Abstract
Purpose
A prototype ultrasound-based probe for use in ureteroscopy was used for in vitro measurements of stone fragments in a porcine kidney.
Methods
Fifteen human stones consisting of three different compositions were placed deep in the collecting system of a porcine kidney. A 2 MHz, 1.2 mm (3.6F) needle hydrophone was used to send and receive ultrasound pulses for stone sizing. Calculated stone thicknesses were compared with caliper measurements.
Results
Correlation between ultrasound-determined thickness and caliper measurements was excellent in all three stone types (r2 = 0.90, p < 0.0001). All 15 ultrasound measurements were accurate to within 1 mm, and 10 measurements were accurate within 0.5 mm.
Conclusion
A 3.6F ultrasound probe can be used to accurately size stone fragments to within 1 mm in a porcine kidney.
Introduction
Stone basketing and extraction represents the most decisive step in ureteroscopic stone treatment influencing its procedural time, effectiveness, and safety.1 Contemporary ureteral access sheaths have a 9.5F to 16F internal lumen, allowing extraction of 3 to 5 mm stone fragments.2 These access sheaths allow repeated basket extraction of stone fragments without need to re-obtain ureteral access with a guidewire. However, operative time is increased and possible ureteral injury may occur if fragments are extracted before stones are small enough to fit through the ureter or access sheath.3
Ureteral avulsion is a rare (0.3–0.5%) but catastrophic injury caused by basketing too large a stone, requiring immediate surgical intervention to restore ureteral continuity.1,4,5 Ureteral intussusception may result in a devitalized segment of ureter necessitating reconstruction if too large a stone fragment is attempted to be extracted.6,7 A trapped basket may be disassembled and removed but can result in injury or stricture formation.8 Current methods to estimate stone fragment size during ureteroscopy remain subjective.
Previously, we demonstrated that an ultrasound-based instrument could be utilized to size in vitro stone fragments.9 A 10F transducer probe was used to send and receive ultrasound pulses to determine the thickness of stone fragments on a kidney tissue phantom. However, for this technology to be clinically viable, the ultrasound probe must work in tissue and be reduced in size to at least 7.5F to 8.5F to work in the tip of a contemporary flexible ureteroscope or ∼2F to 3F to fit through the working channel of a flexible ureteroscope. The purpose of this study was to evaluate this ultrasound-based technology using a 3.6F (1.2 mm) transducer probe deep within the collecting system of a porcine kidney.
Materials and Methods
Stones and experimental setup
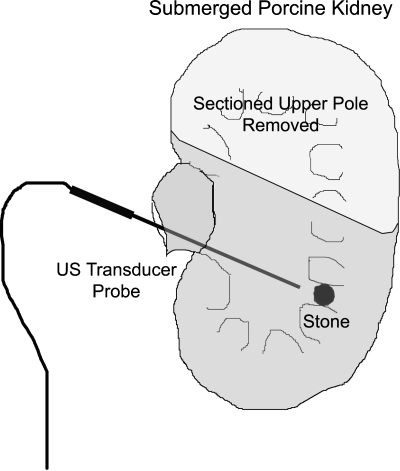
Human urinary calculi from 15 different patients were obtained from a stone reference laboratory and included five stones of each of three different types (calcium oxalate monohydrate, cystine, and calcium hydrogen diphosphate). Stones were a mix of complete stones and stone fragments. All stones were ≥92% pure composition and were rehydrated for more than 24 hours. Stones were a variety of shapes and ranged from 3 to 7 mm. Each stone was placed deep within the collecting system of the lower pole of a freshly sectioned porcine kidney. The kidney was then submerged in 20°C saline. The transducer probe was mounted for experimental convenience and directed visually to the stone surface (Fig. 1).
FIG. 1.
Diagram of the experimental setup. The upper pole of a porcine kidney was sectioned to allow placement of a stone into a lower pole calix. The setup was then submerged. The ultrasound probe was then introduced into the collecting system and pointed to within a few millimeters of the stone of interest and signals were recorded for later determination of stone size.
Ultrasound signal collection
A 2 MHz, 1.2 mm (3.6F) PZT needle hydrophone transducer (Dapco Industries, Ridgefield, CT) was used to size kidney stones. The hydrophone was connected to a pulse/receiver (Model 5072PR; Olympus NDT, Waltham, MA), which provided both the excitation pulse to the hydrophone and was used for obtaining the received signal, and recorded using a digital oscilloscope (Model LT344; LeCroy, Chestnut Ridge, NY).
With no stone present, only the excitation signal was present representing the 2 MHz resonant signal from the transducer. Therefore, the initial pulse consisting of ∼100 cycles was stored using the digital oscilloscope and saved for subtraction from subsequent signals when a stone was present. The transducer was then pointed within a few millimeters of the stone of interest and signals were recorded on the oscilloscope and averaged to improve the signal-to-noise. The initial excitation signal was then subtracted from the signal obtained with a stone present and was stored on a computer for later determination of the stone sizes by a blinded observer.
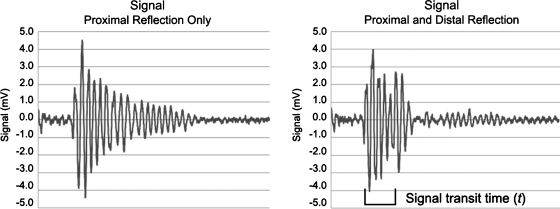
The signals obtained with a stone present contained two reflections: one from the proximal stone surface and the other from the distal stone surface, with the time between them representing the round trip transit time through the stone. The reflected signals from the front surface of the stone were obvious, with a clear deflection and had a distinct reproducible shape. Given the small size of the stones analyzed and the resonant nature of the transducer available, the second reflection overlapped the predictably decaying tail of the first reflection (Fig. 2). The time between the two reflections, t, was determined by the blinded investigator by evaluating the disruption of the normal decay of the first reflected signal from the proximal surface of the stone.10,11
FIG. 2.
Ultrasound signal demonstrating the expected decaying signal from only a proximal reflection (left). The reflected signal from the proximal stone surface had an initial, predictable increase in amplitude followed by a smooth decay. The second ultrasound signal (right) contains both the proximal and distal reflected signal. The second reflection from the distal surface added to the smooth decay of the first signal. The signal transit time (t), the difference between the arrival of the first and second signals, was 1.92 μs, giving a calculated stone size of 4.1 mm compared with 4.0 mm measured by caliper.
Stone thickness determination
Stone thickness, D, was calculated from measured transit time, t, as
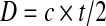 |
where c is the assumed speed of sound in the stone.12,13 Measurements were divided by 2 because the pulse passes through the stone once, reflects from the distal side and passes back through the stone a second time. Measurements with ultrasound and calipers were repeated five times for each stone in the same orientation, and mean ± one standard deviation are reported. Correlation and regression analyses were performed to compare the ultrasound-based measurements to mean caliper measurements.
In clinical use, the exact speed of sound within a stone is unlikely to be known. We selected a representative stone of each of the three types of known thickness and measured the transit time through the stones. The sound speeds were 4270 ± 80 m/s for calcium hydrogen diphosphate, 4320 ± 40 m/s for cystine, and 4330 ± 50 m/s for calcium oxalate monohydrate. We used 4300 m/s for all measurements. Previous experiments have found that the most common types of natural stones have sound speeds ranging from ∼3500 to 4600 m/s. Since we would clinically prefer to overestimate sound size than underestimate stone size, we selected a relatively high sound speed for these experiments.14,15
Results
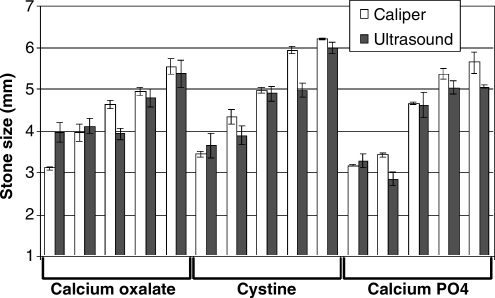
An ultrasound signal and consequent stone size was determined for all 15 stones (Fig. 3). Correlation between ultrasound-determined thickness and caliper measurements was excellent (r2 = 0.90, p < 0.0001). Overall, ultrasound-based measurements led to an underestimation of stone size by 3% and performed equally well in all three stone types. Fifteen of 15 (100%) ultrasound-determined stones' sizes were within 1 mm of the caliper-determined size, and 10 (67%) were within 0.5 mm. All ultrasound measurements had a standard deviation of <0.33 mm (average standard deviation 0.19 mm).
FIG. 3.
Comparison of stone size measurements using calipers (white) versus ultrasound-determined stone size (black) for calcium oxalate monohydrate, cystine, and calcium hydrogen diphosphate stones (calcium PO4). Correlation was excellent (r2 = 0.90, p < 0.0001) with all stone measurements being accurate to within 1 mm.
Role in Endourology
We demonstrate an accurate and precise ultrasound-based method of sizing kidney stone fragments using a 1.2 mm transducer probe deep within the collecting system of a kidney. The goal of this work was ultimately to provide real-time feedback on stone fragment size to assess suitability for extraction or stopping lithotripsy. Our data demonstrate that an ultrasound-based device can be designed either to fit through the working channel or on the tip of the flexible ureteroscope and may provide continuous and instantaneous reading of the stone fragment size. The vibrating element of the transducer is <0.25 mm thick and if included on the tip of a flexible ureteroscope would avoid the need to remove the working laser fiber. The method of sizing may be further improved with better signal processing. Measurements were not automated in these experiments, but that algorithm could be used to automate the measurement even in real time. We are currently incorporating this technology into the tip of a ureteroscope, and future studies will be necessary to evaluate accuracy.
In our previous experiments, we performed ultrasound measurements using two methods using a kidney tissue phantom with a larger ultrasound transducer. We were able to accurately obtain stone sizes.9 In our current experiments, we found that when stones were placed into the kidney we often could not visually see the surface behind the stone. Practically, it was only possible to obtain stone signals that traveled through the stone in line with the ultrasound transducer. Although only one orientation is measured, a system that provided continuous stone size during ureteroscopy could allow the operator to measure the stone from different angles by redirecting the tip of the ureteroscope. This method has the benefit of being able to measure stone size even when the distal end of the stone cannot be seen or is partially imbedded in tissue such as in an impacted stone or a caliceal diverticulum. We also measured and used a different speed of sound in the stone in our current experiments (4300 m/s) compared with the estimated stone speed used in our former experiments (3000 m/s). In the current study, we experimentally determined the speed of sound in each representative stone type. This sound speed may result in less accurate measurements for less common stones with slower sound speeds, but this will result in overestimation of stone size—a more clinically acceptable result than underestimating stone size.
Our data show that measurements are accurate and precise using a 1.2 mm probe and are not affected by placing stones deep in the collecting system. Overall, ultrasound measurements tended to underestimate stone size by 3%. From a clinical standpoint, all of the stones measured in this study had ultrasound-determined stone sizes that were accurate to within 1 mm. We feel this would provide acceptable accuracy for clinical application especially in comparison to current methods of subjective size estimation. Practitioners using ballistic or mechanical lithotripsy, such as electrohydraulic, pneumatic, or pulse dye laser, may find this technology especially useful as these energy sources tend to result in larger stone fragments than when using the holmium laser.16–19
Our method of ultrasound measurement offers consistent and reliable measurements for most stones. There were two stones where our ultrasound measurements had been inaccurate by almost 1 mm. Both of these stones had a concave proximal surface with a base that tapered to a point. This likely generated multiple internal reflections within the stone and made their signal peaks difficult to select.
The probe might further be refined to make it smaller with consideration of returning to a 10-MHz probe as was used in our previous work. Lower frequency transducers may be more sensitive and easier to build but produces longer wavelength signals, which creates the challenge of separating the overlapping proximal and distal signals. Measurements were more difficult to determine using the 2 MHz transducer and might be easier to observe using a higher frequency transducer though we may trade off greater signal attenuation in the stone.
Acknowledgments
This work was supported by Grants NIH DK43881 and NSBRI SMST01601.
Disclosure Statement
Mathew Sorensen—No disclosures or conflicts of interest.
Anup Shah—No disclosures or conflicts of interest.
Michael Canney—No disclosures or conflicts of interest.
Oleg A. Sapozhnikov—No disclosures or conflicts of interest.
Joel Teichman—Consultant/Advisor for Ortho-McNeil. Unrelated to this project.
Michael Bailey—No disclosures or conflicts of interest.
References
- 1.de la Rosette JJ. Skrekas T. Segura JW. Handling and prevention of complications in stone basketing. Eur Urol. 2006;50:991–998. doi: 10.1016/j.eururo.2006.02.033. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 2.Monga M. Gawlik A. Durfee W. Systematic evaluation of ureteral access sheaths. Urology. 2004;63:834–836. doi: 10.1016/j.urology.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Teichman JM. Kamerer AD. Use of the holmium:YAG laser for the impacted stone basket. J Urol. 2000;164:1602–1603. [PubMed] [Google Scholar]
- 4.Huffman JL. Ureteroscopic injuries to the upper urinary tract. Urol Clin North Am. 1989;16:249–254. [PubMed] [Google Scholar]
- 5.St. Lezin MA. Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497–506. doi: 10.1016/0090-4295(91)80165-4. [DOI] [PubMed] [Google Scholar]
- 6.Bernhard PH. Reddy PK. Retrograde ureteral intussusception: A rare complication. J Endourol. 1996;10:349–351. doi: 10.1089/end.1996.10.349. [DOI] [PubMed] [Google Scholar]
- 7.Park J. Siegel C. Moll M. Konnak J. Retrograde ureteral intussusception. J Urol. 1994;151:997–998. doi: 10.1016/s0022-5347(17)35147-9. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor RC. Gerber GS. Management of entrapped ureteral stone baskets. Tech Urol. 2000;6:231–233. [PubMed] [Google Scholar]
- 9.Sorensen MD. Teichman JM. Bailey MR. Proof of principle in vitro study of a prototype ultrasound technology to size stone fragments during ureteroscopy. J Endourol. 2009;23:1161–1164. doi: 10.1089/end.2009.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crocker MJ. Handbook of Acoustics. New York: Wiley; 1998. pp. 47–59. [Google Scholar]
- 11.Kimball JT. Bailey MR. Hermanson JC. Ultrasonic measurement of condensate film thickness. J Acoust Soc Am. 2008;124:EL196–EL202. doi: 10.1121/1.2968297. [DOI] [PubMed] [Google Scholar]
- 12.Kinsler LE. Frey AR. Coppens AB. Sanders JV. Fundamentals of Acoustics. 3rd. New York: John Wiley and Sons; 1982. pp. 107–112. [Google Scholar]
- 13.Nyland TG. Mattoon JS. Small Animal Diagnostic Ultrasound. 2nd. Philadelphia, PA: W.B. Saunders Co.; 2002. pp. 3–9. [Google Scholar]
- 14.Chuong CJ. Zhong P. Preminger GM. Acoustic and mechanical properties of renal calculi: Implications in shock wave lithotripsy. J Endourol. 1993;7:437–444. doi: 10.1089/end.1993.7.437. [DOI] [PubMed] [Google Scholar]
- 15.Heimbach D. Munver R. Zhong P. Jacobs J. Hesse A. Muller SC, et al. Acoustic and mechanical properties of artificial stones in comparison to natural kidney stones. J Urol. 2000;164:537–544. [PubMed] [Google Scholar]
- 16.Teichman JM. Vassar GJ. Bishoff JT. Bellman GC. Holmium:YAG lithotripsy yields smaller fragments than lithoclast, pulsed dye laser or electrohydraulic lithotripsy. J Urol. 1998;159:17–23. doi: 10.1016/s0022-5347(01)63998-3. [DOI] [PubMed] [Google Scholar]
- 17.Aghamir SK. Mohseni MG. Ardestani A. Treatment of ureteral calculi with ballistic lithotripsy. J Endourol. 2003;17:887–890. doi: 10.1089/089277903772036208. [DOI] [PubMed] [Google Scholar]
- 18.Bapat SS. Pai KV. Purnapatre SS. Yadav PB. Padye AS. Comparison of holmium laser and pneumatic lithotripsy in managing upper-ureteral stones. J Endourol. 2007;21:1425–1427. doi: 10.1089/end.2006.0350. [DOI] [PubMed] [Google Scholar]
- 19.Elashry OM. DiMeglio RB. Nakada SY. McDougall EM. Clayman RV. Intracorporeal electrohydraulic lithotripsy of ureteral and renal calculi using small caliber (1.9F) electrohydraulic lithotripsy probes. J Urol. 1996;156:1581–1585. [PubMed] [Google Scholar]