Correlation of N-OH-ABP O-acetyltransferase activities with SMZ (NAT2-specific) N-acetyltransferase activity (r2 = 0.8457; p < 0.0001) and PABA (NAT1-specific) N-acetyltransferase activities (r2 = 0.00321; p > 0.05). Each dot represents a single human hepatocyte sample.
NAT2-Specific Protein.
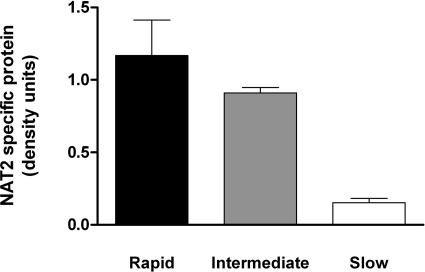
As illustrated in Fig. 5, NAT2-specific protein differed significantly (p < 0.0001) with respect to rapid, intermediate, and slow acetylator genotypes in a small subset of four rapid (three NAT2*4/*4; one NAT2*4/*12A), seven intermediate (two each NAT2*4/*6A and NAT2*4/*7B, one NAT2*4/*5B, one NAT2*5B/*12A, and one NAT2*6A/*13), and seven slow (two each NAT2*5B/*6A and NAT2*6A/*14B, one each NAT2*5A/*5B, NAT2*5B/*5B, and NAT2*6A/*6A) acetylator samples. NAT2-specific protein levels correlated highly (p = 0.0016) with SMZ N-acetyltransferase activities across the three acetylator genotypes.
Fig. 5.
NAT2-specific protein levels in cryopreserved human hepatocyte samples. Black bar illustrates mean ± S.E.M. density units in rapid acetylator genotypes (n = 4), gray bar illustrates mean ± S.E.M. density units in intermediate acetylator genotypes (n = 7), and white bar illustrates mean ± S.E.M. density units in slow acetylator genotypes (n = 7). NAT2-specific protein levels differed significantly (p < 0.0001) with respect to NAT2 acetylator genotype.
Discussion
SMZ N-acetyltransferase catalytic activities differed significantly with respect to NAT2 acetylator genotype (p < 0.0001), whereas PABA N-acetyltransferase catalytic activities did not (p > 0.05). This clearly reflects the fact that at the substrate concentrations used in our assays SMZ is N-acetylated specifically by NAT2, whereas PABA is N-acetylated specifically by NAT1 (Hein et al., 1993).
ABP is a human carcinogen present in cigarette smoke (Stabbert et al., 2003) and cooking oil fumes (Chiang et al., 1999). MeIQx and PhIP are potent and abundant mutagens in the human diet, formed during high-temperature cooking of meats (Keating and Bogen, 2004). They also have been detected in processed food flavorings, beer, wine, cigarette smoke, smoke condensate formed during frying of beef patties and bacon, and in aerosol from cooking of stir-fried fish. Both are designated as “reasonably anticipated to be a human carcinogen” (National Toxicology Program, 2005).
Arylamine DNA adduct formation follows N-hydroxylation, which occurs at relatively high rates in humans (Turesky, 2002). The N-hydroxy-arylamine metabolites are proximate carcinogens that can undergo O-acetylation by NAT2 to acetoxy-derivatives that are highly unstable, leading to electrophilic intermediates that form DNA adducts primarily at dG (Turesky, 2002). dG-C8-ABP, dG-C8-PhIP, and dG-C8-MeIQx adducts have been identified in human tissues and cells (Talaska et al., 1991; Totsuka et al., 1996; Gorlewska-Roberts et al., 2002). Recent results in genetically engineered Chinese hamster ovary cells documented a much greater role for human NAT2 acetylation polymorphism in DNA adduct formation and mutagenesis after exposure to ABP (Bendaly et al., 2009a) and MeIQx (Bendaly et al., 2007; Metry et al., 2010) than PhIP (Metry et al., 2007; Bendaly et al., 2009b).
Although ABP is N-acetylated by both NAT1 and NAT2 (Hein et al., 1993), our results show that the N-acetylation of ABP in human hepatocytes is NAT2 acetylator genotype-dependent regardless of NAT1 phenotype. Nevertheless, the magnitude of differences in ABP N-acetylation activities between the three NAT2 acetylator genotypes was less than that observed for SMZ N-acetylation, most likely reflecting the contribution of NAT1 to ABP N-acetylation. Similar results were observed for the O-acetylation of N-OH-ABP (Fig. 3), reflecting the contribution of NAT1 to N-OH-ABP O-acetylation.
A reduction in the amount of NAT2 protein expressed in human liver from individuals with slow acetylator phenotype has been reported previously (Deguchi et al., 1990; Grant et al., 1990; Blum et al., 1991). Some NAT2 alleles (including NAT2*5B and NAT2*6A) recombinantly expressed in COS-1 cells showed reduced levels of immunoreactive NAT2 protein compared with NAT2*4 (Blum et al., 1991; Zang et al., 2007). Similar findings have been reported after transfection of some of the slow acetylator NAT2 alleles into yeast (Leff et al., 1999). Our results in human liver confirm and are consistent with these previous findings, but are the first to clearly illustrate codominant expression of NAT2 genotype with respect to NAT2 protein level.
Trimodal distributions of N-acetylation capacity in human populations has been shown for SMZ (Chapron et al., 1980; Chen et al., 2006), isoniazid (Deguchi et al., 1990; Parkin et al., 1997), p-phenetidine (Deguchi, 1992), and sulfasalazine (Ma et al., 2009). In congenic mouse (Hein et al., 1988), Syrian hamster (Hein et al., 1991, 1994a), and slow acetylator alleles, N-acetylation capacity and rat (Hein et al., 2008) models wherein all slow acetylators are homozygous for a single slow aceylator allele and obligate heterozygotes all possess the same diplotype of rapid and slow acetylator alleles, N-acetylation capacity clearly segregates into rapid, intermediate, and slow acetylator phenotypes in hepatic and extrahepatic tissues.
In summary, NAT2 genotypes determined in 256 human samples were assigned as rapid (two rapid alleles), intermediate (one rapid and one slow allele), or slow (two slow alleles) acetylator phenotypes based on functional characterization of the NAT2 alleles reported previously in recombinant expression systems (Hein et al., 1994b, 1995; Zang et al., 2007). A robust and significant relationship was observed between deduced NAT2 phenotype (rapid, intermediate, or slow) and N-acetyltransferase activity toward SMZ (p < 0.0001) and the arylamine carcinogen ABP (p < 0.0001), and for O-acetyltransferase-catalyzed metabolic activation of N-OH-ABP (p < 0.0001), N-OH-MeIQx (p < 0.01), and N-OH-PhIP (p < 0.0001). NAT2-specific protein levels also significantly associated with the rapid, intermediate, and slow NAT2 acetylator phenotypes (p < 0.0001). As a negative control, PABA (an NAT1-specific substrate) N-acetyltransferase activities from the same samples did not correlate with the three NAT2 acetylator phenotypes (p > 0.05). These results clearly document codominant expression of human NAT2 alleles resulting in rapid, intermediate, and slow acetylator phenotypes. The three phenotypes reflect levels of NAT2 protein catalyzing both N- and O-acetylation. Our results suggest a significant role of NAT2 acetylation polymorphism in arylamine-induced cancers and are consistent with differential cancer risk and/or drug efficacy/toxicity in intermediate compared with rapid or slow NAT2 acetylator phenotypes.
This study was supported by National Institutes of Health [Grants R01-CA034627, P30-ES014443] and the Susan G. Komen Breast Cancer Foundation [Dissertation Research Award DISS0403147] (to Y.Z.).
Portions of this work represented partial fulfillment by Y.Z. for the PhD in pharmacology and toxicology at the University of Louisville.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168567.
- NAT2
- N-acetyltransferase 2
- NAT1
- N-acetyltransferase 1
- PABA
- p-aminobenzoic acid
- SMZ
- sulfamethazine
- ABP
- 4-aminobiphenyl
- N-OH-ABP
- N-hydroxy-ABP
- HPLC
- high-performance liquid chromatography
- MeIQx
- 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline
- N-OH-MeIQx
- N-hydroxy-MeIQx
- PhIP
- 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- N-OH-PhIP
- N-hydroxy-PhIP
- dG
- deoxyguanosine.
References
- Agúndez JA. (2008) Polymorphisms of human N-acetyltransferases and cancer risk. Curr Drug Metab 9:520–531 [DOI] [PubMed] [Google Scholar]
- Bendaly J, Doll MA, Millner LM, Metry KJ, Smith NB, Pierce WM, Jr, Hein DW. (2009a) Differences between human slow N-acetyltransferase 2 alleles in levels of 4-aminobiphenyl-induced DNA adducts and mutations. Mutat Res 671:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendaly J, Metry KJ, Doll MA, Jiang G, States JC, Smith NB, Neale JR, Holloman JL, Pierce WM, Hein DW. (2009b) Role of human CYP1A1 and NAT2 in 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced mutagenicity and DNA adducts. Xenobiotica 39:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendaly J, Zhao S, Neale JR, Metry KJ, Doll MA, States JC, Pierce WM, Jr, Hein DW. (2007) 2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P4501A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol Biomarkers Prev 16:1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Demierre A, Grant DM, Heim M, Meyer UA. (1991) Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci USA 88:5237–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron DJ, Kramer PA, Mercik SA. (1980) Kinetic discrimination of three sulfamethazine acetylation phenotypes. Clin Pharmacol Ther 27:104–113 [DOI] [PubMed] [Google Scholar]
- Chen B, Zhang WX, Cai WM. (2006) The influence of various genotypes on the metabolic activity of NAT2 in a Chinese population. Eur J Clin Pharmacol 62:355–359 [DOI] [PubMed] [Google Scholar]
- Chiang TA, Pei-Fen W, Ying LS, Wang LF, Ko YC. (1999) Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food Chem Toxicol 37:125–134 [DOI] [PubMed] [Google Scholar]
- Deguchi T. (1992) Sequences and expression of alleles of polymorphic arylamine N-acetyltransferase of human liver. J Biol Chem 267:18140–18147 [PubMed] [Google Scholar]
- Deguchi T, Mashimo M, Suzuki T. (1990) Correlation between acetylator phenotypes and genotypes of polymorphic arylamine N-acetyltransferase in human liver. J Biol Chem 265:12757–12760 [PubMed] [Google Scholar]
- Doll MA, Hein DW. (2001) Comprehensive human NAT2 genotype method using single-nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes. Anal Biochem 288:106–108 [DOI] [PubMed] [Google Scholar]
- Evans DA, Manley KA, McKusick VA. (1960) Genetic control of isoniazid metabolism in man. Br Med J 2:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlewska-Roberts K, Green B, Fares M, Ambrosone CB, Kadlubar FF. (2002) Carcinogen-DNA adducts in human breast epithelial cells. Environ Mol Mutagen 39:184–192 [DOI] [PubMed] [Google Scholar]
- Grant DM. (2008) Structures of human arylamine N-acetyltransferases. Curr Drug Metab 9:465–470 [DOI] [PubMed] [Google Scholar]
- Grant DM, Mörike K, Eichelbaum M, Meyer UA. (1990) Acetylation pharmacogenetics. The slow acetylator phenotype is caused by decreased or absent arylamine N-acetyltransferase in human liver. J Clin Invest 85:968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW. (1988) Acetylator genotype and arylamine-induced carcinogenesis. Biochim Biophys Acta 948:37–66 [DOI] [PubMed] [Google Scholar]
- Hein DW. (2006) N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene 25:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW, Bendaly J, Neale JR, Doll MA. (2008) Systemic functional expression of N-acetyltransferase polymorphism in the F344 Nat2 congenic rat. Drug Metab Dispos 36:2452–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Nerland DE, Fretland AJ. (2006) Tissue distribution of N-acetyltransferase 1 and 2 catalyzing the N-acetylation of 4-aminobiphenyl and O-acetylation of N-hydroxy-4-aminobiphenyl in the congenic rapid and slow acetylator Syrian hamster. Mol Carcinog 45:230–238 [DOI] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, Ferguson RJ. (1995) Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res 55:3531–3536 [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ, Grant DM. (1993) Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis 14:1633–1638 [DOI] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, Gray K, Ferguson RJ, Feng Y. (1994a) Construction of Syrian hamster lines congenic at the polymorphic acetyltransferase locus (NAT2): acetylator genotype-dependent N- and O-acetylation of arylamine carcinogens. Toxicol Appl Pharmacol 124:16–24 [DOI] [PubMed] [Google Scholar]
- Hein DW, Ferguson RJ, Doll MA, Rustan TD, Gray K. (1994b) Molecular genetics of human polymorphic N-acetyltransferase: enzymatic analysis of 15 recombinant wild-type, mutant, and chimeric NAT2 allozymes. Hum Mol Genet 3:729–734 [DOI] [PubMed] [Google Scholar]
- Hein DW, Rustan TD, Bucher KD, Miller LS. (1991) Polymorphic and monomorphic expression of arylamine carcinogen N-acetyltransferase isozymes in tumor target organ cytosols of Syrian hamsters congenic at the polymorphic acetyltransferase locus. J Pharmacol Exp Ther 259:699–704 [PubMed] [Google Scholar]
- Hein DW, Trinidad A, Yerokun T, Ferguson RJ, Kirlin WG, Weber WW. (1988) Genetic control of acetyl coenzyme A-dependent arylamine N-acetyltransferase, hydrazine N-acetyltransferase, and N-hydroxy-arylamine O-acetyltransferase enzymes in C57BL/6J, A/J, AC57F1, and the rapid and slow acetylator A.B6 and B6.A congenic inbred mouse. Drug Metab Dispos 16:341–347 [PubMed] [Google Scholar]
- Keating GA, Bogen KT. (2004) Estimates of heterocyclic amine intake in the US population. J Chromatogr B Analyt Technol Biomed Life Sci 802:127–133 [DOI] [PubMed] [Google Scholar]
- Leff MA, Fretland AJ, Doll MA, Hein DW. (1999) Novel human N-acetyltransferase 2 alleles that differ in mechanism for slow acetylator phenotype. J Biol Chem 274:34519–34522 [DOI] [PubMed] [Google Scholar]
- Ma JJ, Liu CG, Li JH, Cao XM, Sun SL, Yao X. (2009) Effects of NAT2 polymorphism on SASP pharmacokinetics in Chinese population. Clin Chim Acta 407:30–35 [DOI] [PubMed] [Google Scholar]
- Martin RC, Li Y, Liu Q, Jensen NS, Barker DF, Doll MA, Hein DW. (2009) Manganese superoxide dismutase V16A single-nucleotide polymorphism in the mitochondrial targeting sequence is associated with reduced enzymatic activity in cryopreserved human hepatocytes. DNA Cell Biol 28:3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metry KJ, Neale JR, Doll MA, Howarth AL, States JC, McGregor WG, Pierce WM, Jr, Hein DW. (2010) Effect of rapid human N-acetyltransferase 2 haplotype on DNA damage and mutagenesis induced by 2-amino-3-methylimidazo-[4,5-f]quinoline (IQ) and 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline (MeIQx). Mutat Res 684:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metry KJ, Zhao S, Neale JR, Doll MA, States JC, McGregor WG, Pierce WM, Jr, Hein DW. (2007) 2-Amino-1-methyl-6-phenylimidazo [4,5-b] pyridine-induced DNA adducts and genotoxicity in Chinese hamster ovary (CHO) cells expressing human CYP1A2 and rapid or slow acetylator N-acetyltransferase 2. Mol Carcinog 46:553–563 [DOI] [PubMed] [Google Scholar]
- Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, Ilett KF, Kadlubar FF. (1992) N- and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochem Biophys Res Commun 185:839–844 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (2005) National Toxicology Program Report on Carcinogenesis, 11th ed, U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park, NC [Google Scholar]
- Parkin DP, Vandenplas S, Botha FJ, Vandenplas ML, Seifart HI, van Helden PD, van der Walt BJ, Donald PR, van Jaarsveld PP. (1997) Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med 155:1717–1722 [DOI] [PubMed] [Google Scholar]
- Stabbert R, Schäfer KH, Biefel C, Rustemeier K. (2003) Analysis of aromatic amines in cigarette smoke. Rapid Commun Mass Spectrom 17:2125–2132 [DOI] [PubMed] [Google Scholar]
- Talaska G, al-Juburi AZ, Kadlubar FF. (1991) Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci USA 88:5350–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsuka Y, Fukutome K, Takahashi M, Takahashi S, Tada A, Sugimura T, Wakabayashi K. (1996) Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis 17:1029–1034 [DOI] [PubMed] [Google Scholar]
- Turesky RJ. (2002) Heterocyclic aromatic amine metabolism, DNA adduct formation, mutagenesis, and carcinogenesis. Drug Metab Rev 34:625–650 [DOI] [PubMed] [Google Scholar]
- Weber WW, Hein DW. (1985) N-acetylation pharmacogenetics. Pharmacol Rev 37:25–79 [PubMed] [Google Scholar]
- Zang Y, Doll MA, Zhao S, States JC, Hein DW. (2007) Functional characterization of single-nucleotide polymorphisms and haplotypes of human N-acetyltransferase 2. Carcinogenesis 28:1665–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Zhao S, Doll MA, States JC, Hein DW. (2004) The T341C (Ile114Thr) polymorphism of N-acetyltransferase 2 yields slow acetylator phenotype by enhanced protein degradation. Pharmacogenetics 14:717–723 [DOI] [PubMed] [Google Scholar]