Abstract
Background
Fosbretabulin is a novel vascular-disrupting agent that has antitumor activity against anaplastic thyroid cancer (ATC) cell lines, xenografts, and demonstrable efficacy in a phase I trial. This phase II study determined the efficacy and safety of fosbretabulin in patients with advanced ATC and whether fosbretabulin altered the natural history of ATC by virtue of doubling the median survival. A secondary aim evaluated the prognostic value of serum soluble intracellular adhesion molecule-1 (sICAM).
Methods
Twenty-six patients received fosbretabulin 45 mg/m2 as a 10-minute intravenous infusion on days 1, 8, and 15 of a 28-day cycle. sICAM levels were obtained at baseline, over the first two cycles, and end of therapy. Treatment was continued until disease progression.
Results
Fosbretabulin was well tolerated; grade 3 toxicity was observed in nine patients (35%), and grade 4 toxicity in one (4%). QTc prolongation delayed treatment in four causing one to stop treatment. Median survival was 4.7 months with 34% and 23% alive at 6 and 12 months, respectively. Median duration of stable disease in seven patients was 12.3 months (range, 4.4–37.9 months). Baseline serum sICAM levels were measured in 24 patients with a median 253.5 ng/mL. There was a significant difference in event-free survival among tertiles of baseline sICAM levels (p < 0.009).
Conclusions
There were no objective responses seen with single-agent fosbretabulin as administered in this trial, and we did not observe a doubling of survival as our primary endpoint. This is among the largest prospective trials ever conducted for ATC. Fosbretabulin has an acceptable safety profile in patients with advanced ATC, and one-third survived more than 6 months. Despite a small sample size, low baseline sICAM levels were predictive of event-free survival. Further prospective validation of sICAM as a therapeutic biomarker and exploring combination regimens with fosbretabulin are warranted.
Introduction
Anaplastic thyroid cancer (ATC) is among the most lethal solid tumors with a median survival on the order of 12 weeks (1,2). One-year and 10-year survival rates are estimated at 10–20% and less than 5%, respectively (1–3), though some experts question the reliability of diagnosis in these long-term survivors (4). Although ATC accounts for approximately 1–3% of thyroid cancer in the United States, it is responsible for 14–50% of thyroid cancer mortality (1,4–6). The current standard therapeutic approach is to consider the disease as systemic at time of diagnosis and pursue combined modality therapy incorporating cytoreductive surgical resection where feasible and/or chemoradiation either concurrently or sequentially (4). Several studies have shown increased expression of the multidrug-resistant–associated protein with decreased expression of mdr1 mRNA and P-glycoprotein and loss of p53 tumor suppressor gene function in ATC cell lines (7–10). This likely explains the near uniformly poor outcomes with traditional chemotherapy, even with doxorubicin and paclitaxel, which are regarded as the most active single agents in this disease (11–13).
Fosbretabulin (formerly combretastatin A4 phosphate) is the lead compound among a group of novel tubulin-binding compounds originally isolated from the bark of the African bush willow tree, Combretum caffrum (14). The compound is a synthetic, water soluble, phosphorylated prodrug of the active molecule, combretastatin A4 (CA4). CA4 is a vascular-disrupting agent (VDA) that targets existing tumor neovasculature, and causes an acute reduction in tumor blood flow that leads to central necrosis within tumors. In xenograft models, tumor blood flow was reduced 50–60% with an associated 90% loss of functional vascular volume within 6 hours of treatment with fosbretabulin (15,16). In phase I/II human studies, fosbretabulin has been demonstrated to reduce blood flow in many types of solid tumors (17–21). Blood flow reduction was greatest in highly vascular tumors, such as thyroid cancer (19,20).
Although the exact mechanism of action of fosbretabulin has not been fully elucidated, there is evidence to suggest effects on endothelial cells. Fosbretabulin destabilizes microtubules and disrupts cell–cell adhesion mediated by vascular endothelial (VE)-cadherin, leading to collapse of endothelial cells and occlusion of vessels. Endothelial cells in abnormal tumor vessels that lack a full complement of smooth muscle or pericyte support are more sensitive to the effects of CA4, conferring a degree of vascular specificity. In addition, fosbretabulin has cytotoxic effects against several tumor lines in vitro, although the VDA activity is hypothesized to be the predominant mechanism for antitumor activity in vivo (14,22–29).
Initial observations in our fosbretabulin phase I trial provided evidence of a possible correlation between endothelial damage and drug effect (17). At the highest dose levels (60–90 mg/m2) serial measurements of soluble intracellular adhesion molecule-1 (sICAM), soluble vascular cell adhesion molecule, and soluble E-selectin, obtained immediately preinfusion and at hours 1 and 24 postinfusion, showed a significant increase of sICAM after drug treatment (p < 0.025). No statistical difference was seen between 1-hour and 24-hour postinfusion levels. Similarly, no significant change was seen for soluble vascular cell adhesion molecule or soluble E-selectin. A secondary aim of our study was to explore the utility of sICAM as a potential biomarker.
Fosbretabulin has demonstrable activity against ATC in orthotopic xenograft models (30). Phase I/II trials of fosbretabulin in refractory solid tumors included seven patients with ATC (17–21). One patient who received fosbretabulin monotherapy experienced a durable complete response (>9 years) (17). In a study of fosbretabulin in combination with carboplatin/paclitaxel, one subject experienced a partial response and a second experienced stable disease for more than 4 months (20). On the basis of the aforementioned preclinical and clinical rationale, we embarked on a phase II single-agent trial of fosbretabulin in patients with advanced ATC.
Patients and Methods
Selection criteria
Patients were required to have biopsy-proven advanced or metastatic ATC. Hematoxylin and eosin (H&E)–stained tumor slides from all 26 cases were reviewed (J.K.W.) at University Hospitals Case Medical Center. Additionally, patients had assessable disease and no prior therapy for metastatic disease beyond initial combined modality treatment at diagnosis. Patients were eligible if they progressed or relapsed during or after initial combined modality therapy, usually including systemic chemotherapy and radiation, for regionally advanced disease or in the setting of metastatic disease provided therapy was limited to one chemotherapy regimen administered in an uninterrupted primary therapeutic approach. Additional entrance criteria included Eastern Cooperative Oncology Group performance status of ≤2 and adequate cardiac (normal electrocardiogram [ECG] with QTc interval ≤450 milliseconds and left ventricular ejection fraction ≥50% per echocardiogram and no significant wall motion abnormalities), renal (serum creatinine ≤1.5 times upper limit of normal), bone marrow (absolute granulocyte count ≥1500/μL; platelets ≥75,000/μL), and hepatic (total bilirubin ≤1.5×, and aspartate aminotransferase and alanine aminotransferase ≤3.5× the institutional upper limit of normal, respectively) function. Exclusion criteria included patients with serious uncontrolled nonmalignant medical illnesses, grade 2 [NCI Common Toxicity Criteria, version 2.0] (31), or greater preexisting peripheral neuropathy, active brain metastasis, major surgery within the preceding 4 weeks, symptomatic peripheral vascular disease, history of angina, myocardial infarction, heart failure, uncontrolled hypertension, other conditions rendering patients incapable of complying with the requirements of the protocol, hypokalemia (<3.5 mEq/L), hypomagnesemia (<1.5 mg/L), and concurrent investigational or antineoplastic therapy. Signed informed consent was obtained from all patients in keeping with the U.S. Food and Drug Administration and institutional guidelines.
Treatment plan
Patients received fosbretabulin (supplied by Oxigene, Waltham, MA) at a dose of 45 mg/m2 as a 10-minute intravenous infusion weekly for 3 weeks followed by 1 week off treatment (days 1, 8, and 15 of a 28-day treatment cycle). Fosbretabulin was administered in an inpatient/outpatient monitored setting using an infusion pump with frequent blood pressure measurements during the first two cycles of therapy and less frequently thereafter. During each treatment cycle, any medication known to prolong QTc was held a minimum of 72 hours before the administration of fosbretabulin and resumed no earlier than 6 hours after its dosing. All patients were premedicated with appropriate antiemetic regimen; dolasetron was avoided due to its potential to prolong the QTc interval. Treatment was continued until unacceptable toxicity, patient refusal, intercurrent illness preventing continuation of therapy, disease progression, or death occurred.
Baseline and clinical assessments
Before entering the study, all patients underwent a physical examination, complete blood count, serum chemistry including serum magnesium, urinalysis, chest X-ray, 12-lead ECG, echocardiogram, documentation of known or suspected tumor involvement per appropriate imaging technique, and laryngoscopy as warranted to confirm patency of the airway in patients with bulky cervical tumor. Complete blood count and serum chemistry were collected weekly. Toxicity evaluation according to NCI Common Toxicity Criteria (31) (at study closure analysis of QTc data were undertaken with NCI Common Terminology Criteria for Adverse Events [CTCAE], version 3.0, which is more precise) (32), physical examination, performance status, sICAM serum sample collection, and ECG were conducted before drug administration. sICAM levels were measured by ELISA technique (R&D Systems, Minneapolis, MN) per prior published methods (17). Samples were collected 1 and 24 hours after treatment during the first two cycles and at time off-study. On days 1, 8, and 15 of each cycle, three predose 12-lead ECGs were obtained separated by 5 minutes; the mean QTc of the three ECGs served as baseline. Single ECGs were obtained for cycles 1 and 2 at 1-hour increments for 6 hours postadministration of fosbretabulin. If no abnormalities existed, ECG monitoring was reduced for cycles 3 through 6 to one at pretreatment and at hours 2 and 4 posttreatment. ECGs were stopped after cycle 6 in the absence of adverse cardiac events. Assessment of tumor response was performed every two cycles per RECIST criteria (33).
Statistical methods
One of the primary objectives of the study was to determine whether fosbretabulin altered the natural history of ATC by virtue of doubling the median survival of patients with advanced disease from 4–6 months to 8–12 months. With type I error of 0.05, 2-year accrual period and additional 2-year follow-up after enrolling last patient, 24 patients were required for the study to have 80% power to reject the null hypothesis of median survival of 4 months against the alternative hypothesis of median survival of 7 months. The survivor function was estimated by Kaplan–Meier method, and the difference among tertile of baseline sICAM was examined by log-rank test (34). The difference of sICAM measured at baseline and other time points during treatment was examined by paired T-test. All tests are two sided, and p-value ≤0.05 was considered statistically significant.
Results
Patient characteristics
Between April 2003 and December 2006, 26 patients with advanced ATC were enrolled from three cancer centers in Cleveland, Detroit, and Pittsburgh (see Table 1). The majority of patients had prior therapy (92%) and metastatic disease (73%). The clinical characteristics of the seven patients with stable disease can be briefly summarized as follows: there were three men and four women (median age, 64 years; range, 52–76); five had metastatic disease; all seven underwent surgery, and three had radiation, including two with concurrent systemic chemotherapy.
Table 1.
Patient Characteristics
| Sex (male/female) | 16/10 |
| Age, median (range) | 59 years (22–76) |
| ECOG performance status 0/1 | 11/15 |
| Stage (regional disease confined to neck/metastatic) | 7/19 |
| Prior therapy/no prior therapy | 24/2 |
| Combined modality therapy (n = 16) | |
| Surgery + radiation + chemotherapy | 9 |
| Radiation + chemotherapy | 4 |
| Surgery + radiation | 3 |
| Chemotherapy exposure (n = 13) | |
| Paclitaxel-based: single patient each with paclitaxel alone; paclitaxel +doxorubicin; paclitaxel + cisplatin; paclitaxel + doxorubicin + cisplatin | 4 |
| Doxorubicin-based: doxorubicin alone (six patients) and doxorubicin + cisplatin (2) | 8 |
| Cisplatin single agent | 1 |
| Single modality therapy (n = 8) | |
| Surgery | 7 |
| Radiation | 1 |
| Participating centers–patient enrollment: | |
| Cleveland | 20 |
| Detroit | 4 |
| Pittsburgh | 2 |
ECOG, Eastern Cooperative Oncology Group.
Pathology review
Submitted pathology material was independently reviewed for all 26 cases. This included at least one H&E-stained slide in all cases. Additional H&E-stained and immunostained slides were also reviewed when available. All cases were morphologically compatible with the diagnosis of anaplastic (undifferentiated) carcinoma. Nineteen of the 26 cases were entirely comprised of anaplastic (undifferentiated) carcinoma. One of these cases had a previous history of papillary thyroid carcinoma, though residual papillary carcinoma was not identified in the current material. Seven cases contained residual foci of well-differentiated thyroid carcinoma; these included six cases with a papillary thyroid carcinoma component and one case with a Hurthle cell carcinoma component.
Treatment course
A total of 77 cycles of therapy were delivered with a median 2 cycles per patient (range, 1–19). Therapy was delayed in four patients due to QTc interval prolongation. Patient 6 had cycle 1 day 1 (C1D1), C3D15, C4D8, and C4D15 each delayed 1 day due to QTc prolongation average of 461, 491, 467, and 475 milliseconds (grade 1–2 toxicity) (32). Patient 9 had a 1-day delay due to an average QTc of 471 milliseconds during C7D8 (grade 2 toxicity). Patient 13 was delayed twice for a total of 2 weeks during C1D15 due to QTc average of 467 and 457 milliseconds (grade 1 toxicity) (32). Finally, patient 23 had C1D1 delayed 1 week because baseline QTc interval of >450 milliseconds and was finally taken off study when C1D8 was delayed due to recurrent QTc interval prolongation up to 477 milliseconds (grade 2 toxicity) (32).
Toxicity
Therapy was generally well tolerated with the most common adverse effects being mild nausea, vomiting, and headache, all of which resolved within 24 hours of drug administration (Table 2). There was negligible myelosuppression. Of the 26 patients, grade 3 toxicity was observed in 9 (35%) and grade 4 toxicity was observed in 1 (4%). Grade 3 tumor pain was encountered in three patients (12%), and grade 4 tumor pain in 1 (4%). Six subjects experienced seven serious adverse events during the study, all of which were attributable to progression of ATC. Four patients had episodes of QTc interval prolongation in excess of 450 milliseconds that delayed therapy, including one patient who discontinued the study. There was no ECG or clinical evidence of ventricular ectopy, ventricular arrhythmia, or cardiac ischemia.
Table 2.
Composite Summary of Toxicity Per Common Toxicity Criteria (31)
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Bone marrow (leukopenia) | 14 | 0 | 0 | 0 | 0 | 14 |
| Bone marrow (lymphopenia) | 16 | 18 | 11 | 0 | 0 | 45 |
| Cardiovascular (arrhythmia) | 16 | 0 | 0 | 0 | 0 | 16 |
| Cardiovascular (prolonged QTc)a | 28 | 0 | 0 | 0 | 0 | 28 |
| Cardiovascular (sinus tachycardia) | 11 | 0 | 0 | 0 | 0 | 11 |
| Cardiovascular (general/other) | 8 | 5 | 0 | 0 | 0 | 13 |
| Constitutional (fatigue) | 11 | 4 | 0 | 0 | 0 | 15 |
| Constitutional (diaphoresis) | 8 | 2 | 0 | 0 | 0 | 10 |
| Dermatology (flushing) | 10 | 0 | 0 | 0 | 0 | 10 |
| Gastrointestinal (nausea) | 22 | 1 | 0 | 0 | 0 | 23 |
| Gastrointestinal (vomiting) | 10 | 1 | 0 | 0 | 0 | 11 |
| Musculoskeletal (other) | 18 | 1 | 0 | 0 | 0 | 19 |
| Pain (headache) | 36 | 15 | 0 | 0 | 0 | 51 |
| Pain (other) | 27 | 5 | 1 | 0 | 0 | 33 |
| Pain (tumor pain) | 13 | 12 | 3 | 1 | 0 | 29 |
| Pulmonary (voice changes) | 11 | 0 | 0 | 0 | 0 | 11 |
| Subtotal (common AEs >2%) | 259 | 64 | 15 | 1 | 0 | 339 |
| Total (all AEs = 100%) | 371 | 85 | 20 | 1 | 0 | 477 |
There were a total of 477 protocol AEs (371 [78%] grade 1; 85 [18%] grade 2; 20 [4%] grade 3; 1 grade 4 [0.2%]; and no grade 5 treatment-related deaths) that were considered possibly, probably, and definitely related to fosbretabulin. Clinically significant and more common (occurring ≥2% of total) AEs are tabulated above.
At study closure analysis of QTc, data were undertaken with NCI Common Terminology Criteria for Adverse Events (CTCAE, version 3.0), which is more precise (32). Treatment was delayed in four patients because of QTc interval prolongation (see Treatment course section for details). These four patients received 27 cycles of therapy and had four episodes of QTc interval prolongation in excess of 450 milliseconds that delayed therapy, including one instance >500 milliseconds. When cumulative QTc prolongation in these four patients is graded according to CTCAE version 3.0 criteria, there were 11 episodes of grade 1 toxicity, 11 grade 2, and 2 grade 3 toxicity.
AE, adverse event.
Tumor response
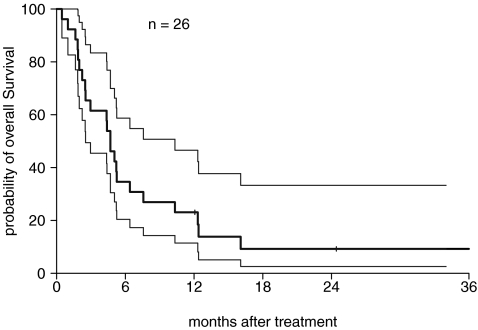
No patient experienced an objective response; a single patient had a near-partial remission after two cycles but rapidly progressed during cycle 3. The initial best response to treatment was stable disease in 7 patients (27%), 15 patients with progressive disease, and response could not be evaluated in 4 patients. At the end of the study, 25 patients were taken off the trial due to disease progression and one due to prolonged QTc interval. Twenty-three of the 26 patients had expired by October 2007, the data cut-off time. Three patients were alive at time of last follow-up at 12.1+, 24.4+, and 37.9+ months. Median survival time was 4.7 months (95% CI, 2.5–6.4 months) (Fig. 1). Overall survival was 34% at 6 months and 23% at 12 months. Event-free survival (EFS) at 3 and 6 months was 23.1% and 7.7%, respectively. The median survival of the seven patients with stable disease was 12.3 months (range, 4.4–37.9 months). One patient experienced prolonged stable disease with progression at 20 months of therapy. In univariate analyses, there was no difference in overall survival outcomes when evaluated by baseline factors, including age, sex, performance status, presence of metastatic disease, and prior therapy.
FIG. 1.
Kaplan–Meier estimation of overall survival with 95% confidence interval. The median survival time was 4.7 months with 95% CI 2.5–6.4 months.
sICAM
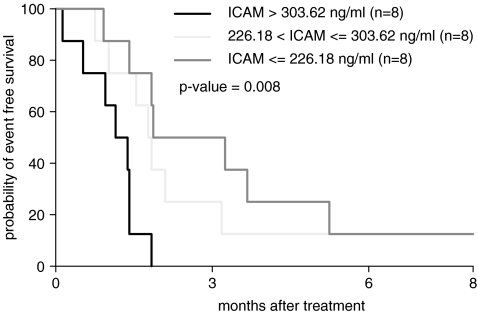
Baseline serum sICAM levels were measured in 24 of the 26 patients with a median of 253.5 ng/mL (range, 172–839.5). The distribution of baseline sICAM was roughly normal with the exception of one patient with an extreme value over 800 ng/mL. sICAM levels did not change significantly over the course of treatment. When patients are grouped by the tertile of the baseline sICAM, there was no significant difference in overall survival among patients with low baseline sICAM levels, those with intermediate levels, and those with high levels (p < 0.8). There was a highly significant difference of EFS stratified by the tertiles of baseline sICAM levels (p < 0.009) (Fig. 2)—the higher baseline sICAM levels, the shorter the EFS.
FIG. 2.
Kaplan–Meier estimation of event-free survival by the tertile of baseline soluble intracellular adhesion molecule-1 (sICAM) level.
Clinical features of the three longest survivors
The clinical features of the three patients with the longest survival (37.9, 24.4+, and 15.9 months) are summarized in Table 3. All of these patients had stable disease.
Table 3.
Summary of Clinical Features of Three Longest-Term Survivors
| Case nos. | Site | Age (years), sex | Stage (metastatic sites) | Prior therapy | No. of cycles | EFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| 9 | Cleveland | 65, M | T4N0M1 (lung) | Surgery | 19 | 20 | 37.9 |
| 18 | Detroit | 60, F | T4NxM1 (lung) | Surgery + dox + XRT | 8.67 | 8.8 | 24.4+ |
| 21 | Cleveland | 53, F | T4N0M1 (lung) | Surgery | 4 | 3.1 | 15.9 |
M, male; F, female; dox, doxorubicin; XRT, radiation; EFS, event-free survival; OS, overall survival.
Discussion
This study is among the largest prospective trials ever conducted for patients with ATC, and there are several notable observations. Despite the absence of any objective response by RECIST criteria and failure to achieve the primary endpoint of the trial, 27% of patients achieved stable disease, which may be noteworthy in a tumor type that can reportedly double in size in as little as a week in extreme cases (4). Median survival was 4.7 months, with 34% and 23% of patients surviving at 6 and 12 months, respectively. This compares favorably with a median survival of 12 weeks for patients captured in the Surveillance, Epidemiology, and End Results (SEER) database (1), particularly because survival is measured from time of diagnosis in the comparator versus from the time of first treatment with fosbretabulin in this study. The findings are comparable to those in a report on 96-hour infusion of paclitaxel in 20 patients, where median survival was 25 weeks and approximately 30% of patients survived a year (12).
It is interesting to briefly comment on the clinical features of the patients treated in our study when compared to the recently reported results from the SEER database and other studies. We observed a median age of 59 years, 38% were female, 73% had metastatic disease, and median survival was 4.7 months or 1-year mortality rate of 77% (with 92% having received some form of therapy before treatment with single-agent fosbretabulin in our trial) versus mean age 71 years, 67% female, 43% metastatic disease, and 3 months median survival or 81% 1-year cause-specific mortality rate, respectively, reported in the SEER database from 1973 to 2000 (1). The largest single-center, identical treatment experience has also been recently reported. In this study, 30 patients between 1990 and 2000 were treated with a combined modality approach including hyperfractionated radiation (35). In this study, the median age was 59 years, 60% female, 20% metastatic disease, and 10 months median survival or 54% 1-year mortality rate. Clearly, survival was most impacted by the presence of metastatic disease in this study. Our results, while not immediately extrapolated to either the SEER or this later report, because nearly all patients had prior treatment and the majority had metastatic disease, 23% of patients survived a year. Additionally, given our observations, the natural history of this disease affecting younger patients may be emerging (35). Most importantly, it is feasible to conduct clinical trials in a reasonable time period for this rare and highly lethal disease.
Fosbretabulin was well tolerated, and the safety profile is consistent with other published observations (17–19,36). Fosbretabulin was devoid of traditional cytotoxic side effects, especially myelosuppression. Adverse effects typical of antiangiogenic agents, such as proteinuria, hemorrhage, and chronic hypertension, were not observed. Adverse effects typical of tubulin depolymerizing agents, such as peripheral neuropathy, were also not observed. This may be due to CA4's short half-life, approximately 4 hours, and reversible binding to its targets, tubulin and VE-cadherin.
Cardiovascular adverse effects consisting of transient hypertension, within 3 hours postinfusion, and cardiac ischemia have previously been reported in clinical trials with fosbretabulin (37,38). In this study, transient hypertension was observed in one patient. There were no episodes of chest pain or cardiac ischemia, which suggests that the entry criteria of normal ECG and echocardiogram were appropriate. Recent investigations in rodents demonstrate that transient hypertension and cardiac strain are due to slight vasospasm, controllable with nitrates or calcium channel blockers (39). These classes of medications have been used successfully for management and prevention of transient hypertension in other clinical trials (40).
QTc prolongation has also been reported in prior clinical trials with fosbretabulin (17–19). The ATC patients in the study are at risk for several factors known to prolong QTc, including hypothyroidism, hypocalcemia, and hypokalemia (secondary to renal effects of cisplatin). Nonetheless, we encountered QTc interval prolongation in only 4 (15%) patients that was readily manageable in all but one. There was no evidence of any ventricular ectopy or arrhythmias throughout the course of the study. Vigilant electrolyte replenishment, particularly with potassium and magnesium, achieving high normal serum levels with outpatient oral regimens before days of therapy with fosbretabulin is particularly helpful in patient management. In our phase I trial, we observed a statistically significant but clinically insignificant (defined as QTc interval prolongation of 500 milliseconds or greater) increase in QTc interval of 27.2 and 30.8 milliseconds at 3- and 4-hour postinfusion, respectively (17,36). At the same time, we also observed a statistically significant increase in heart rate also at the 3- and 4-hour postinfusion time points of 13.2 and 15.1 beats per minute, respectively (36). This increase in heart rate may fortuitously afford protection against proarrhythmia effects associated with prolongation of ventricular repolarization.
With the clinical development of several drugs that target endothelial cell survival mechanisms (e.g., SU5416 and bevacizumab) (41–45) and tumor vasculature (e.g., fosbretabulin) (17), our group hypothesized that drug-induced damage and/or apoptosis of vascular endothelial cells would be associated with release of endothelial cell-specific markers such as sICAM. In this study, we did not observe any change in sICAM levels from baseline to 1 and 24 hours postinfusion. Reasons for this are unclear, but this could be a function of the higher dose (60–90 mg/m2) in the phase I trial versus the 45 mg/m2 used in the current study. The time points for sample collection, collection methods, and analytic methods were identical in the two studies. It remains unclear whether the drug-induced acute increase in markers of endothelial damage was due to tumor endothelium density, type of endothelial damage, rates of release from cells, and/or clearance after drug exposure in our initial study. These data did suggest that acute endothelial damage and/or apoptosis may be reflected in sICAM levels. In any event, the opportunity was afforded by our phase II study to correlate baseline sICAM levels with clinical outcomes.
Theoretically, baseline sICAM level(s) could be used to identify a population that would be responsive to treatment. Our observation that low baseline sICAM serum levels correlate with improved EFS is important and builds on other studies. In our SU5416 breast cancer trial, only sICAM levels correlated with EFS with higher sICAM levels associated with shorter EFS as well (43). These studies provided the framework to further explore baseline endothelial markers in the recently reported large randomized phase III trial by the Eastern Cooperative Oncology Group of combination carboplatin/paclitaxel with or without bevacizumab in nonsmall cell lung cancer. This study confirmed that the addition of an antiangiogenesis agent improved survival for patients with nonsmall cell lung cancer (46). These investigators observed that low sICAM levels were both prognostic and predictive of clinical outcomes; in essence, low sICAM levels may guide the selection of an antiangiogenic or VDA for a chemotherapeutic regimen (47).
There is preclinical data that fosbretabulin improves outcome when combined with carboplatin and paclitaxel (30). Early phase I trials of combination paclitaxel, carboplatin, and fosbretabulin have been completed and borne out these observations. These studies substantiate feasibility, safety, and encouraging clinical activity especially in patients with ovarian cancer (20,21,40). These results, in addition to observations from this study, provide a strong rationale to pursue combination fosbretabulin with this doublet in patients with advanced ATC, which is currently being investigated in an international phase II/III clinical trial. (Protocol OXC4T4-302 under Oxigene sponsorship. The Clinical Trials.gov identifier is NCT00507429.)
In summary, ATC patients treated with fosbretabulin monotherapy had a median survival of 4.7 months, which is comparable to that observed for 96-hour infusions of paclitaxel (12), considered one of the most active single agents for ATC. Although we were unable to demonstrate significant change in the natural history of ATC, one-third of patients survived more than 6 months, which is encouraging. Despite a small sample size, low baseline sICAM levels were predictive of EFS. Further prospective validation of sICAM as a therapeutic biomarker and further exploration of combination regimens with fosbretabulin are warranted.
Acknowledgments
The authors extend their appreciation to Patricia Gallagher in the Case CCC Information Services, and Leigh Kolanz, Susan Bergant, and Kim Rutherford in the Clinical Trials Unit for their vital support. Lastly, we are especially grateful to the patients and families participating in this study. Supported in part by a clinical research grant from Oxigene, Inc. (Waltham, MA) and NIH Grants M01 RR-00080 and P30 CA43703 (Translational Research Shared Resource). Protocol identification: CASE-CWRU-ICC-2302, CASE 2302, and Clinicaltrials.gov identifier: NCT00060242. IND number 67,620.
Disclosure Statement
Since the study closed, SCR received an honorarium from Oxigene over the past two years. The other authors declare that no competing financial interests exist.
References
- 1.Kebebew E. Greenspan FS. Clark OH. Woeber KA. McMillan A. Anaplastic thyroid carcinoma treatment outcome and prognostic factors. Cancer. 2005;103:1330–1335. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- 2.McIver B. Hay ID. Giuffrida DF. Dvorak CE. Grant CS. Thompson GB. van Heerden JA. Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J. Henson DE. Glazer E. Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 4.Are C. Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Hundahl SA. Fleming ID. Fremgen AM. Menck HR. A National Cancer Center Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A. Siegel R. Ward E. Murray T. Xu J. Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Fagin JA. Molecular genetics of human thyroid neoplasms. Annu Rev Med. 1994;45:45–52. doi: 10.1146/annurev.med.45.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Gilliland FD. Hunt WC. Morris DM. Key CR. Prognostic factors for thyroid carcinoma. A population based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–91. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Lehnert M. Clinical multidrug resistance in cancer: a multifactorial problem. Eur J Cancer. 1996;32:912–920. doi: 10.1016/0959-8049(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 10.Satake S. Sugawara I. Watanabe M. Takami H. Lack of point mutation of human DNA topoisomerase II in multidrug-resistant anaplastic thyroid carcinoma cell lines. Cancer. 1997;116:33–39. doi: 10.1016/s0304-3835(97)04742-3. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja S. Ernst H. Chemotherapy of thyroid carcinoma. J Endocrinol Invest. 1987;10:303–310. doi: 10.1007/BF03348135. [DOI] [PubMed] [Google Scholar]
- 12.Ain KB. Egorin MJ. DeSimone PA for the Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Thyroid. 2000;10:587–594. doi: 10.1089/thy.2000.10.587. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb JA. Hill CS., Jr Chemotherapy of thyroid cancer with Adriamycin. N Engl J Med. 1974;290:193–197. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 14.Young SL. Chaplin DJ. Combretastatin A4 phosphate: background and current clinical status. Expert Opin Investig Drugs. 2004;13:1171–1182. doi: 10.1517/13543784.13.9.1171. [DOI] [PubMed] [Google Scholar]
- 15.Chaplin DJ. Pettit GR. Hill SA. Anti-vascular approaches to solid tumour therapy: evaluation of combretastatin A4 phosphate. Anticancer Res. 1999;19:189–195. [PubMed] [Google Scholar]
- 16.Grosios K. Holwell SE. McGown AT. Pettit GR. Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowlati A. Robertson K. Cooney M. Petros WP. Stratford M. Jesberger J. Rafie N. Overmoyer B. Makkar V. Stambler B. Taylor A. Waas J. Lewin JS. McCrae KR. Remick SC. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–3416. [PubMed] [Google Scholar]
- 18.Rustin GJ. Galbraith SM. Anderson H. Stratford M. Folkes LK. Sena L. Gumbrell L. Price PM. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson JP. Rosen M. Sun W. Gallagher M. Haller DG. Vaughn D. Giantonio B. Zimmer R. Petros WP. Stratford M. Chaplin D. Young SL. Schnall M. O'Dwyer PJ. Phase I trial of the antivascular agent combretastatin A-4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21:4428–4438. doi: 10.1200/JCO.2003.12.986. [DOI] [PubMed] [Google Scholar]
- 20.Akerley WL. Schabel M. Morrell G. Horvath E. Yu M. Johnsson B. Arbogast K. A randomized phase 2 trial of combretatstatin A-4 phosphate (CA4P) in combination with paclitaxel, carboplatin to evaluate safety, efficacy in subjects with advanced imageable malignancies. J Clin Oncol 2007 ASCO Ann Meeting Proc. 2007;25:616s. (Abstract no. 14060). [Google Scholar]
- 21.Bilenker JH. Flaherty KT. Rosen M. Davis L. Gallagher M. Stevenson JP. Sun W. Vaughn D. Giantonio B. Zimmer R. Schnall M. O'Dwyer PJ. Phase I trial of combretastatin A-4 phosphate with carboplatin. Clin Cancer Res. 2005;11:1527–1533. doi: 10.1158/1078-0432.CCR-04-1434. [DOI] [PubMed] [Google Scholar]
- 22.Dark GG. Hill SA. Prise VE. Tozer GM. Pettit GR. Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829–1834. [PubMed] [Google Scholar]
- 23.Dorr RT. Dvorakova K. Snead K. Alberts DS. Salmon SE. Pettit GR. Antitumor activity of combretastatin-A4 phosphate, a natural product tubulin inhibitor. Investig New Drugs. 1996;14:131–137. doi: 10.1007/BF00210783. [DOI] [PubMed] [Google Scholar]
- 24.el-Zayat AA. Degen D. Drabek S. Clark GM. Pettit GR. Von Hoff DD. In vitro evaluation of the antineoplastic activity of combretastatin A-4, a natural product from Combretum caffrum (arid shrub) Anticancer Drugs. 1993;4:19–25. doi: 10.1097/00001813-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Griggs J. Metcalfe JC. Hesketh R. Targeting tumour vasculature: the development of combretastatin A4. Lancet Oncol. 2001;2:82–87. doi: 10.1016/S1470-2045(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 26.Grosios K. Loadman PM. Swaine DJ. Pettit GR. Bibby MC. Combination chemotherapy with combretastatin A-4 phosphate and 5-fluorouracil in an experimental murine colon adenocarcinoma. Anticancer Res. 2000;20:229–233. [PubMed] [Google Scholar]
- 27.Landuyt W. Verdoes O. Darius DO. Drijkoningen M. Nuyts S. Theys J. Stockx L. Wynendaele W. Fowler JF. Maleux G. van den Bogaert W. Anne J. van Oosterom A. Lambin P. Vascular targeting of solid tumours: a major “inverse” volume-response relationship following combretastatin A-4 phosphate treatment of rat rhabdomyosarcomas. Eur J Cancer. 2000;36:1833–1843. doi: 10.1016/s0959-8049(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 28.Tozer GM. Prise VE. Wilson J. Locke RJ. Vojnovic B. Stratford MR. Dennis MF. Chaplin DJ. Combretastatin A-4 phosphate as a tumor vascular targeting agent: early effects in tumors and normal tissues. Cancer Res. 1999;59:1626–1634. [PubMed] [Google Scholar]
- 29.Vincent L. Kermani P. Young LM. Cheng J. Zhang F. Shido K. Lam G. Bompais-Vincent H. Zhu Z. Hicklin DJ. Bohlen P. Chaplin DJ. May C. Rafii S. Combretastatin A-4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung SC. She M. Yang H. Pan J. Sun L. Chaplin D. Combination chemotherapy including combretastatin A-4 phosphate and paclitaxel is effective against anaplastic thyroid cancer in a nude mouse xenograft model. J Clin Endocrinol Metab. 2007;92:2902–2909. doi: 10.1210/jc.2007-0027. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute Common Toxicity Criteria (CTC) National Cancer Institute; Bethesda, MD: 1998. Version 2.0. [Google Scholar]
- 32.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) National Cancer Institute; Bethesda, MD: 2006. Version 3.0. [Google Scholar]
- 33.Therasse P. Arbuck SG. Eisenhauer EA. Wanders J. Kaplan RS. Rubinstein L. Verweij J. van Glabbeke M. van Oosterom AT. Christian MC. Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL. Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.de Crevoisier R. Baudin E. Bachelot A. Leboulleux S. Travagli J-P. Caillou B. Schlumberger M. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:1137–1143. doi: 10.1016/j.ijrobp.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Cooney M. Radivoyevitch T. Dowlati A. Overmoyer B. Levitan N. Robertson K. Levine SL. DeCaro K. Butcher C. Taylor A. Stambler BS. Remick SC. Cardiovascular safety-profile of combretastatin A4 phosphate (CA4P) in a single-dose phase I pharmacokinetic study in patients with advanced cancer. Clin Cancer Res. 2004;10:96–100. doi: 10.1158/1078-0432.ccr-0364-3. [DOI] [PubMed] [Google Scholar]
- 37.van Heeckeren WJ. Bhakta S. Ortiz J. Duerk J. Cooney MM. Dowlati A. McCrae K. Remick SC. Promise of new vascular-disrupting agents balanced with cardiac toxicity: is it time for oncologists to get to know their cardiologists? J Clin Oncol. 2006;24:1485–1488. doi: 10.1200/JCO.2005.04.8801. [DOI] [PubMed] [Google Scholar]
- 38.van Heeckeren WJ. Sanborn SL. Narayan A. Cooney MM. McCrae KR. Schmaier AH. Remick SC. Complications from vascular-disrupting agents and angiogenesis inhibitors: aberrant control of hemostasis and thrombosis. Curr Opin Hematol. 2007;14:468–480. doi: 10.1097/MOH.0b013e3282a6457f. [DOI] [PubMed] [Google Scholar]
- 39.Ke Q. Bodyak N. Rigor D. Kang P. Exaggerated hypertensive response of combretastatin A-4 phosphate in hypertensive rats. International Conference of Vascular Targeted Therapies Oncology, Mandelieu, Cannes; France. 2007. (Abstract no. 25). [Google Scholar]
- 40.Rustin GJ. Nathan PD. Boxall J. Saunders L. Ganesan TS. Shreeves GE. A phase Ib trial of combretastatin A-4 phosphate (CA4P) in combination with carboplatin or paclitaxel chemotherapy in patients with advanced cancer. J Clin Oncol ASCO Ann Meeting Proc. 2005;23:217s. (Abstract no. 3103). [Google Scholar]
- 41.Cooney MM. Tserng KY. Makar V. McPeak RJ. Ingalls ST. Dowlati A. Overmoyer B. McCrae K. Ksenich P. Laavertu P. Ivy P. Hoppel CL. Remick S. A phase IB clinical and pharmacokinetic study of the angiogenesis inhibitor SU5416 and paclitaxel in recurrent or metastatic carcinoma of the head and neck. Cancer Chemother Pharmacol. 2005;55:295–300. doi: 10.1007/s00280-004-0871-5. [DOI] [PubMed] [Google Scholar]
- 42.Dowlati A. Robertson K. Radivoyevitch T. Waas J. Ziats NP. Hartman P. Abdul-Karim FW. Wasman JK. Jesberger J. Lewin J. McCrae K. Ivy P. Remick SC. Novel phase I dose de-escalation design trial to determine the biological modulatory dose of the anti-angiogenic agent SU5416. Clin Cancer Res. 2005;11:7938–7944. doi: 10.1158/1078-0432.CCR-04-2538. [DOI] [PubMed] [Google Scholar]
- 43.Overmoyer B. Fu P. Hoppel C. Radivoyevitch T. Shenk R. Persons M. Silverman P. Robertson K. Ziats NP. Wasman JK. Abdul-Karim FW. Jesberger JA. Duerk J. Hartman P. Hanks S. Lewin J. Dowlati A. McCrae K. Ivy P. Remick SC. Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clin Cancer Res. 2007;13:5862–5868. doi: 10.1158/1078-0432.CCR-07-0688. [DOI] [PubMed] [Google Scholar]
- 44.Lyons JA. Silverman P. Remick S. Chen H. Leeming R. Shenk R. Fu P. Dumadag L. Escuro K. Overmoyer B. Toxicity results, early outcome data on a randomized phase II study of docetaxel ± bevacizumab for locally advanced, unresectable breast cancer. J Clin Oncol 6 ASCO Ann Meeting Proc. 2006;24:133s. (Abstract no. 3049). [Google Scholar]
- 45.Baar J. Silverman P. Lyons J. Fu P. Abdul-Karim F. Ziats N. Wasman J. Hartman P. Jesberger J. Dumadag L. Hohler E. Leeming R. Shenk R. Chen H. McCrae K. Dowlati A. Remick S. Overmoyer B. A vasculature-targeting regimen of pre-operative docetaxel with or without bevacizumab for locally advanced breast cancer: impact on angiogenic biomarkers. Clin Cancer Res. 2009. (In press). [DOI] [PMC free article] [PubMed]
- 46.Sandler A. Gray R. Perry MC. Brahmer J. Schiller JH. Dowlati A. Lilenbaum R. Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 47.Dowlati A. Gray R. Sandler AB. Schiller JH. Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern Cooperative Group study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]