Abstract
Human hepatic stem cells (hHpSCs), identifiable by a unique antigenic profile, have been isolated from human livers and established ex vivo under expansion conditions permissive for self-replication. The conditions consist of a substratum of type III collagen, ideally on Transwell inserts, and Kubota's medium, a serum-free medium developed for hepatic progenitors. Under these conditions the cells demonstrated a doubling time of ∼24 h, generating at least a 16-fold increase in cell number within 7–10 days; were stable at confluence for up to 2 weeks; could be passaged, if on type III collagen, to initiate colonies that went through log-phase growth and saturation density kinetics; and expressed telomerase, indicative of regenerative capacity. The hHpSC colonies remained morphologically and phenotypically stable throughout expressing epithelial cell adhesion molecule, neural cell adhesion molecule, albumin, cytokeratins 8, 18, and 19, but not α-fetoprotein, or intercellular adhesion molecule-1 (ICAM-1). Those maintained under self-replication conditions for more than a month were transplanted and found to engraft in the livers of SCID/nod mice yielding human liver tissue expressing adult liver–specific proteins. The conditions for self-replication should offer ideal culture conditions for generating large numbers of hHpSCs for use in commercial and clinical programs.
Introduction
Human hepatic stem cells (hHpSCs) have been identified in livers of all donor ages, and can be purified by immunoselection for epithelial cell adhesion molecule (EpCAM) and neural cell adhesion molecule (NCAM).1–5 They are 7–9 μm in diameter, form morphologically uniform cell colonies expressing EpCAM, CD133/1, NCAM, E-cadherin, claudin 3, albumin +/− , cytokeratins (CK) 8, 18, and 19, and are negative for α-fetoprotein (AFP), hemopoietic, endothelial, and mesenchymal cell markers.1,2 They are found in vivo in ductal plates of fetal and neonatal livers, and canals of Hering in pediatric and adult livers.6 The hHpSCs are precursors to hepatoblasts (hHBs) that have key distinctions in their phenotypic profile including strong expression of AFP, ICAM-1, and P450-A7 and loss of NCAM. We have hypothesized they are the liver's transit amplifying cells.1,2,6
Ability to expand hHpSCs or hHBs is desired to generate cells for clinical and commercial programs and for bioartificial livers.7 A bioartificial liver capable of supporting a patient must have up to 20% of an adult liver mass of ∼2000 g, a percentage requiring billions of cells.8 The only liver parenchymal cells capable of such expansion are stem/progenitor cells.9,10
Past methods for expanding hHpSCs1,2 comprised tissue culture plastic (TCP) with Kubota's medium (KM).11 In the present study, we show that a matrix component dominant in the liver's stem cell niche, type III collagen, elicits self-replication of the hHpSCs. These findings complement prior investigations indicating distinctions in matrix chemistry between the stem cell niche and the different zones within the liver acinus.12
Materials and Methods
Most of the methods, analytical strategies, and all of the sourcing of antibodies and reagents are given in the Online Supplement (available online at www.liebertonline.com/ten).
Culture medium
KM was prepared as described previously.11,13 During the first 10 h of culture, KM was supplemented with 10% fetal bovine serum to inactivate liver processing enzymes and facilitate cell attachment. Thereafter, media changes used only serum-free KM.
Collagen substrata
Culture dishes (Falcon, Franklin Lakes, NJ) or inserts were coated with 6.25 or 60 μg/cm2 of Sigma's (St. Louis, MO) type III collagen, with 1.0 μg/cm2 of Becton Dickinson's (Franklin Lakes, NJ) highly purified type III or type IV collagen, or 0.4 mL of type I collagen.
Passaging techniques
The hHpSCs proliferated rapidly in the first 10–12 days of culture and then underwent saturation density kinetics. Subsequently and to prevent saturation kinetics, the cells were passaged. Three protocols for passaging of the cells were tried: (1) standard trypsinization, (2) treatment with phosphate-buffered saline (PBS) without calcium, and (3) mechanical manipulation. When passaging with mechanical manipulation and gentle suction, a 200 μL mechanical pipette was employed. Passaged cells were evaluated by image analysis using Metamorph tracking software (Universal Imaging, Downingtown, PA).
Telomerase
Indirect measurements of telomerase activities were accomplished using assays described previously.14–16 For further details, see the Online Supplement, available online at www.liebertonline.com/ten.
Results
Phenotype of pluripotent human hepatic progenitors
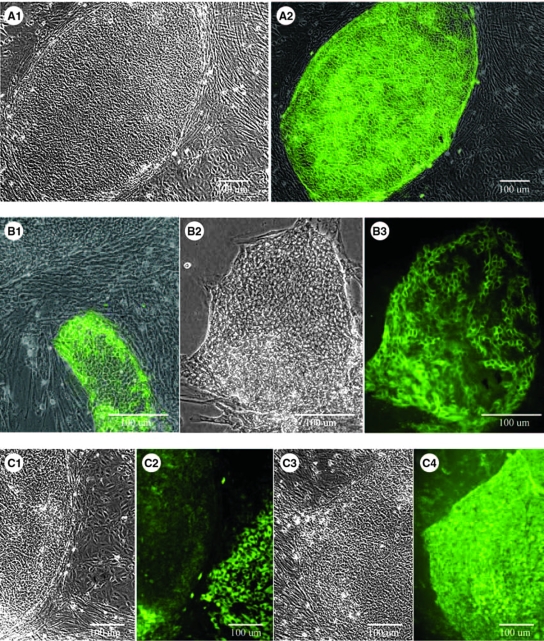
The hHpSC colonies were culture selected on tissue culture plates (TCP) and in KM. As shown in Figure 1, hHpSC colonies under these conditions have a morphology similar to that of embryonic stem cell colonies in that they are tightly and densely packed; they strongly express EpCAM (green) and NCAM (red) (Fig. 1F, G). The average cell diameter is 8 ± 1 μm with the nucleus occupying as much as ∼90% of the cell. Up to four nucleoli were observed; each cell maintained discrete borders. In the first few days, there were also hHBs (not shown), recognizable as being larger (10–12 μm) and more three dimensional, producing colonies with cord-like morphology interspersed by clear channels assumed to be bile canaliculi, and expressing AFP and ICAM-1, but not NCAM or claudin 3. The hHBs survived for only a few days on TCP and were wholly absent from all cultures by 10–14 days after seeding. In prior studies we have shown that hHBs require either embryonic mesenchymal feeders, such as embryonic mouse stromal cell line (STO) feeders, or specific matrix components, such as hyaluronans, to survive ex vivo.1,4,5
FIG. 1.
Phase contrast day-7 images (A1, B2, C1, and C3) paired with immunofluorescent staining (A2, B1, B3, C2, and C4) of hHpSC colonies and demonstrating some of the most well-studied markers found in the hHpSCs. The hHpSCs are highly positive for EpCAM (A2), albumin (B1), and CK19 (C4). NCAM is strongly positive especially at the edges of colonies and in bands of cells through the colonies (B3). The level of expression of CK19 is strongest in committed biliary progenitors seen in cells found to the right of an hHpSC colony in (C2). Morphology of hHpSC colonies and expression of signature antigens. Phase contrast (D) and differential interference contrast microscopy (DIC) image (E) of hHpSC colonies on TCP and in KM. Confocal phase images (F, G) of hHpSC colonies. (F) Cells stained for EpCAM (green). (G) Cells stained for EpCAM (green), NCAM (red). Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
Immunofluorescence studies on day 7 of cultures
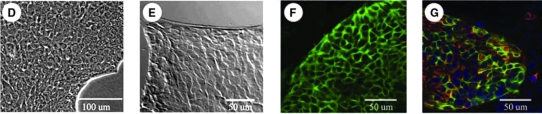
Immunofluorescence for markers representative of hHpSCs was done on day 7 cultures on TCP (Fig. 1A–G). The hHpSCs have strong expression of EpCAM (Fig. 1A2), positive expression of albumin (Fig. 1B1), NCAM (Fig. 1B2, B3), and CK19 (Fig. 1C1–C4). The levels of CK19 are even higher in committed biliary cells (colony on right side of Fig. 1C2). No expression was found for AFP and ICAM-1 in the hHpSC colonies. E-cadherin expression paralleled that of EpCAM except that its levels were lower (data not shown). The hHpSCs plated onto type III collagen (Sigma; 6.25 μg/cm2) retained the morphology and antigenic profile of the hHpSCs on TCP with tightly compact colonies and with individual hHpSC diameters of ∼8 ± 1 μm (Fig. 2A, B). Those plated onto type IV collagen or laminin rapidly converted to cells with an hHB phenotype (Fig. 2C, D) that included expression of AFP, ICAM-1, and loss of NCAM and claudin 3 (data not shown).
FIG. 2.
Phase contrast images of hHpSC colonies plated onto TCP (A), type III collagen (B), laminin (C), type IV collagen (D), and on type III collagen for more than a month (E, F). Note that the hHpSCs plated onto laminin or type IV collagen become more irregular in shape with clear channels found between cells, distinctions found associated with hHBs. The cells on laminin or type IV collagen also expressed AFP and ICAM (data not shown).
Colony formation by hHpSC occurs more quickly with cells on type III collagen
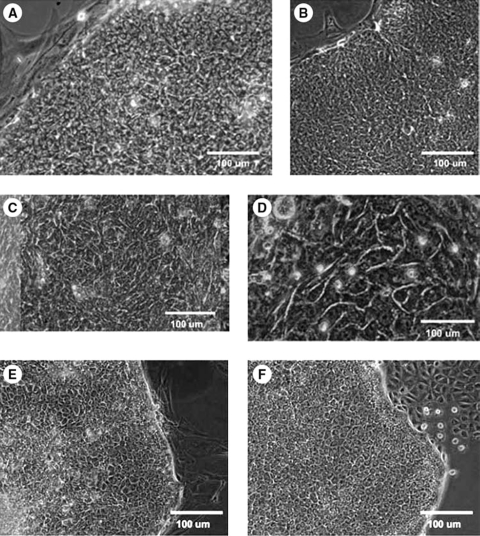
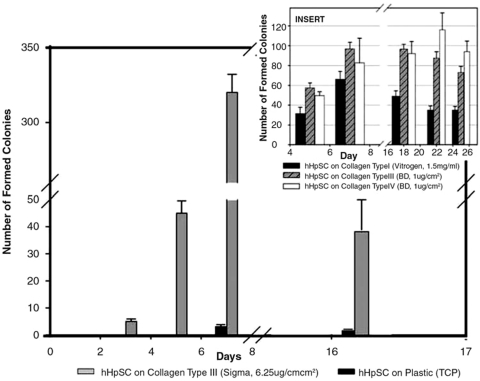
Colony formation of hHpSCs occurs more quickly when cells are seeded on collagen type III (Sigma; 6.25 μg/cm2) due, in part, to improved attachment efficiency; attachment was at least 40% better on type III collagen relative to that on TCP (data not shown). The early colonies that formed consisted of small numbers of cells, typically three to four cells per colony. Quantification of the improved ability to form colonies is documented in Figure 3; the histogram's vertical axis indicates the number of colonies, whereas the x-axis denotes days 2–17 of culture. The histograms indicating hHpSCs plated on TCP are labeled black and those for colonies on Sigma's type III collagen are labeled with gray bars. The hHpSC colonies appeared by day 3 when on Sigma's type III but not until day 7 for those seeded onto TCP. For cell cultures seeded onto Sigma's type III (6.25 μg/cm2), there were over 40 and 333 colonies by days 5 and 9, respectively. In contrast, the maximum number of colonies observed on TCP was less than 10 during the 16-day culture period. The drop in total hHpSC colony numbers on Sigma's type III (6.25 μg/cm2) at day 16 was due to merging of colonies into single large colonies. The insert also depicts colony formations for hHpSCs seeded onto type I collagen or onto purified type III or IV collagens from Becton Dickinson. For cultures with cells plated onto collagen I, hHpSC colony formation peaks at day 7 with over 65 colonies. However, the cells were not stable on this collagen and underwent a steady decline through day 25. By contrast, when cells were seeded onto purified collagen III or IV, the number of colonies peaked by day 20, with 95–120 hHpSC colonies, and the numbers of colonies remained uniform through the study end by day 25.
FIG. 3.
Colony formation and behavior of hHpSCs on various substrata. Number of hHpSC colonies that formed when cells were seeded onto collagen type III (Sigma, 6.25 μg/cm2) versus TCP. The insert illustrates hHpSC colony formation when seeded onto type I collagen versus purified type III or type IV collagen obtained from Becton Dickinson.
Behavior and stability of cells at confluence on different substrata
The hHpSCs on TCP achieved saturation density kinetics by day 7, but never achieved confluence over the entire dishes. Moreover, they were not stable at confluence, losing cells steadily into the media, especially when the colonies underwent contraction, resulting in only ∼15% of the dish surface area covered by cells by day 30. The cells were unable to be passaged successfully from TCP to TCP. On type III collagen, they achieved confluence by days 10–12, were stable for up to 2 weeks and then began to decline in cell numbers resulting in a loss of up to ∼20% of the cells by day 30. The loss in cell numbers could be avoided, if the cells were passaged to new dishes or inserts coated with type III collagen. Comparisons were made to other forms of extracellular matrix including type I collagen and purified type III or type IV collagens from Becton Dickinson (Fig. 3, Insert). Cells seeded onto collagen I (dark gray) demonstrated proliferation beginning by day 3, reached a plateau by day 17, and declined in numbers thereafter. On purified type III collagen from Becton Dickinson (slanted lines), the behavior was similar to that on type III from Sigma, with colony formation evident by day 3, increasing through day 25 and with relative stability at confluence. The cells on type IV collagen from Becton Dickinson (white) demonstrated colony formation by day 5 with sustained colonies through day 25. However, they were less stable at confluence. A summary of the behavior of the cells on the different matrix substrata is given in Table 1.
Table 1.
Comparison of the Effects of Various Purified Extracellular Matrix Components on hHpSCs (Note That Rapid Growth and Sustained Self-Replication Occur Only on Type III Collagen)
| |
Substrata |
|||
|---|---|---|---|---|
| |
|
Collagen types |
||
| Cell properties | TCP | I | III | IV |
| Colony formation (cell #/colony when first formed) | Within 7–10 days (∼10 cells) | By 3 days (5–10 cells) | By 3 days (3–4 cells) | |
| Doubling times: | ||||
| During log-phase growth (7–10 days) | ∼36–40 h | An estimate of >60 h | ∼24–30 h | |
| Day after seeding at which confluence observed | ∼10 days | hHpSCs never reached confluence during the experiment | ∼Day 20 | |
| Saturation density at confluence | 28,000 cells/dish or 2909 cells/cm2 | 43,000 cells/dish or 4468 cells/cm2 | Similar to cells on type III collagen | |
| Behavior: | ||||
| During early confluence (days 10–20) | No growth; slow loss of cells into the medium | hHpSC colonies grew even more slowly in the last 2 weeks of the study | Cells slowing in growth and then stable at confluence for ∼2 weeks | |
| Post confluence (days 20–30) | No growth; loss of half the cells in 8 days | No growth; loss of half the cells in 10 days | ||
| Cell phenotype | Remained as hHpSCs | n.d. | Remained as hHpSCs | Lineage restriction to hHBs |
Properties of hHpSCs on various substrata and in KM. Seeding density was 3820 parenchymal cells/cm,2 of which the majority were hHBs and only ∼1% were hHpSCs.1 The parenchymal cells (both hHBs and hHpSCs) did not attach to fibronectin, and those that did attach rapidly died. The hHBs attached and survived under all conditions tested, but they disappeared within 7 days in cultures on TCP, within 18 days in those on type I collagen, and within 12 days in cultures on all other substrata. The hHpSC responses on laminin were similar to those on type IV collagen. More characterization of the cells on type I collagen is required to define the phenotype(s) of the cells. For further characterization of the cells under these distinct conditions see McClelland et al.12 n.d., not done.
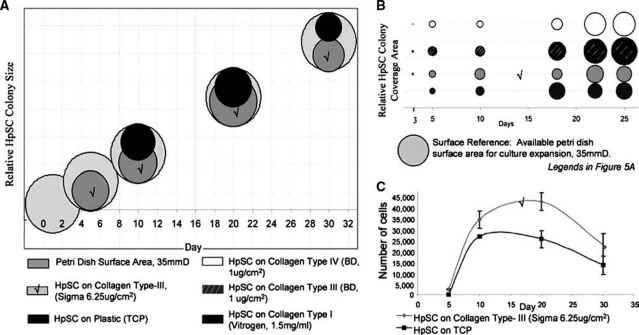
Colony sizes versus total culture surface areas
To correlate proliferation dynamics, bubble plots were used to illustrate “relative cell coverage areas” (Fig. 4). For this analysis, the maximum culture surface area is 9.62 cm2 (35 mm dishes) and is illustrated as large, light gray circles (Fig. 4A). As hHpSCs proliferated to cover portions of the surface, colony area summations were evaluated to indicate changing growth dynamics. The hHpSCs on TCP are symbolized as black circles. Those on Sigma's type III collagen (6.25 μg/cm2) are symbolized as dark gray circles. The data from cells on different substrata are indicated by the distinct bullet points; the data are dispersed over the x-axis to indicate the results from assays over time. On day 1, hHpSCs are not visualized in cultures on either TCP or Sigma's type III collagen (6.25 μg/cm2). By day 5, the hHpSCs plated onto Sigma's type III collagen (6.25 μg/cm2) had expanded to cover ∼35% of the surface, whereas hHpSCs are still not evident in cultures on TCP. By day 10, those on Sigma's type III collagen (6.25 μg/cm2) had expanded to cover ∼40% of the surface, whereas those on TCP indicate only ∼27% coverage. By day 20, hHpSC colonies on Sigma's type III collagen had expanded to be essentially confluent, covering more than 80% of the surface area, compared with those on TCP on which the hHpSCs covered only about ∼30% of the surface area. Proliferation of hHpSCs on type I collagen was compared to that on Becton Dickinson's purified type III or type IV collagens (see Fig. 4B). As shown, cells seeded on collagen I (dark gray) demonstrate proliferation beginning by day 3, peaking near day 17, and waning thereafter. On purified type III collagen from Becton Dickinson (slanted lines), proliferation is evident by day 3 and increases through day 25. hHpSCs on type IV collagen from Becton Dickinson (white) demonstrate proliferation by day 5, and it increases through day 25.
FIG. 4.
Quantification of proliferation responses. (A) Summation of hHpSC proliferation patterns for cells seeded on TCP (black) or Sigma's type III collagen (gray). Large, light gray circles represent the total seeding area (35 mmD dish). (B) The hHpSC colony growth patterns seeded on collagens I and BD collagens III and IV contrasted against total available surface area (35 mmD), and against hHpSC cells seeded on Sigma collagen type III. (C) Quantified numerical variations for cells seeded on TCP (black) and Sigma type III collagen (6.25 μg/cm2) cultures (gray). “√” labels duplicate cultures analyzed in different formats.
Cell division rates
Quantitative analyses of the division rates are given in Figure 4C that depicts the growth curves of hHpSCs on TCP (black) versus those on type III collagen (Sigma; 6.25 μg/cm2) (gray) over culture days 5–30. The first observation of hHpSC colonies occurs by day 5 with cells seeded onto collagen type III (Sigma; 6.25 μg/cm2). At this time, ∼2500 hHpSCs are evident, considered the initial “0” data set. By day 10, cell numbers are ∼34,000 in cultures on collagen type III (Sigma; 6.25 μg/cm2) versus ∼26,000 in cultures on TCP. Both graphs illustrate a “near linear” growth rate through day 10, with a mathematical slope being steeper for hHpSCs in cultures on collagen type III (Sigma; 6.25 μg/cm2). There is a 38% increase rate in cells on collagen type III (Sigma; 6.25 μg/cm2) versus TCP. After day 10, the cultures demonstrate saturation density kinetics with those on collagen type III (Sigma; 6.25 μg/cm2) displaying maximum cell counts of 43,000 at day 18, whereas maximum counts in cultures on TCP approached 26,000 cells at day 12. The hHpSC colonies are not stable after achieving confluence and decline thereafter to ∼22,500 in cultures on collagen type III (Sigma; 6.25 μg/cm2) versus ∼14,000 in cultures on TCP.
The division rates were distinct for log-phase growth (days 1–10) versus saturation density kinetics (days 10–20) versus postconfluence (days 20–30). The collagen substrata resulted in increases (or losses) of the numbers of colonies depending on the collagen type. During log-phase growth, days 5–10, hHpSC doubling rates on collagen type III (Sigma; 6.25 μg/cm2) (gray) versus TCP (black) are 1.2 and 2.5 days, respectively. During saturation density kinetics, days 10–20, the hHpSCs on collagen type III (Sigma; 6.25 μg/cm2) demonstrated doubling rates that had slowed significantly; the calculated doubling time is 40 days. Postconfluence phase occurred from days 20 to 30. The cells on TCP were slowly losing cells into the medium, whereas those on collagen type III were relatively stable.
The culture conditions select for hHpSCs
The hHpSC colonies plated onto TCP versus type III collagen were compared morphologically (Fig. 2A, B) and for production of secreted, liver-specific proteins throughout a 30-day culture period (Fig. 5A). Immediately after plating, the cultures on TCP or type III collagen were predominantly hHBs, recognizable by their size (10–12 μm), by the cord-like structures of the colonies that they form, and by expression of α-fetoprotein and albumin. Within ∼7 days on TCP and by ∼12 days on type III collagen, the hHBs had disappeared leaving behind only the hHpSC colonies. The expansion of the hHpSCs continued until confluence at which point the cells entered into saturation density kinetics in which their growth rate slowed or stopped. Those on TCP were not stable at confluence, undergoing contraction and loss from the plates. Those on type III collagen were stable for 2 weeks or more and only then showed signs of cell loss. Merging of colonies was variable depending on the cell numbers in the dishes. By day 30, the hHpSCs on TCP had formed hollow, circular bands of tissue that were undergoing contraction resulting in the bands of tissue lifting off into the medium. Those on type III collagen (Sigma; 6.25 μg/cm2) also formed contracting bands but were able to remain on the dishes, though the contractions did pull the cells away from some portions of the dishes leaving bare regions.
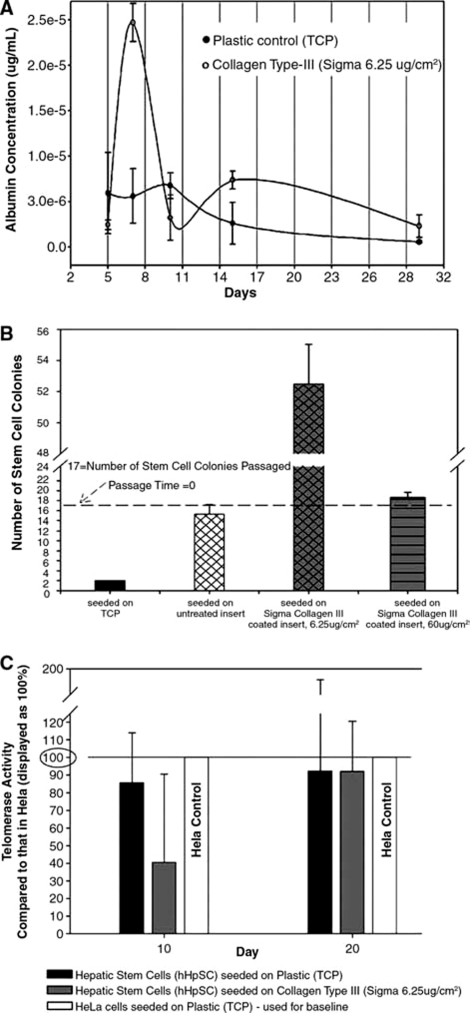
FIG. 5.
(A) Albumin secretion of hHpSCs in primary cultures was always significantly higher in cells on type III collagen than on TCP and went through a distinctive pattern over the 30 days of the culture, paralleling the process of transition from cultures dominated by hepatoblasts, with high albumin production, in the first week to ones occupied entirely by hHpSCs, with lower but stable albumin production, by 11–12 days. (B) Passaging effects on colony formation. The number of colonies that formed when hHpSCs were passaged from TCP to TCP was only two. Passaging was more successful on inserts with 16 colonies forming and was most successful if the insert was coated with type III collagen. There was a collagen concentration effect such that 18 colonies formed with collagen concentration of 60 μg/cm2 and 55 colonies with 6.25 μg/cm2. (C) Telomerase activity of hHpSCs on culture plastic (black bar) versus Sigma's type III collagen (6.25 μg/cm2) (light gray) and given as a percentage of that in HeLa cells (white). The activities are normalized per cell.
Albumin secretion
The morphological changes in the long-term cultures were paralleled by changes in the production of secreted, liver-specific proteins such as albumin (Fig. 5). Albumin expression was analyzed in cultures of hHpSCs seeded on TCP (•) versus on substrata of 6.25 μg/cm2 Sigma's type III collagen (o). Albumin levels were normalized per cell and given as albumin concentrations (μg/mL) per 24-h interval. Three phases are established to segment divisions on the x-axis: (1) the initial phase occurred when hHBs dominated the cultures correlating with high levels of albumin in the medium (with levels reaching 5.2E-6 μg/mL on TCP vs. ∼2.5E-6 μg/cm2 on type III collagen); (2) a second phase (days 7–11 on TCP and days 9–15 on type III collagen), there was a mixture of hHBs and hHpSCs correlated with declining levels of albumin secretion (low to negligible on TCP; peaking at 6.25E-6 μg/mL for cells on type III collagen but then declining); and (3) a third phase (days 11–30) in which hHpSCs were the only hepatic progenitors evident, correlating with low but stable levels of albumin. By day 30 the cultures were found to express little to no albumin under all culture conditions tested.
Passaging of the cells
Cells are well known to undergo rapid division rates initially during log-phase growth, and then to slow their growth at higher densities as they become confluent, a phenomenon called saturation density kinetics; normal cell growth arrest at lower densities than do tumor cells. The ability to passage cells permits one to reactivate repeatedly the log-phase growth kinetics and, thereby, optimize the expansion potential of the cells. Initial attempts to passage hHpSCs utilizing trypsin, other enzymes, or PBS and all methods in which cells were passaged to TCP failed; most cells did not reattach and rapidly loss viability. Mechanical disruption of the cells proved more beneficial but still resulted in limited success until cells were passaged onto Transwells coated with type III collagen (Fig. 5B). The hHpSCs were cultured for 2 weeks on TCP and then passaged under one of four conditions: TCP (black), untreated Transwell inserts (crosshatch white background), collagen type III (Sigma; 6.25 μg/cm2)–coated Transwell inserts (crosshatch gray background), and Sigma III collagen (60 μg/cm2)–coated Transwell inserts (gray with horizontal lines). Two weeks after passaging, the number of hHpSC colonies was counted. Those passaged from TCP to TCP resulted in only 2 colonies, from TCP to untreated Transwells resulted in 15 colonies, from TCP to Transwells with type III collagen were the best with 18 colonies at 60 μg/cm2, and 55 colonies if on inserts with 6.25 μg/cm2. This is a 310% improvement over colony formation on TCP.
Telomerase activity
Telomerase activity in hHpSC colonies was assessed using HeLa cells, a customary standard for telomerase assays. The telomerase activity was measured in hHpSC on culture plastic (black) versus on Sigma's type III collagen (6.25 μg/cm2) (gray) and expressed as a percentage of the HeLa controls (white). The data were normalized to cell numbers (Fig. 5C) and then given as a percentage of that found in HeLa cells. Telomerase activities for cells on TCP were 85% (day 10) and 92% (day 20) of that of HeLa cells; the levels for cells on type III collagen were 40% (day 10) and 92% (day 20) of HeLa controls.
Transplantation of hHpSCs cultured on type III collagen substratum and in KM gives rise to human liver tissue in vivo
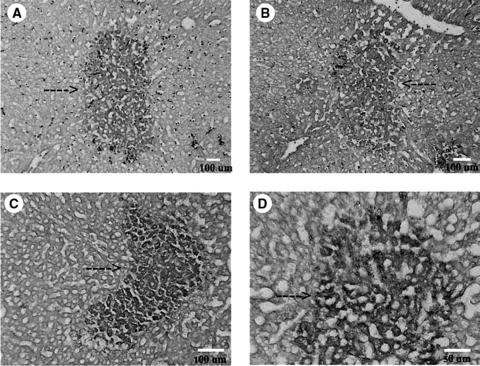
Our prior studies indicated that transplantation of freshly isolated EpCAM+ cells or of hHpSC colonies, derived from fetal, neonatal, or postnatal livers, into livers of SCID/nod mice resulted in engraftment and the formation of mature human liver parenchymal cells.1 We did similar experiments with hHpSC colonies maintained for more than 2 months on type III collagen. Liver sections from mice transplanted with hHpSCs from cultures contained cells strongly expressing human-specific proteins (Fig. 6). The phenotype of the cells changed with time after transplantation. The cells prior to transplantation were EpCAM+, CK19+, NCAM+, and AFP−. Within the first week after transplantation, the engrafted cells were EpCAM+, ALB+, CK19+, and AFP+, a profile indicative of differentiation to hHBs. Two weeks after transplantation, the sections contained cells that were ALB+ and CK19+ but negative for AFP and EpCAM, a profile indicative of further differentiation toward mature human liver cells. The expression of human albumin and CK19 persisted for more than 40 days in the murine livers. In data not shown, the liver sections were negative for late genes such as P450s. The data suggest that the engrafted hHpSCs gave rise to maturing human liver tissue that expressed some, but not yet all, of the adult hepatocyte functions.
FIG. 6.
Liver sections from SCID/nod mice transplanted with hHpSCs from human fetal livers. Stained sections demonstrate cells expressing human-specific proteins within the murine liver sections (arrows indicate human cells). (A) AFP after 7 days, (B) albumin after 40 days, and (C, D) CK19 after 14 days and 40 days, respectively.
Discussion
We studied hHpSCs, found in livers from all donor ages,1–5,17 to learn the conditions for expansion ex vivo. Our studies are the only ones on cells with this phenotypic profile. The only other report of presumptive hHpSCs identified subpopulations with mesodermal (e.g., CD34) and endodermal (e.g., albumin) markers, and claimed that they are capable of lineage restricting to both endodermal and mesodermal fates.18 We suggest that this finding is incorrect and due to coselection for hepatic progenitors with tightly clinging mesenchymal companion cells. Rigorous multiparametric flow cytometric analyses1 on the hHpSCs and on the hHpSCs in long-term cultures studied here have failed to identify any hemopoietic, endothelial, or mesenchymal markers on the cells.1
Most markers defining hHpSCs were expressed at similar levels in all cultures. The only one showing variability was NCAM, expressed by both hHpSCs1 and by angioblasts.19 Its levels were always highest at the edges of colonies at which angioblasts have been found previously.1 The variability in NCAM is hypothesized to be due to variations in angiogenesis within the colonies, a hypothesis corroborated by prior findings of hedgehog signaling in hHpSCs, a signaling pathway known to be important for angiogenesis.3
Conditions for self-replication of the hHpSCs have been identified permitting significant expansion of the cells with retention of the stem cell phenotype. They consist of KM used in combination with type III collagen, ideally coated onto Transwell inserts. The usefulness of KM has been demonstrated previously for rodent and human hepatic progenitors.1–3,11,12,17,20 These conditions proved successful for hHpSCs but not for their immediate descendents, hHBs, that disappeared from cultures in 7–10 days under all conditions except type I collagen on which they persisted for 14–16 days. Conditions required to sustain them long-term ex vivo include special mesenchymal feeders or embedding them into hyaluronan hydrogels.1,4,5 The behavior of hHBs has led to our hypothesis that they are the liver's transit amplifying cells.6
Under self-replication conditions, hHpSCs remained phenotypically stable for weeks to months and yet were able to give rise to mature liver tissue when transplanted into livers of immunocompromised hosts. Further evidence for self-replication and proliferation is that hHpSCs have significant telomerase activity. Telomerase is a ribonucleoprotein enzyme complex composed of RNA and proteins and is present in proliferating cells in which it stabilizes chromosome length.
Passaging proved challenging technically, as it has been for embryonic stem (ES) cells,13 and was accomplished if small cell aggregates were passaged onto Transwells coated with type III collagen but not if onto TCP or onto other matrix substrata such as type I collagen. Being able to passage the cells was essential in order to achieve the maximal expansion potential of the cells given that they obey saturation density kinetics.
The epithelial-mesenchymal relationship, long known to be central to regulation of tissues, is mediated by paracrine signals comprised of soluble factors and insoluble extracellular matrix factors. Newly recognized is that the relationship is maturationally lineage dependent. The exact composition of the paracrine signals changes with each of the maturational lineage stages. The matrix components, part of the paracrine signals produced by the parenchymal–mesenchymal interactions, change with the maturational lineage stage. The stem cell niche comprises a partnership between hHpSCs, angioblasts, and hepatic stellate cells.1,3,20,21,22 The known extracellular matrix components produced by angioblasts and hepatic stellate cells include laminin, type III and IV collagens,23–27 and hyaluronans.28,29 To achieve optimal ex vivo expansion of hHpSCs requires that one mimic the paracrine signaling occurring between hHpSCs and their mesenchymal cell partners.30,31 We surveyed the effects of the known matrix components found in the liver's stem cell niche and learned that self-replication of hHpSCs occurs with use of type III collagen. In prior studies, we reported that seeding hHpSCs onto or into hyaluronans results in lineage restriction to hHBs.4
More than 25 types of collagens have been identified,32,33 of which three of the primary ones in liver include type I collagen, [a1(I)]2[a(I)]; type III collagen, [a1(III)]3, referred to in the older literature as reticulin and known to be produced by endothelial cell precursors34,35; and type IV, [a1(IV)]2[a2(IV)], which yields collagen scaffolds that are nonfibrillar.26,36 In fetal livers, collagen III and IV are two of the dominant collagen types.37 By contrast, the matrix chemistry associated with mature parenchymal cells contains type I collagen mixed with small amounts of type III; no type IV collagen is present.38,39 Limited and very slow growth or even apoptosis was observed with hHpSCs plated onto type I collagen or on fibronectin. This would seem surprising given that type I and III collagens are so similar in their amino acid sequences.32 However, fibril formation is quite distinct between the two due to modifications of the collagen molecules during posttranslational processing in the Golgi.34,35 The distinctions result in long fibrils made of large numbers (five to hundreds) of molecules for type I collagen and in thin, short fibrils with few (exact number unknown) molecules for type III collagen40,41 (M. Yamauchi, personal communication). Empirically, the ratio of collagen III/I is very high in fetal tissues; the ratio shifts with type I collagen dominating in mature tissues.42,43 The distinctions in numbers of collagen molecules/fibril and their relative proportions in fetal versus adult tissues could explain the differences in response of the stem cells if one assumes that turnover of the collagen is a prerequisite for cell division. The sheer mass of collagen molecules to be turned over would be greater for type I collagen than type III collagen and could serve to stabilize tissues during development.
The use of growth-permissive collagen substrata in combination with the defined medium eliminates the need for embryonic stromal feeders for the stem cells, at least in short-term experiments of up to 2 months. Long-term cultures of the stem cells are likely to require soluble paracrine signals from the feeders, and several such signals have been identified and are the focus of ongoing studies.
The value of these findings is that they enable one to expand ex vivo hHpSCs under essentially self-replication conditions that are wholly defined, facilitating their use in clinical and industrial programs.
Supplementary Material
Acknowledgments
Funding derived from a sponsored research grant from Vesta Therapeutics; by NIH grants RO1 DK52851, RO1 AA014243, and RO1 IP30-DK065933; and by a U.S. DOE grant DE-FG02-02ER-63477. We wish to thank Lucendia English for technical support and Dr. Mitsuo Yamauchi (UNC School of Dentistry) and his student, Marnisa Sricholpech, for analyses to check on the collagen types in the commercial preparations from Sigma and Becton Dickinson. Core service support included the Michael Hooker Confocal Microscope Facility (director: Dr. Michael Chua).
References
- 1.Schmelzer E. Zhang L. Bruce A. Eliane W. Ludlow J. Yao H. Moss N. Melhem A. McClelland R.E. Turner W. Kulik M.l. Sherwood S. Tallheden T. Cheng N. Furth M.E. Reid L.M. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmelzer E. Wauthier E. Reid L.M. Phenotypes of pluripotent human hepatic progenitors. Stem Cell. 2006;24:1852. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 3.Sicklick J.K. Li Y.X. Melhem A. Schmelzer E. Zdanowicz M. Huang J. Caballero M. Fair J.H. Ludlow J.W. McClelland R.E. *Reid L.M. *Diehl A.M. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2005;290:G859. doi: 10.1152/ajpgi.00456.2005. [*co-senior authors] [DOI] [PubMed] [Google Scholar]
- 4.Turner W.S. Schmelzer E. McClelland R. Wauthier E. Chen W. Reid L.M. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J Biomed Mater. 2007;82:156. doi: 10.1002/jbm.b.30717. [DOI] [PubMed] [Google Scholar]
- 5.Turner W.S. Seagle C. Galanko J. Favorov O. Prestwich G.D. Macdonald J.M. Reid L.M. Metabolomic Footprinting of human hepatic stem cells and hepatoblasts cultured in engineered hyaluronan-matrix hydrogel scaffolds. Stem Cell. 2008;26:1547. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L. Theise N. Chua M. Reid L.M. Human hepatic stem cells and hepatoblasts: symmetries between liver development and liver regeneration. Hepatology. 2008. (In press). [DOI] [PubMed]
- 7.McClelland R.E. Reid L.M. Bioartificial livers. In: Attala A., editor; Lanza R., editor. Principles of Regenerative Medicine. San Diego, CA: Elsevier Academic Press; 2007. pp. 928–945. [Google Scholar]
- 8.Brusse B. Gerlach J. Bioreactors for hybrid liver support: historical aspects and novel designs. Ann NY Acad Sci. 1999;875:326. doi: 10.1111/j.1749-6632.1999.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmelzer E. Zhang L. Melhem A. Yao H. Turner W. McClelland R. Wauthier E. Furth M. Gerber D. Gupta S. Reid L. Hepatic stem cells. In: Potten C.S., editor; Clarke R.B., editor; Wilson J., editor; Renehan A.G., editor. Tissue Stem Cells. New York: Taylor and Rancis Group; 2006. pp. 161–214. [Google Scholar]
- 10.Cheng N. Yao H. Reid L.M. Human hepatic stem cells and regenerative medicine. In: Attala A., editor; Lanza R., editor. Principles of Regenerative Medicine. San Diego, CA: Elsevier Publishers; 2007. pp. 344–384. [Google Scholar]
- 11.Kubota H. Reid L.M. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA. 2000;97:12132. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland R. Wauthier E. Uronis J. Reid L.M. Gradient in extracellular matrix chemistry from periportal to pericentral zones: regulation of hepatic progenitors. Tissue Eng. 2008;14:59. doi: 10.1089/ten.a.2007.0058. [DOI] [PubMed] [Google Scholar]
- 13.Wauthier E. McClelland R. Turner W. Schmelzer E. Kubota H. Zhang L. Ludlow J. Bruce A. Yao H. Furth M.E. LeCluyse E. Moss N. Turner R. Merrick P. Barbier C. Lozoya O. Ruiz J. Reid L.M. Hepatic stem cells and hepatoblasts: identification, isolation and ex vivo maintenance. In: Mather J., editor. Methods Cell Biology. Vol. 86. Elsevier Press; San Diego, CA: 2008. p. 137. Methods for Stem Cells. [DOI] [PubMed] [Google Scholar]
- 14.Greider C.W. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8:R178. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 15.Wege H. Le H.T. Shui M.S. Liu L. Wu J. Giri R.K. Malhi H. Sappal B.S. Kumaran V. Gupta S. Zern M.A. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology. 2003;124:432. doi: 10.1053/gast.2003.50064. [DOI] [PubMed] [Google Scholar]
- 16.Goodell M.A. Rosenzweig M. Kim H. Marks D.F. DeMaria M. Paradis G. Grupp S.A. Sieff C.A. Mulligan R.C. Johnson R.P. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 17.Cheng N. Reid L.M. Mature human hepatocytes from ex vivo differentiation of alginate-encapsulated hepatic stem cells and hepatoblasts. Tissue Eng. 2008;14:1. doi: 10.1089/ten.a.2007.0131. [DOI] [PubMed] [Google Scholar]
- 18.Dan Y.Y. Riehle K.J. Lazaro C. Teoh N. Haque J. Campbell J.S. Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA. 2006;103:9912. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bussolati B. Grange C. Bruno S. Buttiglieri S. Deregibus M.C. Tei L. Aime S. Camussi G. Neural-cell adhesion molecule (NCAM) expression by immature and tumor-derived endothelial cells favors cell organization into capillary-like structures. Exp Cell Res. 2006;312:913. doi: 10.1016/j.yexcr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Kubota H. Yao H. Reid L.M. Identification and characterization of vitamin A-storing cells in fetal liver. Stem Cell. 2007;25:2339. doi: 10.1634/stemcells.2006-0316. [DOI] [PubMed] [Google Scholar]
- 21.Sigal S.H. Brill S. Fiorino A.S. Reid L.M. The liver as a stem cell and lineage system. Am J Physiol. 1992;263:G139. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- 22.Reid L.M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990;2:121. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- 23.Bender B.L. Jaffe R. Carlin B. Chung A.E. Immunolocalization of entactin, a sulfated basement membrane component, in rodent tissues, and comparison with GP-2 (laminin) Am J Pathol. 1981;103:419. [PMC free article] [PubMed] [Google Scholar]
- 24.Terada T. Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994;25:143. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 25.Blakolmer K. Jaskiewicz K. Dunsford H.A. Robson S.C. Hematopoietic stem cell markers are expressed by ductal plate and bile duct cells in developing human liver. Hepatology. 1995;21:1510. [PubMed] [Google Scholar]
- 26.Ruebner B.H. Blankenberg T.A. Burrows D.A. SooHoo W. Lund J.K. Development and transformation of the ductal plate in the developing human liver. Pediatr Pathol. 1990;10:55. doi: 10.3109/15513819009067096. [DOI] [PubMed] [Google Scholar]
- 27.Alessandri G. Girelli M. Taccagni G. Colombo A. Nicosia R. Caruso A. Baronio M. Pagano S. Cova L. Parati E. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- 28.Cheung W.-F. Cruz T.F. Turley E.A. Receptor for hyaluronan-mediated motility (RHAMM), a hyaladherin that regulates cell responses to growth factors. Biochem Soc Trans. 1999;27:135. doi: 10.1042/bst0270135. [DOI] [PubMed] [Google Scholar]
- 29.Culty M. Miyake K. Kincade P.W. Sikorski E. Butcher E.C. Underhill C. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990;111:2765. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu A. Luntz T. MacDonald J. Kubota H. Hsu E. London R. Reid L.M. Liver stem cells and lineage biology. In: Lanza R., editor; Langer R., editor; Vacanti J., editor. Principles of Tissue Engineering. San Diego: Academic Press; 2000. pp. 559–597. [Google Scholar]
- 31.Brill S. Holst P.A. Zvibel I. Fiorino A. Sigal S.H. Somasundaran U. Reid L.M. Extracellular matrix regulation of growth and gene expression in liver cell lineages and hepatomas. In: Arias I.M., editor; Boyer J.L., editor; Fausto N., editor; Jakoby W.B., editor; Schachter D., editor; Shafritz D.A., editor. Liver Biology and Pathobiology. New York: Raven Press; 1994. pp. 869–897. [Google Scholar]
- 32.Nimni M.E. Fibrillar collagens: their biosynthesis, molecular structure, and mode of assembly. In: Zern M., editor; Reid L.M., editor. Extracellular Matrix: Chemistry, Biology, and Pathobiology with Emphasis on the Liver. New York: Marcel Dekker; 1993. pp. 121–148. [Google Scholar]
- 33.Bornstein P. Sage H. Structurally distinct collagen types. Ann Rev Biochem. 1980;49:957. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- 34.Bulleid N.J. Wilson R.B. Lees J.F. Type III procollagen assembly in semi-intact cells: chain association, nucleation and triple-helix folding do not require formation of interchain disulphide bonds but triple-helix nucleation does require hydroxylation. Biochem J. 1996;317:195. doi: 10.1042/bj3170195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamberg A. Helaakoski T. Myllyharju J. Peltonen S. Notbohm H. Pihlajaniemi T. Kivirikko K. Characterization of human type III collagen expressed in a baculovirus system. Production of a protein with a stable triple helix requires co-expression with the two types of recombinant prolyl 4-hydroxylase subunit. J Biol Chem. 1996;271:11988. doi: 10.1074/jbc.271.20.11988. [DOI] [PubMed] [Google Scholar]
- 36.Furthmayr H. Basement membrane collagen: structure, assembly, and biosynthesis. In: Zern M., editor; Reid L.M., editor. Extracellular Matrix: Chemistry, Biology, and Pathobiology with Emphasis on the Liver. New York: Marcel Dekker; 1993. pp. 149–185. [Google Scholar]
- 37.Martinez-Hernandez A. Amenta P.S. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Archiv A Pathol Anat Histopathol. 1993;423:77. doi: 10.1007/BF01606580. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984;51:57. [PubMed] [Google Scholar]
- 39.Martinez-Hernandez A. Amenta P.S. Morphology, Localization, and Origin of the Hepatic Extracellular Matrix. New York: Marcel Dekker; 1993. [Google Scholar]
- 40.Liu X. Wu H. Byrne M. Krane S. Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA. 1997;94:1852. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konomi H. Sano J. Nagai Y. Immunohistochemical localization of type I, III and IV (basement membrane) collagens in the liver. Acta Pathol Jpn. 1981;31:973. doi: 10.1111/j.1440-1827.1981.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Hernandez A. Amenta P.S. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 43.Clement B. Rissel M. Peyrol S. Mazurier Y. Grimaud J.A. Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985;33:407. doi: 10.1177/33.5.3886779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.