Abstract
Alcohol-sensitive type 1 equilibrative nucleotide transporter (ENT1) is known to regulate glutamate signaling in the striatum as well as ethanol intoxication. However, it was unclear whether altered extracellular glutamate levels in ENT1−/− mice contribute to ethanol-induced behavioral changes. Here we report that altered glutamate signaling in ENT1−/− mice is implicated in the ethanol-induced locomotion and ataxia by NMDA receptor antagonist, CGP37849. ENT1−/− mice appear less intoxicated following sequential treatment with CGP37849 and ethanol, compared to ENT1+/+ littermates on the rotarod. These results indicate that inhibiting NMDA glutamate receptors is critical to regulate the response and susceptibility of alcohol related behaviors. Interestingly, a microdialysis experiment showed that the ventral striatum of ENT1−/− mice is less sensitive to the glutamate-reducing effect of the NMDA receptor antagonist compared to the dorsal striatum. Our findings suggest that differential glutamate neurotransmission in the striatum regulates ethanol intoxication.
Keywords: alcoholism, ENT1, glutamate neurotransmission, CGP37849, microdialysis, ataxia, caudate-putamen, nucleus accumbens
INTRODUCTION
Ethanol alters the homeostasis between excitatory and inhibitory neurotransmitters and the subsequent receptor-mediated signaling cascade in the brain [15, 24]. Adenosine regulates neuronal activity as a neuromodulator or a non-classical neurotransmitter [4, 5] and has been implicated in the pathophysiology of several central nervous system disorders including sleep disorders [2, 35], anxiety [7, 22], and alcoholism [16, 28]. Type 1 equilibrative nucleoside transporter (ENT1) encodes an ethanol-sensitive adenosine transporter, which is known to regulate adenosine levels in response to acute ethanol treatment in cultured cells [29]. Mice lacking ENT1 exhibit reduced ataxic and hypnotic effects of acute ethanol exposure and consume more alcohol compared to their wild-type littermates [8]. Conversely, mice overexpressing human ENT1 in neurons are more sensitive to the intoxicating effects of ethanol [31], indicating that ENT1 gene expression is positively correlated with ethanol sensitivity. Our previous study indicates that increased resistance to acute ethanol intoxication is related to increased glutamate signaling in ENT1−/− mice [6]. Increased glutamate levels regulate several attributes of alcoholism including ethanol withdrawal seizures [37] and excessive ethanol drinking [19, 33, 34]. Since our previous electrophysiological data suggests that one phenotype of ENT1−/− mice is increased baseline glutamate levels [8], a hyperglutamate status might be related to increased tolerance to the ataxic effect of ethanol. Importantly, glutamate levels involved in the cortico-striatal circuit play an important role in the formation of addiction related behaviors including ethanol seeking and locomotor sensitization. We hypothesized that ENT1−/− mice are more tolerant to the ataxic effect of ethanol compared to ENT1+/+ littermates when mice are treated with an NMDA receptor antagonist, which might alter glutamate levels in the striatum.
Here we report that ENT1−/− mice are less sensitive to ethanol-induced ataxia following an NMDA receptor antagonist, CGP37849, treatment compared to ENT1+/+ littermates. Also, using microdialysis, we identified differential glutamate levels in response to CGP37849 between the dorsal striatum and ventral striatum of mice, which may regulate ethanol intoxication through the cortico-striatal neuronal circuit.
METHODS
Animals
ENT1−/− mice were generated as described [8]. Only F2 homozygote (ENT1+/+ and ENT1−/−) mice were used for this study. To generate F2 ENT1−/− mice, heterozygous ENT1 mice in a 129X1/SvJ background (> 10 generations) were crossed with C57BL/6J wild-type mice, and then crossed between F1 heterozygous mice. We obtained approximately 25% wild-type, 25% ENT1 null, and 50% heterozygous mice. We used 8-16 week old male mice for all experiments. Mice were housed in standard Plexiglas cages with food and water ad libitum. The colony room was maintained on a 12 h light/dark cycle with lights on at 6:00 a.m. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with NIH guidelines.
Locomotor activity
Locomotor activity was measured in open field chambers (27 × 27 cm) that automatically recorded activity via photo beam breaks (Med Associates, VT) as described [9, 25]. Spontaneous locomotor activity and habituation were measured for three consecutive time points. At the beginning of the locomotion experiment, mice were placed in the center of open field chambers and the horizontal distance traveled (cm) was recorded per each of the three consecutive 20 min sessions. First, locomotor activity was measured by injecting CGP37849 (1.0, 5.0 or 10 mg/kg) 15 min prior to the placement of the mice in the open field chamber. Then, for the ethanol-stimulated locomotor activity test, mice were injected with CGP37849 (10 mg/kg, i.p., in saline) 30 min before the open field experiment followed by a 1.0 g/kg ethanol injection 15 min prior to the experiment the placement of the mice in the open field chamber.
Ataxia
Motor incoordination (ataxia) was measured using a mouse rotarod treadmill (UGO Basile, Italy) rotating at a fixed speed of 20 rpm as described [6, 14]. Mice were acclimatized to the rotarod treadmill by placing them on the apparatus 2-3 times prior to the actual experiment. Only mice that were able to pass this initial screening (remained on the treadmill for 180 sec; time point 0 min) were used for the rotarod experiments. First, ataxia was measured by injecting CGP37849 (1.0, 5.0 or 10 mg/kg) 15 min prior to the placement of the mice on the rotarod. We also examined the ataxic effect of ethanol. Mice were first injected with an NMDA receptor antagonist (CGP37849; 10 mg/kg, i.p.) 30 min prior to the rotarod experiment and then injected with 1.0 g/kg ethanol (i.p. injection) 15 min prior to the rotarod test. Mice were placed on the rotarod 15 min following the ethanol treatment and every sequential 15 min for 2 h to measure latency to fall. All drugs were dissolved in isotonic saline and injected intraperitoneally.
Microdialysis
To measure extracellular glutamate levels in the brain, a guide cannula was implanted into the nucleus accumbens shell (AP: 1.3 mm; ML: 0.5 mm; DV: −3.5 mm) or caudate-putamen (AP: 0.5 mm; ML: 2.0 mm; DV: −2.0 mm). Mice were given 7 to 10 days for recovery. During this period, mice were placed in the test chamber for 1 h daily to habituate the handling procedure and experimental environment as described [7]. A microdialysis probe with a 2.0 mm cellulose membrane (Eicom, Kyoto, Japan; MW cut off: 50,000 Da) was connected to a micro-syringe pump (Eicom, Kyoto, Japan) to continuously deliver Ringer’s solution (145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, pH 7.4) at a 1.0 μl/min flow rate. To measure glutamate level changes in response to an NMDA receptor antagonist (CPG37849, 10 mg/kg) dialysates were collected, after a 2 h stabilization period, every 20 min immediately following an i.p. injection of the NMDA receptor antagonist. The starting time point of each 20 min interval was used for comparisons (time point 0 min represents dialysate glutmate levels during the time from when the drug was injected to 20 min following the drug injection; time point 20 represents glutamate levels from 20 to 40 min after the drug injection and so on). Dialysates were frozen and stored at −80 °C until analyzed. The glutamate concentration was quantified in each sample by HPLC. Dialysates were assayed with HPLC-ECD (HTEC-500, Eicom) coupled with an autosampler (719D, Alcott, GA). These data were collected through an EPC-500 processor (Eicom) and peak areas were calculated using the PowerChrom software (Eicom)
Statistical analysis
All data are presented as the mean ± SEM (standard error of the mean). Data were analyzed by two-tailed t-test or ANOVA (one-way or two-way) followed by a Tukey post hoc test for individual comparisons. Results were considered significantly different when p < 0.05.
RESULTS
The effect of NMDA glutamate receptor antagonist, CGP37849, on ethanol-induced locomotion and ataxia
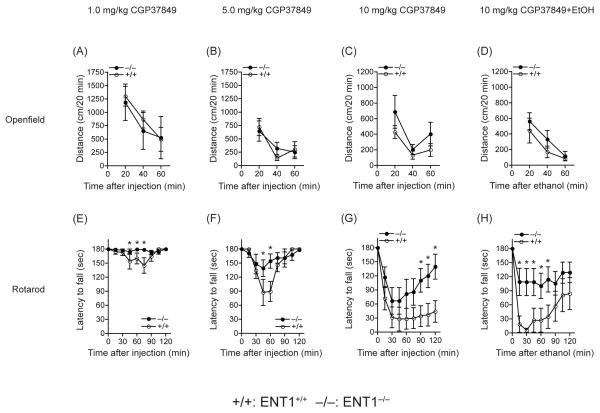
Our previous study showed that ENT1−/− mice are significantly more tolerant to the ataxic effect of ethanol, especially 15 min after the 1.0 or 1.5 g/kg ethanol injection (i.p.) compared to wild-type littermates, while no differences were detected 30-60 min after the ethanol injection [8]. Interestingly, in the present study we found that ENT1−/− mice appear more tolerant to 10 mg/kg CGP37849 from 90-120 min after the injection (i.p.) [6]. Thus, we investigated whether CGP37849 treatment could alter ethanol-induced effects on locomotion or ataxia. First, we examined whether CGP37849 alone has a locomotor stimulating effect in an open field chamber. Locomotor activity for three different doses of CGP37849 (1.0, 5.0 or 10 mg/kg) was similar between genotypes. Two-way ANOVA showed no significant difference in genotype effect for 1.0 mg/kg (F1,42=0.18, p=0.68), 5.0 mg/kg (F1,42=0.03, p=0.87) or 10 mg/kg (F1,42=3.31, p=0.08) of CGP37849. Also, there are no statistical interactions between genotype and time for 1.0 mg/kg (F2,42=0.09, p=0.91), 5.0 mg/kg (F2,42=0.55, p=0.58) or 10 mg/kg (F2,42=0.32, p=0.73) of CGP37849 (Figure 1A-C). When mice were treated by 1.0 g/kg ethanol 15 min after the 10 mg/kg CGP3789 injection, there was no significant change in spontaneous locomotion stimulation between genotypes. Two-way ANOVA showed no locomotion activity difference in genotype (F1,42=3.78, p=0.06) or interaction between genotype and time (F2,42=0.69, p=0.51) (Figure 1D). We also examined CGP37849 induced ataxia to investigate the altered resistance to glutamate neurotransmission of ENT1−/− mice. As shown in Figure 1E-G, different doses of CGP37849 (1.0, 5.0 or 10 mg/kg) induced less ataxia in ENT1−/− mice compared to wild-type littermates. Two-way ANOVA showed a statistical significant effect of genotype for 1.0 mg/kg (F1,135 = 8.47, p=0.004), 5.0 mg/kg (F1,135 = 4.15, p= 0.044) and 10 mg/kg (F1,130 = 22.31, p < 0.001) of CGP37849. Then, we examined the ataxic effect of 1.0 g/kg ethanol, 15 min after the injection of an NMDA receptor antagonist CGP37849 (10 mg/kg, i.p.), since 10 mg/kg CGP37849 induces severe ataxia and has a different recovery pattern than 1.0 g/kg ethanol (Figure 1F). Tukey post-hoc analysis showed that a difference between genotypes was apparent 15-75 min after the ethanol injection (30-90 min after CGP37849 injection; Figure 1H) while CGP37849 alone induced a genotype difference 90-120 min after the injection (Figure 1G). In ENT1+/+ mice, the co-treatment of ethanol and CGP37849 appear to be associated with faster intoxication than with just CGP37849 treatment alone; the recovery rate from the ataxic behavior was also slower.
Figure 1.
Effect of an NMDA glutamate receptor antagonist (CGP37849) on ethanol-induced locomotion and ataxia in ENT1−/− mice (−/−). (A-C) Locomotor activity was similar between genotypes in response to 1.0, 5.0 or 10 mg/kg CGP37849. (D) Ethanol-induced locomotion in response to CGP37849 was also similar between genotypes. (E-G) CGP37849 induced significant ataxic effects at 1.0, 5.0 or 10 mg/kg doses. (H) The ataxic effect of 1.0 g/kg ethanol 15 min following an NMDA receptor antagonist (CGP37849; 10 mg/kg, i.p.) was measured using a rotarod. ENT1−/− mice (n = 10) are less intoxicated than ENT1+/+ littermates (+/+; n = 7) (*p < 0.05 compared to ENT1+/+ mice by Tukey tests). Data are presented as mean ± SEM.
Glutamate levels in response to CGP37849 in the CPu and NAc
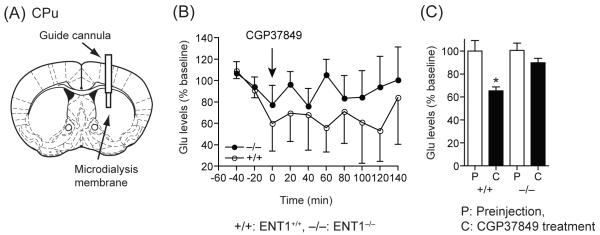
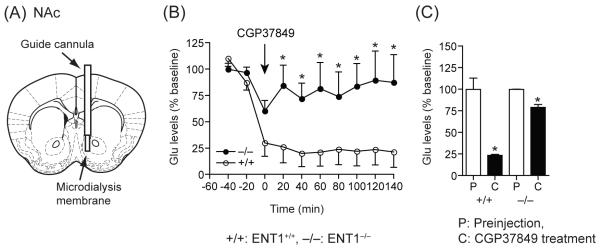
We investigated the extracellular glutamate levels in both the dorsal (CPu) and ventral (NAc) striatum in response to an NMDA receptor antagonist. We performed a microdialysis experiment using NMDA receptor antagonist CGP37849 (10 mg/kg, i.p.). As shown in Figure 2, in the CPu, extracellular glutamate levels were decreased significantly in ENT1+/+ mice compared to basal levels, but were not altered in ENT1−/− mice (Figure 2B and C), suggesting that ENT1−/− mice are more significantly resistant to the inhibitory effect of the NMDA receptor antagonist compared to that of ENT1+/+ mice. Two-way ANOVA showed no significant difference in the effect of genotype for a 10 mg/kg (F1,99=3.44, p=0.07) CGP37849 injection. No time points were statistically significant via Tukey post hoc test (Figure 2B), however the accumulating values between pre-injection (P) and CGP37849-injection (C) showed a significant change between genotypes in the CPu with glutamate levels of ENT1 null mice being less sensitive to the antagonistic effect of the drug. This difference might account for the reduced ataxic effect of the NMDA receptor antagonist in ENT1−/− mice [6]. Interestingly, in the NAc, the NMDA receptor antagonist dramatically reduced extracellular glutamate levels in ENT1+/+ mice while only a moderate effect was observed in ENT1−/− mice (Figure 3B). Two-way ANOVA showed a statistically significant effect of genotype for a 10 mg/kg (F1,103 = 35.72, p< 0.001) CGP37849 injection. ENT1−/− mice appear to be more resistance to the inhibitory effect of the NMDA receptor antagonist than ENT1+/+ mice. Tukey post hoc test for individual comparisons indicated a significant difference from 20 min to 140 min and glutamate levels were also different between genotypes across these time points. (Figure 3B and C). These results support the hypothesis that differential glutamate levels in response to NMDA receptor antagonism may be due to different glutamatergic afferents projecting to the NAc, which regulates the motivational effect of ethanol and is more sensitive to the CGP37849 treatment.
Figure 2.
Effect of an NMDA glutamate receptor antagonist (CGP37849) on glutamate levels in the CPu analyzed by microdialysis. (A) An illustration showing the location of a microdialysis probe inserted into the CPu. (B) There were no significant differences between genotypes. (C) Average glutamate levels of ENT1+/+ mice (+/+) were significantly decreased, whereas no changes were detected in ENT1−/− mice (−/−). n = 4 for each genotype; *p < 0.05 compared to ENT1+/+ mice by Tukey test (B) or to pre-injection periods by unpaired two-tailed t-test (C). P; pre-injection period, C; CGP37849 injection period. Data are presented as mean ± SEM.
Figure 3.
Effect of an NMDA glutamate receptor antagonist (CGP37849) on glutamate levels in the NAc analyzed by microdialysis. (A) An illustration showing the location of a microdialysis probe inserted into the NAc. (B, C) The glutamate levels were dramatically decreased in ENT1+/+ mice compared to ENT1−/− mice. Both genotypes showed decreased glutamate levels in response to the NMDA receptor antagonist. n = 4 for each genotype; *p < 0.05 compared to the ENT1+/+ mice by Tukey test (B) or to pre-injection periods by unpaired two-tailed t-test (C). P; pre-injection period, C; CGP37849 injection period. Data are presented as mean ± SEM.
DISCUSSION
Our results indicate that altered adenosine mediated glutamate homeostasis of ENT1−/− mice is implicated in the differential resistant to both ethanol and NMDA receptor antagonist, CGP37849, induced ataxia. Compared to wild-type mice, ENT1−/− mice are more resistant to ethanol-induced ataxia when mice were given CGP37849 (10 mg/kg, i.p.) 15 min before an ethanol injection in the rotarod. The combination of NMDA receptor antagonism and ethanol treatment appears to induce faster intoxication compared to when mice were given either ethanol [8] or CGP37849 [6] independently, suggesting that NMDA receptors might interact with ethanol in the regulation of motor coordination. Because NMDA receptors are known to bind ethanol directly, especially the NR1 subunit [32], it is possible to postulate that CGP37849 and ethanol bind to different NMDA receptors, but increase the ataxic effect of ethanol. Adenosine-regulated glutamate signaling is known to regulate ataxia in both the striatum and cerebellum [13, 27]. Interestingly, NMDA receptors in cerebellar granule cells play an important role in ethanol intoxication including ataxia, which might also regulate ethanol consumption independent of the cortico-striatal-VTA reward circuit [30]. Thus, increased glutamate levels in the striatum or cerebellum might diminish the antagonistic effect of ethanol on NMDA receptors directly, which may lead to a decrease in ethanol-induced ataxia. However, ethanol is also known to bind to several ligand-gated ion-channel including GABAA receptors [21] which are also implicated in ethanol-induced ataxia [18, 20, 26].
Our microdialysis results strongly support a possible distinction between glutamate neurotransmission in the ventral (NAc) and dorsal (CPu) striatum. These differences in glutamate levels in response to NMDA receptor antagonism revealed the dysregulation of glutamate signaling caused by the ablation of an ethanol-sensitive adenosine transporter in the NAc. Ethanol-induced alterations of glutamate levels regulate locomotion and ataxia through cortico-striatal glutamate neurotransmission [38, 39]. Our previous study using ENT1−/− mice demonstrated that increased extracellular glutamate levels in CPu contribute to ethanol-mediated behaviors including resistance to ataxia and reduced aversive effects of ethanol [6]. However, altered glutamate neurotransmission by CGP37849 was mainly detected in NAc of ENT1−/− mice. Thus, we reasoned that the antagonistic effect on glutamate levels appears to be more substantial in the NAc. Importantly, the NAc receives glutamatergic input from several different brain regions including the dorsal (precentral motor cortex and anterior cingulate) and ventral (prelimbic and infralimbic cortex) sub-divisions of the medial prefrontal cortex [12, 23] as well as the hippocampus and amygdala [17], while the CPu receives glutamatergic input mainly from the motor cortex. These differential glutamate levels in response to an NMDA receptor antagonist or ethanol might be due to different glutamatergic afferents projecting to the CPu and the NAc.
As recommended by a consortium on mice genetic background for neuro-behavioral studies [1, 11], we used F2 generation hybrid mice with C57BL/6J × 129X1/SvJ genetic background to minimize the risk of false positives or negatives in behavioral phenotypes that could be influenced by a genetic background. Nevertheless, there are still potential caveats of using this mixed genetic background since flanking allele or co-segregated genes near the knockout locus could contribute to the phenotypes attributed to the null allele [3]. Interestingly, inbred C57BL/6J mice are less sensitive to the ataxic and sedating effects of ethanol and display higher ethanol consumption than inbred 129X1/SvJ mice [10, 36]. Our previous findings indicate that complications from genetic backgrounds or possible co-segregated genes nearby the ENT1 gene are less likely to influence the phenotypes because ENT1−/− mice prefer more ethanol compared to wild-type littermates [8].
In summary, our study suggests that the ethanol-induced motor-stimulating effects and/or initial sensitivity to the ataxic effects of ethanol can be regulated by NMDA receptor antagonism.
ACKNOWLEDGMENTS
We thank S. Choi for mouse husbandry and D. Frederixon for preparing the manuscript. This research was supported by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic and by grants from the National Institutes of Health (NIH) to D.-S. C. (R01 AA015164 and R01 AA018779). H.W.N. received postdoctoral fellowship from Korea Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Banbury Conference on Genetic Background in Mice, Mutant Mice and Neuroscience: Recommendations Concerning Genetic Background. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- [2].Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog. Neuroniol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [3].Berry ML, Linder CC. Breeding Systems: Considerations, Genetic Fundamentals, Genetic Background, and Strain Types. Vol. 1. Elsevier; Burlington: 2007. [Google Scholar]
- [4].Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [5].Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Dis. 2008:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- [6].Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi D-S. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav. Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, Choi DS. The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav. 2007;6:776–783. doi: 10.1111/j.1601-183X.2007.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat. Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- [9].Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crabbe JC. Use of genetic analyses to refine phenotypes related to alcohol tolerance and dependence. Alcohol. Clin. Exp. Res. 2001;25:288–292. [PubMed] [Google Scholar]
- [11].Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- [12].Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [13].Dar MS. Mouse cerebellar adenosine-glutamate interactions and modulation of ethanol-induced motor incoordination. Alcohol. Clin. Exp. Res. 2002;26:1395–1403. doi: 10.1097/01.ALC.0000030564.69414.74. [DOI] [PubMed] [Google Scholar]
- [14].Dar MS. Mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination: possible involvement of cAMP. Brain Res. 1997;749:263–274. doi: 10.1016/s0006-8993(96)01263-2. [DOI] [PubMed] [Google Scholar]
- [15].Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol. Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- [16].Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- [17].Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- [18].Fidecka S, Langwinski R. Ethanol and benzodiazepines. The influence of CGS 8216 on the ethanol-induced hypothermia and motor incoordination in mice and rats. J. Physiol. Pharmacol. 1995;46:429–437. [PubMed] [Google Scholar]
- [19].Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacol. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- [21].Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Houchi H, Warnault V, Barbier E, Dubois C, Pierrefiche O, Ledent C, Daoust M, Naassila M. Involvement of A2A receptors in anxiolytic, locomotor and motivational properties of ethanol in mice. Genes Brain Behav. 2008;7:887–898. doi: 10.1111/j.1601-183x.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- [23].Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- [24].Koob GF, Le Moal M. Neurobiology of Addiction. Elsevier; London: 2006. [Google Scholar]
- [25].Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi D-S. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharm. Biochem. Behav. 2010;95:235–241. doi: 10.1016/j.pbb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luddens H, Pritchett DB, Kohler M, Killisch I, Keinanen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- [27].Meng ZH, Dar MS. Possible role of striatal adenosine in the modulation of acute ethanol-induced motor incoordination in rats. Alcohol. Clin. Exp. Res. 1995;19:892–901. doi: 10.1111/j.1530-0277.1995.tb00964.x. [DOI] [PubMed] [Google Scholar]
- [28].Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J. Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- [30].Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D’Angelo E, Frassoni C, Amadeo A, Tocchetti A, Pozzi B, Disanza A, Guarnieri D, Betsholtz C, Scita G, Heberlein U, Di Fiore PP. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- [31].Parkinson FE, Xiong W, Zamzow CR, Chestley T, Mizuno T, Duckworth ML. Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J. Neurochem. 2009;109:562–572. doi: 10.1111/j.1471-4159.2009.05991.x. [DOI] [PubMed] [Google Scholar]
- [32].Smothers CT, Woodward JJ. Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol. Clin. Exp. Res. 2006;30:523–530. doi: 10.1111/j.1530-0277.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- [33].Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol. Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [34].Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat. Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- [35].Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–880. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- [36].Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J. Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu. Rev. Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- [38].Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- [39].Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]