Abstract
There is limited information on the prevalence of sexually transmitted infections and the prevalence of cervical neoplasia in rural sub-Saharan Africa. This study describes the prevalence and the etiology of STIs and the prevalence of cervical neoplasia among women in southern Mozambique. An age-stratified cross-sectional study was performed where 262 women aged 14 to 61 years were recruited at the antenatal clinic (59%), the family-planning clinic (7%), and from the community (34%). At least one active STI was diagnosed in 79% of women. Trichomonas vaginalis was present in 31% of all study participants. The prevalence of Neisseria gonorrhea and Chlamydia trachomatis were 14% and 8%, respectively, and Syphilis was diagnosed in 12% of women. HPV DNA was detected in 40% of women and cervical neoplasia was diagnosed in 12% of all women. Risk factors associated with the presence of some of the STIs were being divorced or widowed, having more than one sexual partner and having the partner living in another area. A higher prevalence was observed in the reproductive age group and some of the STIs were more frequently diagnosed in pregnant women. STI control programs are a priority to reduce the STIs burden, including HIV and cervical neoplasia.
1. Introduction
Sexually transmitted infections (STIs) remain a major public health problem in developing countries [1–5]. The HIV/AIDS pandemia [6] has increased the awareness about other STIs. However, their true scope and impact are still mostly unknown. Most of the burden of STIs is borne by women, yet women are less likely than men to seek treatment for STIs [7, 8]. In developing countries, complications related to STIs are a major cause of mother and child mortality and morbidity during pregnancy [9–11] as well as to the high incidences of cervical cancer [12, 13]. Previous data from Mozambique [14–16] shows alarmingly high incidences of cervical cancer and congenital syphilis which suggests that STIs prevalence is likely to be high. In the context of a steady increase of HIV prevalence [17] and in an era where access to health care and prevention systems are still weak in most parts of sub-Saharan Africa, further information to guide effective STIs prevention strategies as well as diagnostic and management policies is needed.
Accordingly, a descriptive study on the prevalence and risk factors for STIs and cervical neoplasia among women living in rural Mozambique was carried out in order to contribute to generate further information.
2. Methods
2.1. Study Area and Population
The study took place in the Manhiça District, southern Mozambique, where the Centro de Investigação em Saúde de Manhiça (CISM) is located and approximately at 80 Km from the capital, Maputo.
The population in the Manhiça District lives in a semi-urban and rural setting and crop farming is the main activity in the area. Illiteracy is prevalent, being 24% among men and 47% among women. While 66% of men and 49% of women have had primary education, only 9% of men and 4% of women have had secondary education, and less than 1% of both men and women have gone beyond their secondary education [18]. An ongoing demographic surveillance system, that has been described in detail elsewhere is in place, covering a population of around 82,000 inhabitants, at the time of the study it covered a population of 36.000. Population data is collected on a semestral basis with a biannual census of the population [19].
The population structure is similar to that of many developing countries with 54% of the population being less than 20 years old.
The adjacent Manhiça District Hospital (MDH) is a 110-bed health facility with antenatal care clinic (ANC) services as well as a family planning clinic.
At the time when the study was carried out, screening and treatment for syphilis were provided at the ANC. Residents are mostly subsistence farmers or employees in a nearby sugar cane processing factory. A high number of the adult male population of the area migrates to South Africa to work at the mines and many other move to the capital of Mozambique for job purposes as well.
2.2. Study Design
This is a cross-sectional, age-stratified study of STIs and cervical neoplasia in women. Two hundred and sixty two women aged 14–61 were recruited between August and October 2000. Fifty-nine percent were enrolled at the ANC and 7% at the family planning clinic (FPC) of the MDH. The remaining 34% were randomly selected from the community using the DSS census from the older than 50-year-old female population. Women recruited at the community were invited by the field workers to the hospital to complete the study visit. Attendance to the ANC is high and it is estimated that approximately 90% of pregnant women are visited at least once at the ANC. Women were offered an informed consent and no study procedures were performed before acceptance to the study was given by either signing or thumb printing the document. The refusal rate to participate in the study was 5%. The inclusion criteria were to attend the ANC or the FPC and for the community participants to be in the list randomly produced from the census. Exclusion criteria were exclusively their refusal to sign the informed consent. The study visit consisted of a gynecological exam, collection of cervical samples, and 5 mL of blood by venipuncture. During the visit, relevant information regarding the participant education, socioeconomic status (see Table 1), reproductive history and lifetime sexual behavior was collected as well. In accordance with the Ministry of Health (MOH) guidelines, unlinked HIV testing was done anonymously since, at the time of the study, there were no voluntary counseling and testing services, nor were antiretroviral drugs available. Women did not receive STIs presumptive treatment and they were offered free treatment according to national guidelines if diagnosed with an active treatable STI.
Table 1.
Characteristics of the study women.
| n | % | ||
|---|---|---|---|
| Age group | 14–20 | 51 | 19 |
| 21–30 | 62 | 24 | |
| 31–40 | 54 | 21 | |
| 41–50 | 53 | 20 | |
| 51–61 | 42 | 16 | |
|
| |||
| Ethnic group | Shangana | 208 | 79 |
| Other | 54 | 21 | |
|
| |||
| Education | Reads and/or writes | 81 | 31 |
| Neither reads nor writes | 181 | 69 | |
|
| |||
| Marital status | Never married | 20 | 8 |
| Currently married | 212 | 81 | |
| Divorced or widowed | 30 | 11 | |
|
| |||
| Number of women living with husband | 1 | 185 | 71 |
| >1 | 72 | 27 | |
| Unknown | 5 | 2 | |
|
| |||
| Husband's job | Farmer | 59 | 23 |
| Driver | 8 | 3 | |
| Miner | 51 | 19 | |
| Manual worker | 44 | 17 | |
| Shop keeper | 7 | 3 | |
| Civil servant | 10 | 4 | |
| Other | 45 | 17 | |
| Unknown | 38 | 15 | |
|
| |||
| Husband's place of residence | Manhiça | 133 | 51 |
| Maputo | 27 | 10 | |
| South Africa | 52 | 20 | |
| Unknown | 50 | 19 | |
|
| |||
| Parity | None | 46 | 18 |
| 1 | 29 | 11 | |
| 2 to 5 | 99 | 38 | |
| >5 | 85 | 32 | |
| Unknown | 3 | 1 | |
|
| |||
| Pregnant now | No | 111 | 42 |
| Yes | 151 | 58 | |
|
| |||
| Age at first sexual intercourse | 12–15 | 52 | 20 |
| 16-17 | 43 | 16 | |
| 18–20 | 26 | 10 | |
| Unknown | 141 | 54 | |
|
| |||
| Number of sexual partners in lifetime | Never had | 3 | 1 |
| 1 | 149 | 57 | |
| >1 | 103 | 39 | |
| Unknown | 7 | 3 | |
Women with a Pap smear compatible with cervical intraepithelial dysplasia/neoplasia (CIN) or carcinoma were referred for histological confirmation and appropriate clinical management to the Maputo Central Hospital.
2.3. Laboratory Methods
The rapid plasma reagin (RPR, Syphacard, Wellcome, USA) test was used for syphilis diagnosis and results were confirmed using an enzyme immunoassay (CAPTIA Syphilis-G, Trinity Biotech, Dublin, Ireland). CT DNA was detected on cervical samples by the CT-ID assay (Digene Corporation, Silver Spring, MD, USA). Neisseria gonorrhea (NG) was identified by Gram stain and culture (Thayer-Martin, Bethesda, MD, USA) of the cervical mucosa. Trichomonas vaginalis was detected by direct microscopic examination of went-mount preparations of the vaginal discharge. Anti HSV-2 antibodies were determined using an ELISA kit from MRL Diagnostics (Cypress, CA, USA). Anti HIV antibodies were detected by Imx HIV-1/HIV-2 III Plus EIA (Abbot Diagnostics, IL, USA). Antibodies against Hepatitis B virus (anti-HBc) and HBsAg were detected using the ELISA technique ETI-AB-COREK-2 and ETI-MAK-3 (DiaSorin). HPV DNA was determined from a cervical swab by the reverse line-blot (RLB) strip-based detection system [20–22] and further improved by using the PGMY09-PGMY11 primer system. Cervical smears were processed and read following standard procedures. Antibiotic sensitivity was assessed by standard techniques using disc diffusion methods and interpreted according to the NCCLS criteria (National Committee for Clinical Laboratory Standards, 2000).
2.4. Statistical Methods and Definitions
Syphilis infection was defined as a RPR positive test confirmed by ELISA for T. Pallidum IgG antibodies. The variable “any treatable STI” was created for women with at least one of the following: syphilis, gonoccocal infection, Trichomonas vaginalis or CT. The variable “any active STI” included women with any of the following: syphilis, gonoccocal infection, Trichomonas vaginalis, CT, HPV or HIV infection. HSV was not included in this latter group because no confirmation of active disease laboratory analysis was performed during the study. Percentages were compared by the uncorrected χ 2 test and the Fisher's exact test. Odds ratio (OR), and 95% Confidence Intervals (CI) were estimated by logistic regression models. Adjustments for age and ethnic group were performed in the multivariate models. Age was categorized in tertiles (14–26, 27–42, and 43–61) for the analysis of risk factors to allow for small numbers or absence of cases in some of the outcomes.
Multivariate models for STs and cervical neoplasia were estimated using a forward-stepwise procedure, with 0.05 from the Likelihood-ratio test as significance level for addition to the model. Potential independent variables for such models were the participant education, socioeconomic status, reproductive history and lifetime sexual behavior as well as other coinfections (STIs other than the one used as dependent variable for each model). Age and ethnic group were forced to be part of all multivariate models in order to adjust for them.
The confidence intervals for the proportion of STIs in Table 2 were calculated by the Exact (or Clopper-Pearson) method, based on the binomial distribution.
Table 2.
Prevalence of STIs and cervical neoplasia in women from Manhiça, Mozambique.
| n/total | Prevalence (%) | 95% Confidence interval | |
|---|---|---|---|
| Gonococcal infection | 34/250 | 14 | (10; 19) |
| Syphilis (RPR & IgG) | 31/258 | 12 | (8; 17) |
| HPV DNA | 100/253 | 40 | (33; 46) |
| HIV antibodies | 30/256 | 12 | (8; 16) |
| HSV-2 antibodies | 213/257 | 83 | (78; 87) |
| Chlamydia trachomatis | 19/253 | 8 | (5; 11) |
| Trichomona vaginalis | 78/254 | 31 | (25; 37) |
| HBsAg antibodies | 20/255 | 8 | (5; 12) |
| Anti-HBc antibodies | 160/256 | 63 | (56; 68) |
| Any treatable STI* | 128/252 | 51 | (44; 57) |
| Any active STI** | 179/254 | 70 | (64; 76) |
| Cervical neoplasia | 30/245 | 12 | (8; 17) |
*Any treatable STI: syphilis (RPR confirmed by IgG), gonococcal infection, trichomona vaginalis or chlamydia trachomatis.
**Any active STI: syphilis (RPR confirmed by IgG), gonococcal infection, trichomona vaginalis, chlamydia trachomatis, HPV or HIV infection.
The analysis was performed using STATA software (Stata, East College Station, TX, USA).
3. Results
3.1. Demographic Characteristics
The median age at first sexual intercourse was 16, and 43% initiated sexual intercourse at age 15 years or younger (Table 1). About 11% of women had been married more than once and a third of their husbands had previously lived with 2 or more different women. The husbands were, on average, 5 years older than their wives (data not shown).
Women recruited at the FPC had a higher literacy level (47% versus 30% and 15% from the ANC and the community, resp.; P ≤ .001), and had more than one sexual partner in a higher percentage in comparison to the other groups (58% versus 41% and 33% from the ANC and the community, resp.; P = .047).
3.2. Prevalence of STIs and Cervical Neoplasia
Vaginal discharge was observed in 68% of cases, cervical mucous in 35%, anogenital warts in 5%. Overall prevalence of STIs is shown in Table 2.
Nearly 70% (179/254) of all women and 75% (112/148) of pregnant women had at least one active STI. This prevalence reached 82% in the 14–20 years age group, and decreased significantly with age, dropping to 44% in the oldest group (51–61 years). Over half (51%) of the participants had at least one treatable STIs. We defined treatable taking into account what was available for treatment as per national policy guidelines at the time of the study.
The most common STI was Trichomonas vaginalis found in 31% of women.
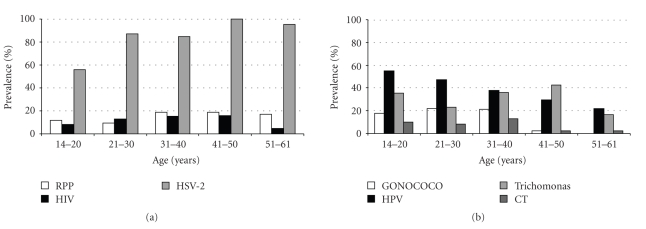
The overall prevalence of gonoccocal infection was 14% (34/250). It was 19% (27/145) in pregnant women and 33% (6/18) among women attending the FPC. Prevalence was associated with age (P < .001), being more prevalent in the younger age group. Antimicrobial sensitivity of the NG was 61% for erythromycin, 18% for penicillin and ampicillin, 15% for chloramphenicol, 9% for gentamicin and 3% for cotrimoxazol. Sensitivity to ceftriaxone, spectinomycin and azythromycin was not assessed. CT DNA was detected in 7.5% (19/253) of cervical samples. The prevalence was lower in the 41–61 age group, but there was no statistically significant association with age. Among pregnant women, the prevalence was 10% (15/151). Thirty-nine women had a RPR positive test (15%), of which 31 (75%) were confirmed by EIA, giving an overall syphilis prevalence of 12% (31/258). The prevalence increased with age, but this relationship was not statistically significant (P = .67). Among pregnant women, syphilis prevalence was 10% (15/149). Overall prevalence of IgG antibodies against T pallidum was 39% (100/257), increasing significantly with age (P < .001). Overall seroprevalence of HIV was 12% (30/256) and 21% (4/19) among women attending the FPC. The highest prevalence was found in the 31–50 age group (15%; 16/104), but there was no significant relationship with age. Anti-HSV-2 antibodies were found in 83% (213/257) of all participants. This frequency increased significantly with age (test for trend P < .001). The lowest prevalence (56%; 28/50) was in the youngest age group. HBsAg was detected in 8% (20/255) and anti-HBc in 62.5% (160/256) of women, respectively. The overall prevalence of HPV DNA was 40% (100/253), the highest was in the youngest age group (test for trend P < .001), and decreased with age (see Figure 1). HPV genotypes have been already published [23] Cervical neoplasia was detected in 30 women (12.2%): 5.3% (13/245) with LSIL (low-grade squamous intraepithelial lesions that include CIN I), 6.5% (16/245) with HSIL (high-grade squamous intraepithelial lesions that include CIN II and III), and one woman (0.4%) with carcinoma.
Figure 1.
Seroprevalence of syphilis, HIV and HSV-2 (a) and prevalence of gonococcal, HPV, trichomonal and chlamydia trachomatis (CT) infections (b).
3.3. Sociodemographic, Behavioral Risk Factors and STIs Coinfection
Table 3 shows the multivariate analysis results for sociodemographic and sexual behavior-related risk factors for each STI and cervical neoplasia.
Table 3.
Significant variables according to the multivariate analysis of risk factors for STIs and cervical neoplasia.
| Infection | Variable | Multivariate OR* | (95% CI) | P-value** | |
|---|---|---|---|---|---|
| Gonococcal infection | Number of sexual partners | 1 | 1 | .014 | |
| >1 | 2.8 | (1.2; 6.2) | |||
|
| |||||
| Syphilis (RPR & IgG) | HPV | No | 1 | .005 | |
| Yes | 3.2 | (1.4; 7.5) | |||
|
| |||||
| Syphilis (RPR & IgG) | Marital status | Never married | 1 | .005 | |
| Married now | 0.7 | (0.1; 3.9) | |||
| Divorced or widowed | 3.6 | (0.6; 21.9) | |||
|
| |||||
| HPV | Cervical neoplasia | No | 1 | <.0001 | |
| Yes | 9.8 | (3.7; 26.3) | |||
| Unknown | 2.7 | (0.6; 12.0) | |||
|
| |||||
| HPV | RPR | No | 1 | <.0001 | |
| Yes | 4.1 | (1.9; 9.1) | |||
|
| |||||
| HPV | Anogenital warts | No | 1 | .054 | |
| Yes | 4.2 | (1.0; 17.8) | |||
|
| |||||
| HIV | Marital status | Never married | 1 | .005 | |
| Married now | 0.5 | (0.1; 2.3) | |||
| Divorced or widowed | 2.7 | (0.5; 16.0) | |||
|
| |||||
| HIV | Parity | None | 1 | .02 | |
| 1 child | 0.7 | (0.1; 3.6) | |||
| 2 to 5 | 0.6 | (0.1; 3.0) | |||
| more than 5 | 4.3 | (0.7; 26.9) | |||
|
| |||||
| HSV-2 | Age at first child | Never had | 1 | .014 | |
| 13–18 | 3.7 | (1.1; 12.9) | |||
| 19 | 48.7 | (3.0; 792.7) | |||
| 20–28 | 27.3 | (3.1; 241.2) | |||
| Unknown | 2.3 | (0.5; 9.7) | |||
|
| |||||
| HSV-2 | Selection of women | Antenatal clinic | 1 | .006 | |
| Family planning | 0.1 | (0.0; 0.8) | |||
| Other | 0 | (0.0; 0.3) | |||
|
| |||||
| Trichomona vaginalis | Husband's place of residence | Manhiça | 1 | .002 | |
| Maputo | 2.5 | (1.0; 6.3) | |||
| South Africa | 1.2 | (0.6; 2.4) | |||
| Unknown | 0.2 | (0.1; 0.6) | |||
|
| |||||
| Trichomona vaginalis | Anogenital warts | No | 1 | .018 | |
| Yes | 7.1 | (1.4; 36.5) | |||
|
| |||||
| HBsAg | Number of women living with husband | 1 | 1 | .028 | |
| >1 | 0.1 | (0.0; 0.8) | |||
|
| |||||
| Any treatable STI# | Anogenital warts | No | 1 | .007 | |
| Yes | 11 | (1.9; 63.1) | |||
|
| |||||
| Any active STI## | HSV-2 antibodies | No | 1 | .0029 | |
| Yes | 2.4 | (1.1; 5.4) | |||
|
| |||||
| Any active STI## | Chlamydia trachomatis | No | 1 | .042 | |
| Yes | 2.3 | (1.0; 5.1) | |||
|
| |||||
| Cervical neoplasia | HPV | No | 1 | <.0001 | |
| Yes | 10.1 | (3.7; 27.5) | |||
|
| |||||
| Cervical neoplasia | RPR | No | 1 | .068 | |
| Yes | 0.2 | (0.1; 1.1) | |||
*Adjusted by age and ethnic group.
**Likelihood Ratio Test versus model with variable removed.
#Any treatable STI: syphilis (RPR confirmed by IgG), gonococcal infection, trichomona vaginalis or Chlamydia trachomatis.
##Any active STI: syphilis (RPR confirmed by IgG), gonococcal infection, trichomona vaginalis, Chlamydia trachomatis, HPV or HIV infection.
Reporting more than one lifetime sexual partner was the only factor statistically significant related to cervical gonoccocal infection. Observation of vaginal secretion or cervical mucous during clinical exam was not associated with gonoccocal infection. The number of lifetime sexual partners and the marital status were significantly associated with the diagnosis of syphilis, and the presence of HPV DNA was associated with a nearly three-fold higher risk (OR = 3.2; 95% CI 1.4, 7.5). Abnormal cervical cytology was associated with HPV DNA detection in cervical cells (OR = 10.1; 95% CI 3.7, 27.5). The presence of anogenital warts was also related to HPV infection (OR = 4.2; 95% CI 1.0, 17.8). A positive test for syphilis was associated with HPV DNA detection (OR = 4.1; 95% CI 1.9, 9.1). In the adjusted analysis, being divorced or widowed was significantly associated with HIV infection. HIV seropositivity prevalence rose with increasing severity of cervical abnormalities: 9% in women with normal cytology, 16% in women with ASCUS, 17% in women with LSIL, and 19% in women with HSIL, and the only woman with carcinoma (test for trend P = .02). HIV seropositive women were more likely to have HPV DNA detected in their cervices, but the differences were not statistically significant (47% versus 37%; OR = 1.6; 95% CI 0.5, 3.5). CT infection was not significantly associated with any social or behavioral risk factor. No relationship between detection of this infection and either vaginal secretion or cervical mucous was identified in the multivariate analysis.
3.4. STIs Risk by HIV Status
Except for HBsAg, there was a consistent association trend for the presence of f all STIs in HIV positive women. However, only CT DNA detection and presence of cervical abnormalities reached borderline statistical significance (Table 4).
Table 4.
Prevalence and risk of STIs and cervical neoplasia by HIV status.
| STD by HIV | HIV (−) | HIV (+) | Adjusted* OR | 95% Cl | P | ||
|---|---|---|---|---|---|---|---|
| N = 226 | N = 30 | ||||||
| n | % | n | % | ||||
| Gonococcal infection | 27 | 12 | 6 | 20 | 1.9 | (0.7; 5.5) | .219 |
| Syphilis (RPR & IgG) | 26 | 12 | 5 | 17 | 1.6 | (0.5; 4.5) | .408 |
| HPV DNA | 83 | 37 | 14 | 47 | 1.6 | (0.7; 3.5) | .262 |
| HSV-2 antibodies | 185 | 82 | 27 | 90 | 2.1 | (0.6; 7.6) | .259 |
| Chlamydia trachomatis DNA | 14 | 6 | 5 | 17 | 3.1 | (1.0; 9.6) | .051 |
| Trichomona vaginalis | 67 | 30 | 9 | 30 | 1.0 | (0.4; 2.4) | .966 |
| HBsAg anribodies | 19 | 8 | 1 | 3 | 0.4 | (0.1; 3.0) | .359 |
| Anti-HBc antibodies | 137 | 61 | 22 | 73 | 1.8 | (0.8; 4.3) | .172 |
| Any treatable STI** | 107 | 47 | 18 | 60 | 1.7 | (0.8; 3.8) | .190 |
| Cervical neoplasia | 23 | 10 | 6 | 20 | 2.5 | (0.9; 7.0) | .088 |
*Adjusted by age and ethnic group.
**Any treatable STI: syphilis (RPR confirmed by IgG), gonococcal infection, trichomona vaginalis or chlamydia trachomatis.
4. Discussion
Although none of the participants were selected based on their STIs seeking behavior, 70% and 50% of women presented with at least one active or treatable STI. It is generally assumed that STI prevalence is higher in urban residents [24]. However, these findings show that the burden of STIs in rural areas may be underestimated. The STI prevalences found were higher than those reported from other rural areas in sub-Saharan Africa [25, 26]. The high migration rate might partially explain the high frequency of STIs in these women [4].
Vaginal discharge showed no correlation with the presence of infection, which suggests that syndromic management alone is unlikely to have a major public health impact in controlling STIs and HIV transmission in women [27–29].
On the contrary, the presence of anogenital warts was significally correlated with the presence of any treatable STI. Most pregnant women had either an active (75.6%) or treatable (55%) STI. These frequencies were higher than what has been reported in the region [7, 30] and in a urban area of northern Mozambique (51%) [31]. The most frequent STI was Trichomonas vaginalis which was found in 31% of women, and taking into account that the direct examination only detects approximately a 60%–70% of infections it is reasonable to assume that we are underestimating its prevalence. The high prevalence of gonorrhea, CT, and syphilis are particularly alarming due to their potential impact on the newborn. At the time of the study, the only control program in place was that of syphilis prevention. These results call for different approaches to STIs prevention of the main STIs in both pregnant women and their partners. In Mozambique, current guidelines recommend ciprofloxacin in cases of vaginal discharge [32]. However, this drug is rarely available and kanamycin plus erythromycin is the combination still most frequently used. Sensitivity for kanamycin was not tested but it can be assumed to be similar to that of gentamicin (9%), which may partly explain the high frequency of gonoccocal infection observed. These are still the only available results on NG sensitivity in the country, and reflect the difficulties that countries with limited resources face in reconciling their health policies with some recommendations.
CT infection prevalence was 8% overall and the 14–20 years old group had a higher prevalence of CT infection (10%) than that reported from rural Tanzania (2.4% of female adolescents) [33]. This discrepancy may be explained by the recruitment sources of our study being more likely for our participants to have had more sexual partners. These data are consistent with the evidence that girls and young women are more susceptible to CT infection than older women, with the consequent risk, among others, of infertility [34].
Syphilis prevalence between 1.6% and 9.8% in rural areas [16] to 18.3% in Maputo has been reported among women in Mozambique [11]. In this study, syphilis was detected in 12% of all women and in 10% in the youngest age group. These figures are also higher than those from rural areas of South Africa (8%) [8] and Tanzania (9.1% of all women, 6.6% of adolescents) [35]. Again, the different selection of individuals may explain the different rates found in the current study. However, syphilis prevalence was also high in the oldest age groups, where most women were recruited from the community and therefore not exposed to the potential selection bias.
The overall prevalence of HSV-2 antibodies was high (83%). Among 14–20 year old women the prevalence was 56%, higher than that reported in another study from rural Africa (27%) [36]. HSV-2 is of much interest in Africa because it increases the risk of HIV transmission [37]. Although the information on HSV-2 is based on seroprevalence of antibodies, HSV-2 seropositivity has been proposed as a marker of sexual risk behavior among adolescents [36]. These observations support the latter, and suggest that there is high-risk sexual behavior at very young ages.
The overall prevalence of HPV infection was high, especially in the youngest age group where over 50% were infected. HIV and co infection with other STIs are highly prevalent in this population, and this is known to increase the risk of progression from cervical HPV infection to cervical neoplasia and invasive cancer [38]. The HIV seroprevalence (12%) found was still lower than that reported from other areas of Southern Africa [39]. However, it could be foreseen that with this high STIs burden, the epidemic could rise dramatically if no effective STIs prevention is implemented. HIV prevalence estimates among ANC attendees in this area have reached 24% confirming this suspicion [40]. The risk factors for STI detection identified are consistent with those observed in other studies [41]. However, the indirect information obtained through questionnaires makes the data on behavioral risk factors subject to bias and difficult to reproduce [42, 43]. The main limitation of this study is that many women were not selected from the community and that the size and age distribution of the three recruitment sources are not comparable. These may limit the extrapolation of these findings to the general population. However, the similar rate of syphilis found among the community-based recruited women suggests that the potential selection bias may not be relevant.
Our data is still to date the only available information on the prevalence of STIs and cervical cancer among women in Mozambique. A study performed in 2004 in an urban area of Mozambique reported a remarkable decline in STIs, which was attributed to successful implementation of STIs prevention strategies in that area [31].
We have to mention that one of the limitations of the study is that it may be underpowered for some of the tested associations.
In conclusion, the burden of disease associated with STIs borne by African women in rural areas, and the implications for the acquisition and transmission of HIV and cervical neoplasia, are enormous. Reproductive health programs do not easily reach adolescents and older women, so specific approaches to target them should be envisioned and implemented. In pregnant women, there is a need for a more aggressive approach to avoid the harmful effects associated with STIs on mothers and children. For HPV infections and cervical cancer, recent development of vaccines [44] offers great hope, but the challenge is that they become affordable for developing countries. There is an urgent need to develop interventions in order to avoid the burden of morbidity due to STIs and the further spread of HIV in rural African populations.
Funding
The financial support was provided by the Spanish Fondo de Investigación Sanitaria (FIS01/1236). The “Centro de Investigação em Saúde da Manhiça” (CISM), receives major core funding from the Spanish Agency for International Cooperation.
Conflict of Interest
None declared.
Ethical Approval
Ethical clearance was provided by the Mozambican Ministry of Health, the Ciutat Sanitaria i Universitaria de Bellvitge and the Hospital Clinic Ethics Committees, Barcelona.
Author's Contributions
All the authors contributed to the design of the paper. Jahit Sacarlal, Betuel Sigauque, Montse Renom, and Clara Menéndez carried out the clinical work. Llorenç Quinto led the statistical analysis. Belen Lloveras, Joellen Klaustermeier, and Janet R Kornegay were responsible of the laboratory tests. Xavier Castellsague, Xavier Bosch, and Pedro Alonso contributed to the overall supervision of the paper. All authors contributed to the writing up of the paper that was led by Clara Menendez and Xavier Castellsague.
Acknowledgments
The authors are grateful to the participating women. The authors want to thank M. Arañó for her dedicated follow-up and treatment of affected women, M. J. Quintana and J. Muñoz for coordinating data management and shipment of material and biological samples, J. Vidal for performing the HIV and HSV-2 serology, and E. García-Recio for assisting in the preparation and reading of the cytological smears. The authors are grateful to the technical support provided by the laboratory, the nursing staff and all field workers involved of the Manhiça Health Center and the Manhiça Health Research Center (CISM), as well as to the technical staff of the Ministry of Health of Mozambique. Without their work and support, this paper would have not been possible.
References
- 1.Agaçfidan A, Kohl P. Sexually transmitted diseases (STDs) in the world. FEMS Immunology and Medical Microbiology. 1999;24(4):431–435. doi: 10.1111/j.1574-695X.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 2.Genuis SJ, Genuis SK. Managing the sexually transmitted disease pandemic: a time for reevaluation. American Journal of Obstetrics and Gynecology. 2004;191(4):1103–1112. doi: 10.1016/j.ajog.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Gerbase AC, Rowley JT, Heymann DHL, Berkley SFB, Piot P. Global prevalence and incidence estimates of selected curable STDS. Sexually Transmitted Infections. 1998;74(1):S12–S16. [PubMed] [Google Scholar]
- 4.Mabey D. Sexually transmitted diseases in developing countries. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(2):97–99. doi: 10.1016/s0035-9203(96)90097-8. [DOI] [PubMed] [Google Scholar]
- 5.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sexually Transmitted Infections. 2004;80(3):174–182. doi: 10.1136/sti.2002.004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallings LO. The first postmodern pandemic: 25 tears of HIV/AIDS. Journal of Internal Medicine. 2008;263(3):218–243. doi: 10.1111/j.1365-2796.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 7.Sturm AW, Wilkinson D, Ndovela N, Bowen S, Connolly C. Pregnant women as a reservoir of undetected sexually transmitted diseases in rural South Africa: implications for disease control. American Journal of Public Health. 1998;88(8):1243–1245. doi: 10.2105/ajph.88.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson D, Abdool Karim SS, Harrison A, et al. Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bulletin of the World Health Organization. 1999;77(1):22–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Moodley P, Sturm AW. Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Seminars in Neonatology. 2000;5(3):255–269. doi: 10.1053/siny.2000.0026. [DOI] [PubMed] [Google Scholar]
- 10.Mullick S, Watson-Jones D, Beksinska M, Mabey D. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sexually Transmitted Infections. 2005;81(4):294–302. doi: 10.1136/sti.2002.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstrand A, Bergstrom S, Bugalho A, Zanconato G, Helgesson A-M, Hederstedt B. Prevalence of syphilis infection in Mozambican women with second trimester miscarriage and women attending antenatal care in second trimester. Genitourinary Medicine. 1993;69(6):431–433. doi: 10.1136/sti.69.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of Oncology. 2007;18(3):581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 13.Sankaranarayanan R. Overview of cervical cancer in the developing world. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. International Journal of Gynecology and Obstetrics. 2006;95(supplement 1):S205–S210. doi: 10.1016/S0020-7292(06)60035-0. [DOI] [PubMed] [Google Scholar]
- 14.Vuylsteke B, Bastos R, Barreto J, et al. High prevalence of sexually transmitted diseases in a rural area in Mozambique. Genitourinary Medicine. 1993;69(6):427–430. doi: 10.1136/sti.69.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossa HA, Gloyd S, Vaz RG, et al. Syphilis and HIV infection among displaced pregnant women in rural Mozambique. International Journal of STD and AIDS. 1994;5(2):117–123. doi: 10.1177/095646249400500208. [DOI] [PubMed] [Google Scholar]
- 16.Liljestrand J, Bergstrom S, Nieuwenhuis F, Hederstedt B. Syphilis in pregnant women in Mozambique. Genitourinary Medicine. 1985;61(6):355–358. doi: 10.1136/sti.61.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Report on the global AIDS epidemic. 2008.
- 18.Loscertales M. Epidemiology and clinical presentation of RSV infection in a rural area of southern Mozambique. Paediatric Infectious Diseases Journal. 2002;(21):148–155. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Nhacolo A. Levels and trends of demographic indices in southern rural Mozambique: evidence from demographic surveillance in Manhiça district. BMC Public Health. 2006;(6):p. 291. doi: 10.1186/1471-2458-6-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. Journal of Clinical Microbiology. 1998;36(10):3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. Journal of Clinical Microbiology. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravitt PE, Burk RD, Lorincz A, et al. A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiology Biomarkers and Prevention. 2003;12(6):477–484. [PubMed] [Google Scholar]
- 23.Castellsagué X, Menéndez C, Loscertales M-P, et al. Human papillomavirus genotypes in rural Mozambique. Lancet. 2001;358(9291):1429–1430. doi: 10.1016/S0140-6736(01)06523-0. [DOI] [PubMed] [Google Scholar]
- 24.Gerbase AC, Rowley JT, Mertens T. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351:2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson D, Rutherford G. Population-based interventions for reducing sexually transmitted infections, including HIV infection. Cochrane Database of Systematic Reviews. 2001;(2) doi: 10.1002/14651858.CD001220. Article ID CD001220. [DOI] [PubMed] [Google Scholar]
- 26.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson D. Syndromic management of sexually transmitted diseases in developing countries: what role in the control of the STD and HIV epidemics? Genitourinary Medicine. 1997;73(6):427–428. doi: 10.1136/sti.73.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson D, Harrison A, Lurie M, Abdool Karim SS. STD syndrome packets: improving syndromic management of sexually transmitted diseases in developing countries. Sexually Transmitted Diseases. 1999;26(3):152–156. doi: 10.1097/00007435-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Vuylsteke B. Current status of syndromic management of sexually transmitted infections in developing countries. Sexually Transmitted Infections. 2004;80(5):333–334. doi: 10.1136/sti.2004.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayaud P, Grosskurth H, Changalucha J, Todd J, West B, Gabone R, et al. Risk assessment and other screening options for gonorrhoea and chlamydial infections in women attending rural Tanzanian antenatal clinics. Bulletin of the World Health Organization. 1995;73(5):621–630. [PMC free article] [PubMed] [Google Scholar]
- 31.Luján J, de Oñate WA, Delva W, et al. Prevalence of sexually transmitted infections in women attending antenatal care in Tete province, Mozambique. South African Medical Journal. 2008;98(1):49–51. [PubMed] [Google Scholar]
- 32.Direccao Nacional de Saúde. Guia para o tratamento e controle das DTS (Niveís primários e secundárius) Maputo, Mozambique: 2007. [Google Scholar]
- 33.Obasi AI, Balira R, Todd J, et al. Prevalence of HIV and Chlamydia trachomatis infection in 15-19-year olds in rural Tanzania. Tropical Medicine and International Health. 2001;6(7):517–525. doi: 10.1046/j.1365-3156.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- 34.Hillis SD, Nakashima A, Marchbanks PA, Addiss DG, Davis JP. Risk factors for recurrent Chlamydia trachomatis infections in women. American Journal of Obstetrics and Gynecology. 1994;170(3):801–806. doi: 10.1016/s0002-9378(94)70286-1. [DOI] [PubMed] [Google Scholar]
- 35.Todd J, Munguti K, Grosskurth H, et al. Risk factors for active syphilis and TPHA seroconversion in a rural African population. Sexually Transmitted Infections. 2001;77(1):37–45. doi: 10.1136/sti.77.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obasi A, Mosha F, Quigley M, et al. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. Journal of Infectious Diseases. 1999;179(1):16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- 37.Todd J, Grosskurth H, Changalucha J, Obasi A, Mosha F, Balira R, et al. Risk factors influencing HIV infection incidence in a rural African population: a nested case-control study. Journal of Infectious Diseases. 2006;193(3):458–466. doi: 10.1086/499313. [DOI] [PubMed] [Google Scholar]
- 38.Castellsagué X, Bosch FX, Muñoz N. Environmental co-factors in HPV carcinogenesis. Virus Research. 2002;89(2):191–199. doi: 10.1016/s0168-1702(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 39.Stanecki KA. The AIDS pandemic in the 21st century. US Census Bureau, International population Reports WP 02-2 U 2004.
- 40.Naniche D, Bardají A, Lahuerta M, et al. Impact of maternal human immunodeficiency virus infection on birth outcomes and infant survival in rural Mozambique. American Journal of Tropical Medicine and Hygiene. 2009;80(5):870–876. [PubMed] [Google Scholar]
- 41.Zuma K, Lurie MN, Williams BG, Mkaya-Mwamburi D, Garnett GP, Sturm AW. Risk factors of sexually transmitted infections among migrant and non-migrant sexual partnerships from rural South Africa. Epidemiology and Infection. 2005;133(3):421–428. doi: 10.1017/s0950268804003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moodley P, Sturm PDJ, Connolly C, Sturm AW. Identification of women at high STD risk among STD clinic attendees: implications for STD programmes. International Journal of STD and AIDS. 2003;14(8):526–531. doi: 10.1258/095646203767869138. [DOI] [PubMed] [Google Scholar]
- 43.Thomas T, Choudhri S, Kariuki C, Moses S. Identifying cervical infection among pregnant women in Nairobi, Kenya: limitations of risk assessment and symptom-based approaches. Genitourinary Medicine. 1996;72(5):334–338. doi: 10.1136/sti.72.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Sanjosé S, Alemany L, Castellsagué X, Bosch FX. Human papillomavirus vaccines and vaccine implementation. Women’s Health. 2008;4(6):595–604. doi: 10.2217/17455057.4.6.595. [DOI] [PubMed] [Google Scholar]