Abstract
The GTP-binding protein RhoA regulates microfilament dynamics in many cell types and mediates the inhibition of axonal regeneration by myelin and chondroitin sulfate proteoglycans. Unlike most other nonsteroidal anti-inflammatory drugs, ibuprofen suppresses basal RhoA activity (Zhou et al., 2003). A recent report suggested that ibuprofen promotes corticospinal axon regeneration after spinal cord injury (Fu et al., 2007). Here, we confirm that ibuprofen reduces ligand-induced Rho signaling and myelin-induced inhibition of neurite outgrowth in vitro. Following 4 weeks of subcutaneous administration of ibuprofen, beginning 3 days after spinal cord contusion, animals recovered walking function to a greater degree, with twice as many rats achieving a hind limb weight-bearing status. We examined the relative role of tissue sparing, axonal sprouting, and axonal regeneration in the action of ibuprofen. Histologically, ibuprofen-treated animals display an increase in spared tissue without an alteration in astrocytic or microglial reaction. Ibuprofen increases axonal sprouting from serotonergic raphespinal axons, and from rostral corticospinal fibers in the injured spinal cord, but does not permit caudal corticospinal regeneration after spinal contusion. Treatment of mice with complete spinal cord transection demonstrates long-distance raphespinal axon regeneration in the presence of ibuprofen. Thus, administration of ibuprofen improves the recovery of rats from a clinically relevant spinal cord trauma by protecting tissue, stimulating axonal sprouting, and allowing a minor degree of raphespinal regeneration.
Key words: axon outgrowth, axon regeneration, ibuprofen, RhoA, myelin, spinal cord injury
Introduction
Many neurological conditions are characterized by the interruption of axonal connectivity. For example, although spinal cord trauma leads to segmental neuronal death, the majority of clinical sequelae result from severedconnections between surviving neurons (Harel and Strittmatter, 2006; Liu et al., 2006). The ability of axons to grow and restore connectivity in the adult central nervous system (CNS) is prevented in part by the presence of inhibitory molecules in the extracellular milieu. These inhibitors include the myelin-derived molecules, Nogo, myelin-associated glycoprotein (MAG), OMgp, and ephrinB3, as well as the astrocyte-scar-enriched chondroitin sulfate proteoglycans (CSPGs) (Buchli and Schwab, 2005; Harel and Strittmatter, 2006; Liu et al., 2006). Remarkably, neuronal signaling for both myelin-derived inhibitors and CSPGs converge upon activation of RhoA (Dergham et al., 2002; Fournier et al., 2003; Jin and Strittmatter, 1997; Lehmann et al., 1999; Monnier et al., 2003; Niederost et al., 2002), an intracellular GTP-binding protein that plays a prominent role in regulating actin filament polymerization and organization (Nobes and Hall, 1994).
These observations have motivated several attempts to promote recovery from spinal cord injury by reducing RhoA signaling (Dergham et al., 2002; Fournier et al., 2003). The Clostridium botulinum bacterium–derived protein, C3 exoenzyme, can inhibit cytoplasmic RhoA by ADP-ribosylation and stimulate axonal growth in vitro. Intracellular delivery of the C3 exoenzyme macromolecule in vivo can be problematic (Dergham et al., 2002; Fournier et al., 2003; Winton et al., 2002), but a modified version has entered clinical trials for spinal cord injury (SCI). There is evidence that Rho inhibitors have both protective and regenerative benefits after CNS injury (Dubreuil et al., 2003). Downstream of RhoA, Rho-associated kinase (ROCK) mediates, in part, the actions of RhoA (Borisoff et al., 2003; Chan et al., 2005; Fournier et al., 2003; Monnier et al., 2003; Nakagawa et al., 1996). ROCK inhibitors promote axonal growth in vitro, and improve behavioral recovery from spinal injury at certain doses when administered shortly after injury (Borisoff et al., 2003; Chan et al., 2005; Dergham et al., 2002; Fournier et al., 2003; Harper et al., 2004; Mukhopadhyay et al., 1994; Ramer et al., 2004; Schwab et al., 2004; Tanaka et al., 2004; Yamagishi et al., 2005). It remains possible that more complete in vivo inhibition of Rho activation might provide a valuable and effective approach to treat SCI (Baptiste and Fehlings, 2006; McKerracher and Higuchi, 2006; Mueller et al., 2005). One study demonstrated that ibuprofen and several other nonsteroidal anti-inflammatory drugs (NSAIDs) reduce RhoA activation (Zhou et al., 2003). Recently, it was reported that ibuprofen prevents myelin inhibition of outgrowth by reducing Rho activation, and also stimulates corticospinal axonal regeneration after spinal cord injury (Fu et al., 2007).
Here, we re-examined ibuprofen's potential to promote recovery from spinal cord injury and investigated the mechanism of potential benefit. We confirm that ibuprofen reduces, but does not eliminate, RhoA signaling and myelin inhibition of axonal outgrowth in vitro. When administered beginning 3 days after spinal cord contusion in rats, ibuprofen improves the recovery of locomotion with both neuroprotective and axon growth–stimulating activity. Though ibuprofen failed to support corticospinal regeneration, the widely used and relatively safe compound has potential therapeutic value for promoting recovery after subacute neurotrauma.
Methods
NIH 3T3 luciferase assay
NIH 3T3 fibroblasts (3–5 million cells) were transfected (Amaxa, program O-13) with a pSRE-luciferase reporter plasmid (2 μg; Promega, Madison, WI) and plated into a six-well plate (Fischer, ) in DMEM and 10% fetal bovine serum for 12–24 h. Following a subsequent 24-h serum starvation, NSAIDs were added for 3–4 h prior to a 6-h LPA stimulation (10 μM). The cells were then lysed and luciferase activity measured from triplicate wells using a luciferase reporter kit (Promega) following the manufacturer's instructions (n = 3–6 separate experiments).
Growth cone collapse and neurite outgrowth assays
E13 chick dorsal root ganglia explants were isolated and cultured as previously described (Fournier et al., 2001; Fournier et al., 2003; GrandPre et al., 2000). Explants were treated with 50 μM of ibuprofen and 10 μM Y-27632 (Millipore, Billerica, MA) 15 min before addition of clustered 100 nM MAG-Fc (Niederost et al., 2002). After 30 min, the explants were fixed and stained with rhodamine phalloidin (Molecular Probes, Eugene, OR). Growth cone morphology was scored as spread or collapsed by an observer unaware of the treatment. Data are presented as mean ± SEM from separate experiments (n = 3).
For SH-SY5Y neuroblastoma outgrowth assays, 96-well plates (Fisher) were coated with fibronectin (10 μg/mL, 1 h, 37°; Sigma, St. Louis, MO). Control or MAG-expressing CHO cells were plated at 30,000 cells/well 24–36 h prior to adding pre-differentiated neuroblastoma cells. The SH-SY5Y cells were pre-differentiated for 6 days with 2% fetal bovine serum and 10 μM retinoic acid and added to the CHO cell monolayers. Neurons were grown at 37° for 20–24 h. Neurite outgrowth per cell was normalized to the control outgrowth for each experiment and presented as mean ± SEM from separate experiments (n = 5–9).
CGN outgrowth was assessed on culture plates prepared by sequentially coating with nitrocellulose (0.8 cm2/mL methanol, 2 min, RT), MAG-Fc (0–800 ng/well, 2 h, RT; Sigma), PDL (100 μg/mL, overnight, 4°), BSA (10 μg/mL, 1 h, 23°), and laminin (3 μg/mL, 2 h, 37°; Invitrogen, Carlsbad, CA). P7-P12 rat CGNs were dissected and dissociated as described elsewhere (Fournier et al., 2003) and plated (3000–5000 cells/well) onto MAG or control plates. Neurons were cultured at 37° for 16 h. Neurite outgrowth per cell was normalized to control wells for each experiment and presented as mean ± SEM from separate experiments (n = 7).
For neuronal outgrowth assays, ibuprofen (500 μM; Sigma), naproxen (400 μM; Sigma), or Y-27632 (10 μM; EMD Chemicals, Gibbstown, NJ) were dissolved in PBS and added at the time of plating of the neuronal cells. After the outgrowth period, the neurons were fixed and stained with anti-βIII tubulin antibody (Covance, Princeton, NJ). Neurite outgrowth per neuron was quantified using an automated cellular imaging and analysis system (Axon Instruments, Union City, CA).
Rat spinal contusion with subacute subcutaneous therapy
Female Sprague-Dawley rats (11–12 weeks of age, 250–270 g) were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). A laminectomy was conducted at the caudal portion of T6 and all of T7 spinal levels. A T7 moderate contusion injury (10 g, 25 mm) was produced with the Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor (Young, 2002). Three days after the contusion injury, animals were re-anesthetized and an Alzet 2ML4 osmotic minipump (Alza Scientific Products, Palo Alto, CA) was implanted subcutaneously on each animal's back. The pumps delivered 2.5 μL/h for 28 days and were filled with 500 mg ibuprofen (70 mg/kg/d) or 150 mg naproxen (25 mg/kg/d) in 2 mL PBS or PBS alone. For the rat behavioral testing, the locomotor scale developed by Basso, Beatty, and Bresnahan (BBB) was used (Basso et al., 1995; Basso et al., 1996). All behavioral tests were performed by researchers unaware of the identity of the compound in the minipump.
Rho pull-down assay
GST-Rho binding domain was expressed in E. coli and bound to glutathione-agarose beads (Ren and Schwartz, 2000). The coupled beads were washed and stored in wash buffer (50 mM Tris pH 7.5, 0.5% Triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.1 mM PMSF). Spinal cord segments (∼2 cm) were weighed and mechanically homogenized in modified RIPA buffer (50 mM Tris pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 500 mM NaCl, 10 mM MgCl2, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM PMSF) at a concentration of 100 μg tissue (wet weight)/mL. The homogenates were clarified by two centrifugation steps (10 min, 18,000 × g at 4°C) and incubated for 1 h at 4°C with 50 μL of GST-Rho binding domain coupled beads. The beads were washed four times, eluted in sample buffer, and run on a 15% SDS-PAGE gel. GTP- bound Rho and total Rho were detected by Western blot using a monoclonal RhoA antibody (Santa Cruz Biotechnology, Santa Cruz, CA), with secondary detection using anti-mouse alkaline phosphatase–conjugated antibody and quantified using Image J software (http://rsweb.nih/gov/ij/index.html).
Anterograde CST tracing
To trace corticospinal tract (CST) axons, a burr hole was made in the skull overlying the left sensorimotor cortex at 36 days after contusion injury. Biotin dextran amine (BDA; MW 10,000; 10% in PBS; Molecular Probes) was applied at seven injection sites at a depth of 1.5 mm from the cortical surface. The rats were sacrificed 2 weeks after BDA injection (49 days after contusion) and spinal sections were processed histologically(GrandPre et al., 2002; Kim et al., 2003; Kim et al., 2004; Li et al., 2004; Li and Strittmatter, 2003).
Histological analysis of rat tissue
The animals were deeply anesthetized and then perfused transcardially with PBS, followed by 4% paraformaldehyde/PBS solution at either 2 weeks or 6.5 weeks after treatment was initiated. The rat spinal cord from 10 mm rostral to the contusion through 10 mm caudal to the lesion's center was embedded in a glutaraldehyde-polymerized albumin matrix, and cut parasagittally (40 μm) on a vibratory microtome. Transverse sections (40 μm) were collected from the spinal cord 11–15 mm rostral to and 11–15 mm caudal to the lesion's center. The sections were incubated with avidin-biotin-peroxidase complex to detect BDA tracer, and/or with an anti-5-HT antibody (1:10,000; Immunostar, Hudson, WI). Labeling was visualized by nickel-enhanced diaminobenzidine HRP reaction or with Extra-Avidin and Alexa Fluor568. Activated microglia/macrophages and astrocytes were detected in parasagittal spinal cord sections (40 μm) extending from 10 mm rostral to 10 mm caudal of the lesion's center with the primary antibodies ED1 (1:500; Serotec, Oxford, U.K.) and rabbit anti-GFAP (1:500; Sigma). Bound primary antibodies were detected by Alexa Fluor conjugated secondary antibody.
All histological analyses were accomplished with NIH image version 1.62 as described elsewhere (GrandPre et al., 2002; Kim et al., 2003; Kim et al., 2004; Li et al., 2004; Li and Strittmatter, 2003). For analysis of serotonin innervation, immunoreactive serotonin fibers in the ventral horn of transverse sections rostral or caudal to the lesion's center were selected by the thresholding, and then the length of serotonin fiber per cross-sectional area was measured with the skeletonize function. Ten serial longitudinal sections from each animal were analyzed for tissue damage. The widths of spared tissue at the lesion's center were measured, and the values from these sections were averaged. For analysis of CST sprouting, longitudinal sections containing the injury or transverse sections from 11–15 mm rostral to the lesion's center were assessed. In parasagittal sections, the number CST fibers crossing a dorsal/ventral line at various distances rostral to the lesion's center of the injured site was measured in each section for each rat. In transverse sections, the optical density of the dorsal CST and the dorsolateral quadrant of the ipsilateral spinal cord without the dorsal CST proper were determined. A densitometric measure for CST sprouting was obtained by dividing the value of the ipsilateral dorsolateral quadrant area by the density of dorsal CST. For camera lucida–style tracing of BDA-labeled CST fibers, serial longitudinal sections were digitally photographed, and fibers were traced using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). For camera lucida tracing of 5-HT-immunoreactive fibers, 10 sections collected at 200-μm intervals from each animal were traced by the method described for CST fibers. For microglia/macrophage invasion and astrocyte reaction analysis, three serial longitudinal sections containing the center of the injury site were analyzed for each animal. The area of ED1 or GFAP immunoreactive cellular staining was measured as a ratio to the total area after utilizing the threshold function.
Mouse complete spinal transection
Female C57/Bl6 mice (8–9 weeks of age, 20 g body weight) were anesthetized with an intraperitoneal injection of ketamine and xylazine. A laminectomy was conducted at the T8 spinal level (Young, 2002). A T8 complete transection injury was created by first cutting the spinal cord with microscissors, and then cutting twice with a no. 11 blade scalpel. The cutting instruments were pressed firmly against the bone anterior and lateral to the spinal cord to ensure a complete transection. All mice that exhibited BMS scores of 1 or more at any later time point or had evidence of caudal 5-HT fibers were examined by GFAP immunohistology for any remaining tissue bridges. Of 47 mice studied, one mouse in the PBS group was excluded due to histological evidence of an incomplete transection. If this single incompletely transected mouse is included in the statistical analysis, then the increase in caudal 5-HT fibers and in BMS scores for the ibuprofen versus PBS mice is reduced, but it remains significant (p < 0.05). In the same surgery, an Alzet 2004 osmotic minipump (Alza Scientific Products) was implanted subcutaneously on each animal's back. The pumps delivered 0.25 μL/h for 28 days and were filled with 20 mg ibuprofen (35 mg/kg/d; n = 13 mice) or 40 mg ibuprofen (70 mg/kg/d; n = 22) in 0.2 mL PBS or 0.2 mL PBS alone (n = 12). For the mouse behavioral testing, the Basso Mouse Scale (BMS) scale was used (Basso et al., 1995; Basso et al., 1996). All behavioral assessments were done by two observers who were unaware of the identity of the compound in the minipump.
For histological analysis of the complete transection mice, tissue was processed for anti-5-HT and anti-GFAP staining as for the spinal contused rat samples. Serotonin fibers in the caudal spinal cord were measured from stains of transverse sections 5 mm caudal to the injury site.
Results
Ibuprofen blocks Rho-mediated signaling
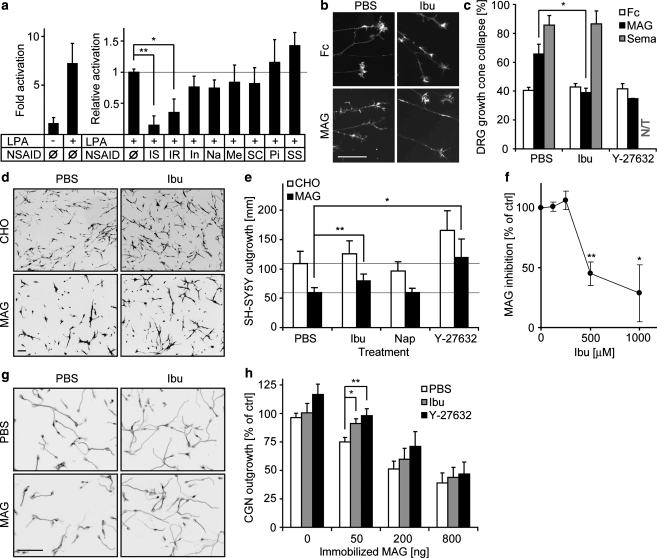
Published work has shown that ibuprofen and a selected subset of NSAIDs can inhibit basal Rho activity via a mechanism independent from cyclooxygenase inhibition (Fu et al., 2007; Jin et al., 2006; Zhou et al., 2003). We examined whether ibuprofen prevents receptor-activated Rho activity, in addition to basal activity. In non-neuronal cells, the lysophosphatidic acid (LPA) receptor is known to activate Rho through a seven transmembrane domain receptor coupled to a heterotrimeric G protein. This Rho activation can be measured by several methods, including increased transcription of a luciferase reporter regulated by the c-Fos gene serum response element (SRE) (Goetzl et al., 1999; Niu et al., 2003; Price et al., 1996) (Fig. 1a). Ibuprofen, but not the majority of cyclooxygenase inhibitors, blocks this LPA-induced Rho activation (Fig. 1a). Interestingly, indomethacin, an NSAID that has been tested in several SCI studies with minimal benefit (Guven et al., 1999; Pantovic et al., 2005; Schwab et al., 2004), does not block Rho signaling in our in vitro studies.
FIG. 1.
Ibuprofen inhibits ligand-induced Rho activation and prevents MAG inhibition. (a) LPA induces a sevenfold activation of pSRE in 3T3 fibroblasts. S-ibuprofen (IS, 500 μM), and R-ibuprofen (IR, 500 μM) significantly reduce SRE activation. Other NSAIDs, including indomethacin (In, 100 μM), naproxen (Na, 400 μM), meloxicam (Me, 250 μM), SC-560 (SC, 50 μM), piroxicam (Pi, 300 μM) and sulindac sulfate (SS, 100 μM), do not prevent SRE activation (*p < 0.05; **p < 0.01 on Student's t-test). (b and c) Ibuprofen abolishes MAG-induced, but not Sema-3A-induced, growth cone collapse of E13 chick DRG (*p < 0.05 on Student's t-test). (d and e) A MAG-expressing CHO feeder layer inhibits SH-SY5Y cell neurite outgrowth by 50%, and ibuprofen (Ibu, 500 μM) and Y-27632 (10 μM), but not naproxen (Nap, 400 μM), reduce this inhibitory effect (*p < 0.05; **p < 0.01 on Student's t-test). (f) Dose-response curve with increasing concentration of ibuprofen (*p < 0.05; **p < 0.01 on Student's t-test). (g and h) Ibuprofen (500 μM) reduces the inhibitory effect of MAG on P7-12 rat CGNs (*p = 0.01; **p < 0.01 on Student's t-test; scale bars are 100 μm).
Ibuprofen protects neurons from MAG inhibition of outgrowth in vitro
To determine if a reduction of Rho-dependent signaling by ibuprofen might mitigate neurite outgrowth inhibition, we examined the responses of neurons treated with ibuprofen to myelin-associated glycoprotein (MAG), one of several proteins inhibiting neurite outgrowth through the NgR signaling pathway (Domeniconi et al., 2002; McGee and Strittmatter, 2003). Among the myelin inhibitors, MAG utilizes both NgR-dependent and NgR-independent pathways, so its mechanism of action is the most diverse of these inhibitors (Chivatakarn et al., 2007; Mehta et al., 2007; Venkatesh et al., 2007). Acutely, MAG induces growth cone collapse in cultured dorsal root ganglion neurons (Liu et al., 2002) (Fig. 1b,c). Ibuprofen suppresses MAG-induced collapse, so the majority of growth cones remain spread. This effect is similar in magnitude to that achieved by the ROCK inhibitor, Y-27632. Another collapsing agent, Sema3A, collapses dorsal root ganglion growth cones through pathways independent of Rho/ROCK. Ibuprofen did not block Sema-3A-mediated growth cone collapse (Fig. 1c).
To determine if ibuprofen, in addition to preventing acute growth cone collapse, also reduces chronic inhibition of outgrowth by myelin-associated inhibitors, differentiated SH-SY5Y neuroblastoma cells were cultured on MAG-expressing CHO cells (Fig. 1d). Ibuprofen does not alter basal outgrowth in control CHO lines, but the compound significantly increases growth on MAG cells (Fig. 1d,e). The apparent EC50 is between 250 and 500 μM ibuprofen (Fig. 1f). In contrast, naproxen does not inhibit Rho signaling (Fig. 1a), and does not promote outgrowth on MAG (Fig. 1e). The Y-27632 ROCK inhibitor increases outgrowth on both MAG and control substrates, suggesting a role in basal axonal extension (Fig. 1e). We also examined the inhibition of neurite outgrowth from primary cultures of rat cerebellar granule neurons (CGN) by substratum-bound MAG-Fc protein (Fig. 1g). MAG-mediated inhibition of outgrowth is significantly reduced by ibuprofen and Y-27632 at moderate, but not at high, levels of MAG. The efficacy of ibuprofen is more complete in growth cone collapse assays than in outgrowth assays. This may relate to acute versus chronic activities, to different inhibitor presentations, or to different cell types (Chivatakarn et al., 2007; Mehta et al., 2007; Venkatesh et al., 2007). The in-vitro data reveal a less complete abolition of Rho signaling than that seen in previous studies (Fu et al., 2007), but they suggest that ibuprofen might promote injury-induced growth of CNS axons in vivo.
Improved recovery from spinal contusion after subacute therapy with ibuprofen
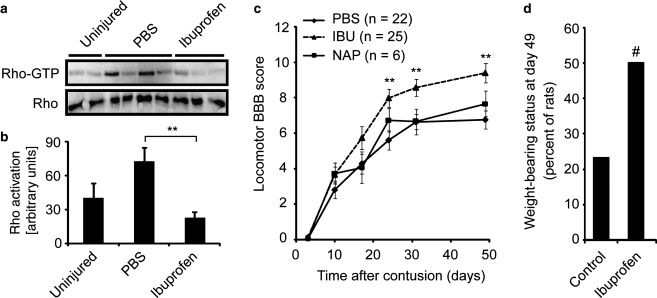
To investigate the potential of ibuprofen-mediated Rho inhibition to promote axonal growth and neurological recovery in vivo, we examined adult rats with moderate spinal contusions produced by the Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor method (Young, 2002). This lesion model is thought to most accurately recreate the multifaceted histopathology of clinical spinal cord trauma. Ibuprofen was administered subcutaneously beginning 3 days after contusion. The maximal adult human U.S. Food and Drug Administration approved dosage is 3200 mg/d without an adjustment for weight, equivalent to 46–64 mg/kg/d in a 50–70 kg person. The maximal tolerated dose in rats is between 240 and 375 mg/kg/d (Antezana et al., 2003; Melarange et al., 1992; Wax et al., 1975), and other neurological conditions have been treated with 56–120 mg/kg/d in rodents (Antezana et al., 2003; Fu et al., 2007; Lim et al., 2000; Lopez et al., 2006; Park et al., 2005; Richardson et al., 2005). In the studies detailed here, treatment continued for 4 weeks at a dosage of 70 mg/kg/d of ibuprofen. Biochemical studies of RhoA activation levels 1 week after a spinal contusion in spinal cord rostral to the lesion site verified that the twofold RhoA activation induced by injury is suppressed by this dose of ibuprofen in vivo (Fig. 2a,b). In addition to a PBS vehicle control group, a naproxen control group was included to test whether any differences associated with ibuprofen are generalizable properties of NSAIDs or a more specialized property, such as Rho inhibition.
FIG. 2.
Ibuprofen treatment improves functional recovery after rat spinal contusion injury. (a) GTP-bound RhoA (Rho-GTP) and total RhoA (Rho) were detected in rostral spinal cords of uninjured animals or injured animals treated with PBS or ibuprofen as in c at day 7. (b) Rho-GTP was quantified from three independent experiments. Ibuprofen treatment significantly decreased RhoA activation in the contused spinal cord (**p < 0.01 on Student's t-test). (c) Subacute subcutaneous treatment with PBS, naproxen (NAP), or ibuprofen (IBU) was provided to rats after a moderate contusion injury at the T7 spinal level from days 3–31 post-injury. The BBB score is reported as a function of time (**p ≤ 0.01 for PBS versus ibuprofen by non-parametric Mann-Whitney two-tailed U test). (d) The fraction of rats with BBB scores >9 (supporting their body with the hind limbs) is reported for the subacute delayed subcutaneous treatment rats at 6.5 weeks after treatment was initiated (#χ2 = 4.1; p = 0.04).
Locomotor performance in an open field was assessed at several time points after injury with the BBB locomotor scale (Basso et al., 1995; Basso et al., 1996) by observers unaware of the treatment. The BBB scores showed a significant improvement in locomotion for the ibuprofen-treated group after 3 weeks of treatment (Fig. 2c [8.0 ± 0.5 in the ibuprofen group]; [5.6 ± 0.6 in the PBS group]; [6.8 ± 0.7 in the naproxen group]; p < 0.01 on the Mann-Whitney two-tailed U test comparing PBS and ibuprofen). The benefit of ibuprofen persisted beyond the cessation of treatment and reached a maximum at day 49, the end point of the experiment (Fig. 2c; [9.4 ± 0.5 in the ibuprofen group]; [6.8 ± 0.5 in the PBS group]; [7.7 ± 0.7 in the naproxen group]; p < 0.002 on the Mann-Whitney two-tailed U test comparing PBS and ibuprofen). At this point more than half (13 of 25) of the ibuprofen-treated rats could support their weight with their hind limbs, while only a quarter (7 of 28) of the rats from the two control groups could do so (Fig. 2d). The persistence of the ibuprofen effect beyond the half-life of the compound demonstrates that the compound promotes a persistent change in neuronal anatomy and/or function, rather than acting in a purely symptomatic mode to enhance activity or performance. Similarly, the observation that ibuprofen, but not naproxen, improves recovery is consistent with the hypothesis that ibuprofen's selective ability to block Rho accounts for the observed functional improvements. These behavioral benefits of ibuprofen therapy for rat spinal contusion largely confirm the results of the study of Fu and colleagues (Fu et al., 2007).
Ibuprofen spares tissue by neuroprotection
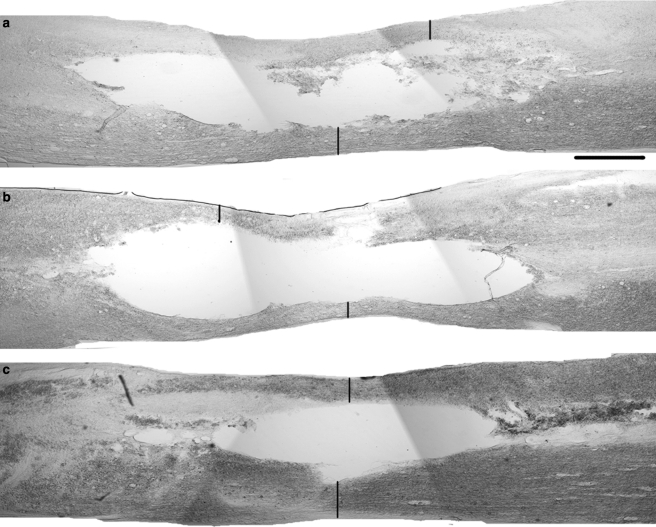
To determine whether the benefits of ibuprofen where primarily at the level of axonal sprouting and regeneration (as suggested by Fig. 1b–h), or limitations on tissue damage (McKerracher and Higuchi, 2006; Smirnova et al., 2001; Trapp et al., 2001), we measured the area of spared tissue at the injury epicenter in animals from each treatment group. Spared tissue was measured at the center of the spinal cord contusion in two groups of rats, one sacrificed after 2 weeks of treatment (17 days post-injury), and another sacrificed at 49 days post-injury (6.5 weeks after the initiation of the 1-month treatment period beginning on post-injury day three) (Fig. 3). In all groups, the majority of spinal cord tissue was destroyed within 2 weeks of the contusion injury. There was a minor degree of further tissue loss between 2 and 6 weeks post-treatment initiation (Fig. 3d). The degree of spared tissue was greater in the ibuprofen-treated group than in the PBS-treated group at 2 weeks. Because there was no significant difference in locomotor scores in this time range, the functional consequence of this sparing may or may not account for the later differences in locomotion. The difference in tissue sparing persisted at 6 weeks (Fig. 3d), by which time the functional recovery of the ibuprofen group had diverged from the PBS and naproxen groups (Fig. 2). Tissue sparing in the naproxen-treated group was identical to that in the PBS group, demonstrating that any limitations on tissue damage were specific to the ibuprofen treatment, and were potentially Rho-dependent.
FIG. 3.
Subcutaneous ibuprofen treatment increases spared tissue at the site of a contusion injury. (a–c) Parasagittal sections of the lesion segments of spinal cords from animals treated with phosphate-buffered saline (PBS) (a), naproxen (b), and ibuprofen (c) show the spared tissue (vertical bars) within the lesion center. Rostral is to the left and dorsal is up (scale bar = 500 μm). (d) There is significantly greater spared tissue in the ibuprofen 2-week-treated (n = 10) and 6-week-treated (n = 9) groups compared to the corresponding naproxen-treated (2-week-treated group n = 7; 6-week-treated group n = 7) and PBS-treated groups (2-week-treated group n = 10; 6-week-treated group n = 9; **p < 0.01 by Student's t-test).
Tissue preservation by ibuprofen might involve effects on numerous cell types, including microglia/macrophage invasion, astroglial proliferation, and neuronal protection. We examined potential ibuprofen effects on inflammatory and astrocytic reactions of the contused spinal cord immunohistologically after 2 weeks of treatment. ED-1 antibodies were employed as markers for activated microglia/macrophages (Fig. 4a–c), and anti-GFAP antibodies for reactive astrocytes (Fig. 4d–f). The area of spinal cord tissue infiltrated with activated microglia/macrophages or GFAP-positive cells was determined as a function of rostral-caudal position (Fig. 4g,h). No significant difference in non-neuronal cell reaction was detected between the groups at 2 weeks post-treatment. Thus, an ibuprofen-specific effect on tissue sparing appears to be most attributable to neuronal protection, as opposed to modulation of inflammatory or tissue reaction mechanisms.
FIG. 4.
Effects of ibuprofen treatment on microglia/macrophage and astrocyte reactions in the lesion area. (a–c) Parasagittal sections from rats treated with phosphate-buffered saline (PBS) (a), naproxen (NAP) (b), and ibuprofen (IBU) (c), stained with the activated microglia/macrophage marker ED1 reveal the cell invasion of the injured area of the spinal cord. Tissue was collected after 2 weeks of treatment beginning at 3 days post-contusion. (d–f) Parasagittal sections from rats treated with phosphate-buffered saline (PBS) (d), naproxen (NAP) (e), and ibuprofen (IBU) (f), stained with anti-GFAP antibodies illustrate the astrocyte reaction in the injured area of the spinal cord. (g) Quantification of the ED1-positive cell area as a fraction of total area shows no significant difference in microglia/macrophage invasion between the three groups. For the x-axis, a positive value is rostral to the center of the lesion, and a negative value is caudal to the center of the lesion. (h) Quantification of GFAP-positive cell area as a fraction of total area demonstrates no significant difference between the astrocytic reactions in the three groups (data are mean ± SEM).
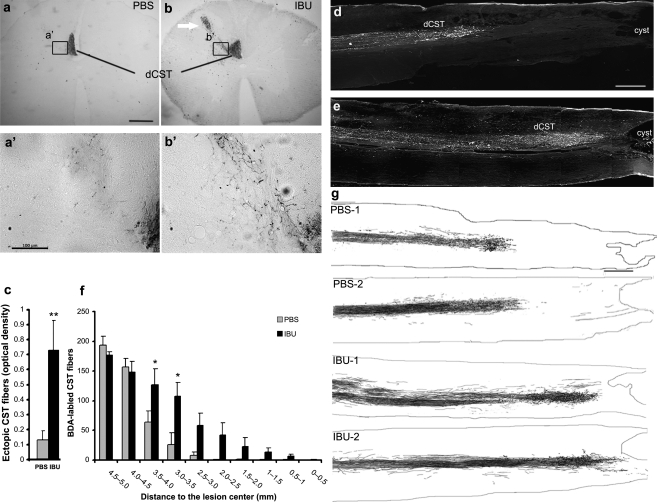
Ibuprofen promotes CST sprouting and increases rostral CST fiber density
Given the role of Rho as a transducer of CSPG- and myelin-associated inhibition of axonal growth, ibuprofen may mediate improved neurological recovery by promoting axonal growth in sprouting and/or regeneration. Such an effect would fit with the delayed behavioral benefit of ibuprofen seen in Fig. 2c, and would be synergistic with a tissue-sparing effect. As a first step to assessing axonal growth, the descending corticospinal tract (CST) was labeled by anterograde axonal tracing with biotin dextran amine (BDA) injection into the left sensorimotor cortex 2 weeks prior to the end point of the experiment (Fig. 5). Transverse spinal sections at 10 mm rostral to the lesion center of ibuprofen-treated animals exhibit increased numbers of non-fasciculated fibers sprouting from the dorsal CST. The density of such fibers is increased fivefold in ibuprofen-treated versus PBS-treated rats (Fig. 5a–c). In parasagittal sections including the contusion site, nearly all CST fibers retract rostrally by 3 mm in PBS-treated animals (Fig. 5d,f,g). In contrast, many BDA-labeled CST fibers in ibuprofen-treated rats reach the rostral edge of the lesion cavity (Fig. 5e–g). Significantly greater numbers of CST fibers are present at 2.5–3.5 mm rostral to the injury in parasagittal sections in these rats (Fig. 5f). This static analysis cannot distinguish whether these fibers retracted and then sprouted, or never retracted to the same degree as fibers in the control group. Because anterograde tracing requires a prolonged survival period, early time points cannot be studied effectively by this method alone. It should be noted that no BDA-labeled CST fibers were observed caudal to the lesion sites in any rats (Fig. 5d–g). Thus, ibuprofen promoted local CST sprouting, but not long-distance growth of CST fibers through or past a spinal contusion. This result differs from that reported previously for dorsal hemisection and contusion (Fu et al., 2007).
FIG. 5.
Subcutaneous ibuprofen treatment increases the sprouting of corticospinal tract (CST) fibers rostral to the injury site. (a and b) Transverse sections at 11–15 mm rostral to the contusion site show a greater number of BDA-labeled CST fibers outside of the dorsal column (arrow) in animals treated with ibuprofen (IBU) than in those treated with phosphate-buffered saline (PBS). The rats were sacrificed 6.5 weeks after the initiation of treatment. The insets shown in a’ and b’ provide higher magnification of the boxed regions from a and b. (c) The ratio of optical densities of sprouting BDA-labeled fibers in the dorsolateral quadrant of the ipsilateral spinal cord relative the density of BDA staining in the dorsal CST was measured from sections like those seen in a and b (**p < 0.01 on Student's t-test). (d and e) Parasagittal micrographs illustrate BDA-labeled dorsal CST (dCST) fibers from PBS-treated animals in d and ibuprofen-treated animals in e. “cyst” indicates the cystic contusion cavity. Rostral is to the left and dorsal is up. (f) Counts reveal a greater number of BDA-labeled CST fibers in the ibuprofen-treated group than in PBS-treated group from 2.5 mm to 3.5 mm rostral to the lesion center (*p < 0.05 on Student's t-test). (g) Camera lucida drawings of CST fibers from four spine-injured rats. Images are generated from all serial parasagittal sections for each rat. Rostral is to the left and dorsal is up. The contusion cavity is at the right (scale bar = 100 μm in a and 500 μm in d and g).
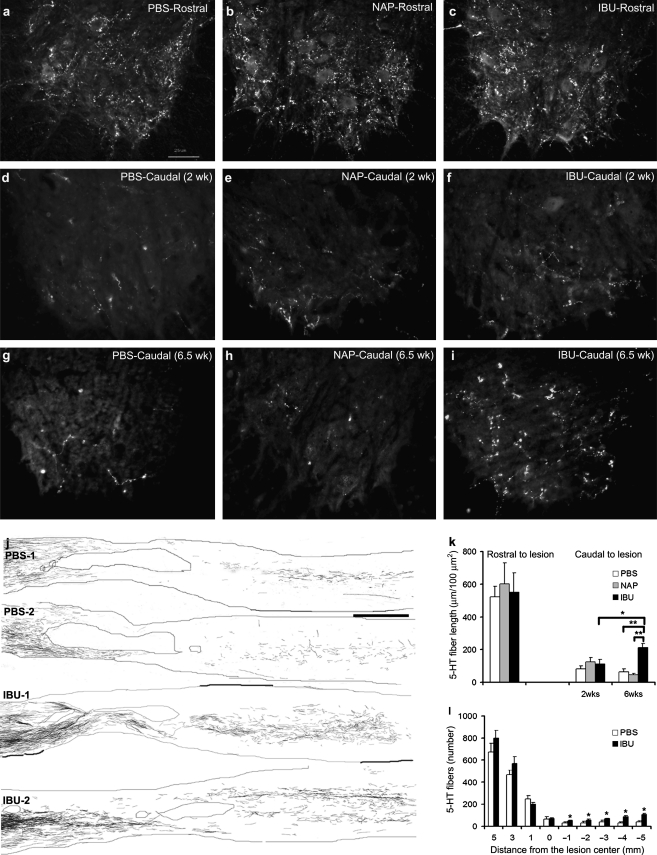
Ibuprofen promotes raphespinal sprouting in the caudal spinal cord
While the CST may play a major role in a range of critical functions after human spinal cord trauma, it has a relatively minor or non-existent role in rodent locomotion (Cafferty and Strittmatter, 2006; Weidner et al., 2001). Among the fiber systems that contribute to a greater extent in rat walking is the serotonergic raphespinal system (Antri et al., 2002; Kim et al., 2004; Li et al., 2004; Saruhashi et al., 1996; Schmidt and Jordan, 2000). The pattern of serotonergic (5-HT) innervation fibers in the ventral horn of a transverse section from 11–15 mm rostral to the lesion center is similar in all groups of injured rats (Fig. 6a–c). In transverse sections caudal to the lesion site, the length of serotonergic fibers in the ventral horn was assessed at 2 weeks or 6.5 weeks after treatment initiation in each group (Fig. 6d–i). At 2 weeks, the density of serotonergic fibers in the caudal ventral horn is reduced compared to rostral levels, and is indistinguishable between groups (Fig. 6d–f,k). Thus there is no evidence for raphespinal neuroprotection by ibuprofen. During an additional 4 weeks of recovery, the ibuprofen and control groups show opposite changes. The naproxen- and PBS-treated groups display a further loss of raphespinal innervation of the caudal spinal cord (Fig. 6g,h,k). In contrast, the ibuprofen-treated group demonstrates a significant increase in serotonergic fiber length during this period (Fig. 6k). Thus ibuprofen treatment supports caudal 5-HT fiber growth 2–6 weeks after spinal contusion.
FIG. 6.
Raphespinal fiber growth is increased by ibuprofen treatment after rat spinal contusion injury. (a–c) Transverse sections of spinal cord stained with anti-5-HT antibodies 11–15 mm rostral to the lesion center from animals treated with phosphate-buffered saline (PBS) (a), naproxen (NAP) (b), and ibuprofen (IBU) (c) have similar numbers of serotonergic fibers in the ventral horn. (d–f) Transverse sections of spinal cord 11–15 mm caudal to the lesion center were obtained from rats at 17 days post-injury, after 2 weeks of treatment with PBS (d), NAP (e), or IBU (f). Anti-5-HT staining demonstrates a marked decrease in the number of serotonergic fibers in the ventral horn compared with sections rostral to the lesion. There is no significant difference between the treated groups at this time point. (g–i) Anti-5-HT immunohistology of transverse sections of spinal cord 11–15 mm caudal to the lesion center was obtained from rats at 6.5 weeks after treatment initiation on day 3 post-contusion. Note that the prevalence of serotonergic fibers in the ventral horn of rats treated with PBS (g) and NAP (h) remains minimal, but that there is an increase in the rats treated with IBU (i). (k) Measurement of serotonergic fiber length per area in the ventral horn caudal to the lesion indicates that there is a significant increase in serotonergic fibers in the ventral horn caudal to the lesion in ibuprofen-treated animals by 6 weeks after treatment initiation (*p < 0.05; **p < 0.01 on Student's t-test). (l) Serotonergic fiber number at various distances rostral and caudal to the center of the lesion from ibuprofen-treated and control animals are shown (*p < 0.05 on Student's t-test). For the x-axis, a positive value is rostral to the center of the lesion, and a negative value is caudal to the center of the lesion. (j) Camera lucida drawings of serotonergic fibers from four separate rats. Each drawing is a composite assembled from a set of 10 parasagittal sections spaced at intervals of 200 μm across the spinal cord. The contusion cavity is encircled near the center of each image. Increased numbers of serotonergic fibers are observed in the caudal spinal cord in the ibuprofen-treated (IBU-1 and IBU-2) animals compared with the PBS-treated animals (PBS-1 and PBS-2) [scale bars = 25 μm (a) and 1000 μm (j)].
The ibuprofen-mediated elevation in 5-HT fiber density caudal to the lesion may be due to stimulation of long-distance fiber growth from rostral segments or segmental sprouting of the few intact raphespinal fibers. We examined the longitudinal pattern of serotonergic innervation in parasagittal sections across the injury site in ibuprofen-treated and PBS-treated animals at 6 weeks post-injury to distinguish between these two mechanisms (Fig. 6j). Camera lucida drawings and fiber counts along the rostral/caudal axis indicate that ibuprofen treatment increases the number of serotonergic fibers in spared tissue bridges at the lesion site to a minor degree (Fig. 6j,L). This is consistent with either tissue-sparing or long-distance axonal regeneration. However, the differences in fiber number are greater between the ibuprofen and control groups in the more caudal segments (Fig. 6j–L), demonstrating that caudal regenerative sprouting plays a prominent role in enhancing the recovery of 5-HT innervation to the distal spinal cord following contusion injury.
Serotonergic regeneration after complete spinal transection
Because spinal contusion creates a partial lesion, it is impossible to unambiguously distinguish between ibuprofen-induced serotonergic axon sprouting in the distal cord from long-distance axonal regeneration. Therefore, we examined the effect of ibuprofen treatment on the mouse raphespinal system 5 weeks after a complete thoracic spinal transection. In vehicle-treated mice, no serotonergic fibers are detected in transverse sections 5 mm caudal to the injury in any mouse with an injury determined to be complete by immunohistological examination of the injury site (Fig. 7a,b,e,f). Camera lucida drawings of sagittal sections illustrate the many rostral raphespinal fibers that abruptly stop at the injury and do not reach into the caudal segment (Fig. 7b). In contrast, a subset of ibuprofen-treated mice (14 of 35) exhibited evidence of serotonergic fibers regenerating through the injury site and extending into the caudal spinal cord (Fig. 7a,b). High-magnification sagittal views of the injury site show serotonergic fibers traversing a complete transection injury (Fig. 7 c,d). At a distance of 5 mm caudal to the lesion site, significant 5-HT fiber length was detectable in ibuprofen-treated complete transection mice (Fig. 7e,f). The 70 mg/kg/d dose of ibuprofen, which was utilized in the contusion studies (Fig. 3) and was shown to inhibit Rho activity in the spinal cord (Fig. 2), showed a trend towards a greater effect here than the 35 mg/kg/d dose (Fig. 7f). It is possible that the weak effect of 35 mg/kg/d of ibuprofen on axonal regeneration (Fig. 7f) occurs through a different, non-Rho mechanism.
FIG. 7.
Raphespinal axon regeneration stimulated by ibuprofen treatment after mouse complete spinal transection. (a) Sagittal sections from three different control PBS mice and three different ibuprofen-treated mice were stained with anti-GFAP antibodies. Note that the spinal transection is complete for the six mice shown in a. All mice included in the study with evidence of raphespinal growth in transverse caudal section or BMS scores of 1 or greater after transection were verified to have complete lesions by this method. The red boxes correspond to the areas magnified in c and d. (b) Camera lucida drawings of 5-HT-immunoreactive fibers are illustrated from one PBS animals and two different ibuprofen-treated mice at 5 weeks after complete spinal transection. In each case, fibers were traced from six 100-μm parasagittal sections spaced at 400-μm intervals across the cord, and the traced fibers are superimposed. The boxed area is shown in d. (c and d) High-magnification images of sagittal sections stained for anti-5-HT (green) and anti-GFAP (red) immunoreactivity from two of the ibuprofen-treated animals shown in a. Note the raphespinal fibers traversing the lesion site (double arrows) and reaching the caudal spinal cord (single arrows). (e) 5-HT immunohistology of the ventral horn in transverse spinal cord sections 5 mm caudal to the complete transection site of a PBS-treated and ibuprofen-treated mouse. (f) The length of raphespinal fibers within the ventral horn of a section as in e is reported for mice treated with PBS (n = 11), 35 mg/kg/d ibuprofen (n = 13), or 70 mg/kg/d ibuprofen (n = 22). Data are mean ± SEM, and the Mann-Whitney U non-parametric test was used to compare the means (*p ≤ 0.05). (g) The BMS locomotor score is reported for the control and ibuprofen-treated mice as a function of time after complete thoracic spinal transection. The data are mean ± SEM. The scores of the 35- and 70-mg/kg/d ibuprofen-treated groups were combined and showed a trend towards higher scores than those of the PBS-treated mice.
The complete transection injury is anatomically more severe than most injuries in clinically “complete” patients. In the ibuprofen-treated group, no mice were able to step effectively and support their weight with the hind limbs. However, a fraction of the ibuprofen-treated mice, but not the control mice, did recover extensive movement of the ankle joint. A comparison of the combined 35- and 70-mg/kg/d ibuprofen treatment groups with the control group revealed a trend towards higher BMS scores from 2–5 weeks after transection (Fig. 7g). This experiment was not designed to distinguish the relative role of axonal regeneration in mediating behavioral improvement, but regeneration and recovery both occurred in this experiment.
Discussion
The principal finding of the current study is that the non-prescription medication ibuprofen promotes recovery from spinal contusion by a complex mechanism that includes axonal sprouting, neuroprotection, and raphespinal regeneration. Several lines of evidence support the hypothesis that inhibition of Rho by ibuprofen supports functional recovery from SCI. Cell culture studies demonstrate that ibuprofen can prevent MAG inhibition and Rho-dependent signaling. After spinal cord trauma, ibuprofen treatment suppresses Rho activity and rats recover a greater degree of neurological function if treated with ibuprofen. This recovery is correlated with neuroprotection and with CST and raphespinal axon growth. The in vitro and in vivo effects are specific to ibuprofen and do not occur with naproxen, a second NSAID, which does not block Rho signaling, but has similar analgesic effects. We conclude that ibuprofen and related Rho-inhibiting compounds have therapeutic potential for SCI.
While ibuprofen therapy was initiated 3 days after contusion, a neurological benefit was not observed until 10–17 days post-injury. Functional improvement persisted for at least 2 weeks after ibuprofen administration ended. The histological studies provide evidence for post-injury axonal sprouting induced by ibuprofen, and the observed time course is consistent with this being a contributory mechanism for the improved behavioral outcome. Moreover, in the complete transection model ibuprofen supports the regeneration of raphespinal axons across a lesion site. There was no evidence for ibuprofen regulation of macrophage/microglial activation or astroglial reaction to injury. However, subacute tissue sparing is enhanced by ibuprofen, demonstrating a multi-modal action of the compound. Neuroprotective effects have been demonstrated for other Rho inhibitors (Dubreuil et al., 2003). The results here reveal that axonal sprouting and neuroprotection are likely to be the primary mechanisms of ibuprofen's action in spinal contusion. In contrast, the corticospinal regeneration highlighted by a previous study (Fu et al., 2007) was not observed here. We find that ibuprofen-dependent axonal regeneration is confined to a small subset of raphespinal fibers after complete transection.
While the current study provides a general validation of a previous study (Fu et al., 2007), the data here are more than a simple replication. There are two issues that we address for the first time here. First, the complete transection work seen in Fig. 7 proves that ibuprofen actually stimulates axonal regeneration. The study by Fu and associates (2007) showed changes in axons that might be either axonal sparing or axonal sprouting or regeneration. Only in a complete transection model can axonal regeneration (rather than sprouting) be convincingly demonstrated. We have provided this in Fig. 7. We also examined serotonin at two time points in the contusion model (2 weeks versus 6 weeks; Fig. 6k) to prove that fibers are lost and then grow. In contrast, the increased 5-HT fiber density seen caudally in the study by Fu and co-workers might have been due to reduced damage to fibers rather than new growth of raphespinal fibers. Second, we show that spinal cord tissue is spared by the ibuprofen treatment after contusion, demonstrating a combined protective and regenerative mechanism. This was not shown by Fu and colleagues, but fits with previous data showing Rho blockade by C3 exoenzyme derivatives from the McKerracher laboratory (Dubreuil et al., 2003).
While our work clearly demonstrates ibuprofen-induced raphespinal axonal growth after SCI, it is quite likely that no single site or axon pathway mediates recovery after systemic ibuprofen therapy. Ibuprofen is likely to increase axonal growth at a number of sites: across the lesion, within the caudal spinal cord's intrinsic circuitry, and rostrally within the brain. Assessing the influence of multiple sites of axonal growth will require anatomical and electrophysiological studies at multiple levels of the neuraxis and at multiple times after injury. The natural history of spontaneous recovery pathways after SCI have begun to be mapped only very recently (Courtine et al., 2008).
Previously, we had found that local delivery of the Rho blocker C3, but not the ROCK blocker Y27632, reduced glial scar formation (Fournier et al., 2003). The ibuprofen result here, showing little change in GFAP reactivity, is more similar to the Y27632 result in the previous work. There are several issues that may explain the differential effects on astrocytosis. First, the previous C3 experiment utilized immediate local treatment of hemisected cord, while the current study had a 3-day delay in systemic ibuprofen treatment of contusion. Second, it is likely that C3 provides complete inhibition of Rho, rather than the partial blockade of receptor-mediated Rho activation achieved by either ibuprofen or Y27632. Third, the action of bacterial-derived C3 on glial scarring in the previous study may have included non-specific, off-target effects of unknown mechanism. Together, the current data do not provide strong support for Rho action in glial scarring.
Previous studies have demonstrated several biochemical activities for ibuprofen at therapeutic doses. Ibuprofen's well-documented anti-inflammatory effects are mediated primarily by cyclooxygenase inhibition that is shared with other clinically useful NSAIDs. More selectively among the NSAIDs, ibuprofen has been shown to inhibit Rho (Jin et al., 2006; Zhou et al., 2003), to alter γ-secretase substrate specificity (Kukar et al., 2005), and to inhibit cellular proliferation (Khwaja et al., 2004; Kokoska et al., 1999; Morre and Morre, 2006; Williams et al., 2001). The inactivity of naproxen in promoting axonal outgrowth on MAG substrates and in enhancing recovery from spinal cord contusion suggests that cyclooxygenase inhibition is not the causal mechanism in our studies. Histological analysis also argued for a mechanism other than combating inflammation.
A major advantage of considering ibuprofen as a subacute therapy for SCI is that the relative tolerability of this inexpensive over-the-counter compound is well documented in humans. While the deleterious side effects can include gastric mucosal erosion and renal insufficiency, simple preventive measures can reduce these risks dramatically. Furthermore, whereas the side effects of ibuprofen and other NSAIDs tend to resolve shortly after withdrawing administration, the beneficial effects of ibuprofen in rat spinal cord trauma persisted well after administration. Thus, ibuprofen administration may only be necessary in the subacute period, further avoiding the risks of chronic administration. If translated into the human setting, these beneficial effects would greatly exceed the expected risks. Other molecules targeting the Rho pathway have also been shown to promote recovery from rodent CNS injury, including variants of the C3 exoenzyme that inactivates RhoA, and compounds that block a downstream signaling molecule, ROCK (Chan et al., 2005; Dergham et al., 2002; Fournier et al., 2003; McKerracher and Higuchi, 2006). Compounds related to ibuprofen might provide a useful starting point to screen for optimal Rho pathway inhibition and future SCI or stroke recovery therapeutics.
Acknowledgments
The authors thank Betty Liu for assistance with growth cone collapse assays, William Cafferty for assistance with immunofluorescence microscopy, and Yiguang Fu for expert animal care and technical assistance. This work was supported by grants to S.M.S. from the Christopher Reeve Paralysis Foundation, the National Institutes of Health, the Falk Medical Research Trust, and the Wings for Life Foundation.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Antezana D.F. Clatterbuck R.E. Alkayed N.J. Murphy S.J. Anderson L.G. Frazier J. Hurn P.D. Traystman R.J. Tamargo R.J. High-dose ibuprofen for reduction of striatal infarcts during middle cerebral artery occlusion in rats. J. Neurosurg. 2003;98:860–866. doi: 10.3171/jns.2003.98.4.0860. [DOI] [PubMed] [Google Scholar]
- Antri M. Orsal D. Barthe J.Y. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Baptiste D.C. Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Anderson D.K. Faden A.I. Gruner J.A. Holford T.R. Hsu C.Y. Noble L.J. Nockels R. Perot P.L. Salzman S.K. Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Borisoff J.F. Chan C.C. Hiebert G.W. Oschipok L. Robertson G.S. Zamboni R. Steeves J.D. Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol. Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Buchli A.D. Schwab M.E. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann. Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Cafferty W.B.J. Strittmatter S.M. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.C. Khodarahmi K. Liu J. Sutherland D. Oschipok L.W. Steeves J.D. Tetzlaff W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp. Neurol. 2005;196:352–364. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chivatakarn O. Kaneko S. He Z. Tessier-Lavigne M. Giger R.J. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J. Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V. R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergham P. Ellezam B. Essagian C. Avedissian H. Lubell W.D. McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M. Cao Z. Spencer T. Sivasankaran R. Wang K. Nikulina E. Kimura N. Cai H. Deng K. Gao Y. He Z. Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Dubreuil C.I. Winton M.J. McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J. Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A.E. GrandPre T. Strittmatter S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier A.E. Takizawa B.T. Strittmatter S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q. Hue J. Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J. Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E.J. Dolezalova H. Kong Y. Zeng L. Dual mechanisms for lysophospholipid induction of proliferation of human breast carcinoma cells. Cancer Res. 1999;59:4732–4737. [PubMed] [Google Scholar]
- GrandPre T. Li S. Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- GrandPre T. Nakamura F. Vartanian T. Strittmatter S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Guven M.B. Cirak B. Yuceer N. Ozveren F. Is indomethacin harmful in spinal cord injury treatment? An experimental study. Pediatr. Neurosurg. 1999;31:189–193. doi: 10.1159/000028860. [DOI] [PubMed] [Google Scholar]
- Harel N.Y. Strittmatter S.M. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.M. Krishnan C. Darman J.S. Deshpande D.M. Peck S. Shats I. Backovic S. Rothstein J.D. Kerr D.A. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc. Natl. Acad. Sci. USA. 2004;101:7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. Mao X.O. Greenberg D.A. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J. Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Jin Z. Strittmatter S.M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja F. Allen J. Lynch J. Andrews P. Djakiew D. Ibuprofen inhibits survival of bladder cancer cells by induced expression of the p75NTR tumor suppressor protein. Cancer Res. 2004;64:6207–6213. doi: 10.1158/0008-5472.CAN-03-3814. [DOI] [PubMed] [Google Scholar]
- Kim J.E. Li S. GrandPre T. Qiu D. Strittmatter S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim J.E. Liu B.P. Park J.H. Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kokoska E.R. Smith G.S. Wolff A.B. Deshpande Y. Miller T.A. Nonsteroidal anti-inflammatory drugs attenuate epidermal growth factor-induced proliferation independent of prostaglandin synthesis inhibition. J. Surg. Res. 1999;84:186–192. doi: 10.1006/jsre.1999.5640. [DOI] [PubMed] [Google Scholar]
- Kukar T. Murphy M.P. Eriksen J.L. Sagi S.A. Weggen S. Smith T.E. Ladd T. Khan M.A. Kache R. Beard J. Dodson M. Merit S. Ozols V.V. Anastasiadis P.Z. Das P. Fauq A. Koo E.H. Golde T.E. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat. Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- Lehmann M. Fournier A. Selles-Navarro I. Dergham P. Sebok A. Leclerc N. Tigyi G. McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Liu B.P. Budel S. Li M. Ji B. Walus L. Li W. Jirik A. Rabacchi S. Choi E. Worley D. Sah D.W. Pepinsky B. Lee D. Relton J. Strittmatter S.M. Blockade of nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Strittmatter S.M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G.P. Yang F. Chu T. Chen P. Beech W. Teter B. Tran T. Ubeda O. Ashe K.H. Frautschy S.A. Cole G.M. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.P. Cafferty W.B. Budel S.O. Strittmatter S.M. Extracellular regulators of axonal growth in the adult central nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.P. Fournier A. GrandPre T. Strittmatter S.M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Lopez J.R. Dominguez-Ramirez A.M. Cook H.J. Bravo G. Diaz-Reval M.I. Deciga-Campos M. Lopez-Munoz F.J. Enhancement of antinociception by co-administration of ibuprofen and caffeine in arthritic rats. Eur. J. Pharmacol. 2006;544:31–38. doi: 10.1016/j.ejphar.2006.06.041. [DOI] [PubMed] [Google Scholar]
- McGee A.W. Strittmatter S.M. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKerracher L. Higuchi H. Targeting Rho to stimulate repair after spinal cord injury. J. Neurotrauma. 2006;23:309–317. doi: 10.1089/neu.2006.23.309. [DOI] [PubMed] [Google Scholar]
- Mehta N.R. Lopez P.H. Vyas A.A. Schnaar R.L. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J. Biol. Chem. 2007;282:27875–27886. doi: 10.1074/jbc.M704055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melarange R. Moore G. Blower P.R. Coates M.E. Ward F.W. Ronaasen V. A comparison of indomethacin with ibuprofen on gastrointestinal mucosal integrity in conventional and germ-free rats. Aliment. Pharmacol. Ther. 1992;6:67–77. doi: 10.1111/j.1365-2036.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Monnier P.P. Sierra A. Schwab J.M. Henke-Fahle S. Mueller B.K. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Morre D.J. Morre D.M. tNOX, an alternative target to COX-2 to explain the anticancer activities of non-steroidal anti-inflammatory drugs (NSAIDs) Mol. Cell Biochem. 2006;283:159–167. doi: 10.1007/s11010-006-2568-z. [DOI] [PubMed] [Google Scholar]
- Mueller B.K. Mack H. Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat. Rev. Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G. Doherty P. Walsh F.S. Crocker P.R. Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa O. Fujisawa K. Ishizaki T. Saito Y. Nakao K. Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Niederost B. Oertle T. Fritsche J. McKinney R.A. Bandtlow C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J. Profirovic J. Pan H. Vaiskunaite R. Voyno-Yasenetskaya T. G protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ. Res. 2003;93:848–856. doi: 10.1161/01.RES.0000097607.14733.0C. [DOI] [PubMed] [Google Scholar]
- Nobes C. Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr. Opin. Genet. Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Pantovic R. Draganic P. Erakovic V. Blagovic B. Milin C. Simonic A. Effect of indomethacin on motor activity and spinal cord free fatty acid content after experimental spinal cord injury in rabbits. Spinal Cord. 2005;43:519–526. doi: 10.1038/sj.sc.3101763. [DOI] [PubMed] [Google Scholar]
- Park E.M. Cho B.P. Volpe B.T. Cruz M.O. Joh T.H. Cho S. Ibuprofen protects against ischemia-induced neuronal injury via up-regulating interleukin-1 receptor antagonist expression. Neuroscience. 2005;132:625–631. doi: 10.1016/j.neuroscience.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Price M.A. Hill C. Treisman R. Integration of growth factor signals at the c-fos serum response element. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:551–559. doi: 10.1098/rstb.1996.0054. [DOI] [PubMed] [Google Scholar]
- Ramer L.M. Borisoff J.F. Ramer M.S. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J. Neurosci. 2004;24:10796–10805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.D. Schwartz M.A. Determination of GTP loading on Rho. Methods Enzymol. 2000;325:264–272. doi: 10.1016/s0076-6879(00)25448-7. [DOI] [PubMed] [Google Scholar]
- Richardson R.L. Kim E.M. Gardiner T. O'Hare E. Chronic intracerebroventricular infusion of lipopolysaccharide: effects of ibuprofen treatment and behavioural and histopathological correlates. Behav. Pharmacol. 2005;16:531–541. doi: 10.1097/01.fbp.0000179278.03868.96. [DOI] [PubMed] [Google Scholar]
- Saruhashi Y. Young W. Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp. Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Schmidt B.J. Jordan L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schwab J.M. Conrad S. Elbert T. Trautmann K. Meyermann R. Schluesener H.J. Lesional RhoA+ cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia. 2004;47:377–386. doi: 10.1002/glia.20031. [DOI] [PubMed] [Google Scholar]
- Smirnova I.V. Citron B.A. Arnold P.M. Festoff B.W. Neuroprotective signal transduction in model motor neurons exposed to thrombin: G-protein modulation effects on neurite outgrowth, Ca(2+) mobilization, and apoptosis. J. Neurobiol. 2001;48:87–100. [PubMed] [Google Scholar]
- Tanaka H. Yamashita T. Yachi K. Fujiwara T. Yoshikawa H. Tohyama M. Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience. 2004;127:155–164. doi: 10.1016/j.neuroscience.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Trapp T. Olah L. Holker I. Besselmann M. Tiesler C. Maeda K. Hossmann K.A. GTPase RhoB: an early predictor of neuronal death after transient focal ischemia in mice. Mol. Cell Neurosci. 2001;17:883–894. doi: 10.1006/mcne.2001.0971. [DOI] [PubMed] [Google Scholar]
- Venkatesh K. Chivatakarn O. Sheu S.S. Giger R.J. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J. Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax J. Winder C.V. Tessman D.K. Stephens M.D. Comparative activities, tolerances and safety of nonsteroidal anti-inflammatory agents in rats. J. Pharmacol. Exp. Ther. 1975;192:172–178. [PubMed] [Google Scholar]
- Weidner N. Ner A. Salimi N. Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.L. Borgo S. Hasan I. Castillo E. Traganos F. Rigas B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer chemoprevention. Cancer Res. 2001;61:3285–3289. [PubMed] [Google Scholar]
- Winton M.J. Dubreuil C.I. Lasko D. Leclerc N. McKerracher L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 2002;277:32820–32829. doi: 10.1074/jbc.M201195200. [DOI] [PubMed] [Google Scholar]
- Yamagishi S. Fujitani M. Hata K. Kitajo K. Mimura F. Abe H. Yamashita T. Wallerian degeneration involves Rho/Rho-kinase signaling. J. Biol. Chem. 2005;280:20384–20388. doi: 10.1074/jbc.M501945200. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog. Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Su Y. Li B. Liu F. Ryder J.W. Wu X. Gonzalez-DeWhitt P.A. Gelfanova V. Hale J.E. May P.C. Paul S.M. Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]