Summary
Recent evidence suggests that the intestine may play a direct facilitative role in reverse cholesterol transport (RCT), independent of hepatobiliary secretion. In order to understand the non-biliary pathway for RCT we created both genetic and surgical models of biliary cholesterol insufficiency. To genetically inhibit biliary cholesterol secretion we generated mice in which Niemann-Pick C1-Like 1 (NPC1L1) was overexpressed in the liver. Compared to controls, NPC1L1Liver-Tg mice exhibit a > 90% decrease in biliary cholesterol secretion, yet mass fecal sterol loss and macrophage RCT is normal. To surgically inhibit biliary emptying into the intestine, we have established an acute biliary diversion model. Strikingly, macrophage RCT persists in mice surgically lacking the ability to secrete bile into the intestine. Collectively, these studies demonstrate that mass fecal sterol loss and macrophage RCT can proceed in the absence of biliary sterol secretion, challenging the obligate role of bile in RCT.
Introduction
Nearly forty years ago, John Glomset (Glomset, 1968) presented the seminal framework of reverse cholesterol transport (RCT), which was described as a process by which peripheral cholesterol is returned to the liver via high-density lipoproteins (HDL) for secretion into bile and excretion through the feces. In the context of atherosclerosis, RCT is thought to involve HDL-mediated efflux of cholesterol from the arterial wall, specifically from cholesterol-laden macrophages (Wang et al., 2007a). In the current view of macrophage RCT, HDL- mediated delivery of peripheral cholesterol to the liver directly promotes biliary and fecal excretion (Lewis et al. 2005). Given this model, plasma HDL levels should accurately predict both biliary sterol secretion and fecal sterol loss. However, in mice with extremely low HDL levels, biliary and fecal sterol loss is normal (Xie et al., 2009; Groen et al., 2001; Jolley et al., 1998). In addition, biliary sterol levels do not accurately predict fecal sterol loss in several mouse models of altered hepatic cholesterol metabolism (Brown et al., 2008a; Plosch et al., 2002; Yu et al., 2003). Collectively, this has led us to believe that fecal cholesterol loss likely originates from two distinct excretory routes: 1) the classic hepatobiliary route, and 2) a non-biliary liver → plasma lipoprotein → small intestine → feces route (Brown et al., 2008a). From a therapeutic standpoint, exploiting the non-biliary route for RCT is a much more attractive option, since excessive augmentation of biliary cholesterol concentrations can promote cholesterol gallstone formation in humans (Cooper, 1991).
Preceding Glomset’s (Glomset, 1968) initial model of RCT by forty years, the existence of a non-biliary pathway for fecal sterol loss was proposed first by Warren Sperry (Sperry, 1927). Sperry’s experiments demonstrated that compared to intact enterohepatic circulation, chronic surgical biliary diversion paradoxically increased fecal neutral sterol loss in dogs. Nearly a half-century later, Pertsemlidis and colleagues (1973) confirmed the results of Sperry by demonstrating that biliary diversion resulted in complete loss of fecal acidic sterol output, yet fecal neutral sterol output actually increased ~7-fold (Pertsemlidis et al. 1973). Based on this observation, the authors speculated “the increased output of fecal neutral steroids could be the result of transfer of plasma cholesterol across the gut wall or due to increased synthesis in the gut.” Similar results have been seen under conditions of biliary diversion in rats (Bandsma et al., 1998) and in patients with familial hypercholesterolemia (Deckelbaum et al., 1997). Actually, as early as 1959, it was suggested that non-dietary fecal sterol loss in humans consists of two distinct fractions: 1) the traditional fraction coming from hepatobiliary secretion, and 2) an elusive fraction directly secreted by the intestine (Cheng et al., 1959). Unfortunately, many of these early observations have been largely ignored, and the theory that fecal sterol loss derives solely from a biliary origin has become well accepted. However, strong evidence for non-biliary fecal sterol loss continues to come to light in the age of genetically modified mice.
In a powerful example of non-biliary fecal sterol loss in mice (Kruit et al., 2005), it was shown that mice lacking the cannicular phospholipid transporter Mdr2, which secondarily have no biliary cholesterol secretion, have normal fecal sterol loss. Furthermore, activation of the liver X receptor (LXR) in Mdr2−/− mice resulted in large increases in fecal sterol output, supporting the presence of an LXR-inducible non-biliary pathway for fecal sterol loss (Kruit et al., 2005). Several studies from this same group have subsequently demonstrated the existence of a nuclear hormone receptor-inducible pathway for non-biliary fecal sterol loss using pioneering approaches such as in situ intestinal perfusion and stable isotope methodologies (van der Veen et al., 2009; Vrins et al., 2009; van der Velde et al., 2007; van der Velde et al. 2008). However, the relative contribution of biliary and non-biliary pathways to the specific process of macrophage RCT is not currently known. Therefore, the purpose of these studies was to determine the direct role of the intestine in macrophage RCT by using novel genetic and surgical mouse models where bile contributes minimally to fecal sterol loss.
Results
NPC1L1-LiverTg Mice Serve As An Excellent Model To Study Non-Biliary Fecal Sterol Loss
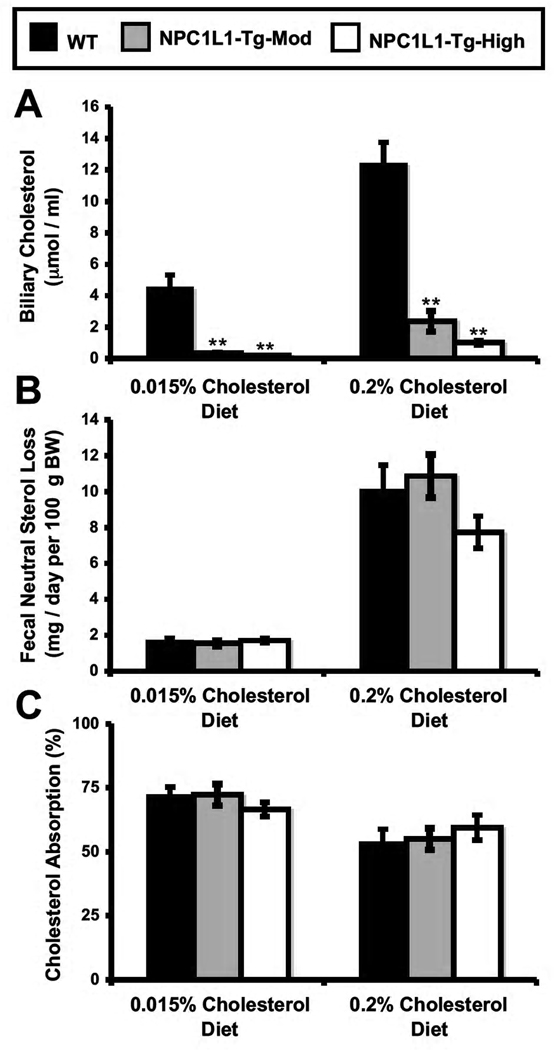
In order to characterize the role of Niemann-Pick C1-Like 1 (NPC1L1) in hepatobiliary sterol secretion and ezetimibe action, our group previously generated two independent lines of mice transgenically overexpressing NPC1L1 in hepatocytes (Temel et al., 2007). As previously reported, NPC1L1 overexpression in the liver results in a > 90% reduction in gall bladder bile cholesterol concentrations (Figure 1A), without affecting biliary phospholipid or bile acid secretion (data not shown; Temel et al., 2007). However, in the face of severely diminished biliary sterol loss, fecal neutral sterol excretion in NPC1L1-LiverTg mice is normal (Figure 1B). This disparity indicates that the non-biliary pathway must completely compensate for biliary sterol insufficiency in NPC1L1-LiverTg mice to maintain normal levels of fecal sterol loss. It is important to note that fractional cholesterol absorption (Figure 1C), and the mRNA expression of cholesterol synthetic genes (Figure S1A) in the liver and intestine was normal in NPC1L1-LiverTg mice.
Figure 1. Mice genetically lacking the ability to secrete cholesterol into bile have normal fecal sterol loss.
Wild type mice (WT) or littermates with moderate (NPC1L1-Tg-Mod) or high level (NPC1L1-Tg-High) overexpression of hepatic NPC1L1 were fed diets containing 0.015% or 0.2% cholesterol (wt/wt) for six weeks.
(A) The concentration of cholesterol in gall bladder bile was determined by gas liquid chromatography.
(B) Fecal neutral sterol excretion was determined by gas liquid chromatography.
(C) Fractional cholesterol absorption was determined using the dual fecal isotope method. Data represent the means ± SEM from 4–7 mice per group, ** = significantly different than WT within each diet group, P < 0.01.
Macrophage RCT Persists in Genetically Modified Mice With Disrupted Biliary Cholesterol Secretion (NPC1L1-LiverTg)
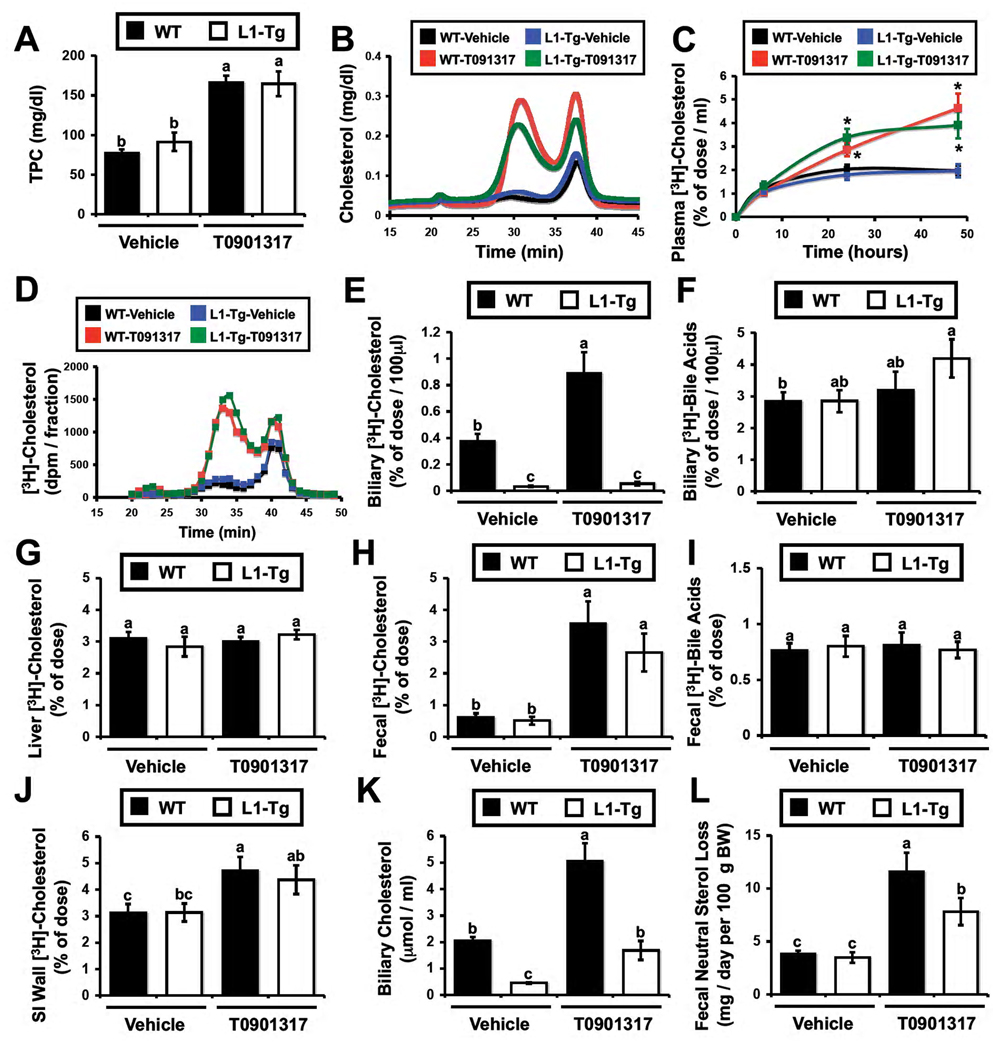
In the current view of macrophage RCT, macrophage-derived cholesterol is delivered to the liver by HDL, which shunts this sterol cargo preferentially into the bile for subsequent fecal excretion (Lewis et al. 2005; deGoma et al., 2008). We set out to test the specific role of bile in this framework using mice genetically lacking the ability to secrete cholesterol into bile (NPC1L1-LiverTg). In these studies, mice were treated with the LXR agonist T0901317, since it is known that LXR activation promotes fecal sterol loss and macrophage RCT (Naik et al., 2006) primarily by augmenting the non-biliary pathway (Kruit et al., 2005; van der Veen et al., 2009). We have previously seen that NPC1L1-LiverTg mice are mildly hypercholesterolemic when fed synthetic diets (Temel et al., 2007). However, when young mice (6–8 weeks old) are maintained on standard rodent chow, NPC1L1-LiverTg mice have similar plasma cholesterol levels as those seen in WT littermates (Figure 2A), and the majority of cholesterol is found in HDL (Figure 2B). When treated with the LXR agonist T0901317, both WT and NPC1L1-LiverTg mice exhibit mild hypercholesterolemia (164 and 166 mg/dl, respectively), with the majority of the cholesterol elevation seen in large HDL particles (Figure 2B), as has been previously described (Grefhorst et al., 2002). Following [3H]-cholesterol labeled macrophage injection the plasma [3H]-cholesterol recovery was significantly higher in T0901317-treated mice (Figure 2C). As expected, the [3H]-cholesterol distribution in plasma (Figure 2D) tracked closely with the cholesterol mass distribution (Figure 2B). Importantly, in WT mice, there was LXR-inducible biliary [3H]-cholesterol secretion (Figure 2E), whereas NPC1L1-LiverTg mice had barely detectable biliary [3H]-cholesterol levels (Figure 2E). However, [3H]-bile acid secretion into bile was not different between WT and NPC1L1-LiverTg mice in the absence or presence of LXR agonist (Figure 2F), which coincides with mass measurements (Temel et al., 2007; data not shown). However, there was no difference in [3H]-cholesterol recovery in the liver between WT and NPC1L1-LiverTg mice on either treatment (Figure 2G). In stark contrast to the biliary [3H]-cholesterol recovery (Figure 2E), fecal [3H]-cholesterol recovery in WT and NPC1L1-LiverTg was identical following treatment with vehicle or the LXR agonist (Figure 2H). As previously reported (Naik et al., 2006), LXR activation did increase fecal [3H]-cholesterol recovery, but it did so to the same extent in mice with normal enterohepatic circulation (WT) and those genetically lacking the ability to secrete cholesterol into bile (NPC1L1-LiverTg) (Figure 2H). This effect seems to be specific to neutral sterol loss, since fecal [3H]-bile acid recovery was not significantly different between any of the groups (Figure 2I).
Figure 2. Macrophage RCT is normal in mice genetically engineered to lack the ability to secrete cholesterol into bile.
Wild type mice (■ in bar graphs) or NPC1L1-LiverTg (□ in bar graphs, L1-Tg) littermates were maintained on a standard chow diet in the absence (Vehicle) or presence of the LXR agonist T0901317 (25 mg/kg per day) for 7 days. During the last 48 hours, mice were singly housed and macrophage to feces RCT was measured as described in the materials and methods.
(A) Total plasma cholesterol (TPC) levels.
(B) Mass cholesterol distribution of pooled plasma (n=4 per pool).
(C) Time course of [3H]-cholesterol recovery in plasma; * = significantly different than WT-vehicle group within each time point, P < 0.05.
(D) [3H]-cholesterol distribution of pooled plasma (n=4 per pool).
(E) [3H]-cholesterol recovery in newly secreted bile.
(F) [3H]-bile acid recovery in newly secreted bile.
(G) [3H]-cholesterol recovery in the liver.
(H) [3H]-cholesterol recovery in the feces.
(I) [3H]-bile acids recovery in the feces.
(J) [3H]-cholesterol recovery in the small intestine (SI) wall.
(K) Mass biliary cholesterol concentrations in newly secreted bile.
(L) Mass fecal neutral sterol loss. Results are combined from two independent experiments. Data in panels A,C,E,F,G,H,I and J represent the means ± SEM from 7–11 mice per group, and data in panel K and L represent means ± SEM from 5–9 mice per group. Means not sharing a common superscript differ significantly, P < 0.05.
Interestingly, the [3H]-cholesterol tracer recovery in the small intestinal (SI) wall was as high as levels seen in the liver (3–5% of the dose; Figure 2G). The recovery in the SI wall was not significantly different between WT and NPC1L1-LiverTg mice, but was modestly increased by LXR activation (Figure 2J). When examining cholesterol mass, vehicle treated NPC1L1-LiverTg mice compared to WT mice had a 78% reduction in cholesterol concentration in bile (Figure 2K), yet fecal neutral sterol loss was similar between the two genotypes (Figure 2L). LXR activation did result in significant increases in biliary cholesterol in both genotypes (Figure 2K), but the levels seen in NPC1L1-LiverTg mice only reached that of vehicle treated WT mice (Figure 2K). In contrast, fecal neutral sterol loss in T091317-treated WT and NPC1L1-LiverTg mice was significantly higher than that of vehicle treated counterparts (Figure 2L), further supporting the disconnect between biliary sterol secretion and fecal neutral sterol loss in these mice.
Surgical Biliary Diversion Reveals The Existence of a Non-Biliary Pathway For Macrophage RCT
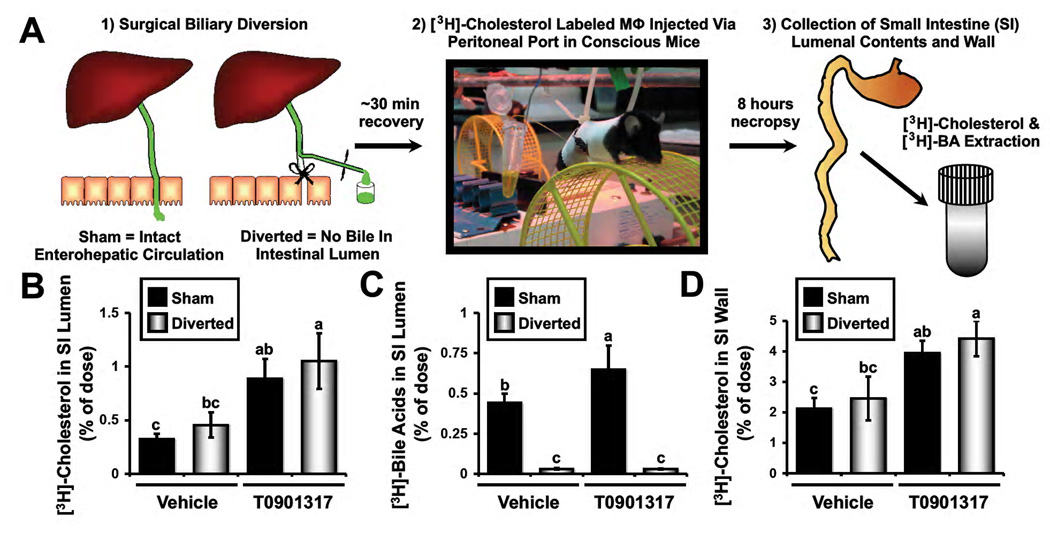
Although NPC1L1-LiverTg mice serve as an excellent model where non-biliary sterol loss predominates, there is a small residual amount of biliary sterol loss in this genetic model (Figure 1A and 2K). In order to more definitively test whether bile is required for macrophage RCT, we set out to create a surgical model where there would be no biliary emptying into the small intestine, without obstructing bile flow. To do this we surgically diverted bile flow away from the small intestine (Figure 3A). Once this biliary diversion, or an appropriate sham surgery, was complete, mice were allowed to recover from anesthesia and received a [3H]-cholesterol labeled macrophage foam cell dose via an externalized port in order to avoid accidental injection directly into the intestine. Quite strikingly, the appearance of [3H]-cholesterol in the intestinal lumen was no different between sham operated and surgically diverted mice (Figure 3B). Furthermore, LXR activation resulted in significant increases in the lumenal appearance of [3H]-cholesterol in both sham and diverted animals (Figure 3B), further supporting the notion that LXR activation promotes primarily the non-biliary pathway for fecal sterol loss (Kruit et al., 2005; Van der Veen et al., 2009). Importantly, the lumenal appearance of [3H]-bile acids was completely blocked by surgical biliary diversion (Figure 3C), which agrees with previous mass data in dogs (Sperry, 1927; Pertsemlidis et al., 1973). These data support the notion that bile is the only route for hepatic bile acids to reach the small intestinal lumen, while cholesterol delivery into the intestine and feces can persist in the absence of biliary return (Sperry, 1927; Pertsemlidis et al. 1973; Dietschy, 1968; Figure 3). Interestingly, at this early 8 hour time point after [3H]-cholesterol labeled macrophage injection, 2–4% of the dose can be found in the small intestinal wall (Figure 3D). There was no difference in the SI wall recovery between sham and diverted animals, yet LXR activation did significantly increase [3H]-cholesterol recovery in the SI wall under conditions of intact and diverted enterohepatic circulation (Figure 3D). Collectively, these data support the notion that macrophage RCT can persist in the complete absence of biliary contributions.
Figure 3. Surgical biliary diversion reveals the existence of a non-biliary pathway for macrophage RCT.
Wild type C57BL/6N mice were maintained on a standard chow diet in the absence (Vehicle) or presence of the LXR agonist T0901317 (25 mg/kg per day) for 7 days.
(A) Experimental design: Following 7 days of vehicle or LXR agonist treatment, mice were either sham operated or underwent complete surgical bile diversion as described in materials and methods. Thereafter, mice received a [3H]-cholesterol labeled macrophage (Mϕ) dose via an externalized peritoneal port in order to ensure no dose was accidentally injected into the intestine. After 8 hours, the small intestinal (SI) lumenal contents and wall were collected, and extracted to separate [3H]-cholesterol and [3H]-bile acids.
(B) [3H]-cholesterol recovered in the SI lumenal contents.
(C) [3H]-bile acids recovered in the SI lumenal contents.
(D) [3H]-cholesterol recovered in the SI wall. Data represent the means ± SEM from 5–9 mice per group, and means not sharing a common superscript differ significantly, P < 0.05.
Discussion
Although it is generally accepted that macrophage RCT depends entirely on the ability of the liver to secrete HDL-delivered cholesterol into bile (Lewis et al. 2005), results from this study suggests that the current conceptual framework of macrophage RCT requires significant modification. The major findings of the current study are: 1) Mice genetically lacking the ability to adequately secrete cholesterol into bile (NPC1L1-LiverTg mice) have normal LXR-inducible macrophage RCT, and 2) Mice surgically lacking biliary contributions to the intestine have normal LXR-inducible macrophage RCT. Importantly, the genetic and surgical models defined in this work should continue to serve as useful tools to further interrogate the molecular mechanisms mediating non-biliary fecal sterol loss and macrophage RCT.
The relative contribution of biliary vs. non-biliary pathways to fecal sterol loss has not been fully defined. Using an intestinal perfusion system, it was estimated that ~44% of total fecal sterol output originated from non-biliary sources in humans (Simmonds et al., 1967). In normal chow-fed C57BL/6J mice, the non-biliary route accounts for 33% (76 µmol/kg/day) of total fecal neutral sterol loss (van der Veen et al., 2009), and roughly 20% of total fecal sterol loss in FVB mice (Kruit et al., 2005). However, it is important to point out that non-biliary fecal sterol loss is quite sensitive to pharmacological manipulation. To this end, LXR activation can dramatically augment non-biliary macrophage RCT (Figure 2 and Figure 3), and increase mass fecal neutral sterol loss (Kruit et al., 2005; van der Veen et al., 2009). In fact, LXR activation in C57BL/6J increases the contribution of non-biliary fecal sterol loss from ~33% of the total (76 µmol/kg/day) in vehicle treated mice to ~63% of the total (442 µmol/kg/day) in T0901317-treated mice (van der Veen et al., 2009). Furthermore, activation of the peroxisome proliferator activated receptor δ (PPAR-δ) promotes non-biliary fecal sterol loss in mice (Vrins et al. 2009). Importantly, now in three independent genetically modified mouse models (ABCG5/G8−/−, Mdr2−/−, and NPC1L1-LiverTg) that have severely diminished biliary cholesterol secretion, fecal sterol loss is only modestly decreased (Yu et al., 2002a) or not altered at all (Kruit et al., 2005; Figure 1). This clearly indicates that the non-biliary pathway must be able to adequately compensate for biliary insufficiency to maintain normal fecal sterol loss in rodents. Collectively, these data support the idea that fecal sterol loss is a mixture of dietary, biliary, and intestinally-derived sterols, and the origins of the latter source likely originates from the plasma compartment (Brown et al., 2008a, Kruit et al., 2005; van der Veen et al., 2009). Given the plasma source of intestinally derived fecal sterols, and the central role of the liver in lipoprotein metabolism, one must consider the liver as a potential site of organization for non-biliary fecal sterol loss.
The classic view of RCT involves the delivery of peripheral cholesterol via HDL to the liver for secretion into bile (Lewis et al. 2005; deGoma et al., 2008). In parallel, we believe that the liver also plays a gatekeeper role for non-biliary fecal sterol loss by re-packaging peripheral cholesterol into nascent plasma lipoproteins that are destined for subsequent intestinal delivery (Brown et al., 2008a). Probably not coincidentally, all of the mouse models described where non-biliary fecal sterol loss is apparent (Figure 1; Brown et al., 2008a; Kruit et al., 2005; Temel et al., 2007) represent conditions where free cholesterol could potentially accumulate in the liver due to defects in normal elimination pathways. It remains possible that under conditions where hepatic free cholesterol burden becomes too excessive for disposal through esterification or biliary secretion [i.e. ACAT2 ASO treatment (Brown et al., 2008a), NPC1L1-Liver-Tg mice (Figure 1; Temel et al., 2007), or Mdr2−/− mice (Kruit et al., 2005),], an alternative plasma-based route for direct intestinal secretion and fecal disposal is utilized. In previous work, we were able to show that the liver can secrete lipoprotein particles that preferentially deliver cholesterol to the proximal small intestine for fecal excretion (Brown et al., 2008a). However, whether these liver-derived lipoproteins represent nascent VLDL particles, nascent HDL particles, or some novel lipoprotein remains to be addressed. Interestingly, unlike the genetic models of biliary cholesterol insufficiency (ABCG5/G8−/−, Mdr2−/−, and NPC1L1-LiverTg), acute surgical diversion of bile is a situation where normal elimination pathways such as esterification or biliary secretion are not obviously impaired, yet de novo cholesterol synthesis is augmented (Figure S1; Dietschy et al., 1968; Deckelbaum et al., 1977; Persemlidis et al., 1973; Bandsma et al., 1998). In this case it has been assumed that the origin of fecal sterols in bile diverted animals comes from the enhanced de novo synthesis (Dietschy et al., 1968; Deckelbaum et al., 1977; Persemlidis et al., 1973; Bandsma et al., 1998). However, our data suggests that a small portion of the fecal sterols present in bile-diverted mice is derived from direct intestinal secretion of macrophage-derived cholesterol. This is supported by the fact that we have added an intact [3H]-cholesterol molecule delivered via J774 macrophages, excluding any contribution from endogenous synthesis.
Although macrophage RCT is agreed to be key to the regression of atherosclerosis, it is important to discuss the quantitative importance of macrophages in RCT and centripetal flux of cholesterol into the feces. To put macrophage RCT in perspective to the total process of centripetal cholesterol flux, it has been estimated that there are only ~ 1 × 108 macrophages in the adult mouse (Lee et al., 1985). Hence, the number of macrophages in the whole body represents only a small fraction of the other cell types present, making the relative contribution of macrophages to mass fecal neutral sterol loss extremely small. Even though macrophages are a minor contributor to mass fecal neutral sterol loss, macrophage-specific RCT is likely highly relevant to atherosclerosis regression in the artery wall (Lewis et al., 2005). Therefore it is also important to discuss whether the macrophage RCT assay used in this work truly recapitulates the process thought to occur in the artery wall. Since the original description (Zhang et al., 2003) of the assay used in our studies, there have been over thirty independent studies described in the literature utilizing this method. Collectively, these studies indicate that once the [3H]-cholesterol labeled J774 macrophages are injected, these cells remain primarily within the peritoneal cavity, and the appearance of [3H]-cholesterol in the plasma and tissues is not likely due to the direct transport of the tracer by intact J774 cells (Zhang et al., 2003; Naik et al., 2006; Wang et al., 2007b; Rader et al., 2009). Instead, the bulk of evidence suggests that a small fraction of [3H]-cholesterol tracer is removed from the J774 macrophages by traditional efflux mechanisms, and once effluxed this tracer is metabolized in a manner comparable to endogenous cholesterol mass (Zhang et al., 2003; Naik et al., 2006; Wang et al., 2007b; Rader et al., 2009). It is important to note that the seemingly modest fecal recovery (1–4%) of injected [3H]-cholesterol (Figure 2H) may actually be substantial, given that only 1–5% was found in the plasma over the 48 period (Figure 2C). Whether this assay truly recapitulates the process of macrophage RCT from the artery wall is still a matter of debate, but there is good evidence that determining the rate of macrophage RCT using this assay accurately predicts atherosclerosis burden in mice (Rader et al. 2009). However, given the assay limitations, and the relatively small contributions of macrophages to fecal sterol loss, it remains critically important for investigators to quantify mass fecal sterol loss to more accurately determine whole body centripetal cholesterol flux in future studies.
It has been over eighty years since Warren Sperry speculated that a non-biliary route for fecal neutral sterol loss must exist in dogs (Sperry, 1927). Unfortunately, this alternative pathway has been largely ignored, and very little progress has been made to identify the molecular mechanisms that define it. There is now strong evidence in mice (Figures 1–3; Kruit et al., 2005; van der Velde et al., 2007; van der Velde et al., 2008; van der Veen et al., 2009; Vrins et al., 2009; Brown et al., 2008a; Plosch et al., 2002), and mounting evidence in man (Simmonds et al., 1967; Cheng et al., 1959; Deckelbaum et al., 1977), that a non-biliary pathway for fecal sterol loss indeed exists. In order to more fully understand this pathway, the following challenges will need to be addressed: 1) Establishing quantitative methods to measure non-biliary fecal sterol loss in primates and man, 2) Identifying the intestinal transport proteins/receptors involved, 3) Characterizing the lipoprotein metabolism that is requisite for non-biliary fecal sterol loss, and 4) Identifying bona fide drug targets to specifically modulate the intestinal component of RCT. Advancement in these areas have the potential to open new therapeutic opportunities targeting the intestine as an inducible sterol excretory organ.
Experimental procedures
In Vivo Macrophage RCT Studies in NPC1L1-LiverTg Mice
In vivo measurement of macrophage RCT was conducted essentially as described by Rader and colleagues (Zhang et al., 2003; Naik et al. 2006), with minor modifications. Extensive descriptions of the mouse models used, cell culture protocols, and RCT method are included in the online supplement.
In Vivo Macrophage RCT Studies With Acute Surgical Biliary Diversion
C57BL/6N mice maintained on standard rodent chow were gavaged with either vehicle or 25 mg/kg T0901317 daily for seven consecutive days prior to surgical biliary diversion. The surgical procedure used to acutely divert biliary emptying into the intestine and macrophage RCT protocol is included in the online supplement.
Plasma Lipoprotein Analyses and Apolipoprotein Distribution
Detailed description of plasma lipid analyses is included in the online supplement.
Quantitative Real-Time PCR (qPCR)
RNA extraction and qPCR was conducted as previously described (Brown et al., 2008a) using the ΔΔ-CT method. Primers used for qPCR are available upon request.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). All data were analyzed using two-way analysis of variance (ANOVA) followed by Student’s t tests for post hoc analysis. Differences were considered significant at p <0.05. All analyses were performed using JMP version 5.0.12 (SAS Institute; Cary, NC) software.
Highlights
Mice lacking biliary sterols have normal reverse cholesterol transport (RCT).
Mice surgically lacking biliary contributions to the intestine have normal RCT.
The conceptual framework of macrophage RCT requires significant modification.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Paul Dawson (Wake Forest University) for critical reading of this manuscript and providing meaningful input into these studies. We also thank George Rothblat (The Children’s Hospital of Philadelphia) for providing J774 macrophages. This work was supported by the National Heart, Lung, and Blood Institute through a pathway to independence grant (1K99-HL096166) to J.M.B. and a program project grant (5P01HL049373) to L.L.R. L.Y. is supported by a Scientist Development Grant (#0635261N) from the American Heart Association. L.L.R. is a member of the Merck speaker’s bureau.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors report that they have no conflicts of interest.
REFERENCES
- Bandsma RHJ, Stellaard F, Vonk RJ, Nagel GT, Neese RA, Hellerstein MK, Kuipers F. Contribution of newly synthesized cholesterol to rat plasma and bile determined by mass isotopomer distribution analysis: bile-salt flux promotes secretion of newly synthesized cholesterol into bile. Biochem. J. 1998;329:699–703. doi: 10.1042/bj3290699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brown JM, Bell TA, III, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 2008a;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, Shah R, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008b;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Stanley MM. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc. Soc. Exp. Biol. Med. 1959;101:223–225. doi: 10.3181/00379727-101-24890. [DOI] [PubMed] [Google Scholar]
- Cooper AD. Metabolic basis of cholesterol gallstone disease. Gastroenterol. Clin. North Am. 1991;20:21–46. [PubMed] [Google Scholar]
- Deckelbaum RJ, Lee RS, Small DM, Hedberg SE, Grundy SM. Failure of complete bile diversion and oral bile acid therapy in the treatment of homozygous familial hypercholesterolemia. N. Engl. J. Med. 1977;296:465–470. doi: 10.1056/NEJM197703032960901. [DOI] [PubMed] [Google Scholar]
- Dietschy JM. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J. Clin. Invest. 1968;47:286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset JA. The plasma lecithin:cholesterol acyltransferase reaction. J. Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- Groen AK, Bloks VW, Bandsma RH, Ottenhoff R, Chimini G, Kuipers F. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 2001;108:843–850. doi: 10.1172/JCI12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley CD, Woollett LA, Turley SD, Dietschy JM. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J. Lipid Res. 1998;39:2143–2149. [PubMed] [Google Scholar]
- Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunohistochemical studies with monoclonal antibody F4/80. J. Exp. Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sukuma N, Pegora R, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- Pertsemlidis D, Kirchman EH, Ahrens EH., Jr Regulation of cholesterol metabolism in the dog I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J. Clin. Invest. 1973;52:2353–2357. doi: 10.1172/JCI107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F. Increased heptobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009;50 Suppl.:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Simmonds WJ, Hofmann AF, Theodor E. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. J. Clin. Invest. 1967;46:874–890. doi: 10.1172/JCI105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry WM. Lipid Excretion IV. A study of the relationship of the bile to the fecal lipids with special reference to certain problems of sterol metabolism. J. Biol. Chem. 1927;71:351–378. [Google Scholar]
- Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentrations and is a target of ezetimibe. J. Clin. Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen JN, Van Dijk TH, Vrins CL, Van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 2009;284:19211–19299. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde AE, Vrins CL, Van den Oever K, Seemann I, Oude Elferink RP, Van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Vrins CL, Van der Velde AE, Van den Oever K, Levels JH, Huet S, Oude Elferink RP, Kuipers F, Groen AK. PPARd activation leads to increased trans intestinal cholesterol efflux. J. Lipid Res. 2009 doi: 10.1194/jlr.M800579-JLR200. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 2007a;22:368–352. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007b;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Turley SD, Dietschy JM. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J. Lipid Res. 2009;50:1316–1329. doi: 10.1194/jlr.M900024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 2002a;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 2002b;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, York J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.