Abstract
Mutations in DKC1, encoding for dyskerin a pseudouridine synthase that modifies rRNA and regulates telomerase activity, are associated with ribosomal dysfunction and increased cancer susceptibility in the human syndrome, X-linked Dyskeratosis Congenita (X-DC). In a mouse model for X-DC, impairments in DKC1 function affected translation of specific mRNAs harboring internal ribosomal entry site (IRES) elements, including the tumor suppressor, p27. However, how this translational deregulation contributes to tumor initiation and progression remains poorly understood. Here, we report that impairment in p27 IRES-mediated translation, due to decreased levels of DKC1 activity markedly increases spontaneous pituitary tumorigenesis in p27 heterozygous mice. Using a new bioluminescent mouse model, we monitor p27 translation in vivo and show that p27 IRES-mediated translation is reduced in the pituitary of DKC1 hypomorphic mice (DKC1m). Furthermore, we show that DKC1 has a critical role in regulating the assembly of the 48S translational pre-initiation complex mediated by the p27 IRES-element. An analysis of human tumors identified a novel mutation of DKC1 (DKC1S485G) in a human pituitary adenoma. We show that this specific amino acid substitution significantly alters DKC1 stability/pseudouridylation activity, and this correlates with reductions in p27 protein levels. Furthermore, DKC1S485G mutation does not alter telomerase RNA levels. Altogether, these findings demonstrate that genetic alterations in DKC1 can contribute to tumorigenesis associated with somatic cancers and establish a critical role for DKC1 in tumor suppression, at least in part, through translational control of p27.
Introduction
Inherited mutations in the X-linked DKC1 gene, which encodes for dyskerin, (a pseudouridine synthase that modifies rRNA) are associated with the pathogenesis of a severe form of Dyskeratosis Congenita (X-DC) characterized by increased susceptibility to cancer (1). In addition, DKC1 expression is found to be deregulated in many human cancers, including a subset of prostate cancers, B-chronic lymphocytic leukemia (B-CLL), and breast carcinomas (2–4). Therefore, somatic mutations in ribosome components, such as DKC1, may have broad implications for tumorigenesis. DKC1 functions within ribonucleoprotein (RNP) complexes in combination with the box H/ACA small nucleolar RNAs (snoRNA) to catalyze the isomerization of specific uridines (U) into pseudouridines (Ψ) on rRNA, a process known as pseudouridylation. Besides its role as pseudouridine synthase, DKC1 is also implicated in telomere maintenance and mRNA splicing through physical association with the RNA component of the human telomerase (TERC) and small Cajal body RNAs (scaRNAs), respectively (5). We have previously generated DKC1 mutant mice (DKC1m) that faithfully recapitulate all the pathological features of X-DC including increased in cancer susceptibility (6). Importantly, DKC1m mice display reductions in rRNA modifications prior to disease when the telomere’s length is unperturbed (6). An outstanding question that remains to be answered is the role of DKC1 as a tumor suppressor in somatic cancers. Indeed, to date, somatic mutations in the DKC1 gene have not been identified.
IRES-dependent translation is a finely-tuned mechanism that regulates the expression of specific mRNAs during distinct cellular processes such as apoptosis, quiescence and differentiation (7). Deregulation of IRES-mediated translation has been associated with tumor initiation and progression (8, 9). We have previously shown that the loss of function in DKC1 results in a defect in the translation of specific mRNAs that all harbor IRES elements in their 5′ untranslated region (5′UTR), including the cell cycle regulator and tumor suppressor gene, p27. These translational defects present in DKC1m cells are also recapitulated in X-DC patient cells (10).
Here, we genetically demonstrate a critical function for p27 translational control in pituitary tumor suppression that is mediated through dyskerin activity. Several cell cycle regulator genes including p27 are lost or aberrantly expressed in pituitary adenomas (11). For example, loss of one copy of the retinoblastoma (Rb) gene is almost invariably associated with the formation of spontaneous pituitary cancers in both mice and humans (12)(13)(14). High levels of the gene encoding the Pituitary Tumor-Transforming Protein (PTTG), important for the mitotic checkpoint, are observed in pituitary adenomas, and also correlate with tumor invasiveness and recurrence (15)(16). Importantly, the expression of p27 is often reduced in pituitary and other human cancers without mutations in the gene locus (17). p27−/− mice, develop spontaneous pituitary tumors (18). In human pituitary tumors, loss of function of p27 occurs at the post-transcription level and without increases in SKP2 expression, which regulates p27 protein stability (19). This suggests that other mechanisms may be involved in controlling p27 expression, which may be important for tumor suppression (19). Indeed, the p27 gene is tightly regulated post-transcriptionally (20–22). For example, translation of p27 is maximal in quiescence and early G1 phase of the cell cycle through an IRES-element positioned in its 5′UTR (23–25).
Using a new bioluminescent mouse model to directly monitor p27 IRES-dependent translation in vivo, we demonstrate that p27 IRES-mediated translation occurs in the pituitary. Moreover, we show that p27 IRES-mediated translation is dramatically reduced in the pituitary of DKC1m animals. We then delineate the molecular mechanism by which reductions in rRNA pseudouridylation impinge on a critical step of p27 IRES-mediated translation control. In addition, we functionally show that DKC1m;p27+/− mice develop a similar spectrum of pituitary malignancies as p27−/− mice. Finally, we report the first mutation of the DKC1 gene in a patient with pituitary adenoma that results in a drastic reduction of DKC1 expression and pseudouridylation activity, which correlates with a significant decrease of p27 protein levels. These findings delineate a critical role of DKC1 as a tumor suppressor gene in controlling gene expression at the translational level as a barrier against tumor development.
Materials and Methods
Generation of p27-IREST mice and luciferase assay
The p27 IREST mice were generated using a pCMV-Myc-RL-p27 IRES-FL construct. The p27 IRES element (10) was subcloned into pCR 2.1 (Invitrogen), digested with EcoRI and inserted into a pRF plasmid. The RL-HCV IRES-FL was amplified, digested using BglII and KpnI and inserted into the pCMV-Myc expression vector. The resulting pCMV-Myc-RL-p27 IRES-FL was linearized using an Alw44I restriction enzyme and microinjected into mouse embryos that were then implanted into female recipient mice. Founder lines were generated, genotyped and crossed with wild type mice to verify germ line transmission. To confirm that there was no splicing of the transgenic p27 IREST dicistronic RNA, retro-transcriptase PCR (RT-PCR) was performed to amplify a 2.3 Kb fragment spanning from the sequence upstream of the Renilla coding region (P1) up to the Firefly coding region (P2). A second RT-PCR was carried out using primers on the Renilla (P3) and Firefly (P2) sequences to amplify a 750 bp fragment containing the p27 IRES element. The following primers were used:
P1 5′-GCTAGCCACCATGACTTCGAAAG,
P2 5′-GATGTTCACCTCGATATGTGC and P3 5′-GTTTATTGAATCGGACCCAGG.
Determination of p27 IRES-mediated translation
Analysis of p27 IRES activity was performed using the Dual Luciferase kit (Promega) protocol with some modifications. Different organs from p27 IREST mice were surgically removed and immediately transferred in suitable amount of Passive Lysis Buffer (PLB, Promega). Subsequently, tissue lysates were subjected to one cycle of freeze-thaw and homogenized using a tissue pulverizer. Lysates were incubated on ice for 30 minutes and an aliquot of 20 – 40μl was used to measure the FLuc (IRES) and RLuc (cap) activities using a single tube luminometer Optocomp1 (MGM instruments). Expression levels of the p27 IRES dicistronic mRNA, which was carried out using quantitative PCR (QPCR), was used to normalize luciferase activities. Pituitary glands from p27 IREST and p27 IREST;DKC1m animals were surgically removed and immediately transferred in 50 μl of Passive Lysis Buffer. The ratio between FLuc (IRES) and RLuc (cap) Fluc activity was measured as described above.
Histological analysis of the pituitary gland
The excised pituitary glands were immediately fixed in 10% buffered formalin. Fixed tissue specimens were subsequently transitioned into ethanol and embedded in paraffin blocks. Tissue blocks were cut into 5 mm thickness on a Leica microtome. Sections were dried onto glass slides; deparaffinized by incubation at 55°C and with the use of xylene; rehydrated in descending grades of alcohol and stained using a standard hemotoxylin and eosin (H&E) protocol.
Cloning of DKC1S485G mutant
The DKC1S485G mutant was generated using the site direct mutagenesis kit (Stratagene) according to the manufacturer’s instructions. Human DKC1 cDNA was prepared from total mRNA isolated from HeLA cells, and was then FLAG-tagged and subcloned into pCDNA3.1 (Invitrogen). Primers for site direct mutagenesis were:
Forward: AGGCCAAAGCTGGTCTGGAGGGCGGGGCCGAGCCTGGAGATGG
Reverse: CCATCTCCAGGCTCGGCCCCGCCCTCCAGACCAGCTTTGGCCT.
Mutagenesis was confirmed by direct sequencing of the plasmid.
Analysis of protein stability
Stability of DKC1S485G mutant was analyzed as described (10) with some modifications. Briefly, HeLa cells were plated on a 6 well plate at a density of 200.000 cells/well and transfected with expression vectors encoding for full-length wild type DKC1 or DKC1S485G along with a pEGFP to normalize transfection efficiency. Twenty-four hours later, cells were cultured in the presence of 50 μg/ml cyclohexamide (Sigma) and harvested at the indicated time points. Protein analysis was carried out using standard protocols. The following antibodies and dilutions were used: anti-FLAG (Sigma, 1:1000), anti-β-actin (Sigma, 1:5000) and anti-GFP (SCBT, 1:250).
Analysis of 48S pre-initiation complex
Analysis of the 48S pre-initiation complex formation was performed as described (26). Briefly, the p27 IRES monocistronic plasmid was linearized, in vitro transcribed and radiolabeled using a MAXIscript T7 kit (Ambion, AM1312) in the presence of 50 μCi [α-32P]-UTP (NEN). Radiolabeled [32P]-p27 IRES RNA was incubated with cytoplasmic extracts prepared from serum starved (0.1% serum) WT and DKC1m. Cells were harvested and lysed in RLN lysis buffer (10 mM Tris-HCl pH 8.0, 140 mM NaCl, 1.5 mM MgCl2, 20 U RNasin, 0.25% NP-40, 150 μg/mL cyclohexamide, 20 mM DTT). Protein extracts were pre-incubated for 2 minutes at 30°C with components necessary for translation initiation (1 mM ATP, 10 mM creatine phosphate, 1 mg/mL creatine phosphakinase, 0.02 mM L-methionine, 12.5 mM HEPES-KOH pH 7.3) as well as the translation initiation inhibitor – 4mM GMP-PNP (Sigma, G0635-5MG), 0.3 mM cyclohexamide and 0.25 mM spermidine. 48S translation initiation complexes were formed by incubating the mixtures with the [32P]-p27 IRES mRNA probe for 15 minutes at 30°C. Following the incubation, the samples were run on 10–30% sucrose density gradients on a SW55 Beckman rotor at 55,000 RPM and 4°C for 2.5 hrs. Gradients were then fractionated into 200 μL fractions; each was immersed into 1mL of 30% scintillation fluid (Fisher, SX23-5) and counted on a Beckman scintillation counter. Counts per minute (cpm) were plotted against the fraction number.
Patients
This study was approved from the ethics committee of the Max Planck Institute. Informed consent was received directly from patients or their relatives. Samples from 35 pituitary tumors and two normal human pituitary glands were obtained from autopsy cases without any evidence of endocrinological disease, with post mortem delay between 8 and 12 hours. The pituitary adenoma specimens analyzed in this study include: 15 acromegaly-associated adenomas (ACRO), 4 corticotrophinomas (CUSH), and 16 NFPA. Adenomas were diagnosed by clinical, radiological, and surgical findings. Adenomas as well as normal tissue samples were collected after surgical resection and immediately snap frozen in liquid nitrogen. Samples were stored at −80°C before mRNA preparation. Specifically, normal tissue specimens were collected at autopsy and RNA quality was analyzed using a spectrophotometer and by gel electrophoresis. RNA preparations were considered pure when the absorbance ratio 260/280 nm was between 1.6 and 2.0 and no degradation was evident from the RNA gel.
Real-time QPCR analysis of DKC1 and p27 mRNA levels from human samples
Total RNA was extracted from frozen samples using Trizol reagent (Invitrogen, Darmstadt, Germany). For each sample, total RNA was reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA), following manufacturer’s instructions. Real-time PCR analysis of cDNA was performed using a Gene Amp 7000 Sequence Detection Systems (Applied Biosystems). Cycling conditions were the following: 50°C for two minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for one minute. Primers and fluorogenic probes for DKC1 (Hs00154737_m1), p27 (Hs00153277_m1) and human β-glucuronidase (4326320E) were purchased from Applied Biosystems. Telomerase RNA analysis was performed as described elsewhere (2). The following primers were used: TERC forward 5′-GGTGGTGGCCATTTTTTGTC-3′ and TERC reverse 5′-CTAGAATGAACGGTGGAAGGC-3′ and TERC taqman probe 5′-CGCGCTGTTTTTCTCGCTGACTTTC-3′. Samples were analyzed in triplicates and the relative amount of mRNA was determined using the −ΔΔCt methods.
p27 and DKC1 western blot analysis from human samples
Normal and tumor specimens were treated by lysis buffer (KH2PO4 0.1M, pH 7.4, 1% Igepal CA 630; Sigma Chemical, St Louis, MO) at 4 °C, homogenized and centrifuged. Supernatants were used for Western blot analysis by standard protocols using anti-p27 and anti-DKC1 antibodies (clone DCS-72; MBL international, Woburn, MA, USA, Atlas antibodies Stockholm, Sweden).
Measure of rRNA pseudouridylation
Evaluation of rRNA pseudouridylation was carried out as previously described (2). Briefly, forty micrograms of total RNA were electrophoresed on a 1.2% agarose-2.2 M formaldehyde gel and 28S and 18S rRNA was purified from the gel using DNA ultrafree DA spin columns (Millipore Corporation, Billerica, MA). The 18S rRNA was treated with RNAse T2 (Sigma-Aldrich, St. Louis, MO) for one hour at 37°C in 10 mM ammonium acetate buffer pH 5.5. The resulting nucleotides were then dephosphorylated using alkaline phosphatase (Fluka, Sigma-Aldrich, St. Louis, MO) for one hour at 37°C after the addition of 0.4 vol. of 50 mM Tris-base and 0.1 vol. of MgCl2 10 mM. The resulting nucleosides were then subjected to high performance liquid chromatographic separation in a Beckman System Gold Programmable Solvent Module 126 equipped with a detector Module 166 set at 254 nm (Beckman-Coulter, Fullerton, CA). The column was a reverse-phase μBondapak C18 (particle size 10 μm) purchased from Waters Associates (Milford, MA). Mobile phase conditions were 0.1 M phosphate buffer pH 6/methanol: 99:1 (v/v) for 12 minutes, 96:4 (v/v) for 13 minutes and 85:15 (v/v) for 25 minutes. Pseudouridine and major nucleosides used as standards were purchased from Berry and associates, Inc. (Dexter, MI, USA)
Results
Bioluminescent mice to monitor p27 IRES-dependent translation in vivo reveal robust translational control in the pituitary, which is reduced in the DKC1m background
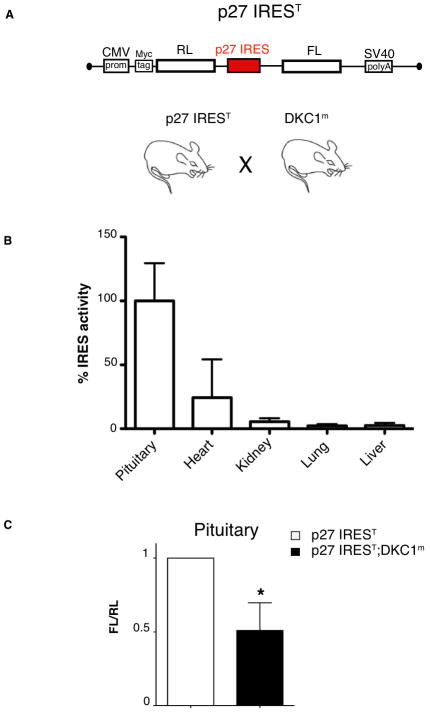
We have previously shown that translational control of p27 gene expression is impaired in DKC1m mice and X-DC patient cells (10). We therefore sought to directly monitor p27 IRES-dependent translation in vivo. To this end, we generated the first bioluminescent transgenic mouse model (p27 IREST) to monitor p27 IRES-dependent translation in vivo. P27 IREST mice harbor a dicistronic reporter gene controlled by the Cytomegalovirus (CMV) ubiquitous promoter. Specifically, the expression of the transgene led to the production of a dicistronic mRNA in which the first cistron (RLuc) is translated via cap-dependent translation, whereas the second cistron (FLuc) is translated under the control of the p27 IRES element (Figure 1A). We ruled out the possibility of aberrant splicing events and cryptic promoters by testing the integrity of the p27 IREST mRNA (Suppl. Figure 1). Employing the p27 IREST mice we detected robust p27 IRES-mediated translation in the pituitary gland while other tissues showed either low or absent activity (Figure 1B).
Figure 1. Analysis of p27 IRES-mediated translation using a new bioluminescent mouse model reveal robust translational control in the pituitary, which is reduced in DKC1m background.
A) Schematic of the dicistronic transgene used to generate bioluminescent p27 IREST animals. B) Analysis of p27 IREST mice. Graph is mean ± SEM of percentage p27 IRES activity (FLuc values) measured in different organs prepared from three p27 IREST mice and normalized by the mRNA dicistronic expression. C) p27 IRES-mediated translation is markedly reduced in DKC1m pituitary glands. Levels of p27 IRES-mediated translation were measured in pituitary glands isolated from p27 IREST and p27 IREST;DKC1m mice. Columns, mean ± SEM of ratio FLuc/RLuc (FL/RL) measured in four pairs of age-matched animals; bars, SEM. Statistical analysis was carried out using Student t test. *P<0.05.
Thus, p27 expression in the pituitary is controlled at least in part by IRES-mediated translation. We therefore focused our attention on the pituitary gland, where p27-IRES dependent translation is active and asked whether this activity may be impaired in DKC1m animals. We observed a strong reduction in p27 IRES-dependent activity within the pituitary of DKC1m mice (Figure 1C). No differences were observed in cap-dependent translation, which is consistent with our previous results (10). These findings suggest that IRES-dependent translation of p27 protein is active within the pituitary reflecting a cap-independent translation mechanism that may be required to maintain accurate protein levels in more differentiated and quiescent cells.
Defining the molecular impairment in p27 IRES-dependent translation in DKC1m cells
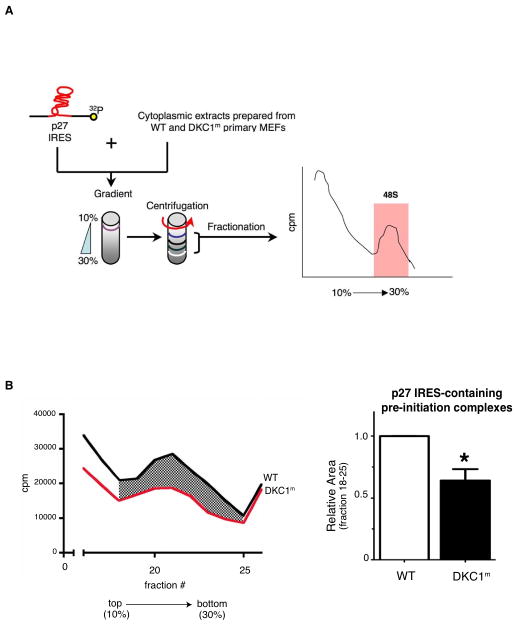
The molecular mechanisms by which defects in IRES-dependent translation occur when rRNA modifications are decreased in DKC1m cells remain unknown. One outstanding question is whether this molecular impairment occurs at the stage of ribosome binding to specific mRNAs, as in the case of p27. To answer this question, we reconstituted the formation of an initiation complex of translation on the p27 IRES element, employing WT and DKC1m cytoplasmic extracts following serum starvation, a condition that strongly induces p27 IRES-mediated translation (10). This enabled us to investigate whether DKC1m cells are impaired in the assembly of the pre-initiation complex (48S), one of the most critical and earliest events in the translation initiation process in which interactions between mRNA-40S subunits occur (Figure 2A) (27). By analyzing the efficiency of the 48S complexes formation in WT and DKC1m cells, we found that the total amount of p27 IRES-containing pre-initiation complexes is markedly reduced in DKC1m compared to WT cells (Figure 2B). These data indicate that rRNA modifications directly impinge on the assembly of a pre-initiation complex of translation that mediates p27 IRES-dependent translation.
Figure 2. Molecular mechanism of p27 IRES-mediated translation impairment in DKC1m cells.
A) Schematic of the biochemical approach used to study the 48S complex formation in WT and DKC1m MEFs. A [32P]-p27 IRES mRNA probe was incubated with cytoplasmic extracts. Newly assembled 48S complexes were separated by sucrose gradient centrifugation and fractionated according to their density. A peak of radioactivity was generated and coincided with fractions containing the 48S complexes. B) Representative of the 48S subunit quantification from total cytoplasmic extracts prepared from serum starved (0.1% FBS) WT and DKC1m cells. Representative profile of a sucrose density gradient reporting the radioactive intensity per fractions in WT (black) and DKC1m (red) extracts, respectively (left). Columns, mean ± SEM of the area under the curve in WT and DKC1m cell extracts measured in 3 independent experiments; bars, SEM (right). Statistical analysis was carried out using Student t test. *P<0.05.
DKC1 and p27 genetically interact in pituitary tumorigenesis
The most prominent spontaneous tumor present in p27−/− mice is pituitary cancer of the intermediate lobe, which occurs after 3 months of age (18). As we observed robust p27 IRES-dependent translation in the pituitary, we reasoned that reduced p27 translation in DKC1m mice might cooperate in pituitary tumorigenesis. We therefore tested for a genetic interaction between DKC1 and p27. Strikingly, histological analysis revealed pronounced hyperplasia of the intermediate lobe (pars intermedia) of p27+/−;DKC1m pituitary glands already evident as early as 8 months of age, while no abnormalities are present in WT, p27+/− and DKC1m mice at this age (Figure 3A). These findings indicate that reductions in DKC1 expression significantly accelerate the onset of intermediate lobe hyperplasia in p27+/−; DKC1m. We next asked whether cooperation between DKC1 and p27 would progress and culminate in full-blown pituitary adenoma in p27+/−;DKC1m animals. By 15 months of age p27+/−;DKC1m mice show a more dramatic intermediate lobe hyperplasia as well as pituitary adenomas (Figure 3B). Indeed, histopathological analysis of these tumors revealed the presence of numerous perivascular pseudorosette formations, typical features of primitive neuroectodermal carcinomas (Figure 3B). Only a mild hyperplasia is present in p27+/− mice and DKC1m mice at this stage (Figure 3B). Taken together these data suggest that p27-IRES dependent translation may be a functionally important mechanism for tumor suppression within the pituitary.
Figure 3. DKC1 and p27 genetically cooperate toward pituitary tumorigenesis.
A) Micrographs of hematoxylin and eosin-stained paraffin sections prepared from pituitary glands of 8 month old WT, p27+/−;DKC1m (hyperplasia) and p27−/− (tumor) animals. Numbers on the pictures indicate: (1) pars nervosa, (2) pars intermedia (intermediate lobe) and (3) pars distalis, and bars correspond to 10 nm. Columns, mean ± SEM of length and area of the intermediate lobe; bars SEM. The number of animals analyzed for each genotype is indicated on the columns. Statistical analysis was performed using unpaired two-tailed t test. P values are ** <0.01 and * <0.05. B) Representative histopathological analysis of a normal pituitary gland (top) and a pituitary adenoma (bottom) from 15 month old WT and p27+/−;DKC1m mice, respectively. Graph shows the percentages of double p27+/−;DKC1m, p27+/− and DKC1m animals with a normal pituitary (blue), pituitary hyperplasia (yellow) and pituitary cancers (red).
A novel mutation in the DKC1 gene is associated with a human pituitary tumor
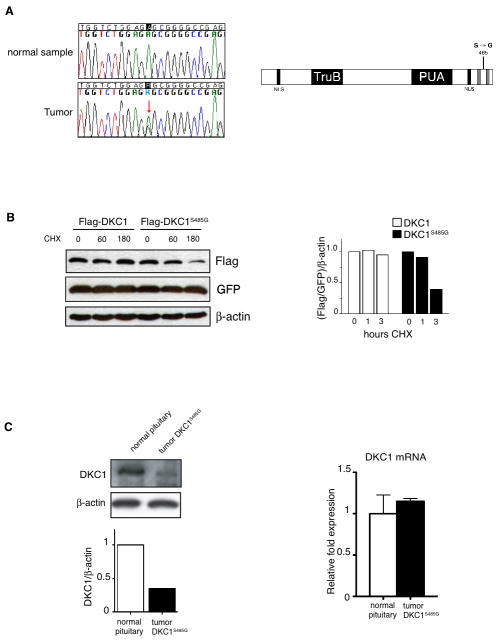
We extended our analysis of DKC1 in human pituitary tumors. To date, cancer-associated mutations in DKC1 have not been identified. We performed direct bidirectional sequencing of DNA isolated from 35 primary human pituitary adenomas. In this context, we identified a novel mutation of the DKC1 gene in a tumor biopsy from a 61-year-old female, diagnosed with Non-Functioning Pituitary Adenoma (NFPA) without previous history of disease. Histological analysis of the tumor showed negative staining for luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), prolactin (PRL), adrenocorticotrophic hormone (ACTH) and growth hormone (GH). The magnetic resonance imaging (MRI) revealed an intrasellar tumor with suprasellar extension and maximum diameters of 26, 24, and 20 mm in the vertical, horizontal, and antero-posterior axes, respectively. The tumor was infiltrating the sellar floor but not the dura mater and was surgically removed. However, the patient relapsed within three years from the initial surgery and radio-surgery was necessary to remove a pathological growth of intrasellar tissue adjacent to the right cavernous sinus. Sequencing analysis of DNA isolated from the primary tumor show a point mutation in one copy of the X-linked DKC1 gene, which corresponded to a novel A to G nucleotide variation, located in exon 14. This results in a non-synonymous serine (S) to glycine (G) substitution at position 485 (henceforth referred to as DKC1S485G) in the C-terminal portion of the protein (Figure 4A). This point mutation in DKC1 has not been previously reported in Dyskeratosis Congenita. Therefore, we next sought to determine whether and how the DKC1S485G alters dyskerin protein expression and/or activity. We engineered the DKC1S485G mutation in an exogenous copy of DKC1 and found that it was significantly less stable than the DKC1 protein (Figure 4B). Consistent with these findings, we observed that in the DKC1S485G primary tumor sample, DKC1 protein levels were significantly reduced, but not affected at the mRNA level (Figure 4C). Decreases in DKC1 activity were also evident as we observed a reduction in 18S rRNA pseudouridylation levels (Figure 5A). Importantly, it has been previously shown that DKC1 mutations affect the accumulation of telomerase RNA (TERC) and alter telomerase complex activity (28). Therefore, we measured TERC RNA levels in the DKC1S485G mutant tumor (Figure 5B). We did not find any significant reduction of TERC RNA levels in the DKC1S485G mutant tumor when compared to normal pituitary tissues (Figure 5B), which suggests that this specific DKC1 mutation may not affect telomerase activity.
Figure 4. Identification of a novel DKC1 mutation in a human pituitary adenoma.
A) Chromatographs showing a novel mutation in the DKC1 locus identified in a human pituitary adenoma (left). Scheme of the mutation, an A1493G variation located on one DKC1 allele leads to the amino acid change S485G in the C-terminal portion of the protein (right). B) Analysis of DKC1S485G protein stability. Densitometric analysis of the DKC1 levels was done at each time point after blockage of protein synthesis as indicated. GFP was co-transfected for transfection efficiency and β-actin was used as loading control. C) DKC1 protein (left) and mRNA (right) levels in a normal pituitary and in the tumor bearing the mutation DKC1S485G. Densitometric analysis of DKC1 over β-actin protein levels in each sample is shown (bottom left).
Figure 5. Impaired rRNA pseudouridylation is associated with reductions of p27 protein levels but not of the telomerase RNA component (TERC) in the DKC1S485G mutant tumor.
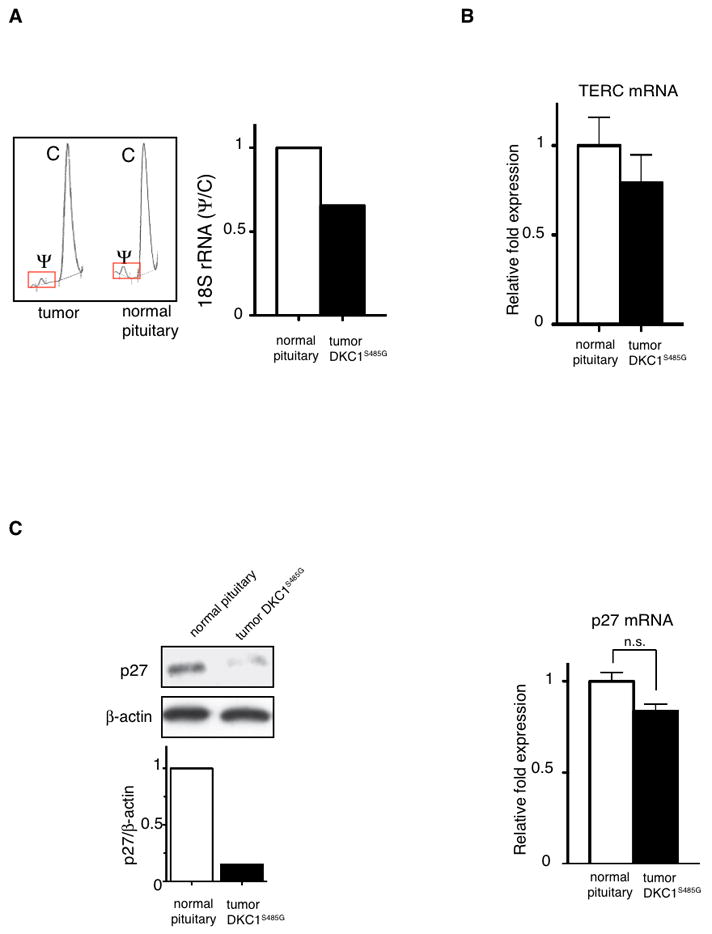
A) Chromatographs of HPLC analysis of the 18S rRNA pseudouridylation are shown. Bar graph shows quantification of the Ψ/C ratio in samples from normal pituitary and the DKC1S485G mutant tumor. B) The levels of the TERC were measured by QPCR in two normal pituitaries and the DKC1S485G pituitary tumor. Analysis was performed in triplicate on two different samples per specimen. No significant differences between the samples were found. P>0.05 was determined employing the unpaired two-tailed t test. C) p27 protein levels are markedly reduced in the tumor carrying the DKC1S485G mutation. Western blot (left) and QPCR analysis (right) of p27 expression was performed using protein extracts and total RNA prepared from a normal pituitary and the tumor carrying the DKC1S485G mutantion. Densitometric analysis of p27 over β-actin is shown (bottom left). No significant differences in p27 mRNA levels were measured between the samples. P>0.05 was determined employing the unpaired two-tailed t test.
Next, we analyzed p27 levels in the primary tumor sample that harbored the DKC1S485G mutation. Consistent with our results showing the importance of dyskerin in p27 translational control, we found that p27 protein expression is significantly diminished while p27 mRNA expression is comparable to normal pituitary tissue (Figure 5C).
Discussion
In this study we employed a novel genetic reporter system to monitor a specific mode of p27 translation control that occurs via an IRES-element positioned in the 5′UTR of the p27 mRNA. We observed robust control of p27-IRES dependent translation in the pituitary and found that this activity is severely impaired in hypomorphic DKC1m mice. At the molecular level we showed that DKC1m ribosomes show a defect in 48S preinitiation complex formation on the p27 IRES element. We functionally extend these findings by identifying a genetic interaction between DKC1m and p27+/− mice leading to the formation of pituitary tumors. Cooperation of other distinct oncogenic lesions has been studied using compound mice in order to elucidate the molecular mechanisms underlying pituitary tumor development (11). For example, compound Rb+/−;Pttg−/− and Rb+/−;Skp2−/− mice display reduced frequency of pituitary tumors (29)(30). In contrast, genetic loss of p21 gene contributes to accelerated pituitary tumor formation in Rb heterozygous mice and similarly mice lacking both p27 and p18 rapidly develop lethal pituitary adenomas (31)(32).
Importantly our results led us to undertake direct sequencing of the DKC1 gene in primary human pituitary tumors, which identified a novel mutation in DKC1. The DKC1S485G mutation leads to a decrease in dyskerin protein stability and activity, and is associated with decreases in rRNA modifications and p27 protein levels.
Furthermore, it would be important to study whether, beside point mutations, additional epigenetic events may deregulate DKC1 expression in pituitary adenomas. In this regard, several studies indicate that hypermethylation of specific CpG island located within promoter regions of genes encoding for tumor suppressors such as Rb, GADD45γ, p16, maternal express gene-3 (MEG3) and pituitary tumor apoptosis gene (PTAG) is associated with loss of protein expression in a number of pituitary tumors (33)(34).
The Multiple Endocrine Neoplasia type 1 (MEN1) gene is mutated in the eponymous syndrome, which is characterized by increased predisposition to pituitary adenoma formation (35). Interestingly, a germ-line point mutation in p27 gene has been identified in a MEN1 patient harboring no mutation in MEN1 gene and presenting with pituitary tumors (36). However, decreased p27 expression has been reported in many human cancers without mutations in the gene (17). Therefore, reductions in p27 expression, by alterations in pathways that control p27 expression, may contribute to the initiation or progression of cancer. It is tempting to speculate that alterations in p27 mRNA translation may represent an early event in the clonal expansion leading to pituitary adenoma phenotype. In support of this hypothesis, ubiquitin-mediated degradation of p27 controlled by SKP2 does not seem to correlate with reduction in p27 protein expression in pituitary tumors (19). At the molecular level we show that impairments in rRNA modifications directly affect a very early step in p27-IRES translation initiation. Interestingly, defects in a second type of rRNA modification, rRNA methylation, have been implicated in tumorigenesis (37, 38). For example, loss of the U50 gene that mediates rRNA methylation has been linked to the pathogenesis of human breast and prostate cancers (39, 40). Therefore, deregulations of rRNA modifications may have broad implications for tumors arising from somatic mutations in ribosome components. Our study sets the rationale for screening for additional somatic DKC1 mutations in human tumors.
Supplementary Material
Acknowledgments
We would like to thank Dr. Barna for critical discussion and reading of the manuscript; Lynn Delos Santos and Anthony Karnezis for technical support; Olivia F. Siegel for editing the manuscript. This work was supported by R01 HL085572 (D.R.) and 3R01HL085572-05S1 (D.R.).
References
- 1.Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet. 2008;73:103–12. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 2.Montanaro L, Brigotti M, Clohessy J, et al. Dyskerin expression influences the level of ribosomal RNA pseudo-uridylation and telomerase RNA component in human breast cancer. J Pathol. 2006;210:10–8. doi: 10.1002/path.2023. [DOI] [PubMed] [Google Scholar]
- 3.Sieron P, Hader C, Hatina J, et al. DKC1 overexpression associated with prostate cancer progression. Br J Cancer. 2009;101:1410–6. doi: 10.1038/sj.bjc.6605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poncet D, Belleville A, t’kint de Roodenbeke C, et al. Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood. 2008;111:2388–91. doi: 10.1182/blood-2007-09-111245. [DOI] [PubMed] [Google Scholar]
- 5.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggero D, Grisendi S, Piazza F, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–62. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 7.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–27. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 8.Silvera D, Arju R, Darvishian F, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–8. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 9.Barna M, Pusic A, Zollo O, et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–5. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon A, Peng G, Brandenburger Y, et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–6. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 11.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikitin A, Lee WH. Early loss of the retinoblastoma gene is associated with impaired growth inhibitory innervation during melanotroph carcinogenesis in Rb+/− mice. Genes Dev. 1996;10:1870–9. doi: 10.1101/gad.10.15.1870. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Chang CY, Hu N, et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–94. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 14.Pei L, Melmed S, Scheithauer B, Kovacs K, Benedict WF, Prager D. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary tumors: evidence for a chromosome 13 tumor suppressor gene other than RB. Cancer Res. 1995;55:1613–6. [PubMed] [Google Scholar]
- 15.Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–41. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- 16.Abbud RA, Takumi I, Barker EM, et al. Early multipotential pituitary focal hyperplasia in the alpha-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol Endocrinol. 2005;19:1383–91. doi: 10.1210/me.2004-0403. [DOI] [PubMed] [Google Scholar]
- 17.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–7. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musat M, Korbonits M, Pyle M, et al. The expression of the F-box protein Skp2 is negatively associated with p27 expression in human pituitary tumors. Pituitary. 2002;5:235–42. doi: 10.1023/a:1025325832698. [DOI] [PubMed] [Google Scholar]
- 20.Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–8. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 21.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–4. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 22.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 23.Gopfert U, Kullmann M, Hengst L. Cell cycle-dependent translation of p27 involves a responsive element in its 5′-UTR that overlaps with a uORF. Hum Mol Genet. 2003;12:1767–79. doi: 10.1093/hmg/ddg177. [DOI] [PubMed] [Google Scholar]
- 24.Miskimins WK, Wang G, Hawkinson M, Miskimins R. Control of cyclin-dependent kinase inhibitor p27 expression by cap-independent translation. Mol Cell Biol. 2001;21:4960–7. doi: 10.1128/MCB.21.15.4960-4967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullmann M, Gopfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 2002;16:3087–99. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa-Mattioli M, Svitkin Y, Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol Cell Biol. 2004;24:6861–70. doi: 10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci U S A. 2004;101:10756–61. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg−/− mice is protective for Rb+/− pituitary tumorigenesis. Mol Endocrinol. 2005;19:2371–9. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Bauzon F, Ji P, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet. 42:83–8. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesnokova V, Zonis S, Kovacs K, et al. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci U S A. 2008;105:17498–503. doi: 10.1073/pnas.0804810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin DS, Godfrey VL, Lee H, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson DJ, Hibberts NA, McNicol AM, Clayton RN, Farrell WE. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res. 2000;60:1211–6. [PubMed] [Google Scholar]
- 34.Farrell WE. Pituitary tumours: findings from whole genome analyses. Endocr Relat Cancer. 2006;13:707–16. doi: 10.1677/erc.1.01131. [DOI] [PubMed] [Google Scholar]
- 35.Marx SJ, Agarwal SK, Kester MB, et al. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent Prog Horm Res. 1999;54:397–438. discussion -9. [PubMed] [Google Scholar]
- 36.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103:15558–63. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belin S, Beghin A, Solano-Gonzalez E, et al. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PLoS One. 2009;4:e7147. doi: 10.1371/journal.pone.0007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munholland JM, Nazar RN. Methylation of ribosomal RNA as a possible factor in cell differentiation. Cancer Res. 1987;47:169–72. [PubMed] [Google Scholar]
- 39.Dong XY, Guo P, Boyd J, et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics. 2009;36:447–54. doi: 10.1016/S1673-8527(08)60134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong XY, Rodriguez C, Guo P, et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet. 2008;17:1031–42. doi: 10.1093/hmg/ddm375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.